Green Hydrogen Value Chain: Modelling of a PV Power Plant Integrated with H2 Production for Industry Application
Abstract
1. Introduction
2. Green Hydrogen Value Chain
2.1. Hydrogen Production through Water Electrolysis
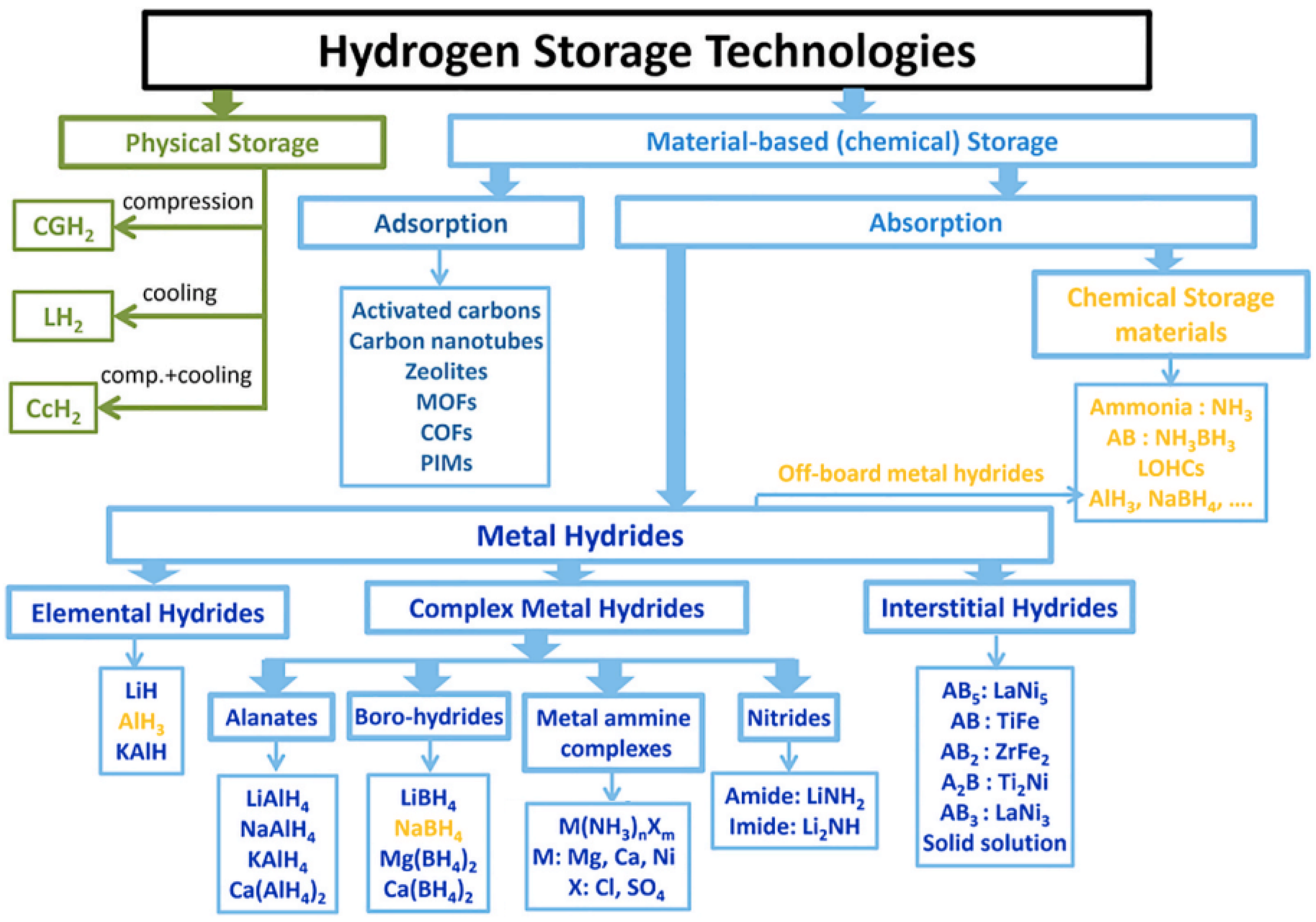
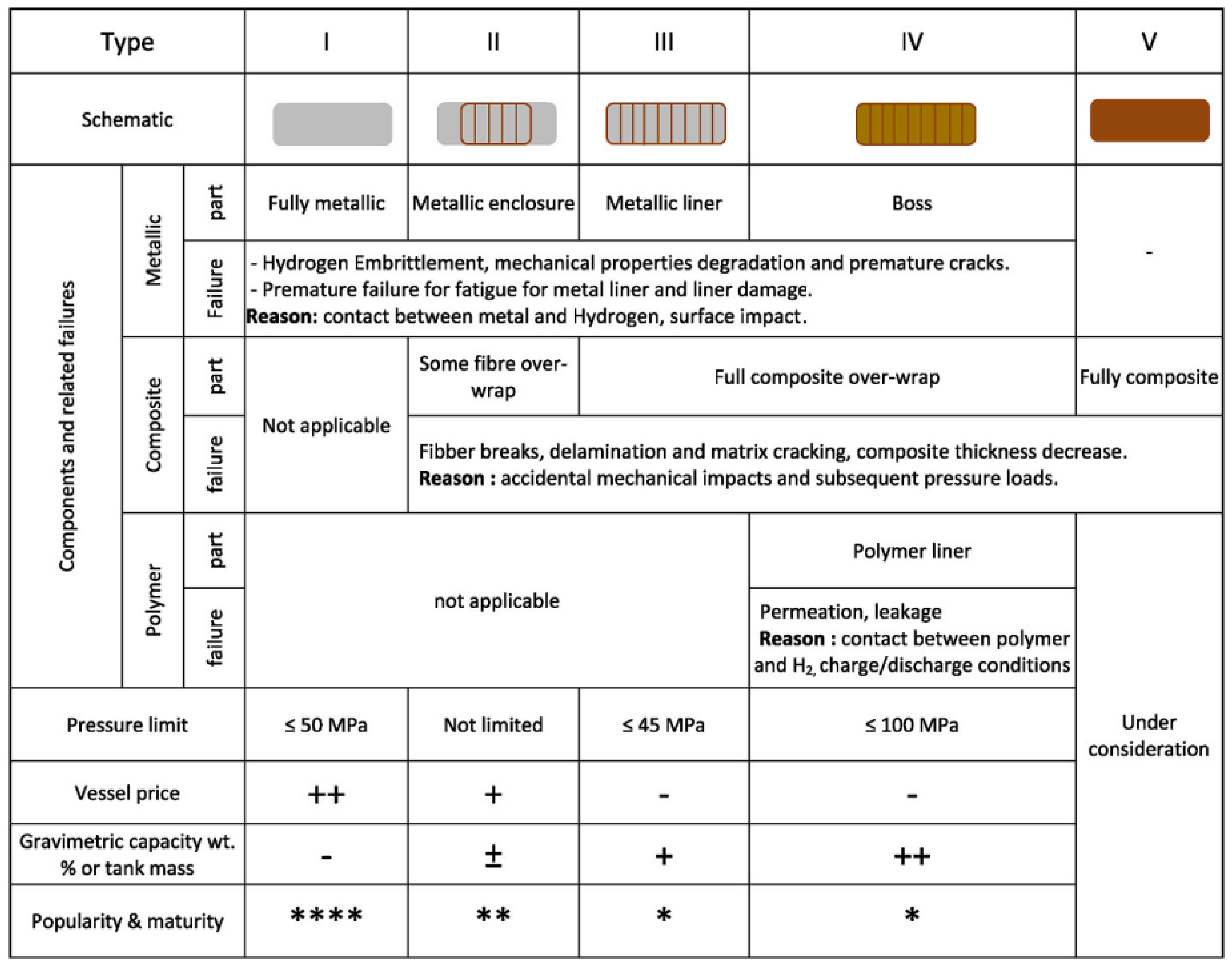
2.2. Hydrogen Storage
2.3. Hydrogen Transportation
2.3.1. Gaseous Hydrogen Transportation
2.3.2. Liquid Hydrogen Transportation
2.4. Hydrogen Applications
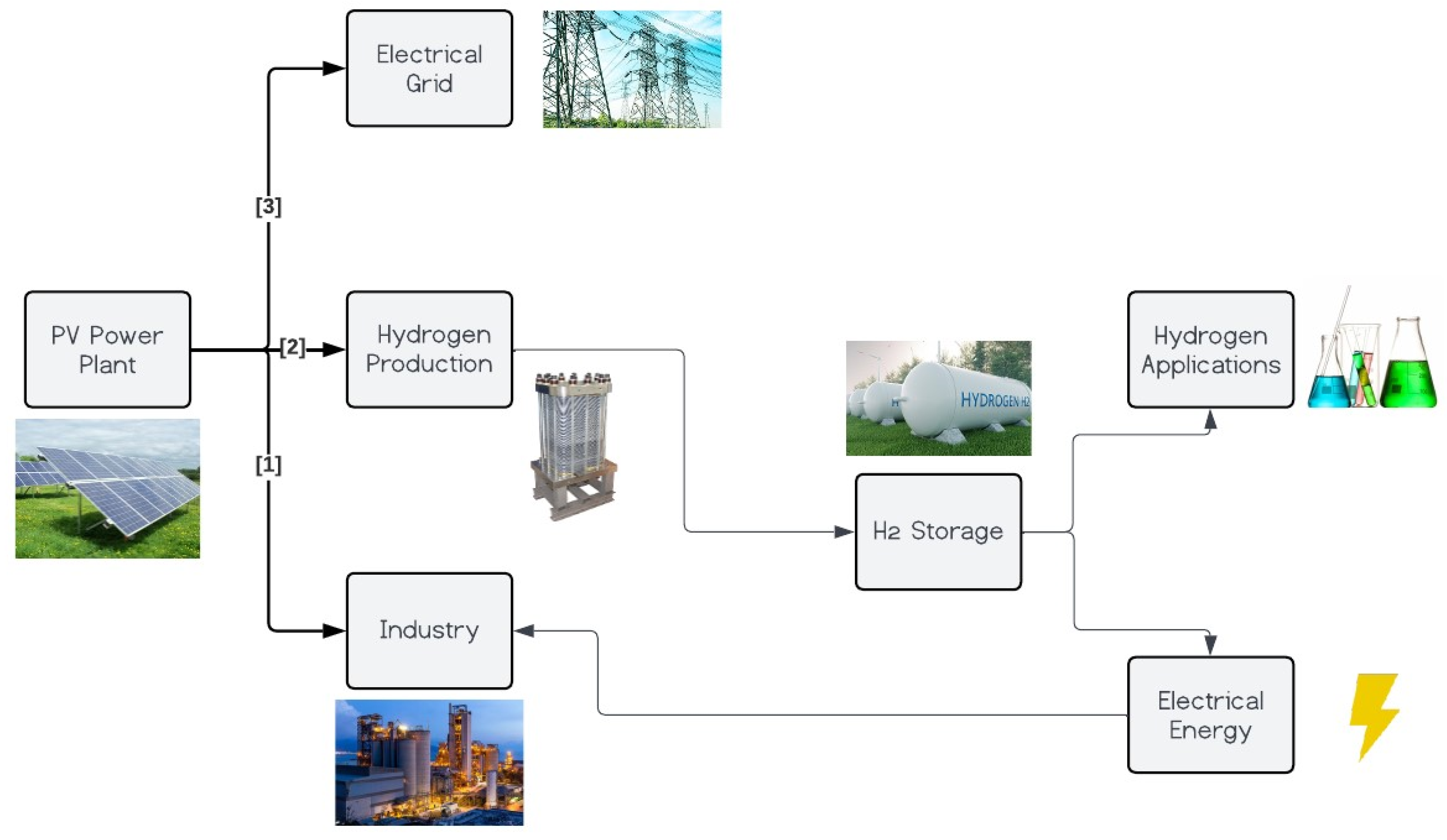
3. Modelling a PV Power Plant Integrated with H2 Production for Industry
3.1. PV Power Production
3.2. Water Electrolyser
3.3. Compressor
3.4. Fuel Cell
3.5. Definition of Conceptual Model Configuration
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- DNV. Energy Transition Outlook 2022-A Global and Regional Forecast to 2050; DNV: Bærum, Norway, 2022. [Google Scholar]
- International Energy Agency. Statistics Report Key World Energy Statistics 2021; International Energy Agency: Paris, France, 2021. [Google Scholar]
- International Energy Agency: IEA. Solar Heat for Industry; International Energy Agency: Paris, France, 2017. [Google Scholar]
- Xu, X.; Zhou, Q.; Yu, D. The Future of Hydrogen Energy: Bio-Hydrogen Production Technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-Year Trends of the Global Burden of Disease Attributable to Ambient Air Pollution: An Analysis of Data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Ehsan, A.; Preece, R. Quantifying the Impacts of Heat Decarbonisation Pathways on the Future Electricity and Gas Demand. Energy 2022, 254, 124229. [Google Scholar] [CrossRef]
- United Nations. The Sustainable Development Goals Report; United Nations: San Francisco, CA, USA, 2022. [Google Scholar]
- IPCC. Global Warming of 1.5 °C; IPCC: Geneva, Switzerlaznd, 2019. [Google Scholar]
- European Commission. A European Strategic Long-Term Vision for a Prosperous, Modern, Competitive and Climate Neutral Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Wappler, M.; Unguder, D.; Lu, X.; Ohlmeyer, H.; Teschke, H.; Lueke, W. Building the Green Hydrogen Market–Current State and Outlook on Green Hydrogen Demand and Electrolyzer Manufacturing. Int. J. Hydrogen Energy 2022, 47, 33551–33570. [Google Scholar] [CrossRef]
- United Nations Conference of the Parties (COP). Available online: https://unfccc.int/process/bodies/supreme-bodies/conference-of-the-parties-cop (accessed on 26 November 2023).
- Fuel Cells and Hydrogen 2 Joint Undertaking. A Sustainable Pathway for the European Energy Transition-Hydrogen Roadmap (Europe); European Comission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. The European Green Deal; European Comission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission A European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 26 November 2022).
- Panarello, D.; Gatto, A. Decarbonising Europe–EU Citizens’ Perception of Renewable Energy Transition amidst the European Green Deal. Energy Policy 2023, 172, 113272. [Google Scholar] [CrossRef]
- Banguero, E.; Correcher, A.; Pérez-Navarro, Á.; García, E.; Aristizabal, A. Diagnosis of a Battery Energy Storage System Based on Principal Component Analysis. Renew. Energy 2020, 146, 2438–2449. [Google Scholar] [CrossRef]
- Cot-Gores, J.; Castell, A.; Cabeza, L.F. Thermochemical Energy Storage and Conversion: A-State-of-the-Art Review of the Experimental Research under Practical Conditions. Renew. Sustain. Energy Rev. 2012, 16, 5207–5224. [Google Scholar] [CrossRef]
- Lagioia, G.; Spinelli, M.P.; Amicarelli, V. Blue and Green Hydrogen Energy to Meet European Union Decarbonisation Objectives. An Overview of Perspectives and the Current State of Affairs. Int. J. Hydrogen Energy 2023, 48, 1304–1322. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, X.; Wen, H.; Pei, A. Hydrogen Production from Offshore Wind Power in South China. Int. J. Hydrogen Energy 2022, 47, 24558–24568. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen Production, Storage, Transportation and Key Challenges with Applications: A Review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, B.; Du, E.; Zhang, N.; Yang, X.; Yang, F.; Wu, Z. Modeling Hydrogen Supply Chain in Renewable Electric Energy System Planning. IEEE Trans. Ind. Appl. 2022, 58, 2780–2791. [Google Scholar] [CrossRef]
- Escamilla, A.; Sánchez, D.; García-Rodríguez, L. Assessment of Power-to-Power Renewable Energy Storage Based on the Smart Integration of Hydrogen and Micro Gas Turbine Technologies. Int. J. Hydrogen Energy 2022, 47, 17505–17525. [Google Scholar] [CrossRef]
- Riera, J.A.; Lima, R.M.; Knio, O.M. A Review of Hydrogen Production and Supply Chain Modeling and Optimization. Int. J. Hydrogen Energy 2023, 48, 13731–13755. [Google Scholar] [CrossRef]
- Frankowska, M.; Rzeczycki, A.; Sowa, M.; Drożdż, W. Functional Model of Power Grid Stabilization in the Green Hydrogen Supply Chain System—Conceptual Assumptions. Energies 2023, 16, 154. [Google Scholar] [CrossRef]
- Forghani, K.; Kia, R.; Nejatbakhsh, Y. A Multi-Period Sustainable Hydrogen Supply Chain Model Considering Pipeline Routing and Carbon Emissions: The Case Study of Oman. Renew. Sustain. Energy Rev. 2023, 173, 113051. [Google Scholar] [CrossRef]
- Pinsky, R.; Sabharwall, P.; Hartvigsen, J.; O’Brien, J. Comparative Review of Hydrogen Production Technologies for Nuclear Hybrid Energy Systems. Prog. Nucl. Energy 2020, 123, 103317. [Google Scholar] [CrossRef]
- Al-Qahtani, A.; Parkinson, B.; Hellgardt, K.; Shah, N.; Guillen-Gosalbez, G. Uncovering the True Cost of Hydrogen Production Routes Using Life Cycle Monetisation. Appl. Energy 2021, 281, 115958. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The Survey of Key Technologies in Hydrogen Energy Storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Sgarbossa, F.; Arena, S.; Tang, O.; Peron, M. Renewable Hydrogen Supply Chains: A Planning Matrix and an Agenda for Future Research. Int. J. Prod. Econ. 2023, 255, 108674. [Google Scholar] [CrossRef]
- Moradpoor, I.; Syri, S.; Santasalo-Aarnio, A. Green Hydrogen Production for Oil Refining–Finnish Case. Renew. Sustain. Energy Rev. 2023, 175, 113159. [Google Scholar] [CrossRef]
- Saur, G.; Ramsden, T. Wind Electrolysis: Hydrogen Cost Optimization; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2011.
- Palys, M.J.; Daoutidis, P. Power-to-X: A Review and Perspective. Comput. Chem. Eng. 2022, 165, 107948. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen Storage Technologies for Stationary and Mobile Applications: Review, Analysis and Perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Bondarenko, V.L.; Ilyinskaya, D.N.; Kazakova, A.A.; Kozlovtsev, P.S.; Lavrov, N.A.; Razenko, E.A. Hydrogen Storage. Chem. Pet. Eng. 2022, 57, 1026–1032. [Google Scholar] [CrossRef]
- Reuß, M.; Grube, T.; Robinius, M.; Preuster, P.; Wasserscheid, P.; Stolten, D. Seasonal Storage and Alternative Carriers: A Flexible Hydrogen Supply Chain Model. Appl. Energy 2017, 200, 290–302. [Google Scholar] [CrossRef]
- He, G.; Mallapragada, D.S.; Bose, A.; Heuberger, C.F.; Gencer, E. Hydrogen Supply Chain Planning with Flexible Transmission and Storage Scheduling. IEEE Trans. Sustain. Energy 2021, 12, 1730–1740. [Google Scholar] [CrossRef]
- Maroufmashat, A.; Fowler, M. Transition of Future Energy System Infrastructure; through Power-to-Gas Pathways. Energies 2017, 10, 1089. [Google Scholar] [CrossRef]
- Guilbert, D.; Collura, S.M.; Scipioni, A. DC/DC Converter Topologies for Electrolyzers: State-of-the-Art and Remaining Key Issues. Int. J. Hydrogen Energy 2017, 42, 23966–23985. [Google Scholar] [CrossRef]
- Kim, A.; Kim, H.; Choe, C.; Lim, H. Feasibility of Offshore Wind Turbines for Linkage with Onshore Green Hydrogen Demands: A Comparative Economic Analysis. Energy Convers. Manag. 2023, 277, 116662. [Google Scholar] [CrossRef]
- Crespi, E.; Colbertaldo, P.; Guandalini, G.; Campanari, S. Energy Storage with Power-to-Power Systems Relying on Photovoltaic and Hydrogen: Modelling the Operation with Secondary Reserve Provision. J. Energy Storage 2022, 55, 105613. [Google Scholar] [CrossRef]
- Palys, M.J.; Daoutidis, P. Optimizing Renewable Ammonia Production for a Sustainable Fertilizer Supply Chain Transition. ChemSusChem 2023, 16, e202300563. [Google Scholar] [CrossRef]
- Kotowicz, J.; Bartela, Ł.; Węcel, D.; Dubiel, K. Hydrogen Generator Characteristics for Storage of Renewably-Generated Energy. Energy 2017, 118, 156–171. [Google Scholar] [CrossRef]
- Moran, C.; Deane, P.; Yousefian, S.; Monaghan, R.F.D. The Hydrogen Storage Challenge: Does Storage Method and Size Affect the Cost and Operational Flexibility of Hydrogen Supply Chains? Int. J. Hydrogen Energy 2024, 52, 1090–1100. [Google Scholar] [CrossRef]
- Chen, F.; Ma, Z.; Nasrabadi, H.; Chen, B.; Mehana, M.; Van Wijk, J.W. Technical and Economic Feasibility Analysis of Underground Hydrogen Storage: A Case Study in Intermountain-West Region USA. arXiv 2022, arXiv:2209.03239. [Google Scholar]
- Kotowicz, J.; Węcel, D.; Jurczyk, M. Analysis of Component Operation in Power-to-Gas-to-Power Installations. Appl. Energy 2018, 216, 45–59. [Google Scholar] [CrossRef]
- Collins Leigh SPECIAL REPORT|Why Shipping Pure Hydrogen around the World Might Already Be Dead in the Water. Available online: https://www.rechargenews.com/energy-transition/special-report-why-shipping-pure-hydrogen-around-the-world-might-already-be-dead-in-the-water/2-1-1155434 (accessed on 22 July 2023).
- Yang, Y.; Ma, C.; Lian, C.; Zhang, Y.; Pang, X. Optimal Power Reallocation of Large-Scale Grid-Connected Photovoltaic Power Station Integrated with Hydrogen Production. J. Clean. Prod. 2021, 298, 126830. [Google Scholar] [CrossRef]
- Chatterjee, S.; Parsapur, R.K.; Huang, K.W. Limitations of Ammonia as a Hydrogen Energy Carrier for the Transportation Sector. ACS Energy Lett. 2021, 6, 4390–4394. [Google Scholar] [CrossRef]
- Grieef-Dickerson, N.; Turner, L.; Hughes, T. Improving Hydrogen Liquefaction Efficiency Via Spin Isomer Separation. SSRN Electron. J. 2023, 1, 4479246. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review 2022; IEA: Paris, France, 2022. [Google Scholar]
- Zou, C.; Li, J.; Zhang, X.; Jin, X.; Xiong, B.; Yu, H.; Liu, X.; Wang, S.; Li, Y.; Zhang, L.; et al. Industrial Status, Technological Progress, Challenges, and Prospects of Hydrogen Energy. Nat. Gas Ind. B 2022, 9, 427–447. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review 2021; IEA: Paris, France, 2021. [Google Scholar]
- Sazali, N. Emerging Technologies by Hydrogen: A Review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Von Zuben, T.W.; Moreira, D.E.B.; Germscheidt, R.L.; Yoshimura, R.G.; Dorretto, D.S.; de Araujo, A.B.S.; Salles, A.G.; Bonacin, J.A. Is Hydrogen Indispensable for a Sustainable World? A Review of H2 Applications and Perspectives for the Next Years. J. Braz. Chem. Soc. 2022, 33, 824–843. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in Energy Transition: A Review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Fonseca, J.D.; Camargo, M.; Commenge, J.M.; Falk, L.; Gil, I.D. Trends in Design of Distributed Energy Systems Using Hydrogen as Energy Vector: A Systematic Literature Review. Int. J. Hydrogen Energy 2019, 44, 9486–9504. [Google Scholar] [CrossRef]
- Li, R.; Kawanami, H. A Recent Review of Primary Hydrogen Carriers, Hydrogen Production Methods, and Applications. Catalysts 2023, 13, 562. [Google Scholar] [CrossRef]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of Renewable Energy Power Fluctuations on Water Electrolysis for Green Hydrogen Production. Int. J. Hydrogen Energy 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Martínez-García, E.; Blanco-Marigorta, E.; Parrondo Gayo, J.; Navarro-Manso, A. Influence of Inertia and Aspect Ratio on the Torsional Galloping of Single-Axis Solar Trackers. Eng. Struct. 2021, 243, 112682. [Google Scholar] [CrossRef]
- Kang, H.; Hong, T.; Jung, S.; Lee, M. Techno-Economic Performance Analysis of the Smart Solar Photovoltaic Blinds Considering the Photovoltaic Panel Type and the Solar Tracking Method. Energy Build. 2019, 193, 1–14. [Google Scholar] [CrossRef]
- Salas, V.; Olías, E.; Barrado, A.; Lázaro, A. Review of the Maximum Power Point Tracking Algorithms for Stand-Alone Photovoltaic Systems. Sol. Energy Mater. Sol. Cells 2006, 90, 1555–1578. [Google Scholar] [CrossRef]
- Węcel, D.; Jurczyk, M.; Uchman, W.; Skorek-Osikowska, A. Investigation on System for Renewable Electricity Storage in Small Scale Integrating Photovoltaics, Batteries, and Hydrogen Generator. Energies 2020, 13, 6039. [Google Scholar] [CrossRef]
- Tajuddin, M.F.N.; Arif, M.S.; Ayob, S.M.; Salam, Z. Perturbative Methods for Maximum Power Point Tracking (MPPT) of Photovoltaic (PV) Systems: A Review. Int. J. Energy Res. 2015, 39, 1153–1178. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An Overview of Water Electrolysis Technologies for Green Hydrogen Production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Hussain, C. Renewable Hydrogen production by Water Electrolysis. In Sustainable Fuel Technologies Handbook; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Nami, H.; Rizvandi, O.B.; Chatzichristodoulou, C.; Hendriksen, P.V.; Frandsen, H.L. Techno-Economic Analysis of Current and Emerging Electrolysis Technologies for Green Hydrogen Production. Energy Convers. Manag. 2022, 269, 116162. [Google Scholar] [CrossRef]
- Krishnan, S.; Koning, V.; Theodorus de Groot, M.; de Groot, A.; Mendoza, P.G.; Junginger, M.; Kramer, G.J. Present and Future Cost of Alkaline and PEM Electrolyser Stacks. Int. J. Hydrogen Energy 2023, 48, 32313–32330. [Google Scholar] [CrossRef]
- Amireh, S.F.; Heineman, N.N.; Vermeulen, P.; Barros, R.L.G.; Yang, D.; van der Schaaf, J.; de Groot, M.T. Impact of Power Supply Fluctuation and Part Load Operation on the Efficiency of Alkaline Water Electrolysis. J. Power Sources 2023, 560, 232629. [Google Scholar] [CrossRef]
- Matute, G.; Yusta, J.M.; Beyza, J.; Correas, L.C. Multi-State Techno-Economic Model for Optimal Dispatch of Grid Connected Hydrogen Electrolysis Systems Operating under Dynamic Conditions. Int. J. Hydrogen Energy 2021, 46, 1449–1460. [Google Scholar] [CrossRef]
- Reksten, A.H.; Thomassen, M.S.; Møller-Holst, S.; Sundseth, K. Projecting the Future Cost of PEM and Alkaline Water Electrolysers; a CAPEX Model Including Electrolyser Plant Size and Technology Development. Int. J. Hydrogen Energy 2022, 47, 38106–38113. [Google Scholar] [CrossRef]
- Brun, K.; Kurz, R. Compression Machinery for Oil and Gas; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Bavane, V.; Nawale, V.; Ingle, N. Fuel Cell Based Future Power Supply for Remote Locations. Int. J. Sci. Res. Eng. Manag. (IJSREM) 2023, 7. Available online: https://www.researchgate.net/profile/Vikas-Bavane/publication/370983255_Fuel_Cell_Based_Future_Power_Supply_for_Remote_Locations/links/646d91d337d6625c002c7a43/Fuel-Cell-Based-Future-Power-Supply-for-Remote-Locations.pdf (accessed on 23 December 2023).
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Rahman Farooqi, Z.U.; Lee, C. Hydrogen Production through Renewable and Non-Renewable Energy Processes and Their Impact on Climate Change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- Brouwer, J. On the Role of Fuel Cells and Hydrogen in a More Sustainable and Renewable Energy Future. Curr. Appl. Phys. 2010, 10, S9–S17. [Google Scholar] [CrossRef]
- Huang, E. Design of Hydrogen Fuel Cell: Methods to Higher Efficiency. In Proceedings of the 2nd International Conference on Green Environmental Materials and Food Engineering (GEMFE 2022), Tianjin, China, 19–20 November 2022; Volume 2022. [Google Scholar]
- Phan Van, L.; Hieu Hoang, L.; Nguyen Duc, T. A Comprehensive Review of Direct Coupled Photovoltaic-Electrolyser System: Sizing Techniques, Operating Strategies, Research Progress, Current Challenges, and Future Recommendations. Int. J. Hydrogen Energy 2023, 48, 25231–25249. [Google Scholar] [CrossRef]
- Guilbert, D.; Sorbera, D.; Vitale, G. A Stacked Interleaved DC-DC Buck Converter for Proton Exchange Membrane Electrolyzer Applications: Design and Experimental Validation. Int. J. Hydrogen Energy 2020, 45, 64–79. [Google Scholar] [CrossRef]
- Garrigós, A.; Lizán, J.L.; Blanes, J.M.; Gutiérrez, R. Combined Maximum Power Point Tracking and Output Current Control for a Photovoltaic-Electrolyser DC/DC Converter. Int. J. Hydrogen Energy 2014, 39, 20907–20919. [Google Scholar] [CrossRef]
- García-Valverde, R.; Miguel, C.; Martínez-Béjar, R.; Urbina, A. Optimized Photovoltaic Generator-Water Electrolyser Coupling through a Controlled DC-DC Converter. Int. J. Hydrogen Energy 2008, 33, 5352–5362. [Google Scholar] [CrossRef]
- Ergin Şahin, M. A Photovoltaic Powered Electrolysis Converter System with Maximum Power Point Tracking Control. Int. J. Hydrogen Energy 2020, 45, 9293–9304. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental Aspects of Fuel Cells: A Review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.L.L.C.C.; Weeda, M.; Detz, R.J.; van der Zwaan, B. Country-Specific Cost Projections for Renewable Hydrogen Production through off-Grid Electricity Systems. Appl. Energy 2022, 309, 118398. [Google Scholar] [CrossRef]


| Input | Reference | Year | Nuclear Electrolysis | PV Electrolysis | Wind Electrolysis | PV + Wind Electrolysis | |
|---|---|---|---|---|---|---|---|
| Onshore | Offshore | ||||||
| Electricity [kWh] | [23] | 2023 | 54.2 | 54.2 | 54.2 | - | |
| Water [kg] | [23] | 2019 | 10 | 10 | 10 | - | |
| LCOH [USD/kg or €/kg] | [23] | 2019 | 4.3 | 9.49 | 5.6 | - | |
| [32] | 2020 | - | (USD) 7.5 | (USD) 4.4 | (USD) 4.2 | (€) 3.5 | |
| Reference | Type of Storage | Type of Compression | Geological Storage | |
|---|---|---|---|---|
| Low Pressure | High Pressure | |||
| [39] | Pressure range [bar] | Up to 200 | Up to 700 | 20 up to 180 |
| Type of vessel | Based on SA516 Grade 70 carbon steel | - | Salt caverns depleted oil and gas fields and aquifers | |
| Application | Stationary applications | Transportation fuel (refuelling stations) | Stationary applications | |
| [22] | Pressure range [bar] | 50 up to 400 | Up to 900 | - |
| Type of vessel | - | Carbon fibre composite pressure vessels | - | |
| Application | Stationary applications | Mobility applications | - | |
| [36] | Pressure range [bar] | Up to 206.65 | - | |
| Type of vessel | Standard steel cylinders | - | ||
| Application | - | - | ||
| [37] | Pressure range [bar] | 200 up to 350 | 350 or 700 | - |
| Type of vessel | - | - | ||
| Application | Stationary tube systems | Automotive applications (on-board hydrogen storage) | - | |
| [43] | Pressure range [bar] | 200 up to 1000 | Up to 200 | |
| Type of vessel | Composite shell associated with a permeation barrier | - | ||
| Application | Transport applications (700 bar) | - | ||
| [25] | Pressure range [bar] | 15 bar up to 250 | 60 up to 150 | |
| Type of vessel | - | Salt cavern | ||
| Application | - | - | ||
| [41] | Pressure range [bar] | 15 bar up to 250 bar | - | |
| Type of vessel | - | - | ||
| Application | - | - | ||
| [42] | Pressure range [bar] | 30 to 200 | - | |
| Type of vessel | - | - | ||
| Application | - | - | ||
| [47] | Pressure range [bar] | - | - | |
| Type of vessel | Cylindrical or spherical pressure tanks | - | ||
| Application | - | - | ||
| [44] | Pressure range [bar] | - | Up to 700 | 60 up to 180 |
| Type of vessel | Cylindrical or spherical tanks | - | ||
| Application | - | Electrical energy production (through fuel cell devices) | Rock caverns, and coal or salt mines | |
| Parameter | Alkaline | PEM | Solid Oxide |
|---|---|---|---|
| Electrolyte | KOH/NaOH (5 M) | Solid polymer electrolyte (PFSA) | Yttria stabilised Zirconia (YSZ) |
| Separator | Asbestos/Zirfon/Ni | Nafion | Solid electrolyte YSZ |
| Electrode/Catalyst (Hydrogen: side) | Nickel-coated perforated stainless steel | Iridium oxide | Ni/YSZ |
| Electrode/Catalyst (Oxygen side) | Nickel-coated perforated stainless steel | Platinum Carbon | Perovskites (LSCF, LSM) (La, Sr, Co, FE) (La, Sr, Mn) |
| Gas Diffusion layer | Nickel mesh | Titanium mesh/carbon cloth | Nickel mesh/foam |
| Bipolar Plates | Stainless steel/Nickel coated stainless steel | Platinum/Gold-coated Titanium or Titanium | Cobalt-coated stainless steel |
| Nominal current density | 0.2–0.8 A/cm2 | 1–2 A/cm2 | 0.3–1 A/cm2 |
| Voltage range (limits) | 1.4–3 V | 1.4–2.5 V | 1.0–1.5 V |
| Operating temperature | 70–90 °C | 50–80 °C | 700–850 °C |
| Cell pressure | <30 bar | <70 bar | 1 bar |
| H2 purity | 99.5–99.9998% | 99.9–99.9999% | 99.9% |
| Efficiency | 50–78% | 50–83% | 89% (laboratory) |
| Lifetime (stack) | 60,000 h | 50,000–80,000 h | 20,000 h |
| Development status | Mature | Commercialised | R & D |
| Electrode area | 10,000–30,000 cm2 | 1500 cm2 | 200 cm2 |
| Capital costs (stack) minimum 1 MW | 270 USD/kW | 400 USD/kW | >2000 USD/kW |
| Capital costs (stack) minimum 10 MW | 500–1000 USD/kW | 700–1400 USD/kW | Unknown |
| Stack specific costs | 262–419 USD/kW | 415–1158 USD/kW | 1100–1300 USD/kW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, H.; Ferreira, A.C.; Teixeira, S.F.; Teixeira, J.C. Green Hydrogen Value Chain: Modelling of a PV Power Plant Integrated with H2 Production for Industry Application. Energies 2024, 17, 1414. https://doi.org/10.3390/en17061414
Machado H, Ferreira AC, Teixeira SF, Teixeira JC. Green Hydrogen Value Chain: Modelling of a PV Power Plant Integrated with H2 Production for Industry Application. Energies. 2024; 17(6):1414. https://doi.org/10.3390/en17061414
Chicago/Turabian StyleMachado, Hugo, Ana Cristina Ferreira, Senhorinha F. Teixeira, and José Carlos Teixeira. 2024. "Green Hydrogen Value Chain: Modelling of a PV Power Plant Integrated with H2 Production for Industry Application" Energies 17, no. 6: 1414. https://doi.org/10.3390/en17061414
APA StyleMachado, H., Ferreira, A. C., Teixeira, S. F., & Teixeira, J. C. (2024). Green Hydrogen Value Chain: Modelling of a PV Power Plant Integrated with H2 Production for Industry Application. Energies, 17(6), 1414. https://doi.org/10.3390/en17061414








