Thermal Evolution of NiFe-NO3 LDH and Its Application in Energy Storage Systems
Abstract
:1. Introduction
2. Materials and Methods
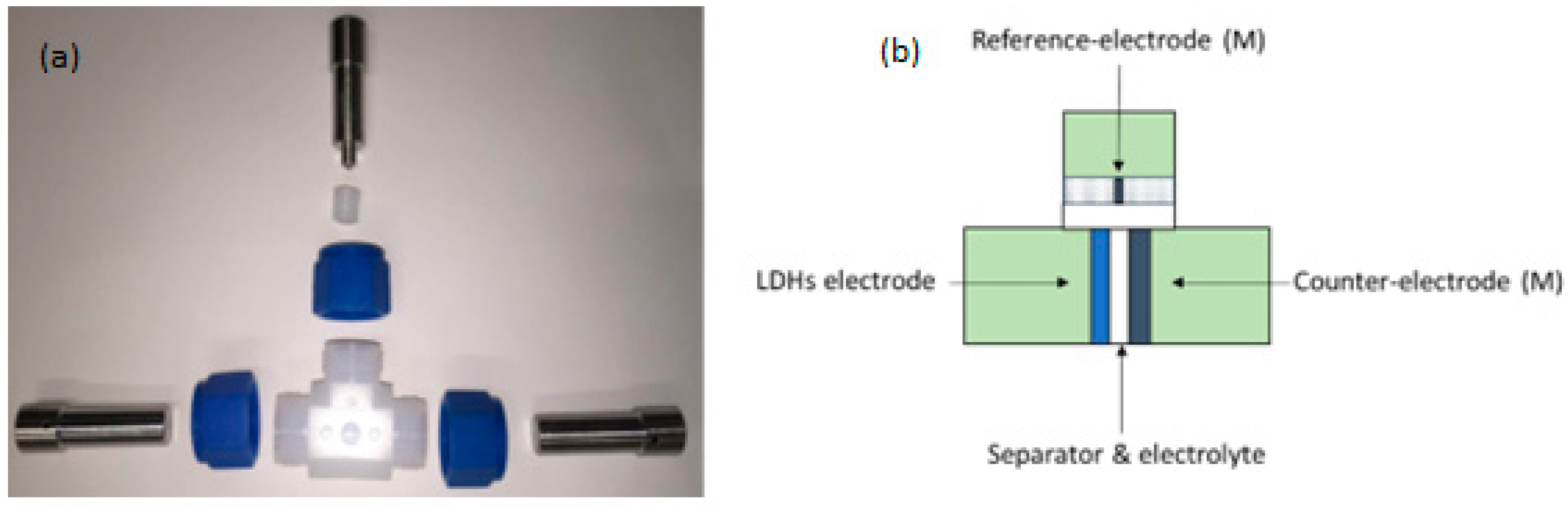
2.1. Reagents Used for LDH Synthesis and Electrochemical Cell Setup
2.2. Synthesis Method
2.3. Characterization Setup
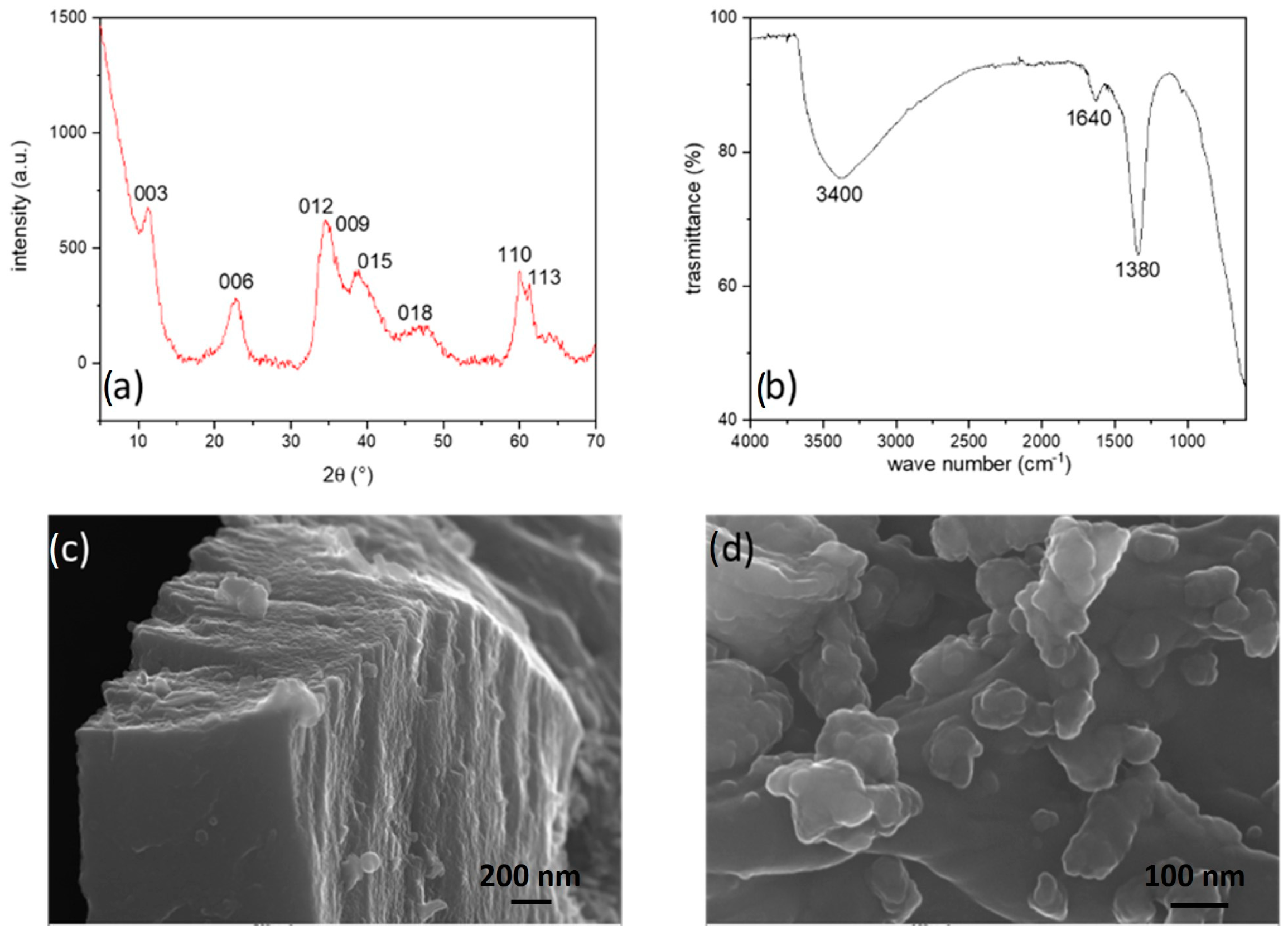
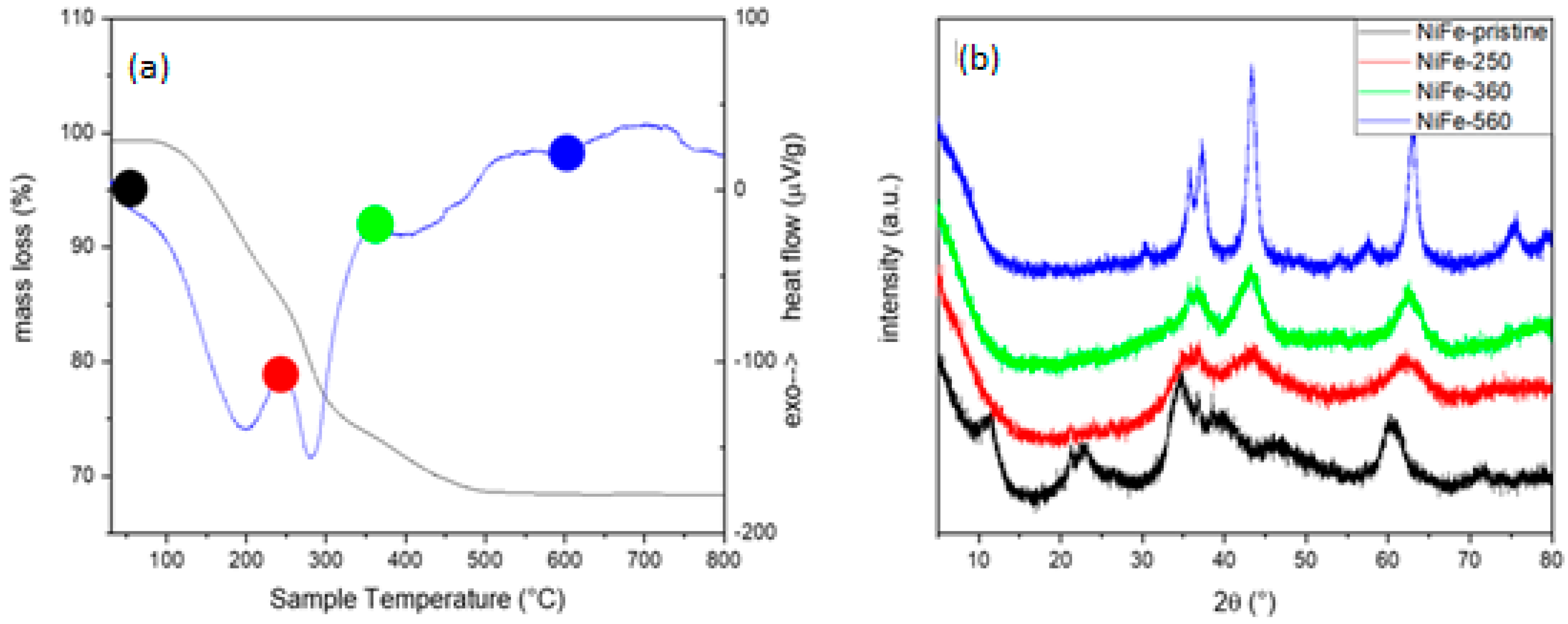
- Powder X-ray diffraction (PXRD): the patterns were recorded using a powder diffractometer (X’Pert MPD, Philips, Almelo, The Netherlands) equipped with a Cu anticathode (Kα1Cu = 1.5406 Å). The data were collected between 10 and 90 2ϑ with a step of 0.001 and a measuring time of 50 s/step. The indexing of the diffraction data was performed in comparison with the literature using the software package [WinPLOT version 2019].
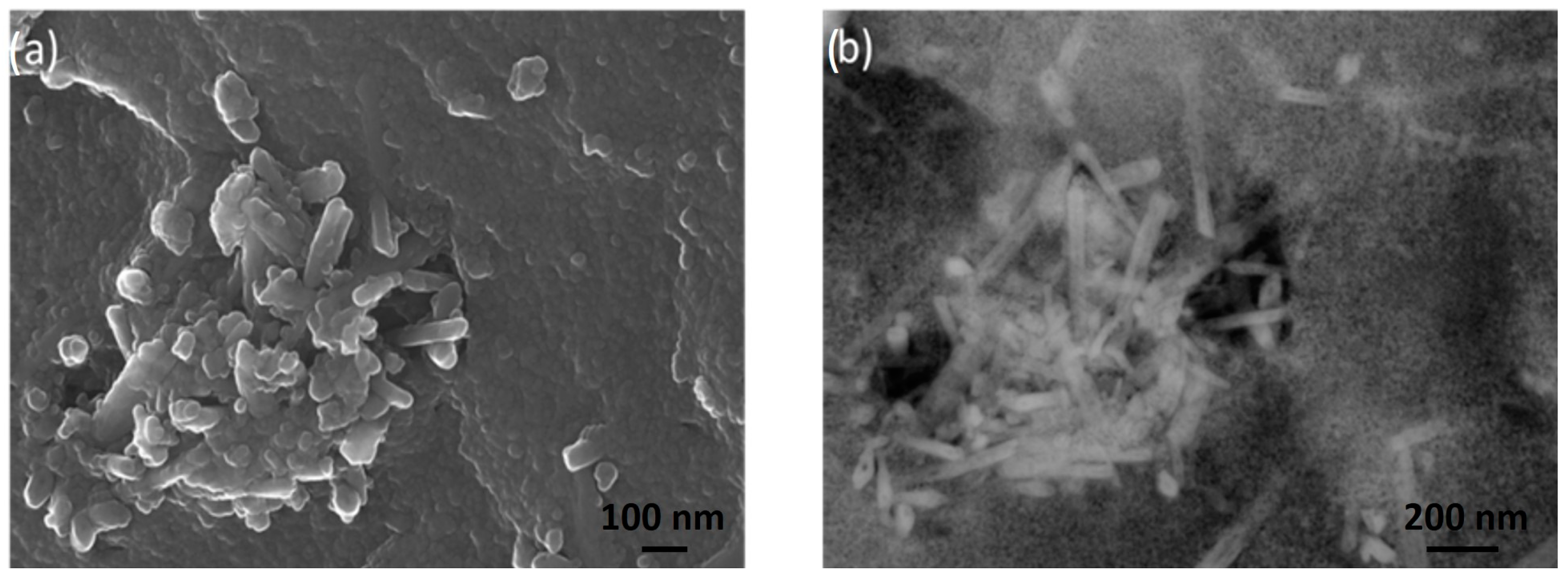
- Field Emission Scanning Electron Microscopy (FE-SEM): the samples were observed with a ZEISS SUPRA 40 V microscope, applying an acceleration voltage of 5 kV for 50 s.
- Fourier Transformed Infra-Red (FT-IR) spectroscopy: the spectra ranging from 4000 to 600 cm−1 were obtained utilizing a Spectrum 65 FT-IR Spectrometer (PerkinElmer, Waltham, MA, USA). The instrument was equipped with a KBr beam-splitter and a DTGS detector, and an ATR accessory with a diamond crystal was employed for data collection.
- Inductively coupled plasma optical emission spectroscopy (ICP-OES): The experiments were conducted utilizing a Varian Vista PRO (Springvale, Australia) with an axially oriented configuration. The sample introduction setup included a pneumatic nebulizer of the glass concentric K-style type (Varian) connected to a glass cyclonic spray chamber (Varian).
- Thermogravimetry Differential Thermal Analysis (TG-DTA): the analyses were conducted using a LabsysEvo 1600–Setaram thermobalance equipped with a double thermocouple Platinum/Platinum–Rhodium 10%. The thermocouples were calibrated by using, as calibration materials, high-purity elements such as Ag, Au. Approximately 20 mg of the sample was loaded into an open alumina crucible and subjected to heating from 30 °C to 1250 °C at a rate of 10 °C/min, under argon flow (60 mL/min). In the temperature range considered, the error on mass loss determination was 0.2% and in temperature determination 0.5%.
2.4. Cell Preparation for Electrochemical Tests
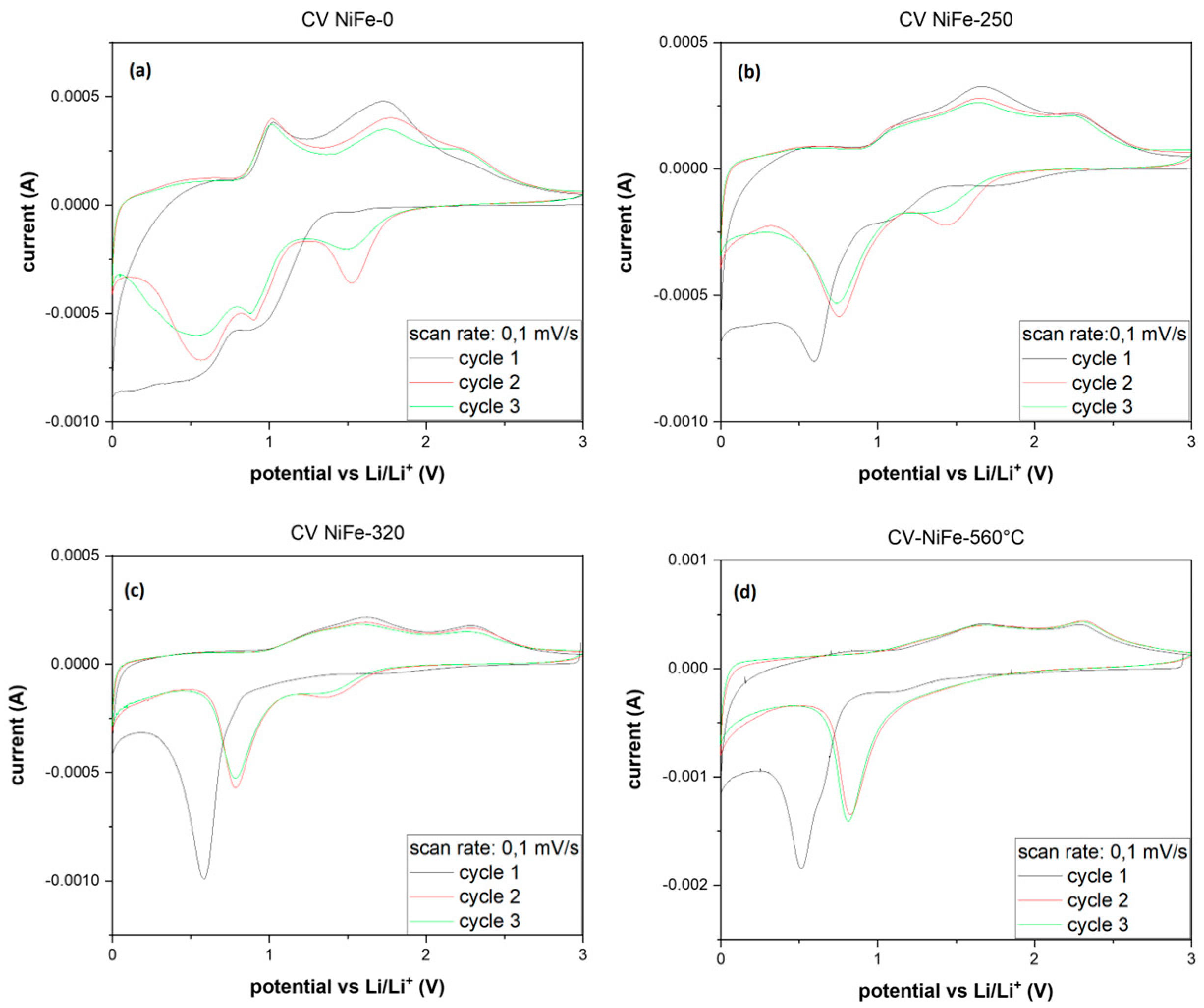
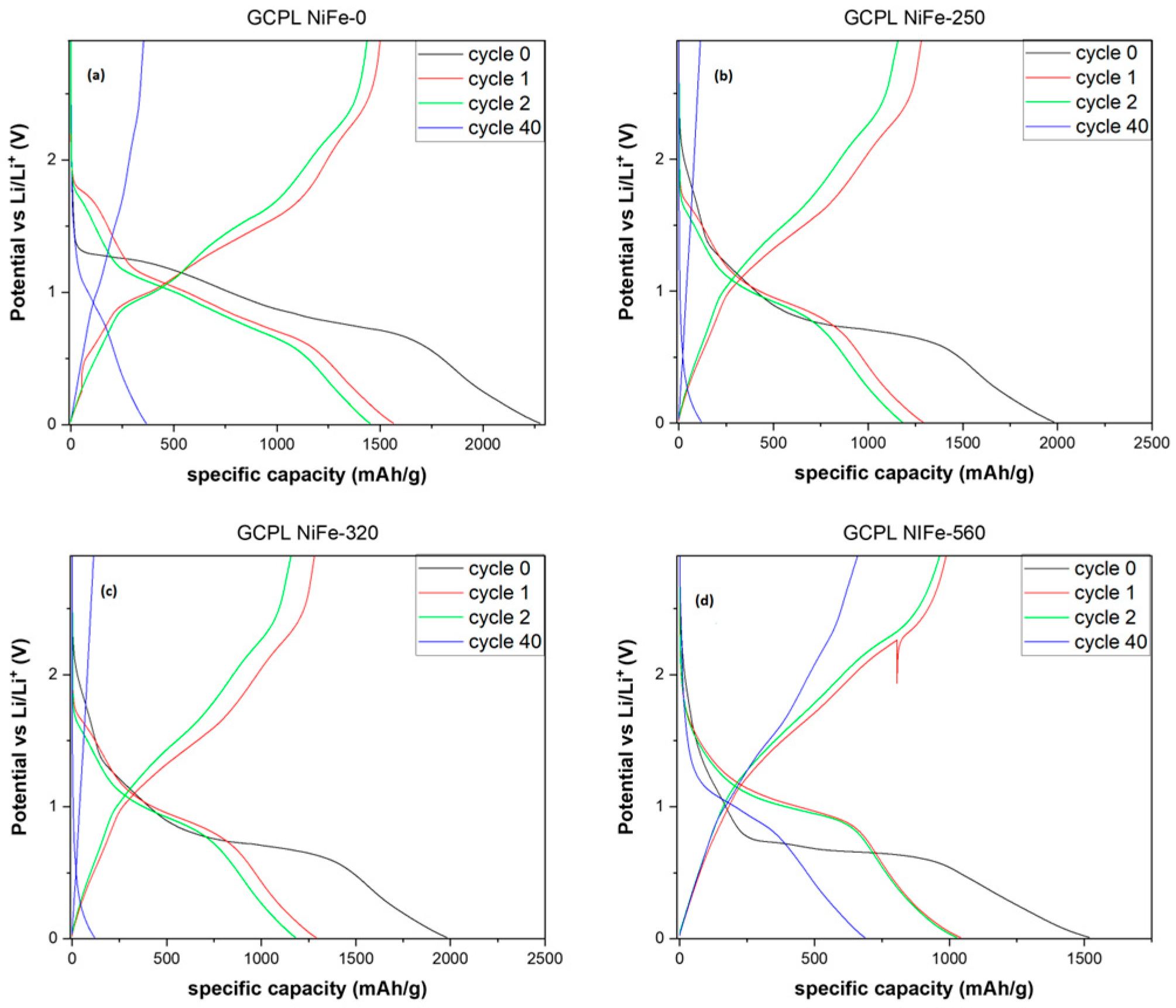
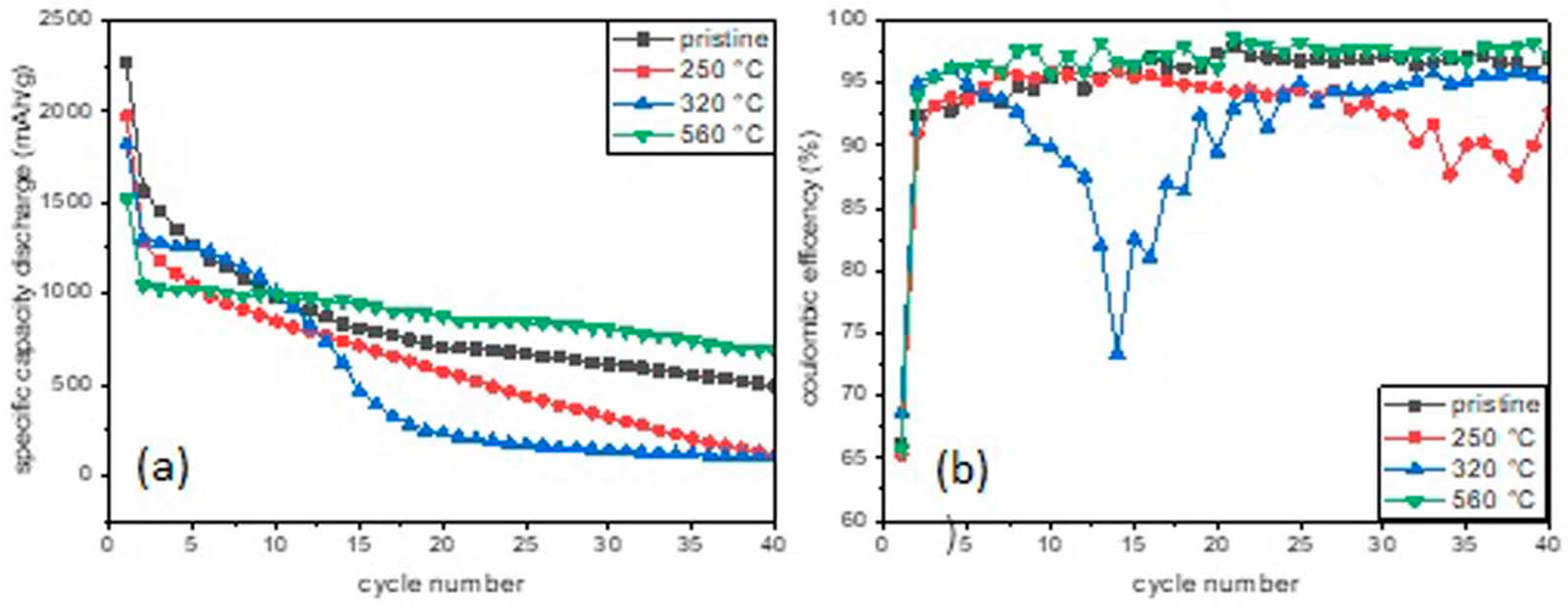
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chavan, S.; Rudrapati, R.; Manickam, S. A comprenhensive review on current advances of thermal energy storage and its applications. Alex. Eng. J. 2022, 61, 5455–5463. [Google Scholar] [CrossRef]
- Hörbe Emanuelsson, A.; Johnsson, F. The cost to consumers of carbon capture and storage—A product value chain analysis. Energies 2023, 16, 7113. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Murtaza, S.Z.M.; Al-Othman, A.; Alami, A.H.; Singh, K.; Olabi, A.G. Ammonia: A versatile candidate for the use in energy storage systems. Renew. Energy 2022, 194, 955–977. [Google Scholar] [CrossRef]
- Navarro-Suárez, A.M.; Shaffer, M.S.P. Designing structural electrochemical energy storage systems: A perspective on the role of device chemistry. Front. Chem. 2021, 9, 810781. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chen, W.; Chen, Y.; Chen, Y.; Chen, Y.; Ding, F.; Fan, C.; Fan, H.J.; Fan, Z.; Gong, C.; et al. Recent Progress on Two-Dimensional Materials. Acta Phys. -Chim. Sin. 2021, 37, 2108017. [Google Scholar] [CrossRef]
- Vairo, T.; Pettinato, M.; Reverberi, A.P.; Milazzo, M.F.; Fabiano, B. An approach towards the implementation of a reliable resilience model based on machine learning. Process Saf. Environ. Prot. 2023, 172, 632–641. [Google Scholar] [CrossRef]
- Deepak Selvakumar, R.; Wu, J.; Ding, Y.; Alkaabi, A.K. Melting behavior of an organic phase change material in a square thermal energy storage capsule with an array of wire electrodes. Appl. Therm. Eng. 2023, 228, 120492. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-ion batteries: Current status and future perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Sarmah, S.; Lakhanlal; Kakati, B.K.; Deka, D. Recent advancement in rechargeable battery technologies. Wiley Interdiscip. Rev. Energy Environ. 2023, 12, e461. [Google Scholar] [CrossRef]
- Patel, M.; Mishra, K.; Banerjee, R.; Chaudhari, J.; Kanchan, D.K.; Kumar, D. Fundamentals, recent developments and prospects of lithium and non-lithium electrochemical rechargeable battery systems. J. Energy Chem. 2023, 81, 221–259. [Google Scholar] [CrossRef]
- Bubulinca, C.; Kazantseva, N.E.; Pechancova, V.; Joseph, N.; Fei, H.; Venher, M.; Ivanichenko, A.; Saha, P. Development of all solid-state Li-ion batteries: From key technical areas to commercial use. Batteries 2023, 9, 157. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Kondo, Y.; Abe, T.; Yamada, Y. Kinetics of interfacial ion transfer in lithium-ion batteries: Mechanism understanding and improvement strategies. ACS Appl. Mater. Interfaces 2022, 14, 22706–22718. [Google Scholar] [CrossRef] [PubMed]
- Tirado, J.L. Inorganic materials for the negative electrode of lithium-ion batteries: State of the art and future prospects. Mater. Sci. Eng. 2003, R40, 103–136. [Google Scholar] [CrossRef]
- Nwachukwu, I.M.; Chinwe Nwanya, A.; Ekwealor, A.B.C.; Ezema, F.I. Recent progress in Mn and Fe-rich cathode materials used in Li-ion batteries. J. Energy Storage 2022, 54, 105248. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Wu, F.; Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 2017, 10, 435–459. [Google Scholar] [CrossRef]
- Manthiram, A. An outlook on lithium battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Xu, W.; Xiao, J.; Cao, X.; Liu, J. Lithium metal anodes with nonaqueous electrolytes. Chem. Rev. 2020, 120, 13312–13348. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, C.; Zhang, L.; Zhang, W.; Gao, L. Comparative study on state of power estimation of lithium-ion battery based on equivalent circuit model. Jixie Gongcheng Xuebao/J. Mech. Eng. 2021, 57, 64–76. [Google Scholar] [CrossRef]
- Kotal, M.; Jakhar, S.; Roy, S.; Sharma, H.K. Cathode materials for rechargeable lithium batteries: Recent progress and future prospects. J. Energy Storage 2022, 47, 103534. [Google Scholar] [CrossRef]
- Shea, J.J.; Luo, C. Organic electrode materials for metal ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 5361–5380. [Google Scholar] [CrossRef]
- Daniel, C.; Mohanty, D.; Li, J.; Wood, D.L. Cathode materials review. Review on Electrochemical Storage Materials and Technology: Proceedings of the 1st International Freiberg Conference on Electrochemical Storage Materials. Proc. AIP Conf. Proc. 2014, 1597, 26–43. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Malode, S.J.; Shetti, N.P.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Electrode materials for lithium-ion batteries. Mater. Sci. Energy Technol. 2018, 1, 182–187. [Google Scholar] [CrossRef]
- Pascariu, V.; Avadanei, O.; Gasner, P.; Stoica, I.; Reverberi, A.P.; Mitoseriu, L. Preparation and characterization of PbTiO 3-epoxy resin compositionally graded thick films. Phase Transit. 2013, 86, 715–725. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Adelhelm, P.; Titirici, M.-M.; Hu, Y.-S. Intercalation chemistry of graphite: Alkali metal ions and beyond. Chem. Soc. Rev. 2019, 48, 4655–4687. [Google Scholar] [CrossRef]
- Kim, H.-J.; Krishna, T.N.V.; Zeb, K.; Rajangam, V.; Muralee Gopi, C.V.V.; Sambasivam, S.; Raghavendra, K.V.G.; Obaidat, I.M. A comprehensive review of Li-ion battery materials and their recycling techniques. Electronics 2020, 9, 1161. [Google Scholar] [CrossRef]
- Cardinale, A.M.; Alberti, S.; Reverberi, A.P.; Catauro, M.; Ghibaudo, N.; Fortunato, M. Antibacterial and photocatalytic activities of LDH-based sorbents of different compositions. Microorganisms 2023, 11, 1045. [Google Scholar] [CrossRef]
- Cardinale, A.M.; Carbone, C.; Fortunato, M.; Fabiano, B.; Reverberi, A.P. ZnAl-SO4 layered double hydroxide and allophane for Cr(VI), Cu(II) and Fe(III) adsorption in wastewater: Structure comparison and synergistic effects. Materials 2022, 15, 6887. [Google Scholar] [CrossRef]
- Li, X.; Fortunato, M.; Cardinale, A.M.; Sarapulova, A.; Njel, C.; Dsoke, S. Electrochemical study on nickel aluminum layered double hydroxides as high-performance electrode material for lithium-ion batteries based on sodium alginate binder. J. Solid State Electrochem. 2022, 26, 49–61. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Vocciante, M.; Salerno, M.; Ferretti, M.; Fabiano, B. Green synthesis of silver nanoparticles by low-energy wet bead milling of metal spheres. Materials 2020, 13, 63. [Google Scholar] [CrossRef]
- Pasman, H.; Sripaul, E.; Khan, F.; Fabiano, B. Energy transition technology comes with new process safety challenges and risks. Process Saf. Environ. Prot. 2023, 177, 765–794. [Google Scholar] [CrossRef]
- Zhang, S.S. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 2020, 24, 247–254. [Google Scholar] [CrossRef]
- Grégoire, B.; Ruby, C.; Carteret, C. Hydrolysis of mixed Ni2+–Fe3+ and Mg2+–Fe3+ solutions and mechanism of formation of layered double hydroxides. J. Chem. Soc. Dalton Trans. 2013, 42, 15687–15698. [Google Scholar] [CrossRef]
- Solchenbach, S.; Pritzl, D.; Kong, E.J.Y.; Landesfeind, J.; Gasteiger, H.A. A gold micro-reference electrode for impedance and potential measurements in lithium ion batteries. J. Electrochem. Soc. 2016, 163, A2265–A2272. [Google Scholar] [CrossRef]
- Ender, M.; Illig, J.; Ivers-Tiffée, E. Three-electrode setups for lithium-ion batteries I. Fem-simulation of different reference electrode designs and their Implications for half-cell impedance spectra. J. Electrochem. Soc. 2017, 164, A71–A79. [Google Scholar] [CrossRef]
- Bodhankar, P.M.; Sarawade, P.B.; Singh, G.; Vinu, A.; Dhawale, D.S. Recent advances in highly active nanostructured NiFe LDH catalyst for electrochemical water splitting. J. Mater. Chem. A 2021, 9, 3180–3208. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríquez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Mater. Sci. Forum 2001, 378–381, 118–123. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, F.; Fang, L.; Hu, J.; Luo, H.; Guan, T.; Hu, B.; Zhou, M. Layered NiFe-LDH/MXene nanocomposite electrode for high-performance supercapacitor. Int. J. Hydrogen Energy 2020, 45, 13080–13089. [Google Scholar] [CrossRef]
- Tian, M.; Liu, C.; Neale, Z.G.; Zheng, J.; Long, D.; Cao, G. Chemically Bonding NiFe-LDH Nanosheets on rGO for Superior Lithium-Ion Capacitors. ACS Appl. Mater. Interfaces 2019, 11, 35977–35986. [Google Scholar] [CrossRef]
- Zhou, G.; Ding, W.; Guan, Y.; Wang, T.; Liu, C.; Zhang, L.; Yin, J.; Fu, Y. Progress of NiO-Based Anodes for High-Performance Li-Ion Batteries. Chemical Record. 2022, 22, e202200111. [Google Scholar] [CrossRef]
- Yang, C.C.; Zhang, D.M.; Du, L.; Jiang, Q. Hollow Ni-NiO nanoparticles embedded in porous carbon nanosheets as a hybrid anode for sodium-ion batteries with an ultra-long cycle life. J. Mater. Chem. A 2018, 6, 12663–12671. [Google Scholar] [CrossRef]
- Iftikhar, M.; Latif, S.; Jevtovic, V.; Ashraf, I.M.; El-Zahhar, A.A.; Saleh, E.A.M.; Abbas, S.M. Current advances and prospects in NiO-based lithium-ion battery anodes. Sustain. Energy Technol. Assess. 2022, 53, 102376. [Google Scholar] [CrossRef]
| Name | Formula | Purity (Mass%) |
|---|---|---|
| Iron (III) nitrate nonahydrate | Fe(NO3)3·9H2O | ACS reagent, ≥98% by Sigma-Aldrich Co., LLC. (St. Louis, MO, USA) |
| Nickel nitrate hexahydrate | Ni(NO3)2·6H2O | ACS reagent, ≥98.5% by Sigma-Aldrich Co., LLC. (St. Louis, MO, USA) |
| Sodium hydroxide | NaOH | Reagent grade, ≥98% (anhydrous) by Carlo Erba srl (Cornaredo MI, Italy) |
| Lithium hexafluorophosphate | LiPF6 | Commercial LP30, 1 M battery grade solution in EC/DMC, ≥99.5% by Sigma-Aldrich Co., LLC. (St. Louis, MO, USA) |
| Isopropanol | CH3CHOHCH3 | Purum p.a., ≥97% by Labbox ITALIA, S.R.L (Cornaredo MI, Italy) |
| Conductive carbon C65 | … | Commercial battery grade, ≥99.5% by TIMCAL. Ltd. (Bodio, Switzerland) |
| Sodium alginate | (NaC6H7O6)n | Purum p.a., ≥99.8% by Sigma-Aldrich Co., LLC (St. Louis, MO, USA) |
| Element | Amount of Element (Mass%) | Amount of Element (mol) |
|---|---|---|
| Ni | 17.0 | 0.30 |
| Fe | 35.8 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, M.; Reverberi, A.P.; Fabiano, B.; Cardinale, A.M. Thermal Evolution of NiFe-NO3 LDH and Its Application in Energy Storage Systems. Energies 2024, 17, 1035. https://doi.org/10.3390/en17051035
Fortunato M, Reverberi AP, Fabiano B, Cardinale AM. Thermal Evolution of NiFe-NO3 LDH and Its Application in Energy Storage Systems. Energies. 2024; 17(5):1035. https://doi.org/10.3390/en17051035
Chicago/Turabian StyleFortunato, Marco, Andrea Pietro Reverberi, Bruno Fabiano, and Anna Maria Cardinale. 2024. "Thermal Evolution of NiFe-NO3 LDH and Its Application in Energy Storage Systems" Energies 17, no. 5: 1035. https://doi.org/10.3390/en17051035
APA StyleFortunato, M., Reverberi, A. P., Fabiano, B., & Cardinale, A. M. (2024). Thermal Evolution of NiFe-NO3 LDH and Its Application in Energy Storage Systems. Energies, 17(5), 1035. https://doi.org/10.3390/en17051035







