Efficient Production of Microalgal Biomass—Step by Step to Industrial Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Strains
2.2. Preincubation
2.3. Experimental Setup
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. Algal Biomass Concentration
3.2. Lipid Content
3.3. Ash Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factories 2018, 17, 173. [Google Scholar] [CrossRef]
- Pruvost, J.; Le Borgne, F.; Artu, A.; Cornet, J.F.; Legrand, J. Chapter Five—Industrial Photobioreactors and Scale-Up Concepts. In Advances in Chemical Engineering; Legrand, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 257–310. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Masojídek, J.; Kopecký, J.; Giannelli, L.; Torzillo, G. Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J. Ind. Microbiol. Biotechnol. 2011, 38, 307–317. [Google Scholar] [CrossRef]
- Sforza, E.; Simionato, D.; Giacometti, G.M.; Bertucco, A.; Morosinotto, T. Adjusted Light and Dark Cycles Can Optimize Photosynthetic Efficiency in Algae Growing in Photobioreactors. PLoS ONE 2012, 7, e38975. [Google Scholar] [CrossRef] [PubMed]
- Zarmi, Y.; Bel, G.; Aflalo, C. Theoretical Analysis of Culture Growth in Flat-Plate Bioreactors: The Essential Role of Timescales. In Handbook of Microalgal Culture; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of Photobioreactors for Mass Cultivation of Photosynthetic Organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Loera-Quezada, M.M.; Angeles, G.; Olguín, E.J. Effect of irradiance on the cell density, size and lipid accumulation of Neochloris oleoabundans. Rev. Latinoam. Biotechnol. Ambient. Algal 2011, 2, 81–92. [Google Scholar]
- González-Camejo, J.; Aparicio, S.; Jiménez-Benítez, A.; Pachés, M.; Ruano, M.V.; Borrás, L.; Barat, R.; Seco, A. Improving membrane photobioreactor performance by reducing light path: Operating conditions and key performance indicators. Water Res. 2020, 172, 115518. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Hu, B.; Min, M.; Chen, P.; Ruan, R.R. Effect of light intensity on algal biomass accumulation and biodiesel production for mixotrophic strains Chlorella kessleri and Chlorella protothecoide cultivated in highly concentrated municipal wastewater. Biotechnol. Bioeng. 2012, 109, 2222–2229. [Google Scholar] [CrossRef]
- Fernández del Olmo, P.; Acién, F.G.; Fernández-Sevilla, J.M. Analysis of productivity in raceway photobioreactor using computational fluid dynamics particle tracking coupled to a dynamic photosynthesis model. Bioresour. Technol. 2021, 334, 125226. [Google Scholar] [CrossRef]
- Rai, M.P.; Gautom, T.; Sharma, N. Effect of salinity, pH, light intensity on growth and lipid production of microalgae for bioenergy application. Online J. Biol. Sci. 2015, 15, 260–267. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M. Production of Chlorella vulgaris biomass in tubular photobioreactors during different culture conditions. Appl. Sci. 2021, 11, 3106. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Rai, S.V.; Rajashekhar, M. Effect of pH, salinity and temperature on the growth of six species of marine phytoplankton. J. Algal Biomass Util. 2014, 5, 55–59. [Google Scholar]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Legrand, J.; Artu, A.; Pruvost, J. A review on photobioreactor design and modelling for microalgae production. React. Chem. Eng. 2021, 6, 1134–1151. [Google Scholar] [CrossRef]
- Schediwy, K.; Trautmann, A.; Steinweg, C.; Posten, C. Microalgal kinetics—A guideline for photobioreactor design and process development. Eng. Life Sci. 2019, 19, 830–843. [Google Scholar] [CrossRef]
- Posten, C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Wang, C.; Lan, C.Q. Effects of shear stress on microalgae—A review. Biotechnol. Adv. 2018, 36, 986–1002. [Google Scholar] [CrossRef]
- Chisti, Y. Fermentation (industrial) Basic Considerations. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 751–761. [Google Scholar]
- Luzi, G.; McHardy, C. Modeling and Simulation of Photobioreactors with Computational Fluid Dynamics—A Comprehensive Review. Energies 2022, 15, 3966. [Google Scholar] [CrossRef]
- Lucas, D.; Tomiyama, A. On the role of the lateral lift force in poly-dispersed bubbly flows. Int. J. Multiph. Flow. 2011, 37, 1178–1190. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 18122:2016-01; Biopaliwa Stałe—Oznaczanie zawartości Popiołu. Polish Committee for Standardisation: Warszawa, Poland, 2016. Available online: https://sklep.pkn.pl/pn-en-iso-18122-2016-01p.html (accessed on 26 April 2021). (In Polish)
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, J.M. Attenuation of monochromatic and polychromatic lights in Chlorella vulgaris suspensions. Appl. Microbiol. Biotechnol. 2001, 55, 765–770. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Elmore, S.; Van Gerven, T.; Stankiewicz, A. Hydrodynamic evaluations in high rate algae pond (HRAP) design. Chem. Eng. J. 2013, 217, 231–239. [Google Scholar] [CrossRef]
- Nguyen, L.; Nguyen, D.K.; Nguyen, T.; Nguyen, B.; Nghiem, T.X. Analysis of Microalgal Density Estimation by Using LASSO and Image Texture Features. Sensors 2023, 23, 2543. [Google Scholar] [CrossRef]
- Han, F.; Pei, H.; Hu, W.; Song, M.; Ma, G.; Pei, R. Optimization and lipid production enhancement of microalgae culture by efficiently changing the conditions along with the growth-state. Energy Convers. Manag. 2015, 90, 315–322. [Google Scholar] [CrossRef]
- de Alva, M.S.; Luna-Pabello, V.M.; Cadena, E.; Ortiz, E. Green microalga Scenedesmus acutus grown on municipal wastewater to couple nutrient removal with lipid accumulation for biodiesel production. Bioresour. Technol. 2013, 146, 744–748. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Sąsiadek, M. Optimization of Microalgal Biomass Production in Vertical Tubular Photobioreactors. Energies 2023, 16, 2429. [Google Scholar] [CrossRef]
- Kim, W.; Park, J.M.; Gim, G.H.; Jeong, S.-H.; Kang, C.M.; Kim, D.-J.; Kim, S.W. Optimization of culture conditions and comparison of biomass productivity of three green algae. Bioprocess Biosyst. Eng. 2012, 35, 19–27. [Google Scholar] [CrossRef]
- Min, M.; Wang, L.; Li, Y.; Mohr, M.J.; Hu, B.; Zhou, W.; Chen, P.; Ruan, R. Cultivating Chlorella sp. in a Pilot-Scale Photobioreactor Using Centrate Wastewater for Microalgae Biomass Production and Wastewater Nutrient Removal. Appl. Biochem. Biotechnol. 2011, 165, 123–137. [Google Scholar] [CrossRef]
- Kazbar, A.; Cogne, G.; Urbain, B.; Marec, H.; Le-Gouic, B.; Tallec, J.; Takache, H.; Ismail, A.; Pruvost, J. Effect of dissolved oxygen concentration on microalgal culture in photobioreactors. Algal Res. 2019, 39, 101432. [Google Scholar] [CrossRef]
- Kishi, M.; Tanaka, K.; Tagawa, M.; Akizuki, S.; Toda, T. Energy-efficient algal culture through aeration-less oxygen removal in a gas-permeable bag photobioreactor. Algal Res. 2023, 69, 102959. [Google Scholar] [CrossRef]
- Camacho, F.G.; Gómez, A.C.; Sobczuk, T.M.; Molina-Grima, E. Effects of mechanical and hydrodynamic stress in agitated, sparged cultures of Porphyridium cruentum. Process Biochem. 2000, 35, 1045–1050. [Google Scholar] [CrossRef]
- Wu, J. Mechanisms of animal cell damage associated with gas bubbles and cell protection by medium additives. J. Biotechnol. 1995, 43, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Dhup, S.; Dhawan, V. Effect of nitrogen concentration on lipid productivity and fatty acid composition of Monoraphidium sp. Bioresour. Technol. 2014, 152, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Kamberovic, F.; Uota, S.T.; Kovan, I.-M.; Viegas, C.S.B.; Simes, D.C.; Gangadhar, K.N.; Varela, J.; Barreira, L. Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Appl. Sci. 2022, 12, 5877. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Amaro, H.M.; Guedes, A.C.; Malcata, F.X. Advances and perspectives in using microalgae to produce biodiesel. Appl. Energy 2011, 88, 3402–3410. [Google Scholar] [CrossRef]
- Xu, L.; Weathers, P.J.; Xiong, X.-R.; Liu, C.-Z. Microalgal bioreactors: Challenges and opportunities. Eng. Life Sci. 2009, 9, 178–189. [Google Scholar] [CrossRef]
- Pham, H.-M.; Kwak, H.S.; Hong, M.-E.; Lee, J.; Chang, W.S.; Sim, S.J. Development of an X-Shape airlift photobioreactor for increasing algal biomass and biodiesel production. Bioresour. Technol. 2017, 239, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, G.C.; Zhou, B.C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef]
- Feng, Y.; Li, C.; Zhang, D. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour. Technol. 2011, 102, 101–105. [Google Scholar] [CrossRef]
- Wong, Y.K.; Ho, Y.H.; Ho, K.C.; Leung, H.M.; Yung, K.K.L. Growth medium screening for Chlorella vulgaris growth and lipid production. J. Aquac. Mar. Biol. 2017, 6, 00143. [Google Scholar] [CrossRef]
- Papapolymerou, G.; Karayannis, V.; Besios, A.; Riga, A.; Gougoulias, N.; Spiliotis, X. Scaling-up sustainable Chlorella vulgaris microalgal biomass cultivation from laboratory to pilot-plant photobioreactor, towards biofuel. Glob. Nest J. 2018, 21, 37–42. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M.; Koniuszy, A. Utilisation of CO2 from Sodium Bicarbonate to Produce Chlorella vulgaris Biomass in Tubular Photobioreactors for Biofuel Purposes. Sustainability 2021, 13, 9118. [Google Scholar] [CrossRef]
- Jerez, C.G.; Malapascua, J.R.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Effect of Nutrient Starvation under High Irradiance on Lipid and Starch Accumulation in Chlorella fusca (Chlorophyta). Mar. Biotechnol. 2016, 18, 24–36. [Google Scholar] [CrossRef]
- Arrojo, M.Á.; Regaldo, L.; Orquín, J.C.; Figueroa, F.L.; Díaz, R.T.A. Potential of the microalgae Chlorella fusca (Trebouxiophyceae, Chlorophyta) for biomass production and urban wastewater phycoremediation. AMB Express 2022, 12, 43. [Google Scholar] [CrossRef]
- Deamici, K.M.; Cardias, B.B.; Costa, J.A.V.; Santos, L.O. Static magnetic fields in culture of Chlorella fusca: Bioeffects on growth and biomass composition. Process Biochem. 2016, 51, 912–916. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Ratomski, P.; Koniuszy, A.; Golimowski, W.; Teleszko, M.; Grygier, A. Fatty Acid Profile of Microalgal Oils as a Criterion for Selection of the Best Feedstock for Biodiesel Production. Energies 2021, 14, 7334. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D.; Ding, K.; Che, R.; Xu, J.-W.; Zhao, P.; Li, T.; Ma, H.; Yu, X. Production of biomass and lipids by the oleaginous microalgae Monoraphidium sp. QLY-1 through heterotrophic cultivation and photo-chemical modulator induction. Bioresour. Technol. 2016, 211, 669–676. [Google Scholar] [CrossRef]
- Guerrero-Cabrera, L.; Rueda, J.A.; García-Lozano, H.; Navarro, A.K. Cultivation of Monoraphidium sp., Chlorella sp. and Scenedesmus sp. algae in batch culture using Nile tilapia effluent. Bioresour. Technol. 2014, 161, 455–460. [Google Scholar] [CrossRef]
- Bogen, C.; Klassen, V.; Wichmann, J.; La Russa, M.; Doebbe, A.; Grundmann, M.; Uronen, P.; Kruse, O.; Mussgnug, J.H. Identification of Monoraphidium contortum as a promising species for liquid biofuel production. Bioresour. Technol. 2013, 133, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M. Sustainable Production of Monoraphidium Microalgae Biomass as a Source of Bioenergy. Energies 2020, 13, 5975. [Google Scholar] [CrossRef]
- Klin, M.; Pniewski, F.; Latała, A. Characteristics of the growth rate and lipid production in fourteen strains of Baltic green microalgae. Oceanol. Hydrobiol. Stud. 2018, 47, 10–18. [Google Scholar] [CrossRef]
- Tang, H.; Chen, M.; Garcia, M.E.D.; Abunasser, N.; Ng, K.Y.S.; Salley, S.O. Culture of microalgae Chlorella minutissima for biodiesel feedstock production. Biotechnol. Bioeng. 2011, 108, 2280–2287. [Google Scholar] [CrossRef]
- Freitas, B.C.B.; Cassuriaga, A.P.A.; Morais, M.G.; Costa, J.A.V. Pentoses and light intensity increase the growth and carbohydrate production and alter the protein profile of Chlorella minutissima. Bioresour. Technol. 2017, 238, 248–253. [Google Scholar] [CrossRef]
- Amaral, M.S.; Loures, C.C.A.; Naves, F.L.; Baeta, B.E.L.; Silva, M.B.; Prata, A.M.R. Evaluation of cell growth performance of microalgae Chlorella minutissima using an internal light integrated photobioreactor. J. Environ. Chem. Eng. 2020, 8, 104200. [Google Scholar] [CrossRef]
- Di Caprio, F.; Altimari, P.; Toro, L.; Pagnanelli, F. Effect of Lipids and Carbohydrates Extraction on Astaxanthin Stability in Scenedesmus sp. Chem. Eng. Trans. 2015, 43, 205–210. [Google Scholar] [CrossRef]
- Wu, Y.H.; Yu, Y.; Li, X.; Hu, H.Y.; Su, Z.F. Biomass production of a Scenedesmus sp. under phosphorous-starvation cultivation condition. Bioresour. Technol. 2012, 112, 193–198. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of Light Intensity and Quality on Growth Rate and Composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.M.; Ross, A.B.; Anastasakis, K.; Hodgson, E.M.; Gallagher, J.A.; Jones, J.M.; Donnison, I.S. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Laurens, L.M.L.; Dempster, T.A.; Jones, H.D.T.; Wolfrum, E.J.; Van Wychen, S.; McAllister, J.S.P.; Rencenberger, M.; Parchert, K.J.; Gloe, L.M. Algal biomass constituent analysis: Method uncertainties and investigation of the underlying measuring chemistries. Anal. Chem. 2012, 84, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. Characterization of ash in algae and other materials by determination of wet acid indigestible ash and microscopic examination. Algal Res. 2017, 25, 307–321. [Google Scholar] [CrossRef]
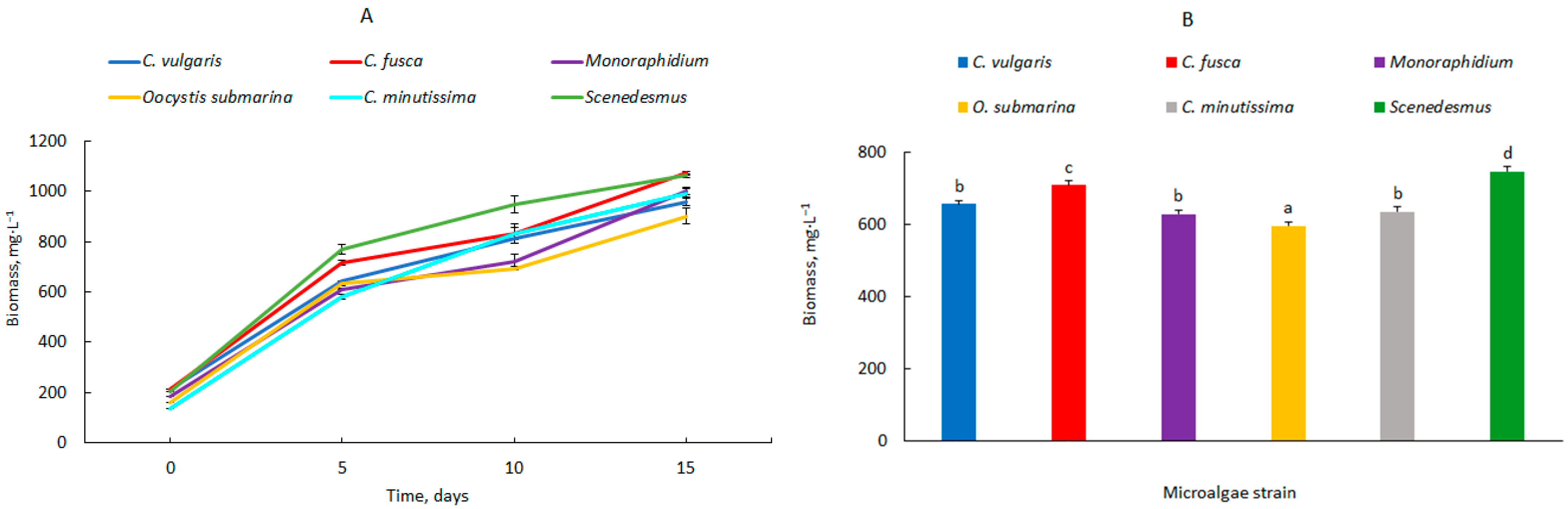

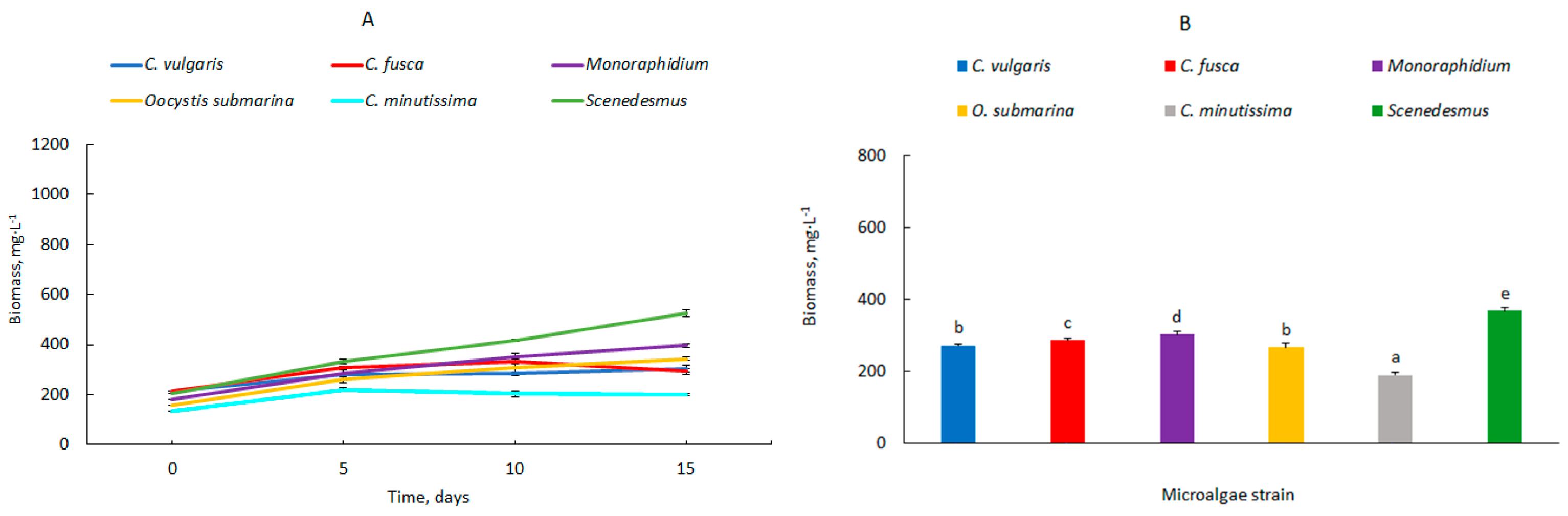
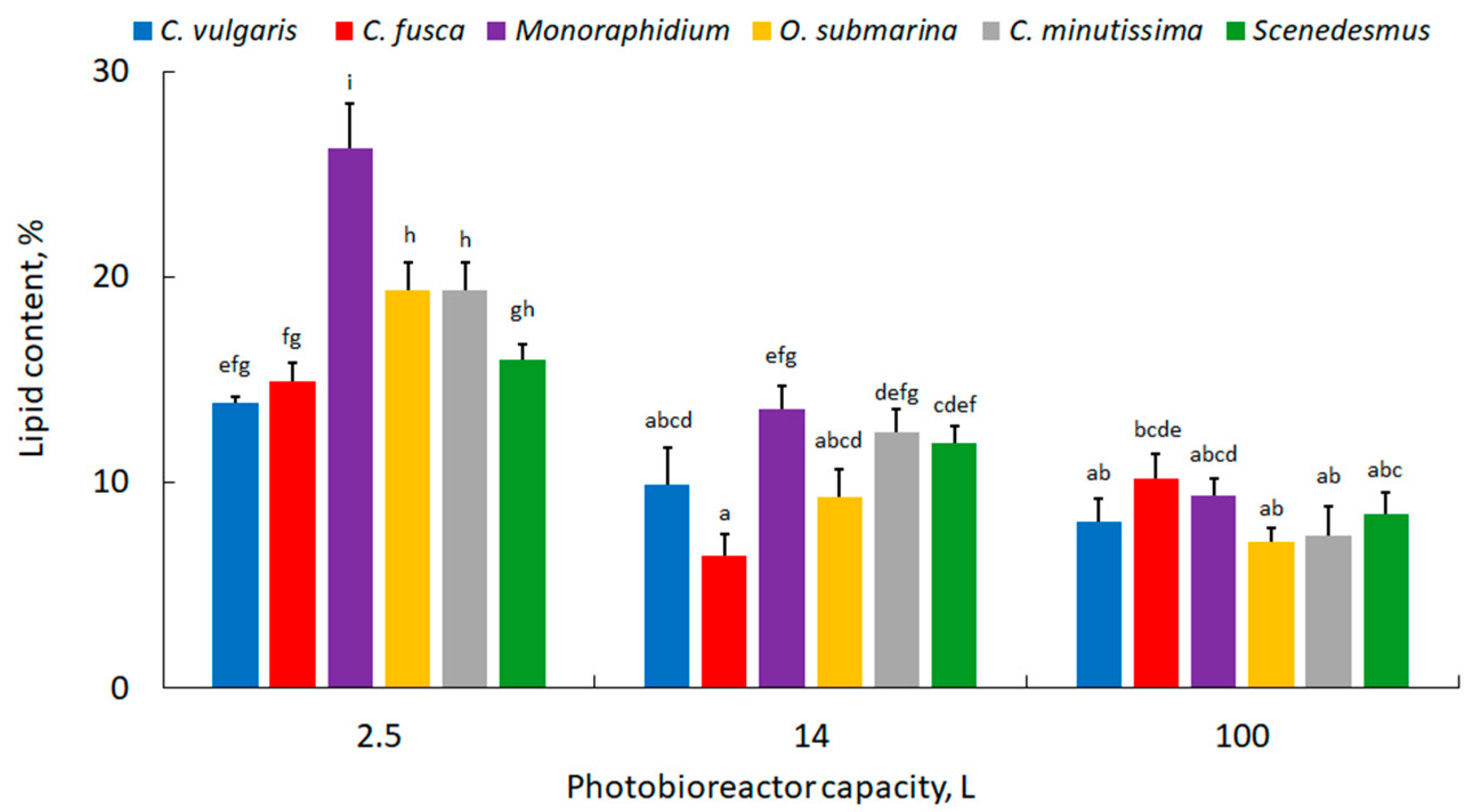

| Photobioreactor Capacity, L | Microalgae | Biomass Productivity (mg·L−1·d−1) | ||
|---|---|---|---|---|
| Day 5 | Day 10 | Day 15 | ||
| 2.5 | Chlorella vulgaris | 86.1 ± 0.2 e* | 60.0 ± 1.9 bc | 49.6 ± 1.0 a |
| Chlorella fusca | 100.8 ± 2.2 g | 62.0 ± 3.9 c | 57.4 ± 0.5 bc | |
| Monoraphidium | 85.6 ± 0.7 e | 53.7 ± 2.9 ab | 54.6 ± 1.0 ab | |
| Oocystis submarina | 94.8 ± 1.2 fg | 53.6 ±0.5 ab | 49.6 ± 2.1 a | |
| Chlorella minutissima | 89.1 ± 1.6 ef | 69.8 ± 2.4 d | 57.2 ± 1.0 bc | |
| Scenedesmus | 113.5 ± 4.1 h | 74.6 ± 3.4 d | 57.4 ± 0.5 bc | |
| 14 | Chlorella vulgaris | 54.0 ± 1.1 h | 46.5 ± 0.9 ef | 41.3 ± 1.6 cd |
| Chlorella fusca | 48.7 ± 1.4 f | 34.6 ± 0.9 b | 29.6 ± 1.0 a | |
| Monoraphidium | 60.5 ± 1.0 i | 40.7 ± 0.8 cd | 44.2 ± 0.6 de | |
| Oocystis submarina | 79.1 ± 2.7 j | 88.1 ± 1.2 j | 53.4 ± 0.4 gh | |
| Chlorella minutissima | 55.7 ±1.0 h | 49.5 ± 1.8 fg | 37.4 ± 2.8 bc | |
| Scenedesmus | 60.1 ± 0.8 i | 54.6 ± 0.9 h | 29.6 ± 1.0 a | |
| 100 | Chlorella vulgaris | 14.0 ± 0.8 bcd | 7.1 ± 0.6 a | 6.2 ± 0.9 a |
| Chlorella fusca | 18.9 ± 1.6 efg | 11.9 ± 0.8 b | 5.3 ± 0.9 a | |
| Monoraphidium | 20.0 ± 2.8 fg | 16.6 ± 1.5 cdef | 14.2 ± 0.5 bcd | |
| Oocystis submarina | 20.7 ± 3.2 fg | 14.9 ± 2.1 bcde | 12.3 ± 0.5 bc | |
| Chlorella minutissima | 16.9 ± 1.4 defg | 6.8 ± 1.1 a | 4.3 ± 0.4 a | |
| Scenedesmus | 25.9 ± 2.2 h | 21.3 ± 0.5 g | 21.4 ± 0.9 gh | |
| Microalgal Strain | Cultivation System and Capacity; L | Max Dry Biomass, mg·L−1 | Max Lipid Content, % | References |
|---|---|---|---|---|
| C. vulgaris | conical flask; 0.2 | - | 56.6 | [48] |
| tubular; 2.0 | 1700 | 42 | [49] | |
| conical flask; 2.0 | 1420 | 17.6 | [50] | |
| tubular; 2.5 | 656 ± 8.7 | 13.9 ± 0.3 | this study | |
| tubular; 14 | 551 ± 9.7 | 9.9 ± 1.8 | this study | |
| flat-plate; 25 | 1200 | - | [51] | |
| tubular; 100 | 271 ± 6.0 | 8.1 ± 1.1 | this study | |
| tubular; 100 | 572 | 26 | [52] | |
| C. fusca | Erlenmeyer flasks; 0.25 | 6500 | 31 | [53] |
| cylindrical reactors; 1.5 | - | 16.7 | [54] | |
| tubular vertical; 2.0 | 1940 | 13.2 | [55] | |
| tubular; 2.5 | 708 ± 14.3 | 14.9 ± 0.9 | this study | |
| tubular; 14 | 470 ± 7.8 | 6.4 ± 1.1 | this study | |
| tubular; 100 | 286 ± 7.3 | 10.2 ± 1.2 | this study | |
| tubular; 100 | 292 | 14 | [56] | |
| Monoraphidium | Erlenmeyer flasks; 0.5 | 1518 | 52.8 | [57] |
| bottles PET; 0.5 | 481 | 17.8 | [58] | |
| tubular; 2.5 | 629 ± 11.8 | 26.3 ± 2.2 | this study | |
| vertical glass botltles; 3 | - | 23.4 | [59] | |
| tubular; 14 | 526 ± 5.2 | 13.6 ± 1.1 | this study | |
| tubular; 14 | 3790 | 18.5 | [60] | |
| tubular; 100 | 303 ± 9.3 | 9.3 ± 0.8 | this study | |
| tubular; 100 | 525 | 14 | [56] | |
| Oocycstis submarina | Erlenmeyer flasks; 0.1 | 2.80·106 [cells mL−1] | 43.47 [mg·L−1] | [61] |
| tubular; 2.5 | 596 ± 10.6 | 19.3 ± 1.4 | this study | |
| tubular; 14 | 677 ± 8.0 | 9.3 ± 1.3 | this study | |
| tubular; 100 | 267 ± 11.0 | 7.1 ± 0.6 | this study | |
| tubular; 100 | 508 | 12 | [56] | |
| C. minutissima | conical flask; 0.65 | 1240 | 50.0 | [62] |
| vertical tubular, 2.0 | 1550 | - | [63] | |
| tubular; 2.5 | 636 ± 11.8 | 19.3 ± 1.4 | this study | |
| tubular; 14 | 469 ± 16.3 | 12.5 ± 1.1 | this study | |
| ILIPBR; 20 | 443 | 21.4 | [64] | |
| tubular; 100 | 369 ± 7.3 | 7.4 ± 1.4 | this study | |
| Scenedesmus | bootles PET; 0.5 | 1264 | 10.3 | [58] |
| bootles; 1 | 560 | 32 | [65] | |
| tubular; 2.5 | 747 ± 15.4 | 16 ± 0.7 | this study | |
| tubular; 14 | 526 ± 7.1 | 12 ± 0.8 | this study | |
| column air-lift; 80 | 900 | 25 | [66] | |
| tubular; 100 | 369 ± 7.3 | 8.5 ± 1.0 | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawrot-Paw, M.; Ratomski, P. Efficient Production of Microalgal Biomass—Step by Step to Industrial Scale. Energies 2024, 17, 944. https://doi.org/10.3390/en17040944
Hawrot-Paw M, Ratomski P. Efficient Production of Microalgal Biomass—Step by Step to Industrial Scale. Energies. 2024; 17(4):944. https://doi.org/10.3390/en17040944
Chicago/Turabian StyleHawrot-Paw, Małgorzata, and Patryk Ratomski. 2024. "Efficient Production of Microalgal Biomass—Step by Step to Industrial Scale" Energies 17, no. 4: 944. https://doi.org/10.3390/en17040944
APA StyleHawrot-Paw, M., & Ratomski, P. (2024). Efficient Production of Microalgal Biomass—Step by Step to Industrial Scale. Energies, 17(4), 944. https://doi.org/10.3390/en17040944







