Abstract
The organic material present at the same depth as the oil in the reservoirs has the potential for conversion, as indicated by analyses conducted before and after heavy oil combustion. Therefore, in this study, we examined the oxidation and pyrolysis reaction pathways of hydrocarbons, specifically benzaldehyde (C7H6O) and naphthalene (C10H8), before and after combustion using molecular dynamics simulations. The results showed that the primary products formed under various temperature conditions included H2O, HO2, CO, and CO2. We determined the number of molecules, such as HO and H, as well as their temperature variations, and found that the activating group functions as an electron donor, while the inactivating group serves as an electron acceptor. The oxidation and pyrolysis reactions of naphthalene and the synthesis pathway of benzaldehyde were also explored. C-C dissociation in the early stages of combustion and the process of C-C bond synthesis in the later stages of the reactions were investigated through dynamic simulations at different temperatures, 3000 K, 3500 K, and 4000 K, with a particular focus on the reaction network at 4000 K. The application of the molecular reaction dynamics method to heavy oil combustion research was the primary objective of this work. This study aims to provide a novel approach to investigating hydrocarbon conversion at high temperatures and offer recommendations for enhanced oil recovery.
1. Introduction
Karamay heavy oil is not only abundant but also renowned for its unique rheological characteristics. In addition to its typical features such as a high density, viscosity, and low freezing point—common in heavy oils from various oil fields—Karamay heavy oil is distinguished by its exceptionally low levels of asphaltene and sulfur. This specific type of heavy oil is highly sought after as a feedstock for producing premium lubricant base oils, resulting in a relatively higher market price compared to other crude oils. Karamay heavy oil reservoirs are typically located at depths of less than 1000 m and at lower temperatures, which contribute to their high viscosity under reservoir conditions. Consequently, thermal techniques such as cyclic steam stimulation, steam drive, and in situ combustion are employed to extract this heavy oil. Notably, the viscosity of Karamay heavy oil exhibits less variation when the temperature is at elevated levels, setting it apart from heavy oils found in other oil fields. While the distinct rheological properties of Karamay heavy oil are believed to be influenced by its chemical composition, further research is necessary to fully understand this phenomenon.
Olalekan and colleagues [1] researched new technologies for heavy oil extraction. The commercial production of heavy oil has heavily relied upon the steam injection method due to its effectiveness at reducing viscosity and improving mobility. However, due to the uncertainty of oil prices, high energy costs, water requirements, heat loss, and environmental issues, attention is being given to new thermal EOR technologies such as in situ steam generation using thermochemical fluid (TCF) injection. This technology essentially involves the downhole generation of heat and pressure from exothermic chemical reactions. In comparison, from the injection of steam generated at 250 °C, the pressure at the inlet of the core was 212 psi, and the recovery factor was 71% OOIP. These results therefore confirm the increase in interest in the application of TCF injection technology in heavy oil production. In order to enhance oil and gas recovery, significant advancements have been made in the use of plugging agents and drilling tool nozzles for the extraction of carbonate rock [2,3].
According to the studies by Zhao [4] and other researchers, as the heating rate increases, the pyrolysis of oil shale occurs at a higher temperature, with this trend being particularly pronounced with higher oil content. The stability of oil shale during pyrolysis is influenced by both oil content and the pyrolysis atmosphere. Specifically, higher oil content correlates with greater stability in the pyrolysis process. In a nitrogen atmosphere, the pyrolysis interval of oil shale is more concentrated, whereas exposure to air extends the pyrolysis interval and reduces the pyrolysis stability index. Furthermore, an increase in the heating rate enhances the release characteristics of the products, which remain largely unaffected by the oil content.
These ferroan carbonates are preferentially concentrated at distances ranging from 0.5 to 1.0 m from sandstone–mudstone contacts. Kinetic modeling effectively reproduces the evolutionary pathways of various diagenetic minerals in the Es4s sandstones, which constitute the upper part of the fourth member of the Eocene Shahejie Formation. More importantly, the modeling results indicate that the dissolution of non-ferroan calcite cement does not improve overall reservoir quality, as it results in the precipitation of more stable ferroan calcite and ankerite cement [5].
Zhang and colleagues [6] revealed that increased pressure enhances the generation of light components and accelerates the conversion of alkenes to alkanes and aromatics. The release temperature of gases increases under pressure, which promotes the production of alkane gas while simultaneously reducing the hydrogen yield. As the pyrolysis temperature rises, the combustion performance of semi-coke deteriorates under low pressures; however, this trend is reversed under high pressures. Finally, a pyrolysis mechanism for the coupling of temperature and pressure in oil shale has been proposed.
High temperatures induced various chemical and physical changes that led to the formation of the coking zone. Additionally, the small-aperture channels within the coking zone became concentrated with residual oil as a result of this formation process, which decreased fluidity and made production more challenging [7].
Lab-scale experiments and the analysis of core samples are the primary methods used to investigate compositional changes associated with in situ combustion [8,9,10,11,12,13].
Furthermore, as shown in Figure 1, benzaldehyde (C7H6O) was detected in the vicinity of the combustion interface (547.8 m), and naphthalene (C10H8) was identified in samples collected from the unswept area (<547.8 m). Zhao and colleagues [14] employed gas chromatography-mass spectrometry (GC-MS) analysis to confirm the presence of naphthalene in the unswept layers and benzaldehyde at the combustion interface. This finding supports the hypothesis that the pyrolysis and oxidation reactions involve C-O and O-H bonds. Collectively, these results demonstrate the potential for the conversion of benzaldehyde and naphthalene. However, there are limited studies in the existing literature that explore the reaction pathways and networks governing the molecular transition between these two compounds (benzaldehyde and naphthalene).

Figure 1.
The morphology of Well J2107a core samples shows that they are oriented up-dip in the Karamay Oil Field. The figure is taken from [14] and used with permission.
Enhancing oil recovery can be accomplished by gaining a deeper understanding of the mechanisms involved in the transformation of chemical molecules during the primary reactions associated with heavy oil thermal recovery. Research plays a crucial role in this process. Analyzing complex chemical networks in polymerization, combustion, and chemical engineering has always been essential for elucidating these mechanisms.
The combustion reaction mechanisms of heavy oil predominantly function on a large scale and are primarily characterized by macroscopic phenomena. The conversion processes among component molecules are largely determined by the number of carbon atoms present; nevertheless, the specific reaction pathways and networks involving individual molecules remain inadequately explored. By utilizing molecular dynamics methodologies to investigate the reaction processes associated with heavy oil combustion, we can enhance our understanding of the various reactions that transpire during combustion at the microscopic molecular level.
Molecular dynamics techniques are capable of simulating extensive networks that encompass thousands of reactions and hundreds of species. However, achieving accurate modeling presents considerable experimental and computational challenges. Reactive molecular dynamics (MD) has emerged as a crucial instrument for elucidating these intricate networks. Here, we present a methodology for deriving both a network of reactions and its kinetic parameters from reactive MD simulations.
ReaxFF-MD, a method capable of addressing reactivity in computational calculations, has been proposed as a result of ongoing research developments [15,16]. In carbon/hydrogen/oxygen (C/H/O) fuel systems, such as polystyrene [17], cellulosic materials [18], styrene-butadiene [19], JP-10 [20], polycyclic aromatic hydrocarbons (PAHs) [21,22], hydrogen [23,24], and various other systems [25,26,27,28,29,30,31], ReaxFF-MD is recognized as a significant supplementary approach to experimental methods.
2. Methodology
Reactive Force Field Molecular Dynamics (ReaxFF MD) and Density Functional Theory (DFT) are both methods employed to study reaction mechanisms. ReaxFF MD is particularly effective for investigating intermolecular interactions and chemical reaction processes, while DFT is better suited for calculating the electronic structure and properties of molecules. ReaxFF MD offers a relatively low computational cost, making it ideal for large-scale simulations. In contrast, DFT, despite its higher computational expense, can provide more accurate electronic structure information.
ReaxFF MD is an empirical force field method used to describe the interactions and chemical reactions among atoms. It is particularly well-suited for investigating large-scale, long-term dynamic processes, such as the complex reaction systems involved in pyrolysis and oxidation. ReaxFF MD can effectively simulate the formation and breaking of chemical bonds, making it ideal for studying intermolecular interactions and chemical reaction mechanisms. Additionally, other methods can be employed to analyze reaction networks.
For the purposes of this research, both methods can be combined or used independently [32]. This paper focuses on investigating the pyrolysis and oxidation reaction network pathways between naphthalene and benzaldehyde in the context of heavy oil thermal recovery. The research objectives will be accomplished by integrating ReaxFF molecular dynamics with other methodologies.
2.1. ReaxFF MD Reactive Force Field
ReaxFF-MD simulates the formation and breaking of chemical bonds by incorporating the concept of bond order, distinguishing it from non-reactive force fields. Each term, including bond order, is dependent on atomic connectivity. Non-bonded interactions, such as van der Waals and Coulomb forces, are considered between every pair of atoms due to their independent connections. Equation (1) [29] outlines the simulated system utilized by ReaxFF-MD to assess atomic charges through the Electronegativity Equalization Method [30].
Esystem = Ebond + Eover + Eangle + Etors + EvdWaals + Ecoulomb
In this case, the symbol Ebond denotes the bond energy; this part of energy is related to bond order. Eover denotes over-coordination energy penalty. Eangle denotes valence angle energy, where the energy contribution from valence angle terms goes to zero as the bond orders in the valence angle goes to zero. Etors denotes torsion angle energy, EvdWaals denotes non-bonded van der Waals interactions, to account for the van der Waals interactions using a distance-corrected Morse-potential. ECoulomb denotes Coulomb interactions, which are considered between all atom pairs. Further evaluation of the force field details is possible [33,34,35].
2.2. Simulation Details
We utilized Material Studio 8.0 to populate a cubic box with 20 naphthalene (C10H8) molecules and 240 oxygen (O2) molecules to investigate the naphthalene oxidation process. The dimensions of the simulation boxes were measured in angstroms (Å). Based on the initial molecular count and the target density of 1.00 g/cm3, we calculated the box volume, whose geometry was preoptimized, which was placed in a 25.00 Å × 25.00 Å × 25.00 Å cubic periodic unit cell. The dimensions of the simulation boxes were measured in angstroms (Å). In the subsequent step, we assigned atomic velocities that conformed to a Gaussian distribution at the target temperature. The NVT ensemble, utilizing the Berendsen thermostat, was employed to maintain the system at the desired temperatures for one second. For molecular recognition, we established a C-C bond order cutoff of 0.55 Å, a C-O bond cutoff of 0.65 Å, and a time step of 0.1 fs. To ensure adequate collisions, the simulation temperatures were artificially elevated to 3000 K, 3500 K, and 4000 K. For each of these 10 configurations, another 100 ps simulation was continued at 4000 K to obtain the detailed reaction mechanism.
The high temperatures facilitated sufficient collisions between molecules and intermediates, thereby enhancing the simulation process. Within the temperature range of 3000 K to 4000 K, we conducted ten annealing simulations.
Compared to naphthalene, atomic charge analysis indicates that the activating group functions as an electron donor, while the inactivating group acts as an electron acceptor. The effects of substituents on naphthalene are primarily assessed through geometric and vibrational analyses [35]. A higher stability energy correlates with increased reactivity of the compound and a smaller band gap [36].
The species was analyzed, and the mechanisms and pathways of the oxidation process were investigated using the LAMMPS package (Large-scale Atomic/Molecular Massively Parallel Simulator) [37,38,39]. By assessing the number of reaction events that modify the chemical composition and current concentration of the respective reactants, these simulations can be utilized to compute rate constants with software such as the Chemical Trajectory Analyzer 2.0 (CTY 2.0), which was employed in this study to analyze changes in chemical composition within a MD trajectory [40,41].
3. Results and Discussion
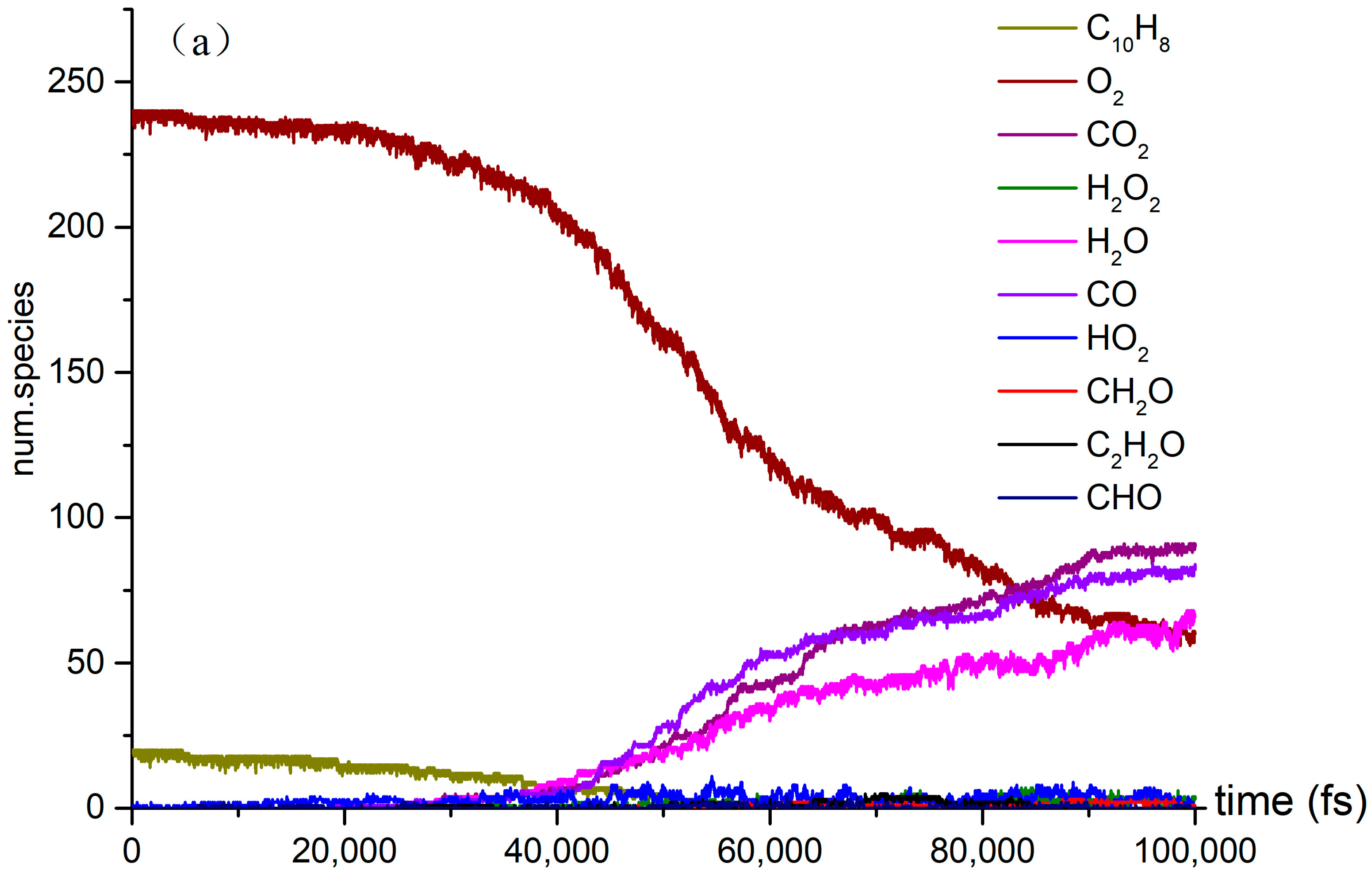
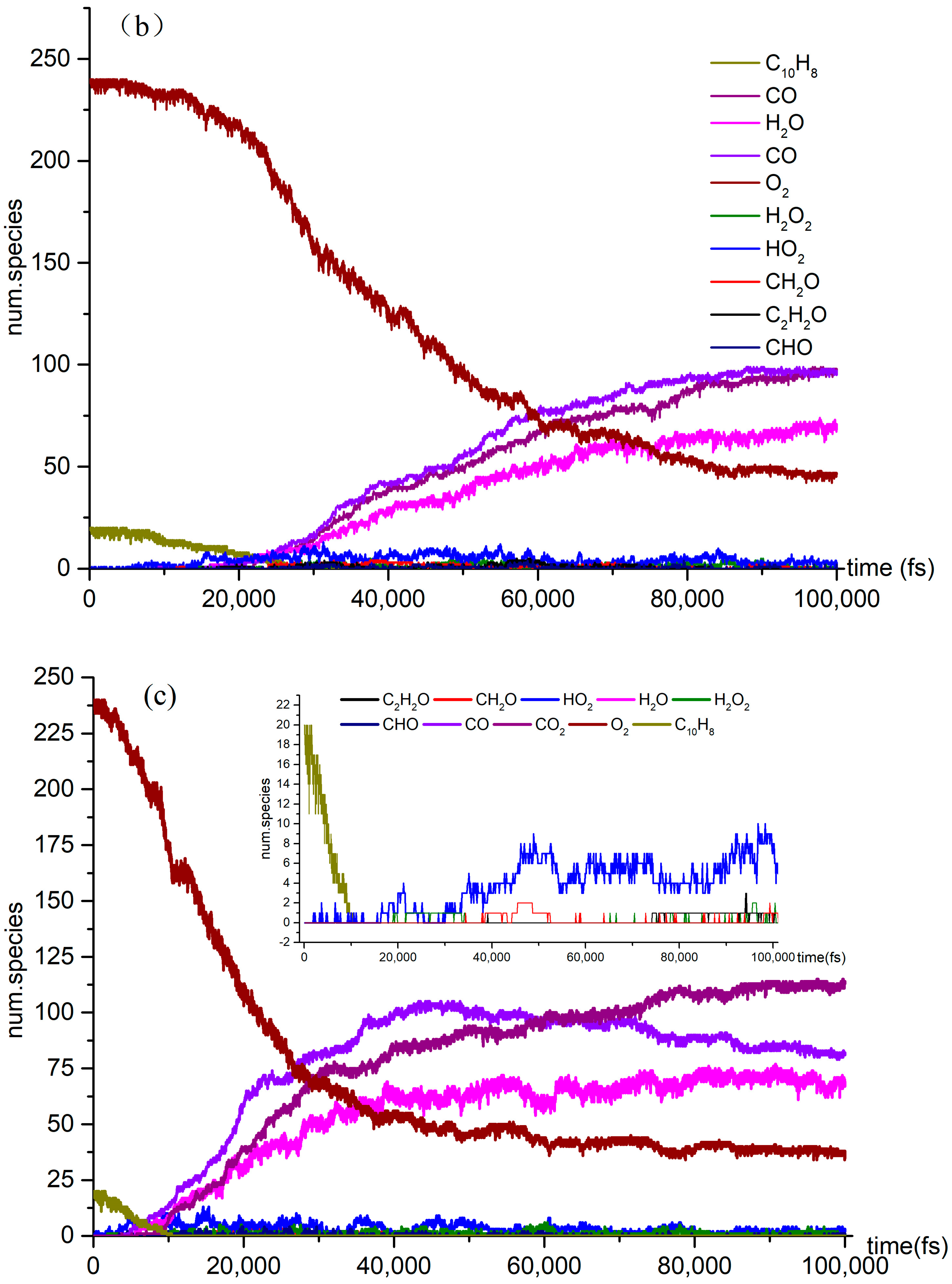
A series of NVE-MD simulations of naphthalene oxidation at elevated temperatures (3000 K, 3500 K, and 4000 K) were conducted as described in Section 2. Additionally, we employed the CHO-2008 force field [22] to simulate the same system in order to investigate the initiation mechanism and combustion dynamics. Figure 2 illustrates the distribution of reactants, products, and intermediate species during the high temperature simulations.
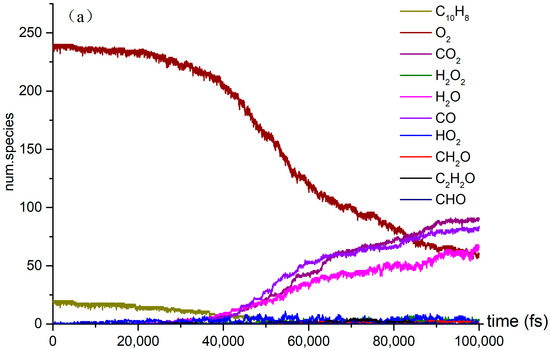
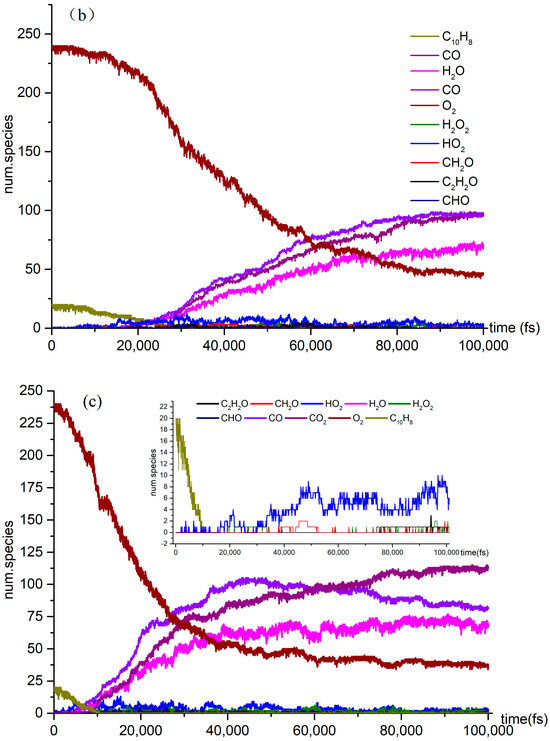
Figure 2.
The time evolution of O2 and C10H8 molecules during the NVT-MD simulations of the oxidation process at the temperatures of (a) 3000 K, (b) 3500 K, and (c) 4000 K.
Several reactions, including pyrolysis, occur concurrently with the naphthalene oxidation reaction. This investigation examined both pyrolysis and oxidation reactions. It focused on the effect of temperature on the variation in the quantities of CO2, CO, H2O, and other products over time. As the temperature rises to 4000 K, the reaction rate of C10H8 in O2 oxidation reaches its peak, and at 60,000 fs, the concentration of CO in the reaction exceeds that of CO2. Additionally, the number of small molecules and the diversity of free radical colors in the oxidation reaction significantly increase at 4000 K.
The three stages of the oxidation process can be identified based on the reaction rate of naphthalene oxidation. In the first stage, from 10,000 fs to 40,000 fs, naphthalene reacted at a relatively slow rate, at temperatures of 3000 K, 3500 K, and 4000 K. Although the production of products is limited at this point, the temperature still influences the slope of the reaction curve. In the second stage, the number of products increases significantly and the reaction rates of the reactants accelerate. Our simulations revealed the presence of various species, including highly unsaturated compounds, unstable radicals, alkanes, and alkenes. While the type of products remains unchanged in the third stage, the reaction rate does vary. The examination of Figure 2 highlights intriguing topics for further research, such as naphthalene’s post-oxidation products and intermediates, their effects on the oxidation reaction, and the transformation of naphthalene into benzaldehyde. At 4000 K, the curve is notably steep due to the abundance of products. We intend to explore the kinetic details of naphthalene’s oxidation reaction, particularly at 4000 K (Figure 2c), given the active products and reactions involved.
3.1. Detailed Analysis of the Kinetic Mechanisms of the Oxidation Reaction of Naphthalene
The understanding of the complex pyrolysis process relies on the initiation of the MCH oxidation reaction, which determines the subsequent distributions of intermediates and products. Therefore, a comprehensive description of the initial reaction pathway is essential for elucidating the mechanisms of the MCH oxidation reaction [42].
Figure 2 illustrates the naphthalene oxidation process, which consists of three distinct stages. As previously mentioned, the time-axis divisions for these stages remain consistent across various reaction temperature conditions. The rapid oxidation observed in Stage 1 can be attributed to the presence of highly reactive free radicals generated during the reactions. In this study, we identified several active free radicals, including H2O2, HO2, and CH2O. However, we were unable to identify the isomers present in the reaction products, which did not impact the overall objectives of the investigation. By analyzing the data collected at each stage, we were able to determine the reaction rates for the different phases of the process.
We analyzed the reaction types and rates of the four principal products obtained from naphthalene oxidation reaction, i.e., carbon dioxide, water, carbon monoxide, and hydrogen peroxide. The highest reaction rate, along with a comparatively high number of reactions, is shown in the reactions listed below.
Naphthalene primarily dissociates through two types of reactions, unimolecular decomposition and hydrogen abstraction reactions, both of which involve the dissociation of C-H and C-C bonds. These reactions are illustrated in Table 1. The reactions listed in the table represent those with the highest frequency that were observed during the simulation process, and they predominantly contribute to the generation of the corresponding products. Naphthalene undergoes an oxidation reaction in which H-O and C-O bonds are formed. During the dehydrogen reaction, the formation of C-O and O-H bonds occurs as part of the main product formation process.

Table 1.
The main reactions in the reaction of naphthalene.
3.2. Intermediate Reactions
Although naphthalene oxidation has been the subject of extensive research, a clear understanding of the intermediate processes has yet to emerge. Comprehending the intermediate mechanisms necessitates a thorough examination of the reactions related to the primary products. The reaction pathways for the main products were studied, and the proportions of reaction times for these products were calculated and documented. The primary forward and reverse reactions in each pathway were discussed, and the key reactions within the overall process were identified.
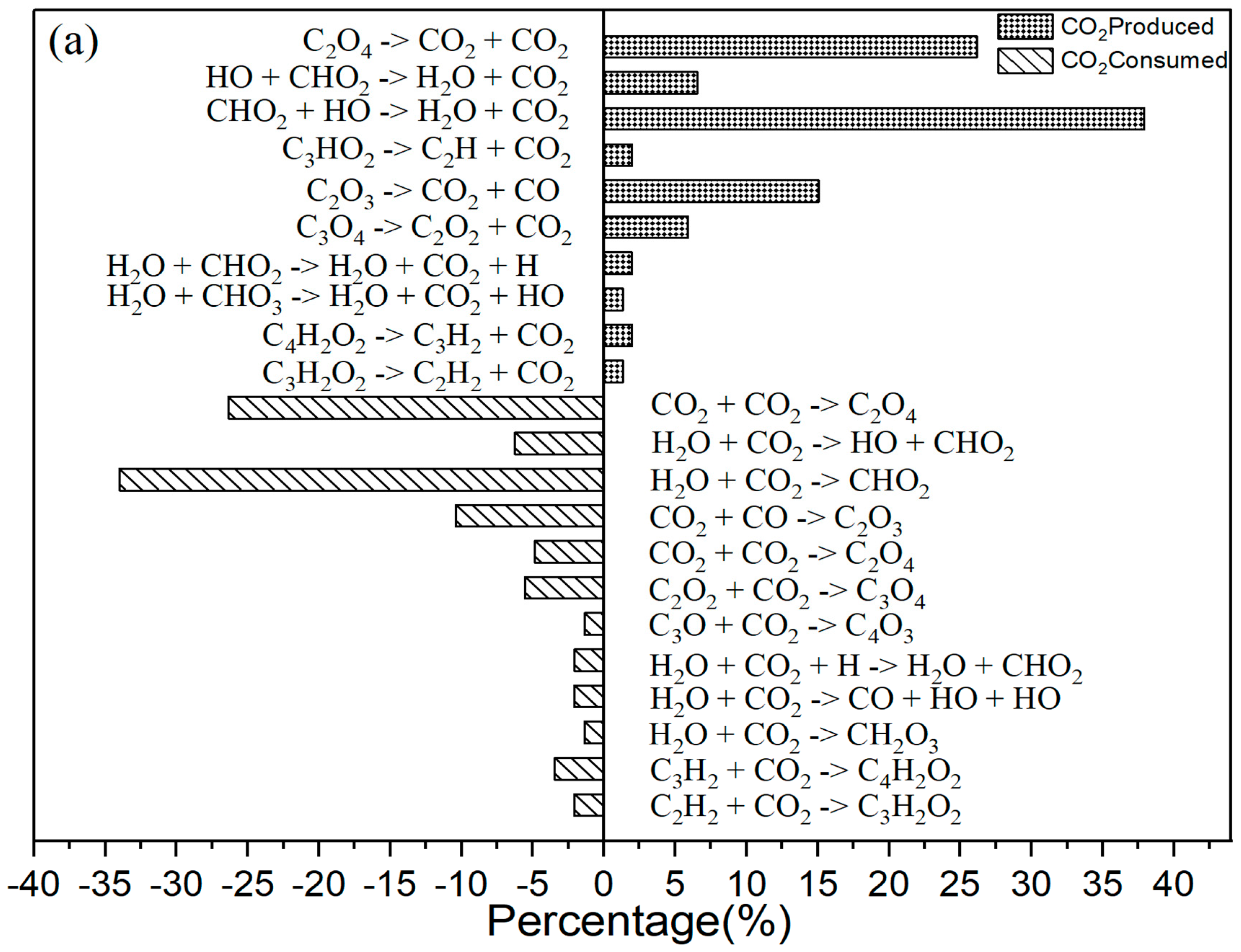
It is explained how free radicals influence the rate of reaction and synthesis activity. In Figure 2c, carbon dioxide is identified as the most prevalent product resulting from oxidation. The changes in carbon dioxide concentrations throughout the reactions are highlighted. The time-dependent distributions of carbon dioxide are illustrated in Figure 2c, showing a dramatic increase in production between 0 and 40,000 femtoseconds (fs) during the reaction process. During this period, the production of carbon dioxide significantly exceeds its consumption. Figure 3a indicates that reversible reactions are present. Among the various positive reactions, the most frequent is the oxidation reaction of free radicals (CHO2 + HO → H2O + CO2), along with several pyrolysis reactions (C2O4 → CO2 + CO2). These reactions involve the dissociation of carbon–carbon (C-C) bonds and hydrogen abstraction. After 80,000 fs, the reaction rate of CO2 approaches equilibrium, at which point the consumption of carbon dioxide surpasses its production. Reversible reactions serve as the primary pathways for the consumption of CO2.
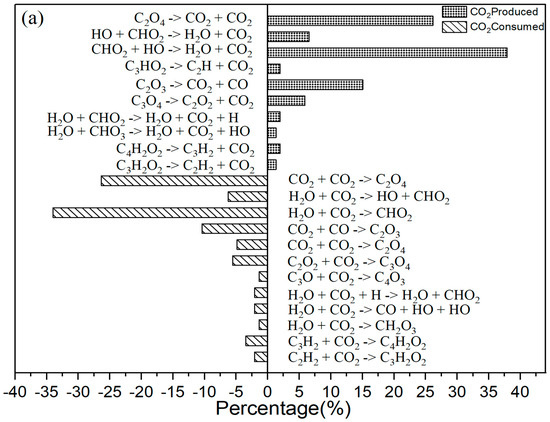
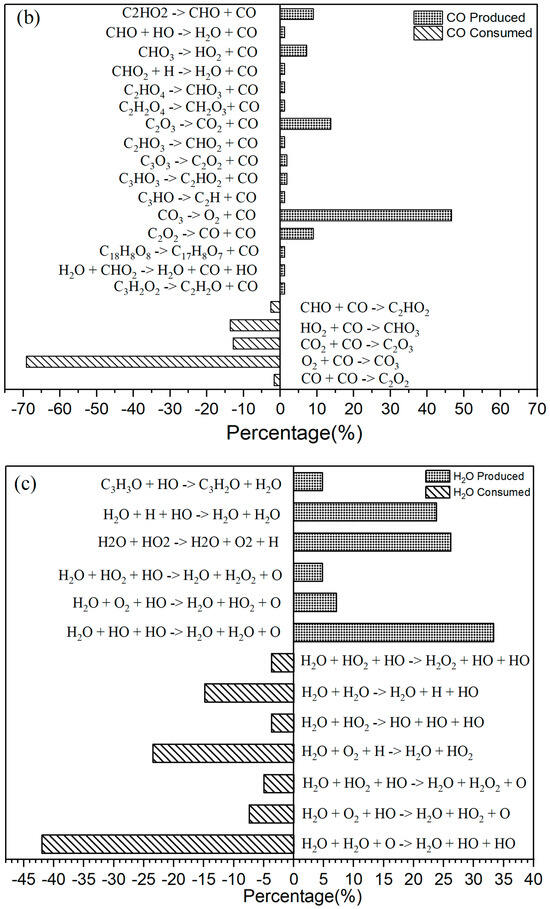
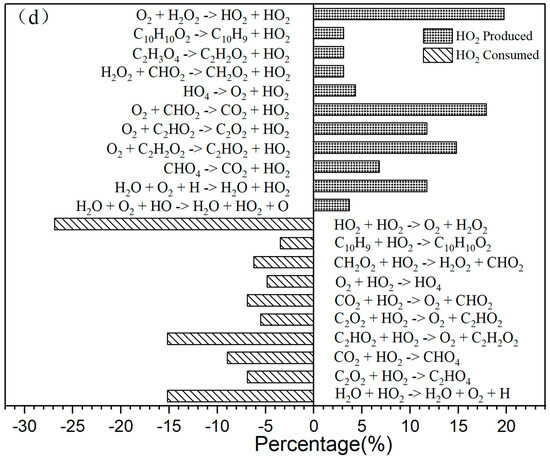
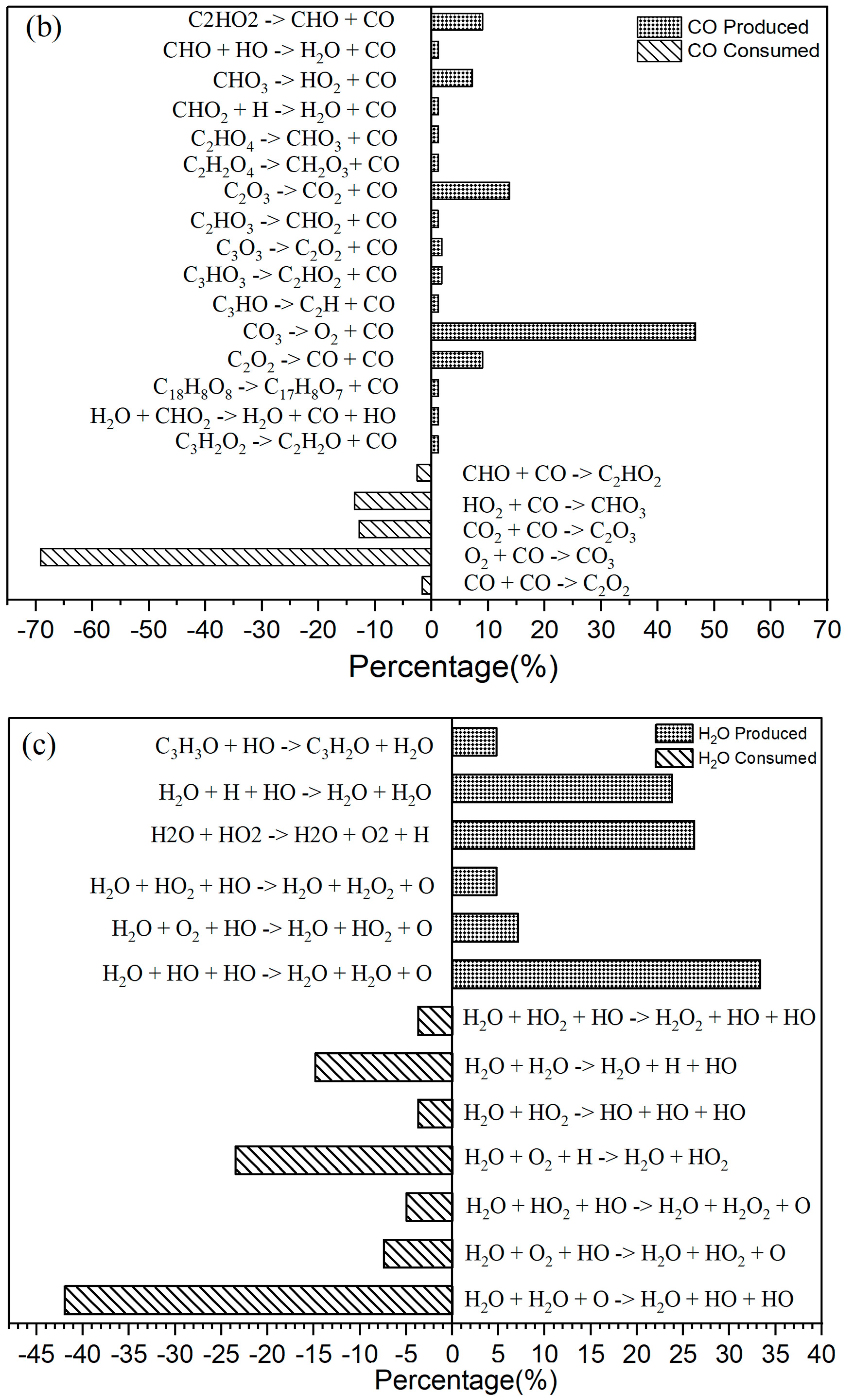
Figure 3.
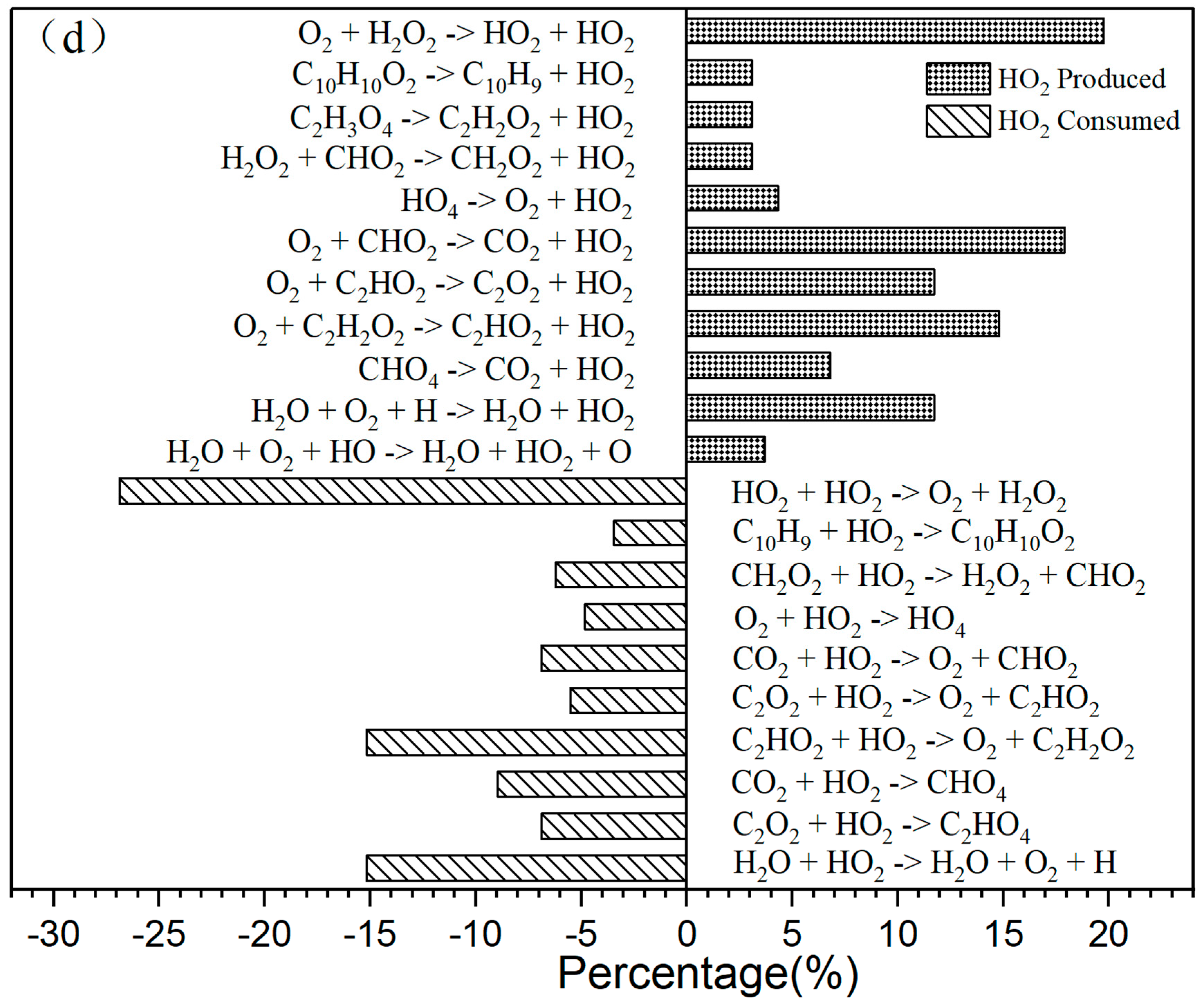
(a) The primary CO2 reaction pathways. The production and consumption channels are represented by solid and medium-sized diamonds, respectively. (b) The primary CO reaction pathways. The production and consumption channels are represented by solid and medium-sized diamonds, respectively. (c) The primary H2O reaction pathways. The production and consumption channels are represented by solid and medium-sized diamonds, respectively. (d) The primary HO2 reaction pathways. The production and consumption channels are represented by solid and medium-sized diamonds, respectively.
As Figure 3a shows, for CO2 there were 10 positive reactions and 12 negative reactions. Among the positive reactions, the reaction with the highest frequency accounts for 38% (CHO2 + HO → H2O + CO2). In the negative reactions, the reaction with the highest frequency accounts for 34% (H2O + CO2 → CHO2).
In the oxidation reaction of naphthalene, the behavior of carbon monoxide (CO) undergoes significant changes. The production of CO has been continuously increasing during the time interval of 0 to 42,000 fs, as illustrated in Figure 2c. The reaction with the highest frequency during the CO generation process is the pyrolysis reaction (CO3 → O2 + CO), as depicted in Figure 3b. Conversely, the production of CO decreases during the period from 42,000 to 89,000 fs, indicating an increase in CO consumption during this stage. The reactions occurring during this interval are predominantly oxidation reactions. A comparison of the curves for CO and carbon dioxide (CO2) reveals an intersection point, where the production of CO2 is lower than that of CO within the range of 0 to 62,000 fs.
As Figure 3b shows, for CO there were 16 positive reactions and 5 reverse reactions. Among the positive reactions, the one with the highest frequency accounts for 47% (CO3 → O2 + CO). In the case of the reverse reactions, the reaction with the highest frequency accounted for 69% (O2 + CO → CO3).
As shown in Figure 2c, there was a significant increase in H2O production during the initial stage of 0–30,000 fs. In the subsequent stages, H2O production remains relatively stable. In the positive reaction channel depicted in Figure 3c, the proportion of reaction counts reached 33% (H2O + HO + HO → H2O + H2O + O). Conversely, the reverse reaction accounts for 42% of the negative reaction channels. Overall, the ratio of positive reactions producing H2O is higher than that of the negative reaction channels. This disparity is one of the reasons for the relative stability of H2O production. Additionally, HO radicals participate in numerous reactions.
The generation curve data for the HO2 reaction are illustrated in the locally enlarged image in Figure 2c. After 35,000 fs, there is a significant increase in HO2 production. An analysis of the reaction channels presented in Figure 3d reveals that HO2 is involved in numerous oxidation reactions, as well as in the thermal decomposition of hydrocarbons and the dissociation of certain free radicals. These findings indicate that HO2 exhibits strong reactivity and can facilitate various reactions. Within the reaction channel for HO2, there are 11 forward reactions and 10 reverse reactions. The most prevalent reaction is the oxidation of hydrogen peroxide by oxygen (O2 + H2O2 → HO2 + HO2), with the reverse reaction occurring at a frequency that accounts for 27% of the total.
3.3. Detailed Reaction Map for Naphthalene Oxidation
Figure 4 illustrates the proposed reaction networks of the oxidation of naphthalene based on the outcomes of our simulation.
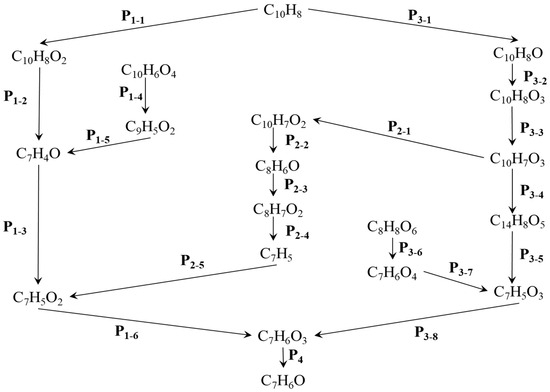
Figure 4.
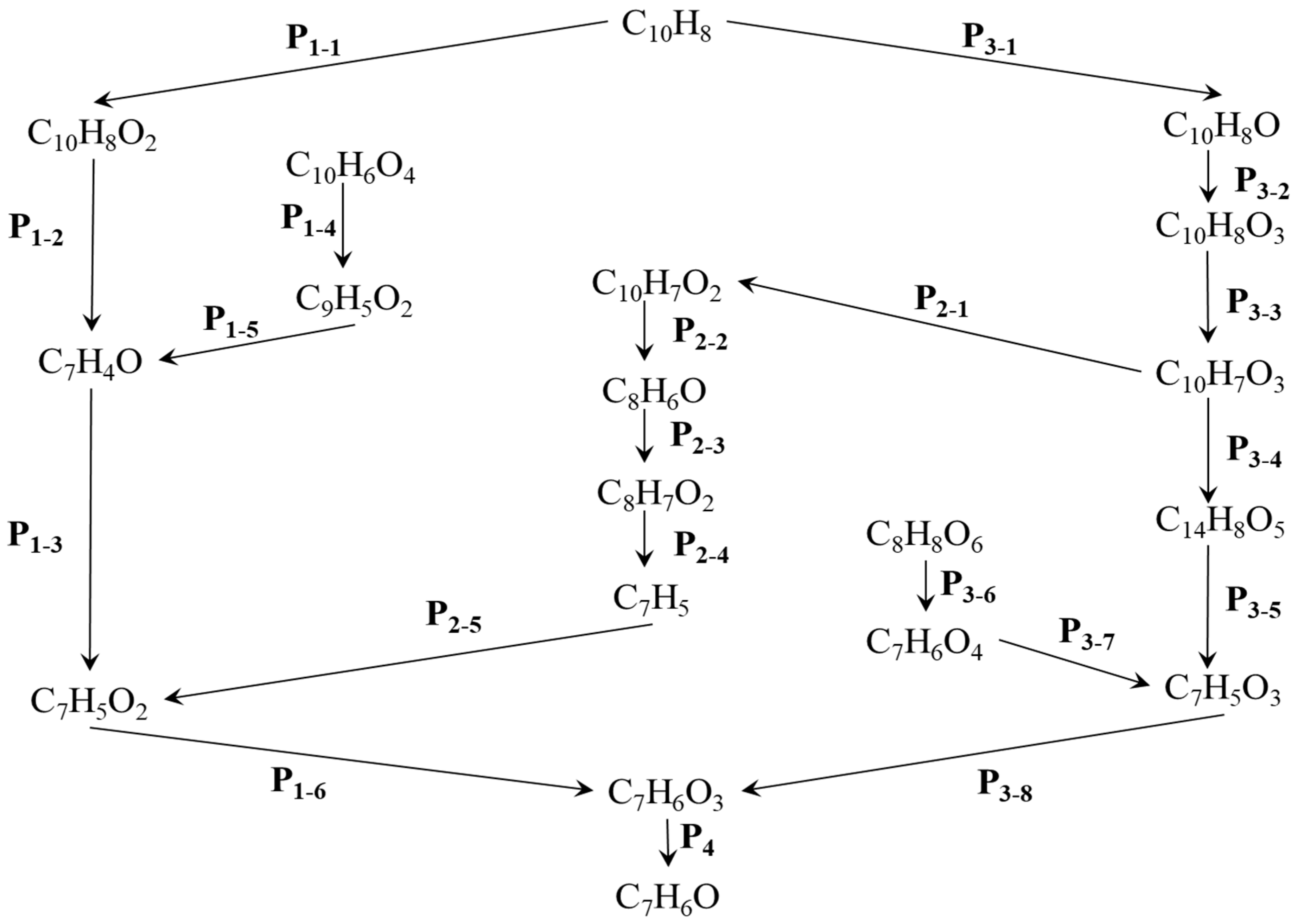
Reaction map for conversion of naphthalene (C10H8) into benzaldehyde (C7H6O). Captions P1–P6 indicate main pathway. Px-y (x = 1, …, 6; y = 1, …, 6) represent various elementary reactions.
The impact of isomers was overlooked during the research on reaction networks. The reaction rate was high, and the reaction time was short, based on the simulation conditions, which included elevated temperatures. The isomers are believed to have minimal influence. These networks provide a comprehensive explanation of the process that converts a C10H8 molecule into C7H6O, for which six potential pathways are proposed.
P1 is the most straightforward of the four reaction pathways, as illustrated in Figure 4. The compound C7H4O is essential to the P1 reaction pathway and is involved in the P1-2, P1-4, and P1-5 reactions, among others. Additionally, C7H5O2 is critical to the P1-3, P1-6, and P2-5 reaction pathways, which presents a similar challenge. C7H5O2 is produced at the intersection of the P1 and P2 reaction pathways.
P2 incorporates products from both P3 and P1. C10H7O3 serves as the initial reactant in P2 and plays a crucial role in the subsequent reactions. P3 is relatively complex, encompassing numerous products, including eight smaller reaction pathways. The product that lies at the intersection of P3 and P2 is C10H7O3.
P4 is relatively short and is the shortest among all reaction pathways. It exhibits the highest conversion efficiency and a high reaction rate. The P1, P2, and P3 reaction pathways encompass various types of reactions, including oxidation reactions, pyrolysis reactions, and hydrogenation reactions (C7H5O3 + H → C7H6O3). In contrast, the P4 reaction pathway consists solely of pyrolysis reactions. The specific reactions involved in these four reaction pathways are detailed in Table 2, Table 3, Table 4, Table 5 and Table 6.

Table 2.
Main reactions of P1 reaction pathway.

Table 3.
Main reactions of P2 reaction pathway.

Table 4.
Main reactions of P3 and P4 reaction pathway.

Table 5.
Main reactions of P5 reaction pathway.

Table 6.
Main reactions of P6 reaction pathway.
The P5 and P6 reaction pathways are relatively simple in the reaction network. P5 has four elementary reactions, and the first node of its reaction is C10H6O4. It intersects with P1 and P2 at points C7H4O and C7H5O2. P6 has three elementary reactions and intersects with the P3 reaction pathway at C7H5O3
Table 2, Table 3, Table 4, Table 5 and Table 6 present the intermediate reactions for each reaction pathway, along with their corresponding reaction rate constants. The reaction map indicates that a total of 20 intermediate reactions are involved. There are three categories of statistical reactions: hydrogenation, pyrolysis, and oxidation. In some of these reactions, hydroxyl and hydrogen radicals play a significant role.
Based on the reaction network map and Table 2, we will discuss the characteristics of the P1 reaction pathway. This pathway consists of four reactions. The initial oxidation reaction, represented by the equation O2 + C10H8 → C10H8O2, exhibits the lowest reaction rate. The intermediate product, C7H4O, is formed through two reactions, P1-2 and P5-2, both of which involve the pyrolysis of C-C bond dissociation. Additionally, two reactions contribute to the formation of the product C7H5O2, specifically P1-3 and P2-5.
As a node in the reaction network, C7H5O2 has an alternative generation pathway, as illustrated in Figure 4. According to Table 3, three of the reactions are classified as pyrolysis reactions. Additionally, there is one oxidation reaction and one activation polymerization reaction, where the former exhibits the lowest rate among these processes. The end products of the pyrolysis reaction (C8H7O2 → C7H5 + CH2O2), which results from the dissociation of C-C bonds, are C7H5 and CH2O2. This particular reaction has the highest reaction rate. A relatively high reaction rate is also observed when the free radical OH is involved.
Compared to the previously mentioned reaction pathway, the P3 reaction pathway is more complex, involving a total of six reactions. Among these, the proportion of pyrolysis reactions is relatively high. The oxidation reaction exhibits the slowest rate among all the reactions. When examining the hydrogenation (C7H5O3 + H → C7H6O3) and dehydrogenation (C10H8O3 → H + C10H7O3) reactions, it is evident that the rate of the hydrogenation reaction is significantly higher than that of the dehydrogenation reaction. Conversely, the rate of the deoxidation reaction P4 (C7H6O3 → C7H6O + O2) is greater than that of the oxidation reaction (O2 + C10H8O → C10H8O3).
From Table 5 and Table 6, it can be seen that the rate of pyrolysis reaction is relatively high. Obviously, the hydrogenation reaction has the highest rate. The dehydrogenation reaction occurs in P5-1, resulting in the production of C9H5O2, as observed.
As illustrated in Figure 4, the transition from C10H8 to C7H6O involves two critical products: C7H5O2 and C7H5O3. A comparison of the two reactions involving C7H5O2 reveals that the oxidation reaction (C7H5 + O2 → C7H5O2) has a relatively low rate, as shown in Table 3. In contrast, the formation of C7H5O3 occurs at a higher rate during the pyrolysis reaction (Table 4 and Table 6), which involves the participation of free radical OH. Specifically, C7H5O2 reacts with free radical OH to produce C7H6O3 (P5-4), while C7H5O3 is produced through a hydrogenation reaction. Ultimately, C7H6O3 yields the target product, C7H6O, via a pyrolysis reaction (C7H6O3 → C7H6O + O2).
4. Conclusions
The ReaxFF MD method was employed to investigate the distribution of products during the oxidative pyrolysis of naphthalene. Additionally, the reaction pathway and characteristics of oxidative pyrolysis were analyzed using the CTY method. The reaction products and rates exhibit distinct characteristics at varying temperatures. The reaction pathway network for the conversion of C10H8 to C7H6O primarily comprises six pathways, which include ring opening, C-C bond cleavage, and hydrogenation, and other related processes.
1. Naphthalene undergoes oxidation at elevated temperatures when using NVT-MD (microcanonical ensemble molecular dynamics) with a reactive force field. To obtain a comprehensive understanding of the oxidation process, simulations were conducted on a model system consisting of 20 naphthalene molecules and 240 oxygen molecules. The primary species identified in the current simulations include CO2, CO, H2O, and HO2. The formation of these products is characterized by a high reaction rate constant. Oxidation reactions are distinguished by the generation of C-O and O-H bonds, whereas pyrolysis reactions primarily involve the dissociation and dehydrogenation of individual molecules. In addition to the oxidative pyrolysis reaction, reactive free radicals such as hydrogen peroxide, hydroperoxyl, and formaldehyde are produced, which further accelerates the reaction rate.
2. The reaction rates of the products in the oxidation and pyrolysis reactions varied in the different time stages. After the intersection of CO and CO2, the yield of the former was greater than that of the latter. This phenomenon can be attributed to insufficient collision contact in the early stages and more favorable reaction conditions in the later stages.
A comparative analysis of the primary reactions reveals the occurrence of both positive and negative reactions across all reaction types, which presents an intriguing aspect for the investigation of reaction mechanisms. In terms of reaction kinetics, the hydrogenation reaction demonstrates the highest rate, followed by the pyrolysis reaction, while the oxidation reaction exhibits the lowest rate.
3. The reaction network from C10H8 to C7H6O was thus also examined to enhance our understanding of the process. Among the reactions in the network, hydrogenation was found to have the highest reaction rate. From the reaction network associated with the conversion of naphthalene to benzaldehyde, six principal reaction pathways can be delineated. Throughout this reaction process, a variety of reactions take place, including oxidation, pyrolysis, dehydrogenation, and hydrogenation. The elucidation of these reaction networks provides a novel molecular-level insight into the oxidation and pyrolysis processes of heavy oil.
Author Contributions
Conceptualization, T.Y.; methodology, T.Y.; molecular dynamic simulation calculations, T.Y., H.W. and Z.S.; validation, T.Y. and Z.S.; investigation, T.Y., L.C. and Z.S.; writing—original draft preparation, T.Y. and H.L.; writing—review and editing, T.Y. and Z.L.; review and editing, T.Y. and R.Z.; supervision, T.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Karamay Science and Technology Bureau under grant no. XQZX20220045.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Hongbing Luo was employed by the company CNPC Xibu Drilling Engineering Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alade, O.S.; Hamdy, M.; Mahmoud, M.; Al Shehri, D.A.; Mokheimer, E.; Patil, S.; Al-Nakhli, A. A preliminary assessment of thermochemical fluid for heavy oil recovery. J. Pet. Sci. Eng. 2020, 186, 106702. [Google Scholar] [CrossRef]
- Belousov, A.; Lushpeev, V.; Sokolov, A.; Sultanbekov, R.; Tyan, Y.; Ovchinnikov, E.; Shvets, A.; Bushuev, V.; Islamov, S. Hartmann–Sprenger Energy Separation Effect for the Quasi-Isothermal Pressure Reduction of Natural Gas: Feasibility Analysis and Numerical Simulation. Energies 2024, 17, 2010. [Google Scholar] [CrossRef]
- Islamov, S.; Islamov, R.; Shelukhov, G.; Sharifov, A.; Sultanbekov, R.; Ismakov, R.; Agliullin, A.; Ganiev, R. Fluid-Loss Control Technology: From Laboratory to Well Field. Processes 2024, 12, 114. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, Y.; Lü, X.; Li, Q. Energy consumption and product release characteristics evaluation of oil shale non-isothermal pyrolysis based on TG-DSC. J. Pet. Sci. Eng. 2020, 187, 106812. [Google Scholar] [CrossRef]
- Jia, Y.; Cao, Y.; Wang, H.; Ma, B. Influence of multiphase carbonate cementations on the Eocene delta sandstones of the Bohai Bay Basin, China. J. Pet. Sci. Eng. 2021, 205, 108866. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Pan, J.; Zhu, C.; Deng, S. In-situ pyrolysis of oil shale in pressured semi-closed system: Insights into products characteristics and pyrolysis mechanism. Energy 2024, 168, 129608. [Google Scholar] [CrossRef]
- Tao, L.; Hu, Z.; Xu, Z.; Zhang, X.; Ding, Y.; Wang, C.; Chen, D.; Li, S. Experimental investigation of in situ combustion (ISC) in heavy oil thermal recovery. Geoenergy Sci. Eng. 2024, 233, 212488. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Lu, N.; Chai, M.; Wang, S.; Feng, Q.; Chen, Z. Integration of ramped temperature oxidation and combustion tube tests for kinetic modeling of heavy oil in-situ combustion. Energy 2023, 274, 127435. [Google Scholar] [CrossRef]
- Jayaraman, K.; Kök, M.V.; Gökalp, I. Combustion mechanism and model-free kinetics of different origin coal samples: Thermal analysis approach. Energy 2020, 204, 117905. [Google Scholar] [CrossRef]
- Mahvelati, E.A.; Forcinito, M.; Fitschy, L.; Maesen, A. Three-dimensional CFD Model Development and Validation for Once Through Steam Generator (OTSG): Coupling Combustion Heat Transfer and Steam Generation. ChemEngineering 2022, 6, 23. [Google Scholar] [CrossRef]
- Askarova, A.; Popov, E.; Ursenbach, M.; Moore, G.; Mehta, S.; Cheremisin, A. Experimental Investigations of Forward and Reverse Combustion for Increasing Oil Recovery of a Real Oil Field. Energies 2020, 13, 4581. [Google Scholar] [CrossRef]
- Huang, S.; Sheng, J.J.; Jiang, Q.; Liu, J. Screening of Spontaneous Ignition Feasibility During Air Injection EOR Process Based on Thermal Experiments. Energies 2019, 12, 3687. [Google Scholar] [CrossRef]
- Zhao, R.B.; Xia, X.T.; Luo, W.W.; Shi, Y.L.; Diao, C.J. Alteration of heavy oil properties under in-situ combustion: A field study. Energy Fuels 2015, 29, 6839. [Google Scholar] [CrossRef]
- Zhao, R.B.; Zhang, C.H.; Yang, F.X.; Heng, M.H.; Shao, P.T.; Wang, Y.J. Influence of temperature field on rock and heavy components variation during in-situ combustion process. Fuel 2018, 230, 244. [Google Scholar] [CrossRef]
- Sun, Z.; Ji, J.; Zhu, W. Effects of Nanoparticle Size on the Thermal Decomposition Mechanisms of 3, 5-Diamino-6-hydroxy-2-oxide-4-nitropyrimidone through ReaxFF Large-Scale Molecular Dynamics Simulations. Molecules 2024, 29, 56. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.W.; Dyatkin, B.; Wang, H.W.; Turner, C.H.; Sang, X.; Unocic, R.R.; Iacovella, C.R.; Gogotsi, Y.; Van Duin, A.C.; Cummings, P.T. An Atomistic Carbide-Derived Carbon Model Generated Using ReaxFF-Based Quenched Molecular Dynamics. J. Carbon. Res. 2017, 3, 32. [Google Scholar] [CrossRef]
- Thompson, M.W.; Dyatkin, B.; Wang, H.-W.; Turner, C.H.; Xiahan; Li, C.; Yang, Z.; Wu, X.; Shao, S.; Meng, X.; et al. Reactive Molecular Dynamics Simulations of Polystyrene Pyrolysis. Int. J. Mol. Sci. 2023, 24, 16403. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Zhang, Y.; Shi, K. Mechanism Analysis of Ethanol Production from Cellulosic Insulating Paper Based on Reaction Molecular Dynamics. Polymers 2022, 14, 4918. [Google Scholar] [CrossRef]
- Deng, S.; Zhuo, H.; Wang, Y.; Leng, S.; Zhuang, G.; Zhong, X.; Wei, Z.; Yao, Z.; Wang, J. Multiscale Simulation on Product Distribution from Pyrolysis of Styrene-Butadiene Rubber. Polymers 2019, 11, 1967. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; He, R.; Li, X.; Zheng, M.; Ren, C.; An, G.; Xu, X.; Zheng, Z. Overall mechanism of JP-10 pyrolysis unraveled by large-scale reactive molecular dynamics simulation. Combust. Flame 2022, 237, 111865. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, Q.; Wang, Z.; Luo, K.H.; Zhou, L.; Wei, H. A ReaxFF molecular dynamics study of polycyclic aromatic hydrocarbon oxidation assisted by nitrogen oxides. Combust. Flame 2023, 248, 112571. [Google Scholar] [CrossRef]
- van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396. [Google Scholar] [CrossRef]
- Cheng, T.; Jaramillo-Botero, A.; Goddard, W.A.; Sun, H. Adaptive accelerated ReaxFF reactive dynamics with validation from simulating hydrogen combustion. J. Am. Chem. Soc. 2014, 136, 9434. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, X.; Li, Z.; Xu, H.; Qing, M.-X.; Jiang, X.; Liu, L. The molecular evolution mechanism of direct pyrolysis and hydropyrolysis of Changqing petroleum coke was compared based on ReaxFF method. J. Mol. Struct. 2023, 1290, 135882. [Google Scholar] [CrossRef]
- Senftle, T.P.; van Duin, A.C.T.; Janik, M.J. Methane Activation at the Pd/CeO2 Interface. ACS Catal. 2017, 7, 327. [Google Scholar] [CrossRef]
- Ostadhossein, A.; Rahnamoun, A.; Wang, Y.; Zhao, P.; Zhang, S.; Crespi, V.H.; van Duin, A.C.T. ReaxFF Reactive Force-Field Study of Molybdenum Disulfide (MoS2). J. Phys. Chem. Lett. 2017, 8, 631. [Google Scholar] [CrossRef]
- Yeon, J.; van Duin, A.C.T.; Kim, S.H. Effects of Water on Tribochemical Wear of Silicon Oxide Interface: Molecular Dynamics (MD) Study with Reactive Force Field (ReaxFF). Langmuir 2016, 32, 1018. [Google Scholar] [CrossRef]
- Yeon, J.; van Duin, A.C.T. ReaxFF Molecular Dynamics Simulations of Hydroxylation Kinetics for Amorphous and Nano-Silica Structure, and Its Relations with Atomic Strain Energy. J. Phys. Chem. C 2016, 120, 305. [Google Scholar] [CrossRef]
- Shin, Y.K.; Gai, L.; Raman, S.; van Duin, A.C.T. Development of a ReaxFF Reactive Force Field for the Pt-Ni Alloy Catalyst. J. Phys. Chem. A 2016, 120, 8044. [Google Scholar] [CrossRef]
- Zhong, Q.; Mao, Q.; Xiao, J.; van Duin, A.C.T.; Mathews, J.P. ReaxFF simulations of petroleum coke sulfur removal mechanisms during pyrolysis and combustion. Combust. Flame 2018, 198, 146. [Google Scholar] [CrossRef]
- Liu, L.; Xu, H.; Zhu, Q.; Ren, H.; Li, X. Soot formation of n-decane pyrolysis: A mechanistic view from ReaxFF molecular dynamics simulation. Chem. Phys. Lett. 2020, 760, 137983. [Google Scholar] [CrossRef]
- Tuo, Y.-X.; Shi, L.-J.; Cheng, H.-Y.; Zhu, Y.-A.; Yang, M.-L.; Xu, J.; Han, Y.-F.; Li, P.; Yuan, W.-K. Insight into the support effect on the particle size effect of Pt/C catalysts in dehydrogenation. J. Catal. 2018, 360, 175. [Google Scholar] [CrossRef]
- Ashraf, C.; Shabnam, S.; Jain, A.; Xuan, Y.; van Duin, A.C.T. Pyrolysis of binary fuel mixtures at supercritical conditions: A ReaxFF molecular dynamics study. Fuel 2019, 235, 194. [Google Scholar] [CrossRef]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF reactive force-field: Development, applications and future directions. Npj Comput. Mater. 2016, 2, 1. [Google Scholar] [CrossRef]
- Huo, E.; Zhang, S.; Xin, L.; Wang, S.; Cai, S.; Zhang, L.; Bai, M. Pyrolysis mechanism study of n-heptane as an endothermic hydrocarbon fuel: A reactive molecular dynamic simulation and density functional theory calculation study. Comput. Theor. Chem. 2022, 1211, 113696. [Google Scholar] [CrossRef]
- Hirai, H. Molecular dynamics simulations for initial formation process of polycyclic aromatic hydrocarbons in n-hexane and cyclohexane combustion. Chem. Phys. 2021, 548, 111225. [Google Scholar] [CrossRef]
- Apebende, C.G.; Magu, T.O.; Asogwa, F.C.; Onyebuenyi, I.B.; Unimuke, T.O.; Gber, T.E. Density functional theory study of the influence of activating and deactivating groups on Naphthalene. Results Chem. 2022, 4, 10669. [Google Scholar] [CrossRef]
- Alamfard, T.; Lorenz, T.; Breitkopf, C. Thermal Conductivities of Uniform and Random Sulfur Crosslinking in Polybutadiene by Molecular Dynamic Simulation. Polymers 2023, 15, 2058. [Google Scholar] [CrossRef]
- Orekhov, N.; Ostroumova, G.; Stegailov, V. High temperature pure carbon nanoparticle formation: Validation of AIREBO and ReaxFF reactive molecular dynamics. Carbon 2020, 270, 606. [Google Scholar] [CrossRef]
- Döntgen, M.; Przybylski-Freund, M.D.; Kröger, L.C.; Kopp, W.A.; Ismail, A.E.; Leonhard, K. Automated discovery of reaction pathways, rate constants, and transition states using reactive molecular dynamics simulations. J. Chem. Theory Comput. 2015, 11, 2517. [Google Scholar] [CrossRef]
- Döntgen, M.; Schmalz, F.; Kopp, W.A.; Kröger, L.C.; Leonhard, K. Automated Chemical Kinetic Modeling via Hybrid Reactive Molecular Dynamics and Quantum Chemistry Simulations. J. Chem. Inf. Model. 2018, 58, 1343. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yuan, W.; Gaïl, S.; Li, W.; Zhao, L.; Yang, J.; Qi, F.; Li, Y.; Dagaut, P. Exploration on the combustion chemistry of p-xylene: A comprehensive study over wide conditions and comparison among C8H10 isomers. Combust. Flame 2024, 262, 113377. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).