Enhancing Biodiesel Production: A Review of Microchannel Reactor Technologies
Abstract
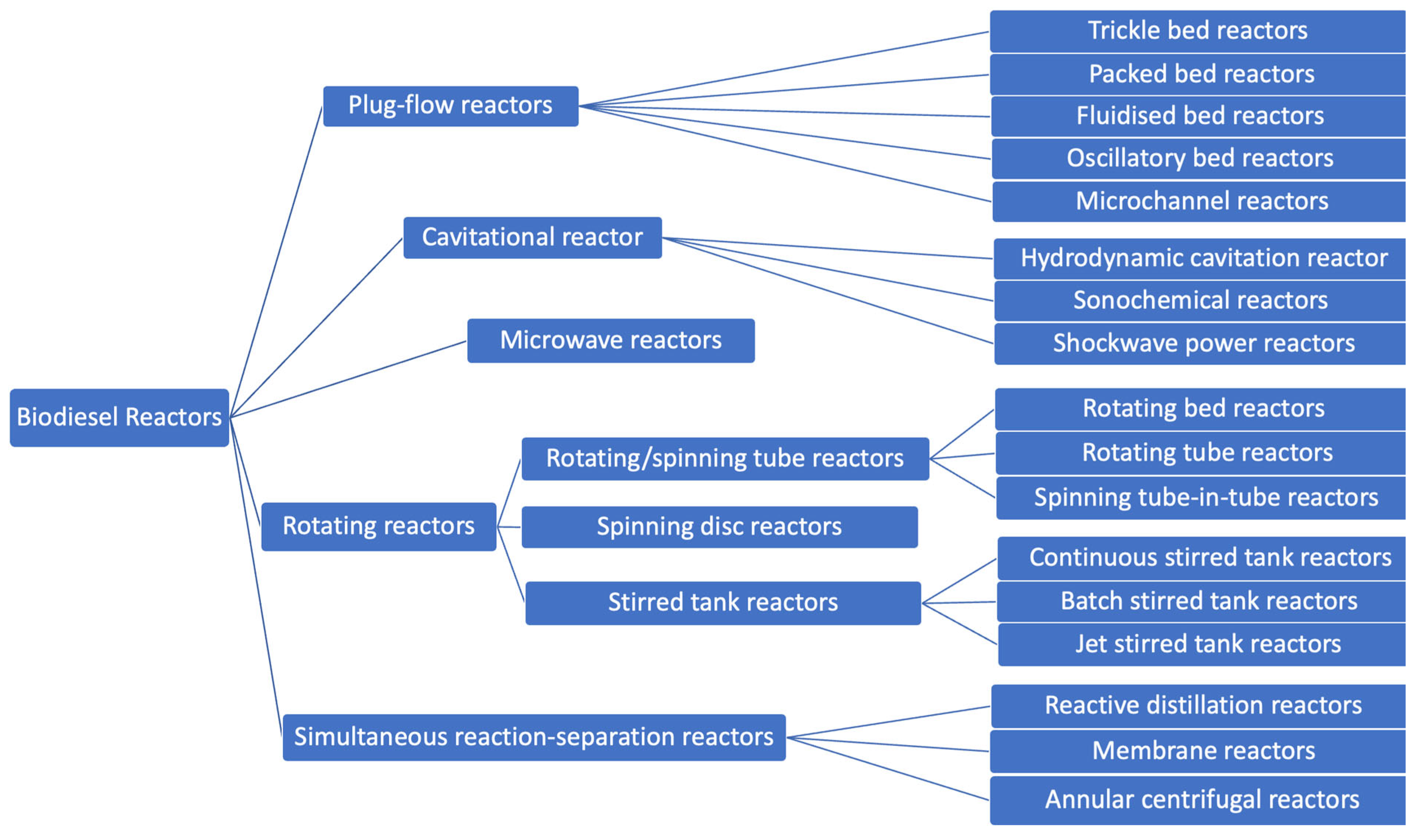
:1. Introduction
2. Microchannel Reactor
2.1. Microchannel Reactors for Biodiesel Production
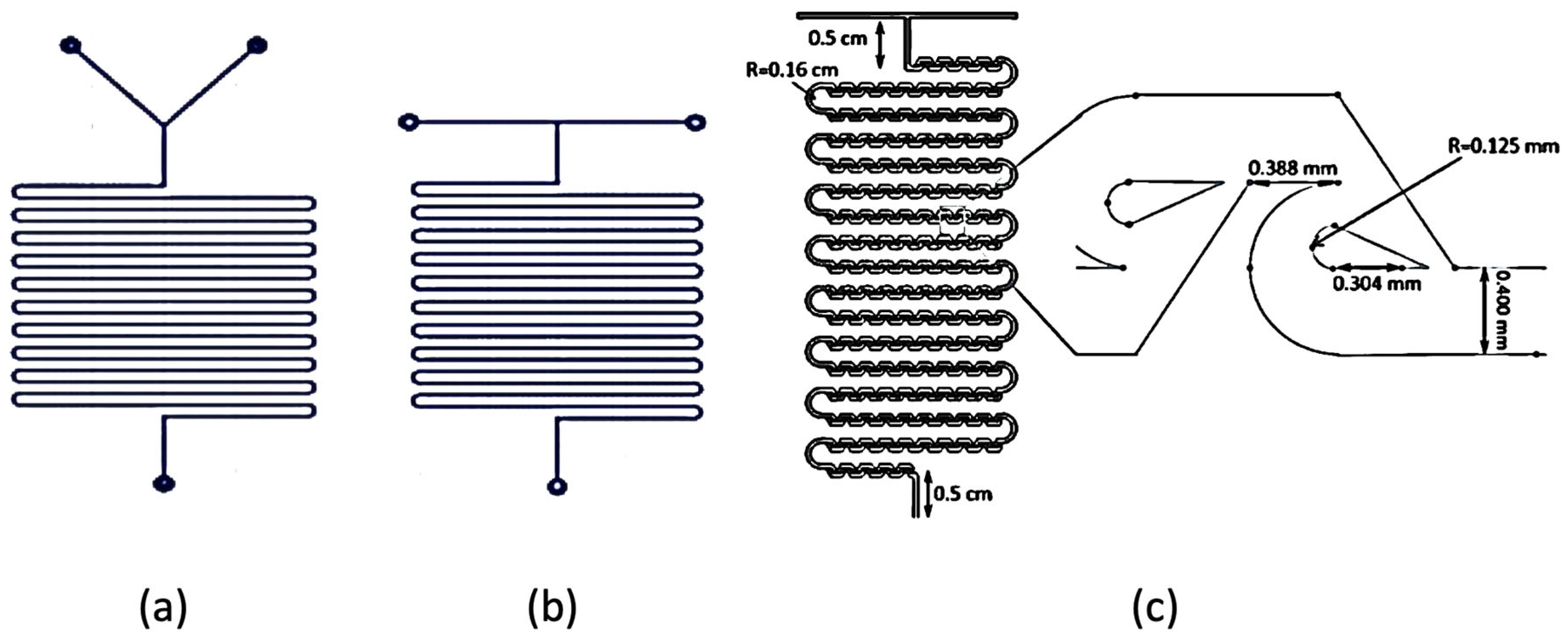
2.2. Microchannel Reactor Design
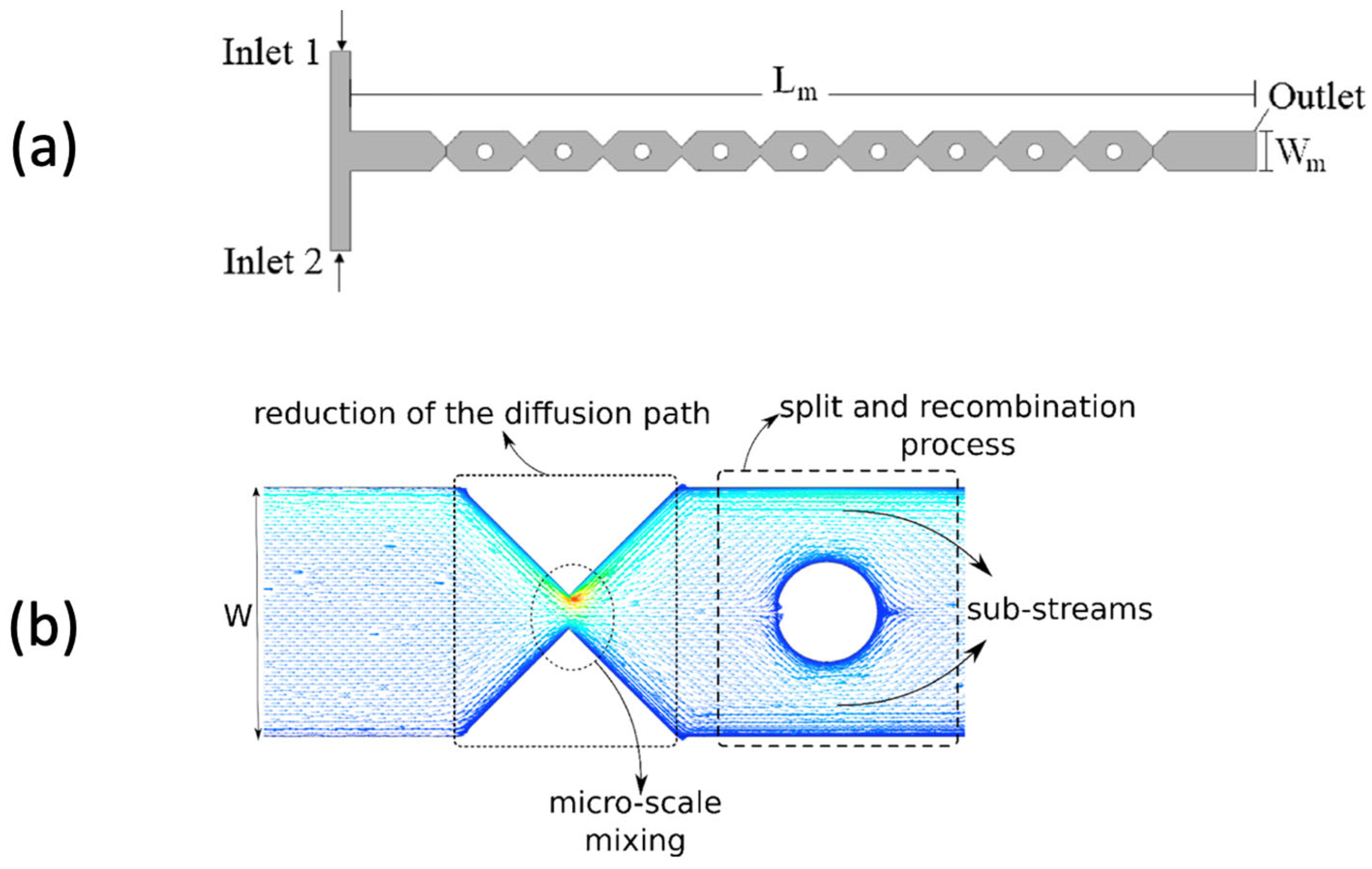
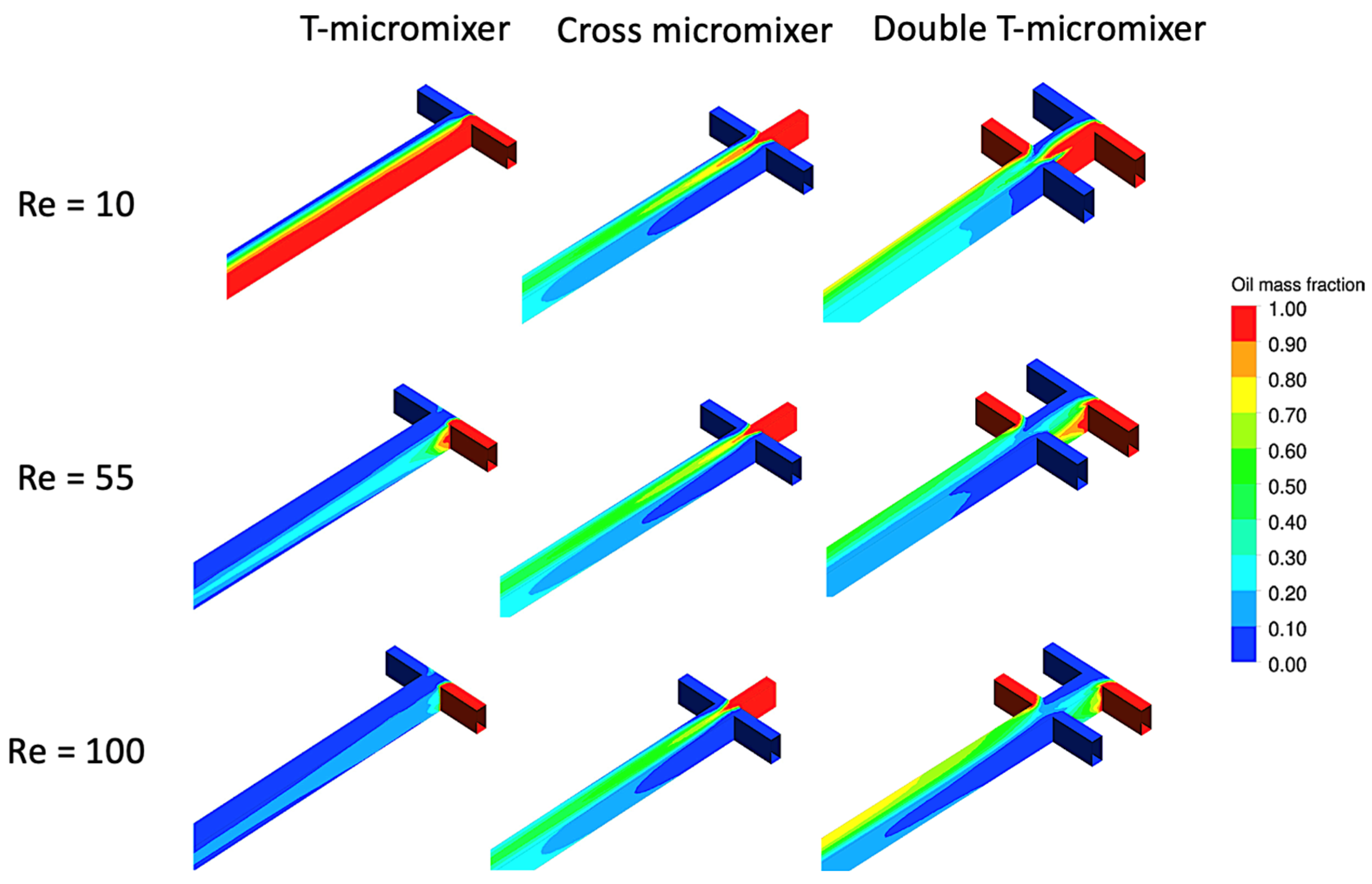
2.3. Micromixers in Microchannel Reactors
2.4. Biodiesel Yield, Reaction Time and Heat Delivery Mechanism in Microchannel Reactors
3. Flow in Microchannel Reactors
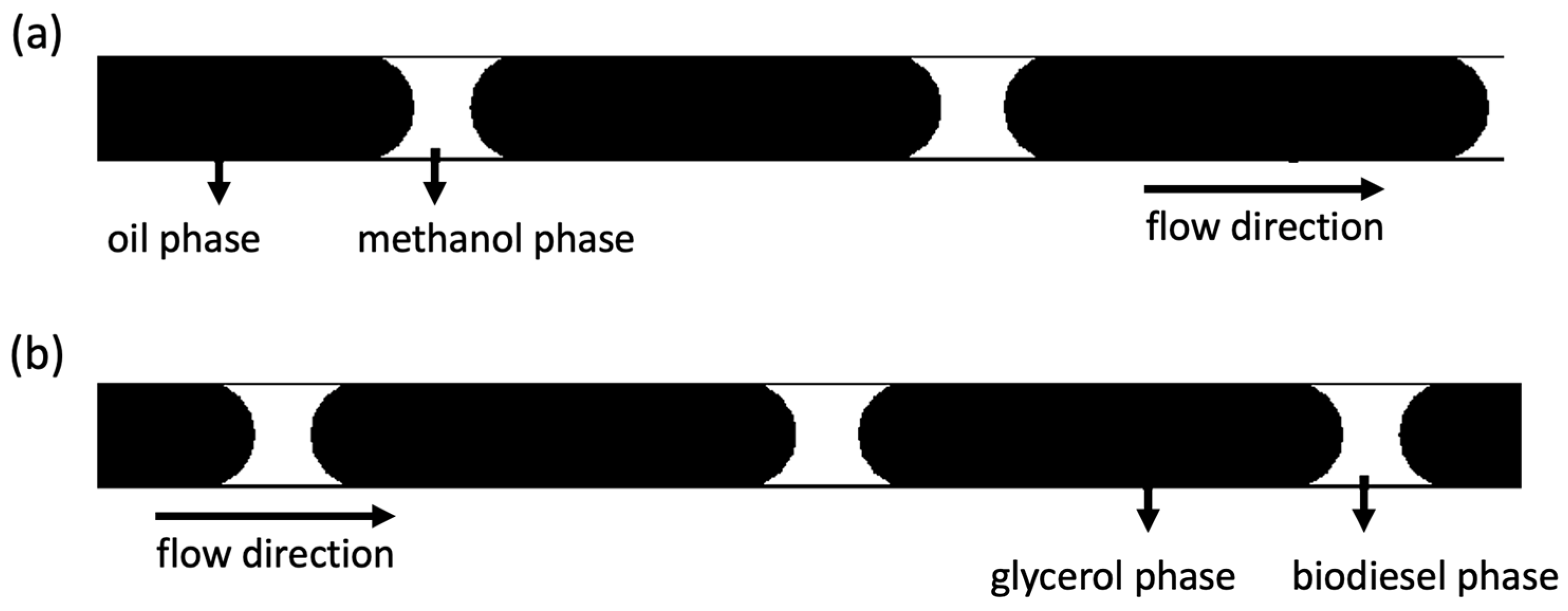
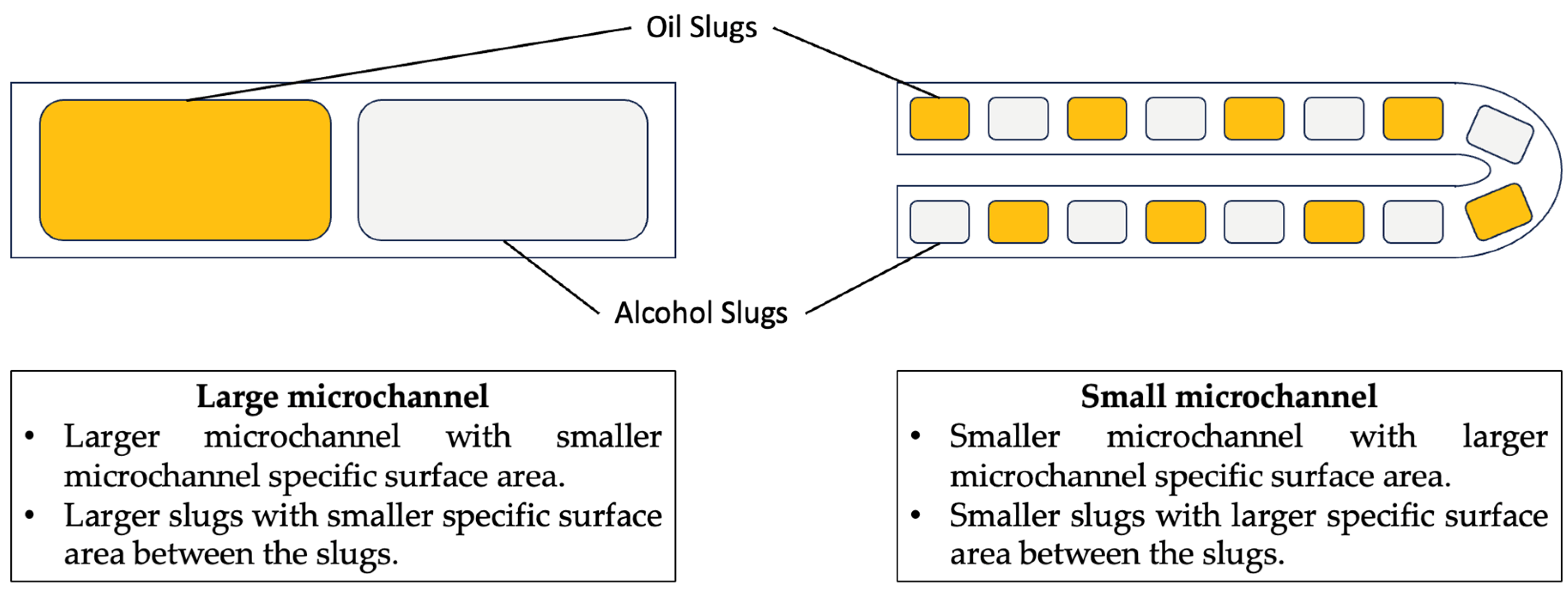
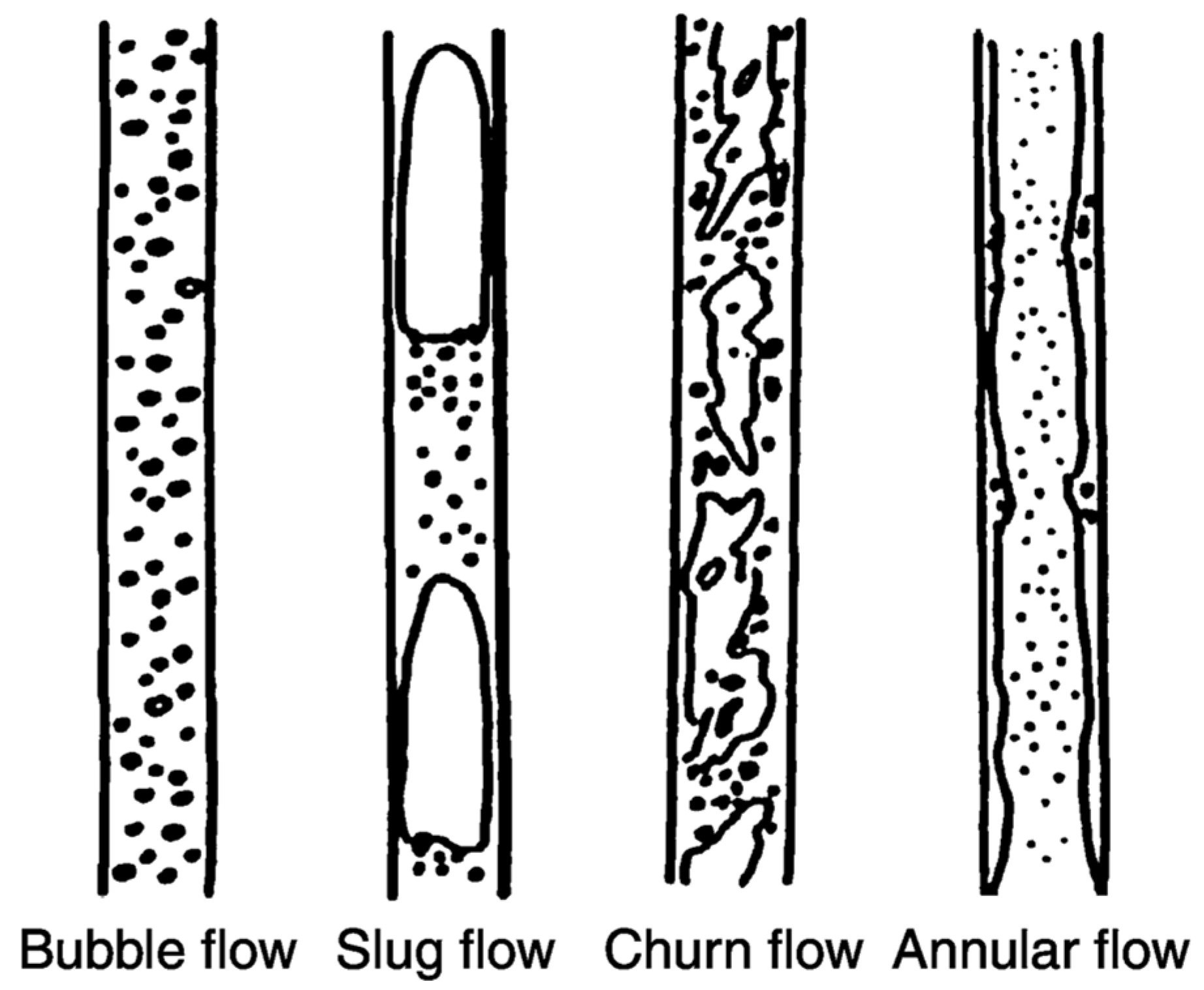
3.1. Slug Flow in a Microchannel Biodiesel Reactor
3.2. Slug Flow Formation
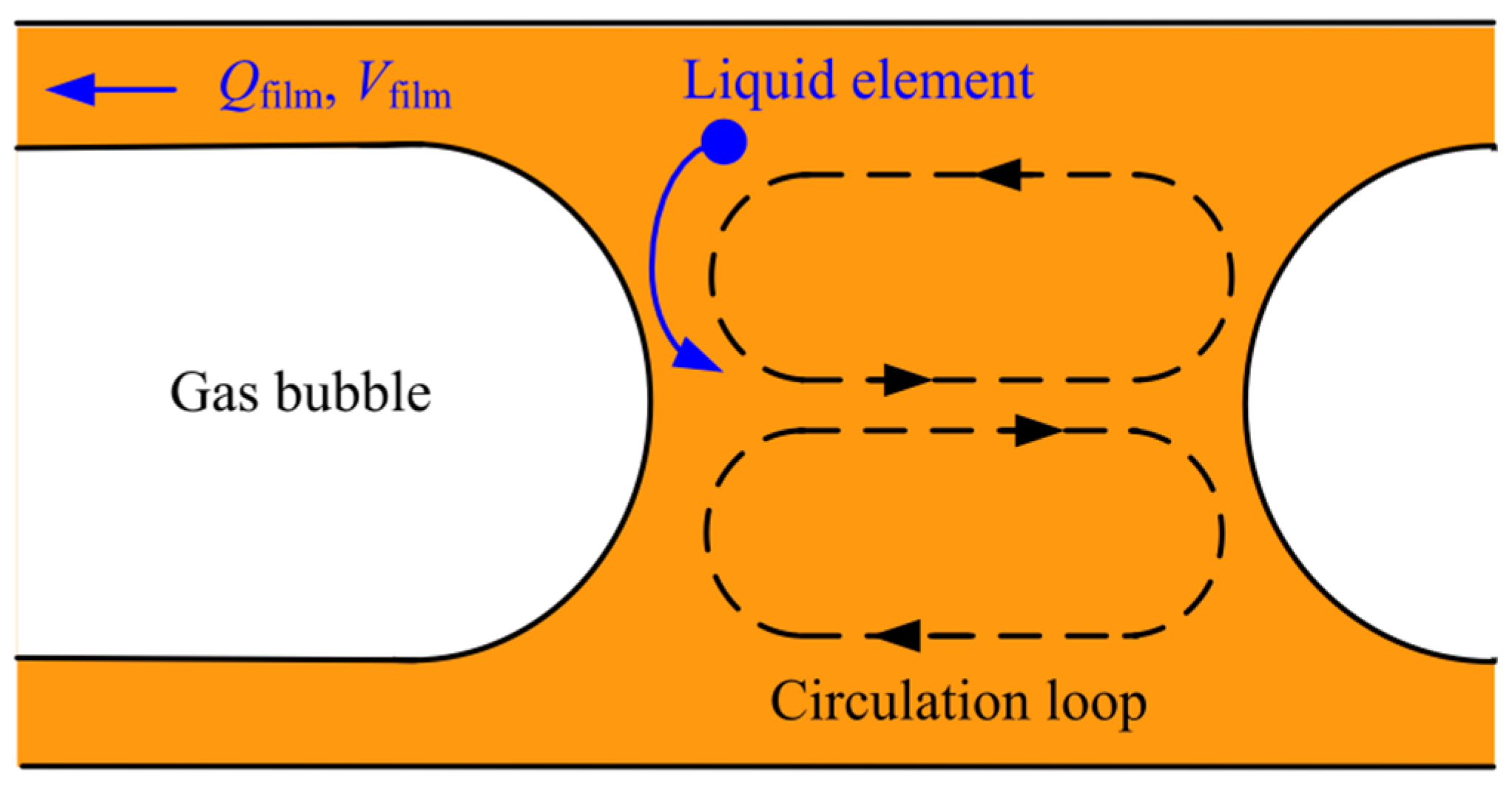
3.3. Mass Transfer of Slug Flow
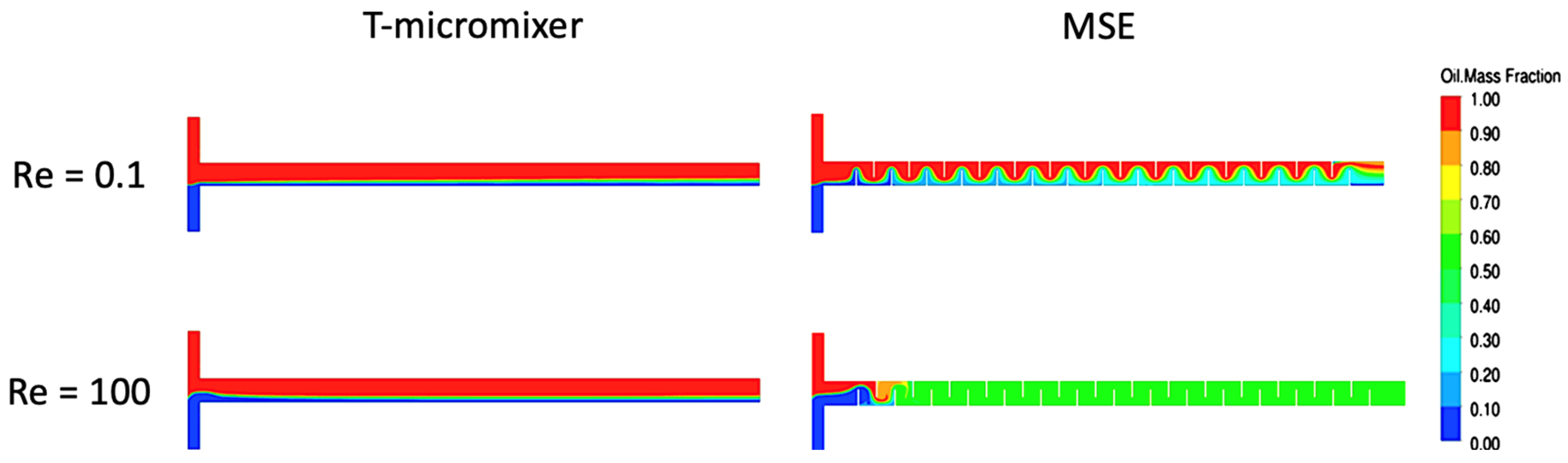
4. Simulation Studies in Microchannel Reactors
5. Challenges, Limitations and the Future of Microchannel Reactors
6. Future Directions
6.1. Microwave-Assisted Heating
6.2. Microwave-Assisted Transesterification for Biodiesel Production
6.3. Combined Microchannel Reactor and Microwave Heating
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohd Laziz, A.; KuShaari, K.; Azeem, B.; Yusup, S.; Chin, J.; Denecke, J. Rapid production of biodiesel in a microchannel reactor at room temperature by enhancement of mixing behaviour in methanol phase using volume of fluid model. Chem. Eng. Sci. 2020, 219, 115532. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Ndeddy Aka, R.J. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Process. Technol. 2022, 227, 107120. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook for Electricity. Available online: https://www.iea.org/reports/world-energy-outlook-2022/outlook-for-electricity (accessed on 19 January 2024).
- IEA. World Energy Outlook for Solid Fuels. Available online: https://www.iea.org/reports/world-energy-outlook-2022/outlook-for-solid-fuels#abstract (accessed on 19 January 2024).
- IEA. World Energy Outlook for Liquid Fuels. Available online: https://www.iea.org/reports/world-energy-outlook-2022/outlook-for-liquid-fuels (accessed on 19 January 2024).
- IEA. World Energy Outlook for the Global Energy Crisis. Available online: https://www.iea.org/reports/world-energy-outlook-2022/the-global-energy-crisis (accessed on 19 January 2024).
- IEA. World Energy Outlook Executive Summary. Available online: https://www.iea.org/reports/world-energy-outlook-2022/executive-summary (accessed on 19 January 2024).
- Pata, U.K.; Ertugrul, H.M. Do the Kyoto Protocol, geopolitical risks, human capital and natural resources affect the sustainability limit? A new environmental approach based on the LCC hypothesis. Resour. Policy 2023, 81, 103352. [Google Scholar] [CrossRef]
- Salman, M.; Long, X.; Wang, G.; Zha, D. Paris climate agreement and global environmental efficiency: New evidence from fuzzy regression discontinuity design. Energy Policy 2022, 168, 113128. [Google Scholar] [CrossRef]
- Chandel, S.S.; Shrivastva, R.; Sharma, V.; Ramasamy, P. Overview of the initiatives in renewable energy sector under the national action plan on climate change in India. Renew. Sustain. Energy Rev. 2016, 54, 866–873. [Google Scholar] [CrossRef]
- Williams, M.L.; Lott, M.C.; Kitwiroon, N.; Dajnak, D.; Walton, H.; Holland, M.; Pye, S.; Fecht, D.; Toledano, M.B.; Beevers, S.D. The Lancet Countdown on health benefits from the UK Climate Change Act: A modelling study for Great Britain. Lancet Planet Health 2018, 2, e202–e213. [Google Scholar] [CrossRef]
- Pastore, L.M.; Lo Basso, G.; Cristiani, L.; de Santoli, L. Rising targets to 55% GHG emissions reduction—The smart energy systems approach for improving the Italian energy strategy. Energy 2022, 259, 125049. [Google Scholar] [CrossRef]
- Kirsten, S. Renewable Energy Sources Act and Trading of Emission Certificates: A national and a supranational tool direct energy turnover to renewable electricity-supply in Germany. Energy Policy 2014, 64, 302–312. [Google Scholar] [CrossRef]
- Attari, A.; Abbaszadeh-Mayvan, A.; Taghizadeh-Alisaraei, A. Process optimization of ultrasonic-assisted biodiesel production from waste cooking oil using waste chicken eggshell-derived CaO as a green heterogeneous catalyst. Biomass Bioenergy 2022, 158, 106357. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Brahma, S.; Nath, B.; Basumatary, B.; Das, B.; Saikia, P.; Patir, K.; Basumatary, S. Biodiesel production from mixed oils: A sustainable approach towards industrial biofuel production. Chem. Eng. J. Adv. 2022, 10, 100284. [Google Scholar] [CrossRef]
- Athar, M.; Imdad, S.; Zaidi, S.; Yusuf, M.; Kamyab, H.; Jaromír Klemeš, J.; Chelliapan, S. Biodiesel production by single-step acid-catalysed transesterification of Jatropha oil under microwave heating with modelling and optimisation using response surface methodology. Fuel 2022, 322, 124205. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. The use of KOH/Clinoptilolite catalyst in pilot of microreactor for biodiesel production from waste cooking oil. Fuel 2020, 263, 116659. [Google Scholar] [CrossRef]
- Chanthon, N.; Ngaosuwan, K.; Kiatkittipong, W.; Wongsawaeng, D.; Appamana, W.; Quitain, A.T.; Assabumrungrat, S. High-efficiency biodiesel production using rotating tube reactor: New insight of operating parameters on hydrodynamic regime and biodiesel yield. Renew. Sustain. Energy Rev. 2021, 151, 111430. [Google Scholar] [CrossRef]
- Banerjee, R.; Kumar, S.P.J.; Mehendale, N.; Sevda, S.; Garlapati, V.K. Intervention of microfluidics in biofuel and bioenergy sectors: Technological considerations and future prospects. Renew. Sustain. Energy Rev. 2019, 101, 548–558. [Google Scholar] [CrossRef]
- Gong, H.; Gao, L.; Nie, K.; Wang, M.; Tan, T. A new reactor for enzymatic synthesis of biodiesel from waste cooking oil: A static-mixed reactor pilot study. Renew. Energy 2020, 154, 270–277. [Google Scholar] [CrossRef]
- Annal, U.N.; Natarajan, A.; Gurunathan, B.; Sahadevan, R. Lipid-derived biofuel: Production methodologies. In Biofuels and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 409–434. [Google Scholar]
- Gul, I.; Khan, S.M.; Nawaz, U.; Haq, Z.U.; Abdullah; Ahmad, Z.; Iqbal, M. Techniques Used in the Process of Biodiesel Production and Its Merits and Demerits from a Historical Perspective. In Zero Waste Biorefinery; Energy, Environment, and Sustainability; Springer: Singapore, 2022; pp. 535–556. [Google Scholar]
- Fatimah, I.; Yanti, I.; Suharto, T.E.; Sagadevan, S. ZrO2-based catalysts for biodiesel production: A review. Inorg. Chem. Commun. 2022, 143, 109808. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Pua, F.-L.; Zhang, F. A review of magnetic solid catalyst development for sustainable biodiesel production. Biomass Bioenergy 2021, 149, 106099. [Google Scholar] [CrossRef]
- Noriega, M.A.; Narváez, P.C. Scale-up and cost analysis of biodiesel production using liquid-liquid film reactors: Reduction in the methanol consumption and investment cost. Energy 2020, 211, 118724. [Google Scholar] [CrossRef]
- Surya Abadi Ginting, M.; Tazli Azizan, M.; Yusup, S. Alkaline in situ ethanolysis of Jatropha curcas. Fuel 2012, 93, 82–85. [Google Scholar] [CrossRef]
- Ajeni, M.O.; Heath, W.P.; Poupard, E. Modelling for Control of Biodiesel Microreactors. IFAC PapersOnLine 2021, 54, 312–317. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, L.; Weatherley, L. Process intensification technologies in continuous biodiesel production. Chem. Eng. Process. Process Intensif. 2010, 49, 323–330. [Google Scholar] [CrossRef]
- Hsieh, L.-S.; Kumar, U.; Wu, J.C.S. Continuous production of biodiesel in a packed-bed reactor using shell–core structural Ca(C3H7O3)2/CaCO3 catalyst. Chem. Eng. J. 2010, 158, 250–256. [Google Scholar] [CrossRef]
- Carlucci, C. An Overview on the Production of Biodiesel Enabled by Continuous Flow Methodologies. Catalysts 2022, 12, 717. [Google Scholar] [CrossRef]
- Akkarawatkhoosith, N.; Bangjang, T.; Kaewchada, A.; Jaree, A. Biodiesel production from rice bran oil fatty acid distillate via supercritical hydrolysis–esterification–transesterification in a microreactor. Energy Rep. 2023, 9, 5299–5305. [Google Scholar] [CrossRef]
- Aghel, B.; Gouran, A.; Parandi, E.; Jumeh, B.H.; Nodeh, H.R. Production of biodiesel from high acidity waste cooking oil using nano GO@MgO catalyst in a microreactor. Renew. Energy 2022, 200, 294–302. [Google Scholar] [CrossRef]
- Abdulla Yusuf, H.; Hossain, S.M.Z.; Aloraibi, S.; Alzaabi, N.J.; Alfayhani, M.A.; Almedfaie, H.J. Fabrication of novel microreactors in-house and their performance analysis via continuous production of biodiesel. Chem. Eng. Process. Process Intensif. 2022, 172, 108792. [Google Scholar] [CrossRef]
- Akkarawatkhoosith, N.; Tongtummachat, T.; Kaewchada, A.; Jaree, A. Non-catalytic and glycerol-free biodiesel production from rice bran oil fatty acid distillate in a microreactor. Energy Convers. Manag. X 2021, 11, 100096. [Google Scholar] [CrossRef]
- Mohadesi, M.; Gouran, A.; Dehghan Dehnavi, A. Biodiesel production using low cost material as high effective catalyst in a microreactor. Energy 2021, 219, 119671. [Google Scholar] [CrossRef]
- Gojun, M.; Šalić, A.; Zelić, B. Integrated microsystems for lipase-catalyzed biodiesel production and glycerol removal by extraction or ultrafiltration. Renew. Energy 2021, 180, 213–221. [Google Scholar] [CrossRef]
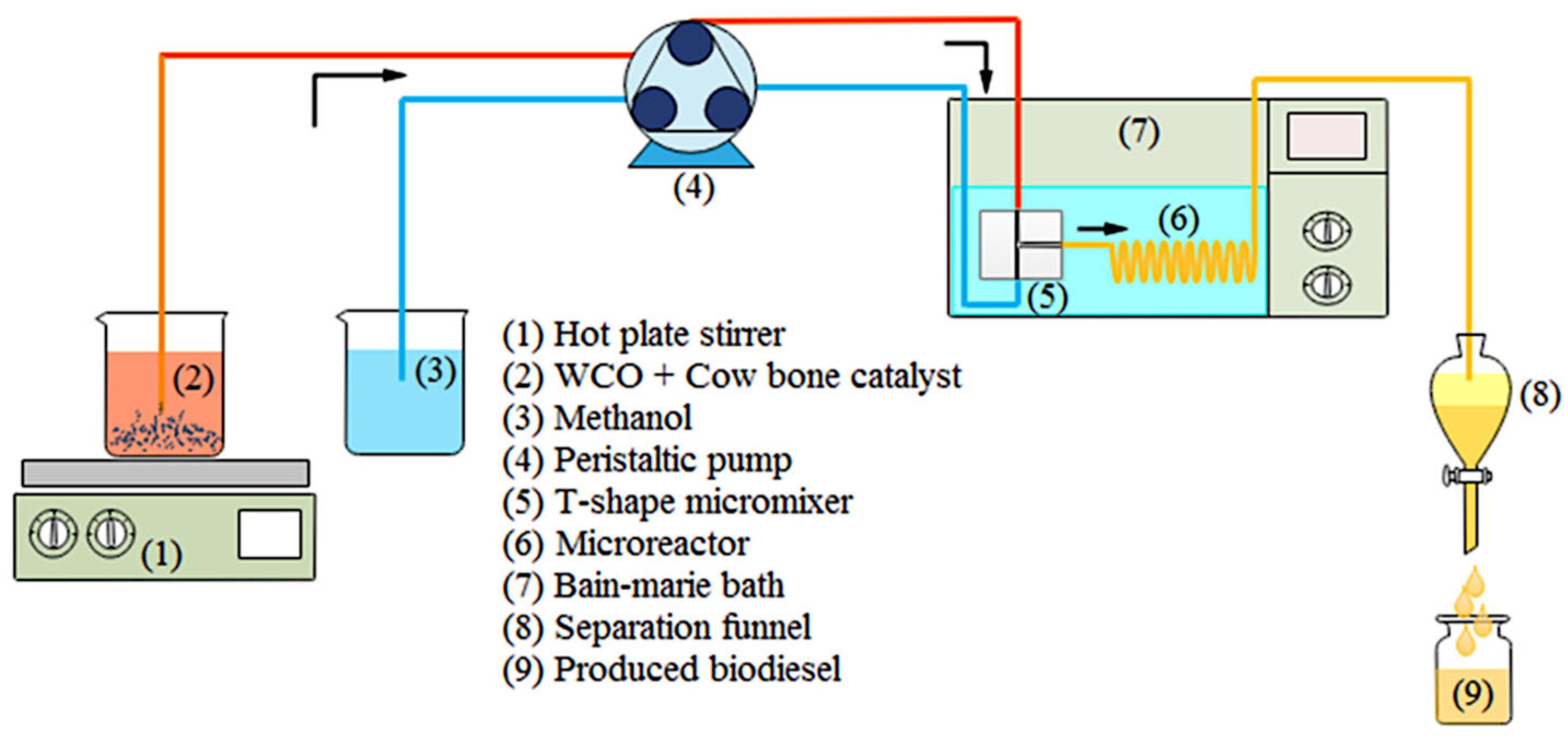
- Aghel, B.; Mohadesi, M.; Razmehgir, M.H.; Gouran, A. Biodiesel production from waste cooking oil in a micro-sized reactor in the presence of cow bone-based KOH catalyst. Biomass Convers. Biorefinery 2022, 13, 13921–13935. [Google Scholar] [CrossRef]
- Pavlovic, S.; Selo, G.; Marinkovic, D.; Planinic, M.; Tisma, M.; Stankovic, M. Transesterification of Sunflower Oil over Waste Chicken Eggshell-Based Catalyst in a Microreactor: An Optimization Study. Micromachines 2021, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. Study of the transesterification of waste cooking oil for the production of biodiesel in a microreactor pilot: The effect of acetone as the co-solvent. Fuel 2020, 273, 117736. [Google Scholar] [CrossRef]
- Akkarawatkhoosith, N.; Kaewchada, A.; Jaree, A. Continuous catalyst-free biodiesel synthesis from rice bran oil fatty acid distillate in a microreactor. Energy Rep. 2020, 6, 545–549. [Google Scholar] [CrossRef]
- Thangarasu, V.; Siddharth, R.; Ramanathan, A. Modeling of process intensification of biodiesel production from Aegle Marmelos Correa seed oil using microreactor assisted with ultrasonic mixing. Ultrason. Sonochem. 2020, 60, 104764. [Google Scholar] [CrossRef] [PubMed]
- Abdulla Yusuf, H.; Elkanzi, E.M.; Hossain, S.M.Z.; Alsaei, A.M.; Alhindy, A.H.; Ebrahem, E. Design and performance assessment of an in-house fabricated microreactor for enzyme-catalysed biodiesel synthesis. Arab. J. Basic Appl. Sci. 2020, 27, 239–247. [Google Scholar] [CrossRef]
- Yuan, S.; Jiang, B.; Peng, T.; Zhou, M.; Drummer, D. Investigation of efficient mixing enhancement in planar micromixers with short mixing length. Chem. Eng. Process. Process Intensif. 2022, 171, 108747. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, H.; Xiao, Y.; Zhang, J.; Qiu, Y.; Wei, J.; Hao, N. Rational design of robust flower-like sharp-edge acoustic micromixers towards efficient engineering of functional 3D ZnO nanorod array. Chem. Eng. J. 2022, 447, 137547. [Google Scholar] [CrossRef]
- Santana, H.S.; Silva, J.L.; Taranto, O.P. Optimization of micromixer with triangular baffles for chemical process in millidevices. Sens. Actuators B Chem. 2019, 281, 191–203. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, C.; Chen, G. Gas–liquid–liquid slug flow and mass transfer in hydrophilic and hydrophobic microreactors. Chin. J. Chem. Eng. 2022, 50, 85–94. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, T.; Yang, Y.; Tang, S. Simulation and design microreactor configured with micromixers to intensify the isobutane/1-butene alkylation process. J. Taiwan Inst. Chem. Eng. 2019, 98, 53–62. [Google Scholar] [CrossRef]
- Yang, M.; Gan, Y.; Gao, L.; Zhu, X. A structural optimization model of a biochemical detection micromixer based on RSM and MOEA/D. Chem. Eng. Process. Process Intensif. 2022, 173, 108832. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Cai, Y.; Wang, B.; Luo, X. A cost-effective serpentine micromixer utilizing ellipse curve. Anal. Chim. Acta 2021, 1155, 338355. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, B.; Tian, Y.; Zhang, Y.; Chen, Y. Analysis of different shapes of cross-sections and obstacles in variable-radius spiral micromixers on mixing efficiency. Chem. Eng. Process. Process Intensif. 2022, 171, 108756. [Google Scholar] [CrossRef]
- Mondal, B.; Mehta, S.K.; Pati, S.; Patowari, P.K. Numerical analysis of electroosmotic mixing in a heterogeneous charged micromixer with obstacles. Chem. Eng. Process. Process Intensif. 2021, 168, 108585. [Google Scholar] [CrossRef]
- Tanimu, A.; Jaenicke, S.; Alhooshani, K. Heterogeneous catalysis in continuous flow microreactors: A review of methods and applications. Chem. Eng. J. 2017, 327, 792–821. [Google Scholar] [CrossRef]
- Yue, J. Green process intensification using microreactor technology for the synthesis of biobased chemicals and fuels. Chem. Eng. Process. Process Intensif. 2022, 177, 109002. [Google Scholar] [CrossRef]
- Agarwal, T.; Wang, L. Numerical analysis of vortex T micromixer with diffuser plates and obstacles. Therm. Sci. Eng. Prog. 2022, 28, 101156. [Google Scholar] [CrossRef]
- Aghasi, M.A.; Hekmat, M.H. A novel design of split and recombination multilayer micromixers with excellent hydraulic and mixing performance based on the baker’s transformation. Chem. Eng. Process. Process Intensif. 2022, 174, 108894. [Google Scholar] [CrossRef]
- Antognoli, M.; Tomasi Masoni, S.; Mariotti, A.; Mauri, R.; Brunazzi, E.; Galletti, C. Investigation on steady regimes in a X-shaped micromixer fed with water and ethanol. Chem. Eng. Sci. 2022, 248, 117254. [Google Scholar] [CrossRef]
- Zhu, S.; Fang, Y.; Chen, Y.; Yu, P.; Han, Y.; Xiang, N.; Ni, Z. Stackable micromixer with modular design for efficient mixing over wide Reynold numbers. Int. J. Heat Mass Transf. 2022, 183, 122129. [Google Scholar] [CrossRef]
- Bai, C.; Zhou, W.; Yu, S.; Zheng, T.; Wang, C. A surface acoustic wave-assisted micromixer with active temperature control. Sens. Actuators A Phys. 2022, 346, 113833. [Google Scholar] [CrossRef]
- Chen, S.; Hao, M.; Shang, J.; Jiang, Y.; Xie, Y.; Ba, Y.; Liu, K. Numerical analysis of modified micromixers with staggered E-shape mixing units. Chem. Eng. Process. Process Intensif. 2022, 179, 109087. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Z.; Zhong, M.; Ma, Y.; Chen, S.; Liu, X.; Jia, S. Numerical and experimental investigation on a planar passive micromixer embedded with omega-shaped obstacles for rapid fluid mixing. Chem. Eng. Process. Process Intensif. 2022, 182, 109203. [Google Scholar] [CrossRef]
- Lv, H.; Chen, X.; Wang, X.; Zeng, X.; Ma, Y. A novel study on a micromixer with Cantor fractal obstacle through grey relational analysis. Int. J. Heat Mass Transf. 2022, 183, 122159. [Google Scholar] [CrossRef]
- Talebjedi, B.; Ghazi, M.; Tasnim, N.; Janfaza, S.; Hoorfar, M. Performance optimization of a novel passive T-shaped micromixer with deformable baffles. Chem. Eng. Process. Process Intensif. 2021, 163, 108369. [Google Scholar] [CrossRef]
- Zhang, C.; Brunet, P.; Royon, L.; Guo, X. Mixing intensification using sound-driven micromixer with sharp edges. Chem. Eng. J. 2021, 410, 128252. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Wang, B.; Cai, Y.; Wan, Y. Vortices degradation and periodical variation in spiral micromixers with various spiral structures. Int. J. Heat Mass Transf. 2022, 183, 122168. [Google Scholar] [CrossRef]
- Liu, G.; Wang, M.; Dong, L.; Zhu, D.; Wang, C.; Jia, Y.; Li, X.; Wang, J. A novel design for split-and-recombine micromixer with double-layer Y-shaped mixing units. Sens. Actuators A Phys. 2022, 341, 113569. [Google Scholar] [CrossRef]
- Sinha, A.; Zunaid, M. Numerical study of 3-D helical passive micromixer having both inlets at offset with blood as fluid. Mater. Today Proc. 2022, 62, 3713–3718. [Google Scholar] [CrossRef]
- Vatankhah, P.; Shamloo, A. Parametric study on mixing process in an in-plane spiral micromixer utilizing chaotic advection. Anal. Chim. Acta 2018, 1022, 96–105. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Xu, C. Vertical chaotic mixing of oscillating feedback micromixer in passive mode. Chem. Eng. Sci. 2022, 263, 118127. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.T.; Yan, J.; Dahlquist, E. Intensification of biodiesel synthesis using zigzag micro-channel reactors. Bioresour. Technol. 2009, 100, 3054–3060. [Google Scholar] [CrossRef]
- Martínez Arias, E.L.; Fazzio Martins, P.; Jardini Munhoz, A.L.; Gutierrez-Rivera, L.; Maciel Filho, R. Continuous Synthesis and in Situ Monitoring of Biodiesel Production in Different Microfluidic Devices. Ind. Eng. Chem. Res. 2012, 51, 10755–10767. [Google Scholar] [CrossRef]
- Shimizu, K.; Kato, K.; Kobayashi, T.; Komoda, Y.; Ohmura, N. Flow and mixing characteristics of gas-liquid slug flow in a continuous Taylor-Couette flow reactor with narrow gap width. Chem. Eng. Process. Process Intensif. 2023, 183, 109226. [Google Scholar] [CrossRef]
- Shah, R.K.; Khandekar, S. Influence of external magnetic manipulation on thermal transport characteristics of the bubble-slug flow of ferro-nanocolloids. Colloids Surf. A Physicochem. Eng. Asp. 2022, 646, 128936. [Google Scholar] [CrossRef]
- Santiago, L.E.P.; Silva, M.G.; Sobrinho, E.V.; Ruiz, J.A.C.; Padilha, C.E.A.; Souza, D.F.S. Extractive desulfurization in microchannels with polyethylene glycol 400: An experimental study and mass transfer evaluation. Chem. Eng. Process. Process Intensif. 2022, 181, 109096. [Google Scholar] [CrossRef]
- Mohd Laziz, A.; KuShaari, K.; Chin, J.; Denecke, J. Quantitative analysis of hydrodynamic effect on transesterification process in T-junction microchannel reactor system. Chem. Eng. Process. Process Intensif. 2019, 140, 91–99. [Google Scholar] [CrossRef]
- López-Guajardo, E.; Ortiz-Nadal, E.; Montesinos-Castellanos, A.; Nigam, K.D.P. Process Intensification of Biodiesel Production Using A Tubular Micro-Reactor (TMR): Experimental and Numerical Assessment. Chem. Eng. Commun. 2017, 204, 467–475. [Google Scholar] [CrossRef]
- Sun, J.; Ju, J.; Ji, L.; Zhang, L.; Xu, N. Synthesis of Biodiesel in Capillary Microreactors. Ind. Eng. Chem. Res. 2008, 47, 1398–1403. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, L.; Li, H.; Xin, G.; Wang, X. Experimental and numerical studies of liquid-liquid two-phase flows in microchannel with sudden expansion/contraction cavities. Chem. Eng. J. 2022, 433, 133820. [Google Scholar] [CrossRef]
- Shareef, N.F.; Abdulwahid, M.A. Experimental and numerical study of slug flow in horizontal perforated wellbore. Geoenergy Sci. Eng. 2023, 221, 111239. [Google Scholar] [CrossRef]
- Ren, W.; Dai, R.; Jin, N. Modeling of liquid film thickness around Taylor bubbles rising in vertical stagnant and co-current slug flowing liquids. Chin. J. Chem. Eng. 2022, 58, 179–194. [Google Scholar] [CrossRef]
- Renken, A.; Kiwi-Minsker, L. Microstructured Catalytic Reactors. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2010; pp. 47–122. [Google Scholar]
- Ghaini, A.; Kashid, M.N.; Agar, D.W. Effective interfacial area for mass transfer in the liquid–liquid slug flow capillary microreactors. Chem. Eng. Process. Process Intensif. 2010, 49, 358–366. [Google Scholar] [CrossRef]
- Yan, P.; Jin, H.; Tao, F.; He, G.; Guo, X.; Ma, L.; Yang, S.; Zhang, R. Flow characterization of gas-liquid with different liquid properties in a Y-type microchannel using electrical resistance tomography and volume of fluid model. J. Taiwan Inst. Chem. Eng. 2022, 136, 104390. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, U.K.A. Hydrodynamics and mass transfer studies of liquid-liquid two-phase flow in parallel microchannels. Int. J. Multiph. Flow 2022, 157, 104248. [Google Scholar] [CrossRef]
- Nie, X.; Zhu, C.; Fu, T.; Ma, Y. Mass transfer intensification and mechanism analysis of gas–liquid two-phase flow in the microchannel embedding triangular obstacles. Chin. J. Chem. Eng. 2022, 51, 100–108. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Zhao, S.; Yao, C.; Chen, G. Hydrodynamics and mass transfer characteristics of liquid–liquid slug flow in microchannels: The effects of temperature, fluid properties and channel size. Chem. Eng. J. 2019, 358, 794–805. [Google Scholar] [CrossRef]
- Ramji, S.; Rakesh, A.; Pushpavanam, S. Modelling mass transfer in liquid-liquid slug flow in a microchannel. Chem. Eng. J. 2019, 364, 280–291. [Google Scholar] [CrossRef]
- Xu, B.; Cai, W.; Liu, X.; Zhang, X. Mass transfer behavior of liquid–liquid slug flow in circular cross-section microchannel. Chem. Eng. Res. Des. 2013, 91, 1203–1211. [Google Scholar] [CrossRef]
- Santana, H.S.; de Sousa, M.R.P.; Silva Júnior, J.L. Intensification of biodiesel production through computational fluid dynamics. In Biofuels and Biorefining; Elsevier: Amsterdam, The Netherlands, 2022; pp. 231–271. [Google Scholar]
- Santana, H.S.; Tortola, D.S.; Silva, J.L.; Taranto, O.P. Biodiesel synthesis in micromixer with static elements. Energy Convers. Manag. 2017, 141, 28–39. [Google Scholar] [CrossRef]
- Santana, H.S.; Jr, J.o.L.S.; Taranto, O.P. Numerical simulation of mixing and reaction of Jatropha curcas oil and ethanol for synthesis of biodiesel in micromixers. Chem. Eng. Sci. 2015, 132, 159–168. [Google Scholar] [CrossRef]
- Santana, H.S.; Júnior, J.L.S.; Taranto, O.P. Numerical simulations of biodiesel synthesis in microchannels with circular obstructions. Chem. Eng. Process. Process Intensif. 2015, 98, 137–146. [Google Scholar] [CrossRef]
- Shah, I.; Aziz, S.; Soomro, A.M.; Kim, K.; Kim, S.W.; Choi, K.H. Numerical and experimental investigation of Y-shaped micromixers with mixing units based on cantor fractal structure for biodiesel applications. Microsyst. Technol. 2020, 27, 2203–2216. [Google Scholar] [CrossRef]
- Om Tapanes, N.C.; Gomes Aranda, D.A.; de Mesquita Carneiro, J.W.; Ceva Antunes, O.A. Transesterification of Jatropha curcas oil glycerides: Theoretical and experimental studies of biodiesel reaction. Fuel 2008, 87, 2286–2295. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification kinetics of soybean oil. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Mazubert, A.; Poux, M.; Aubin, J. Intensified processes for FAME production from waste cooking oil: A technological review. Chem. Eng. J. 2013, 233, 201–223. [Google Scholar] [CrossRef]
- Tiwari, A.; Rajesh, V.M.; Yadav, S. Biodiesel production in micro-reactors: A review. Energy Sustain. Dev. 2018, 43, 143–161. [Google Scholar] [CrossRef]
- Gopi, R.; Thangarasu, V.; Vinayakaselvi, M.A.; Ramanathan, A. A critical review of recent advancements in continuous flow reactors and prominent integrated microreactors for biodiesel production. Renew. Sustain. Energy Rev. 2022, 154, 111869. [Google Scholar] [CrossRef]
- Natarajan, Y.; Nabera, A.; Salike, S.; Dhanalakshmi Tamilkkuricil, V.; Pandian, S.; Karuppan, M.; Appusamy, A. An overview on the process intensification of microchannel reactors for biodiesel production. Chem. Eng. Process. Process Intensif. 2019, 136, 163–176. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Panahi, H.K.S.; Mollahosseini, A.; Hosseini, M.; Soufiyan, M.M. Reactor technologies for biodiesel production and processing: A review. Prog. Energy Combust. Sci. 2019, 74, 239–303. [Google Scholar] [CrossRef]
- Liow, M.Y.; Gourich, W.; Chang, M.Y.; Loh, J.M.; Chan, E.-S.; Song, C.P. Towards rapid and sustainable synthesis of biodiesel: A review of effective parameters and scale-up potential of intensification technologies for enzymatic biodiesel production. J. Ind. Eng. Chem. 2022, 114, 1–18. [Google Scholar] [CrossRef]
- Okolie, J.A.; Ivan Escobar, J.; Umenweke, G.; Khanday, W.; Okoye, P.U. Continuous biodiesel production: A review of advances in catalysis, microfluidic and cavitation reactors. Fuel 2022, 307, 121821. [Google Scholar] [CrossRef]
- Kumar, Y.; Jaiswal, P.; Panda, D.; Nigam, K.D.P.; Biswas, K.G. A critical review on nanoparticle-assisted mass transfer and kinetic study of biphasic systems in millimeter-sized conduits. Chem. Eng. Process. Process Intensif. 2022, 170, 108675. [Google Scholar] [CrossRef]
- Wijakmatee, T.; Hemra, N.; Wongsakulphasatch, S.; Narataruksa, P.; Cheenkachorn, K.; Prapainainar, C. Process intensification of biodiesel production with integrated microscale reactor and separator. Chem. Eng. Process. Process Intensif. 2021, 164, 108422. [Google Scholar] [CrossRef]
- Rokni, K.; Mostafaei, M.; Dehghani Soufi, M.; Kahrizi, D. Microwave-assisted intensification of transesterification reaction for biodiesel production from camelina oil: Optimization by Box-Behnken Design. Bioresour. Technol. Rep. 2022, 17, 100928. [Google Scholar] [CrossRef]
- Gouda, S.P.; Ngaosuwan, K.; Assabumrungrat, S.; Selvaraj, M.; Halder, G.; Rokhum, S.L. Microwave assisted biodiesel production using sulfonic acid-functionalized metal-organic frameworks UiO-66 as a heterogeneous catalyst. Renew. Energy 2022, 197, 161–169. [Google Scholar] [CrossRef]
- Kamel Ariffin, M.F.; Idris, A. Fe2O3/Chitosan coated superparamagnetic nanoparticles supporting lipase enzyme from Candida Antarctica for microwave assisted biodiesel production. Renew. Energy 2022, 185, 1362–1375. [Google Scholar] [CrossRef]
- Qu, S.; Chen, C.; Guo, M.; Jiang, W.; Lu, J.; Yi, W.; Ding, J. Microwave-assisted in-situ transesterification of Spirulina platensis to biodiesel using PEG/MgO/ZSM-5 magnetic catalyst. J. Clean. Prod. 2021, 311, 127490. [Google Scholar] [CrossRef]
- Khan, H.M.; Iqbal, T.; Mujtaba, M.A.; Soudagar, M.E.M.; Veza, I.; Fattah, I.M.R. Microwave Assisted Biodiesel Production Using Heterogeneous Catalysts. Energies 2021, 14, 8135. [Google Scholar] [CrossRef]
- Prajapati, N.; Oza, S.; Kodgire, P.; Singh Kachhwaha, S. Microwave assisted biodiesel production: Assessment of optimization via RSM techniques. Mater. Today Proc. 2022, 57, 1637–1644. [Google Scholar] [CrossRef]
- Xu, C.; Lan, J.; Ye, J.; Yang, Y.; Huang, K.; Zhu, H. Design of continuous-flow microwave reactor based on a leaky waveguide. Chem. Eng. J. 2023, 452, 139690. [Google Scholar] [CrossRef]
- Helmi, F.; Helmi, M.; Hemmati, A. Phosphomolybdic acid/chitosan as acid solid catalyst using for biodiesel production from pomegranate seed oil via microwave heating system: RSM optimization and kinetic study. Renew. Energy 2022, 189, 881–898. [Google Scholar] [CrossRef]
- Nayak, M.G.; Vyas, A.P. Parametric study and optimization of microwave assisted biodiesel synthesis from Argemone Mexicana oil using response surface methodology. Chem. Eng. Process. Process Intensif. 2022, 170, 108665. [Google Scholar] [CrossRef]
- Abusweireh, R.S.; Rajamohan, N.; Vasseghian, Y. Enhanced production of biodiesel using nanomaterials: A detailed review on the mechanism and influencing factors. Fuel 2022, 319, 123862. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Han, Z.; Wang, T.; Wang, Y.; Liu, C.; Guo, M.; Li, G.; Lu, W.; Yu, M.; et al. Nanosheet like CaO/C derived from Ca-BTC for biodiesel production assisted with microwave. Appl. Energy 2022, 326, 120045. [Google Scholar] [CrossRef]
- Thakkar, K.; Kachhwaha, S.S.; Kodgire, P. A novel approach for improved in-situ biodiesel production process from gamma-irradiated castor seeds using synergistic ultrasound and microwave irradiation: Process optimization and kinetic study. Ind. Crops Prod. 2022, 181, 114750. [Google Scholar] [CrossRef]
- Gude, V.G.; Patil, P.; Martinez-Guerra, E.; Deng, S.; Nirmalakhandan, N. Microwave energy potential for biodiesel production. Sustain. Chem. Process. 2013, 1, 5. [Google Scholar] [CrossRef]
- Yang, J.; Cong, W.-j.; Zhu, Z.; Miao, Z.-d.; Wang, Y.-T.; Nelles, M.; Fang, Z. Microwave-assisted one-step production of biodiesel from waste cooking oil by magnetic bifunctional SrO–ZnO/MOF catalyst. J. Clean. Prod. 2023, 395, 136182. [Google Scholar] [CrossRef]
- Prajapati, N.; Kodgire, P.; Singh Kachhwaha, S. Comparison of RSM Based FFD and CCD Methods for Biodiesel Production Using Microwave Technique. Mater. Today Proc. 2022, 62, 6985–6991. [Google Scholar] [CrossRef]
- Amesho, K.T.T.; Lin, Y.-C.; Chen, C.-E.; Cheng, P.-C.; Shangdiar, S. Kinetics studies of sustainable biodiesel synthesis from Jatropha curcas oil by exploiting bio-waste derived CaO-based heterogeneous catalyst via microwave heating system as a green chemistry technique. Fuel 2022, 323, 123876. [Google Scholar] [CrossRef]
- Temur Ergan, B.; Yılmazer, G.; Bayramoğlu, M. Fast, High Quality and Low-Cost Biodiesel Production using Dolomite Catalyst in an Enhanced Microwave System with Simultaneous Cooling. Clean. Chem. Eng. 2022, 3, 100051. [Google Scholar] [CrossRef]
- Pham, E.C.; Le, T.V.T.; Le, K.C.T.; Ly, H.H.H.; Vo, B.N.T.; Van Nguyen, D.; Truong, T.N. Optimization of microwave-assisted biodiesel production from waste catfish using response surface methodology. Energy Rep. 2022, 8, 5739–5752. [Google Scholar] [CrossRef]
- Nazir, M.H.; Ayoub, M.; Zahid, I.; Shamsuddin, R.B.; Zulqarnain; Ameen, M.; Sher, F.; Farrukh, S. Waste sugarcane bagasse-derived nanocatalyst for microwave-assisted transesterification: Thermal, kinetic and optimization study. Biofuels Bioprod. Biorefining 2021, 16, 122–141. [Google Scholar] [CrossRef]
- Zhang, M.; Ramya, G.; Brindhadevi, K.; Alsehli, M.; Elfasakhany, A.; Xia, C.; Lan Chi, N.T.; Pugazhendhi, A. Microwave assisted biodiesel production from chicken feather meal oil using Bio-Nano Calcium oxide derived from chicken egg shell. Environ. Res. 2022, 205, 112509. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.-C.; Liao, P.-H.; Lan, N.V.; Hou, S.-S. Enhancement of Biodiesel Production from High-Acid-Value Waste Cooking Oil via a Microwave Reactor Using a Homogeneous Alkaline Catalyst. Energies 2021, 14, 437. [Google Scholar] [CrossRef]
- Binnal, P.; Amruth, A.; Basawaraj, M.P.; Chethan, T.S.; Murthy, K.R.S.; Rajashekhara, S. Microwave-assisted esterification and transesterification of dairy scum oil for biodiesel production: Kinetics and optimisation studies. Indian Chem. Eng. 2020, 63, 374–386. [Google Scholar] [CrossRef]
- Hsiao, M.-C.; Kuo, J.-Y.; Hsieh, S.-A.; Hsieh, P.-H.; Hou, S.-S. Optimized conversion of waste cooking oil to biodiesel using modified calcium oxide as catalyst via a microwave heating system. Fuel 2020, 266, 117114. [Google Scholar] [CrossRef]
- Athar, M.; Zaidi, S.; Hassan, S.Z. Intensification and optimization of biodiesel production using microwave-assisted acid-organo catalyzed transesterification process. Sci. Rep. 2020, 10, 21239. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Shamsuddin, A.H.; Mahlia, T.M.I.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.H.; Masjuki, H.H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Lawan, I.; Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. Synergies between the microwave reactor and CaO/zeolite catalyst in waste lard biodiesel production. Renew. Energy 2020, 145, 2550–2560. [Google Scholar] [CrossRef]
- Mohd Ali, M.A.; Gimbun, J.; Lau, K.L.; Cheng, C.K.; Vo, D.N.; Lam, S.S.; Yunus, R.M. Biodiesel synthesized from waste cooking oil in a continuous microwave assisted reactor reduced PM and NOx emissions. Environ. Res. 2020, 185, 109452. [Google Scholar] [CrossRef] [PubMed]
- Phromphithak, S.; Meepowpan, P.; Shimpalee, S.; Tippayawong, N. Transesterification of palm oil into biodiesel using ChOH ionic liquid in a microwave heated continuous flow reactor. Renew. Energy 2020, 154, 925–936. [Google Scholar] [CrossRef]
- Rahimi, M.; Aghel, B.; Alitabar, M.; Sepahvand, A.; Ghasempour, H.R. Optimization of biodiesel production from soybean oil in a microreactor. Energy Convers. Manag. 2014, 79, 599–605. [Google Scholar] [CrossRef]
- Azcan, N.; Danisman, A. Microwave assisted transesterification of rapeseed oil. Fuel 2008, 87, 1781–1788. [Google Scholar] [CrossRef]







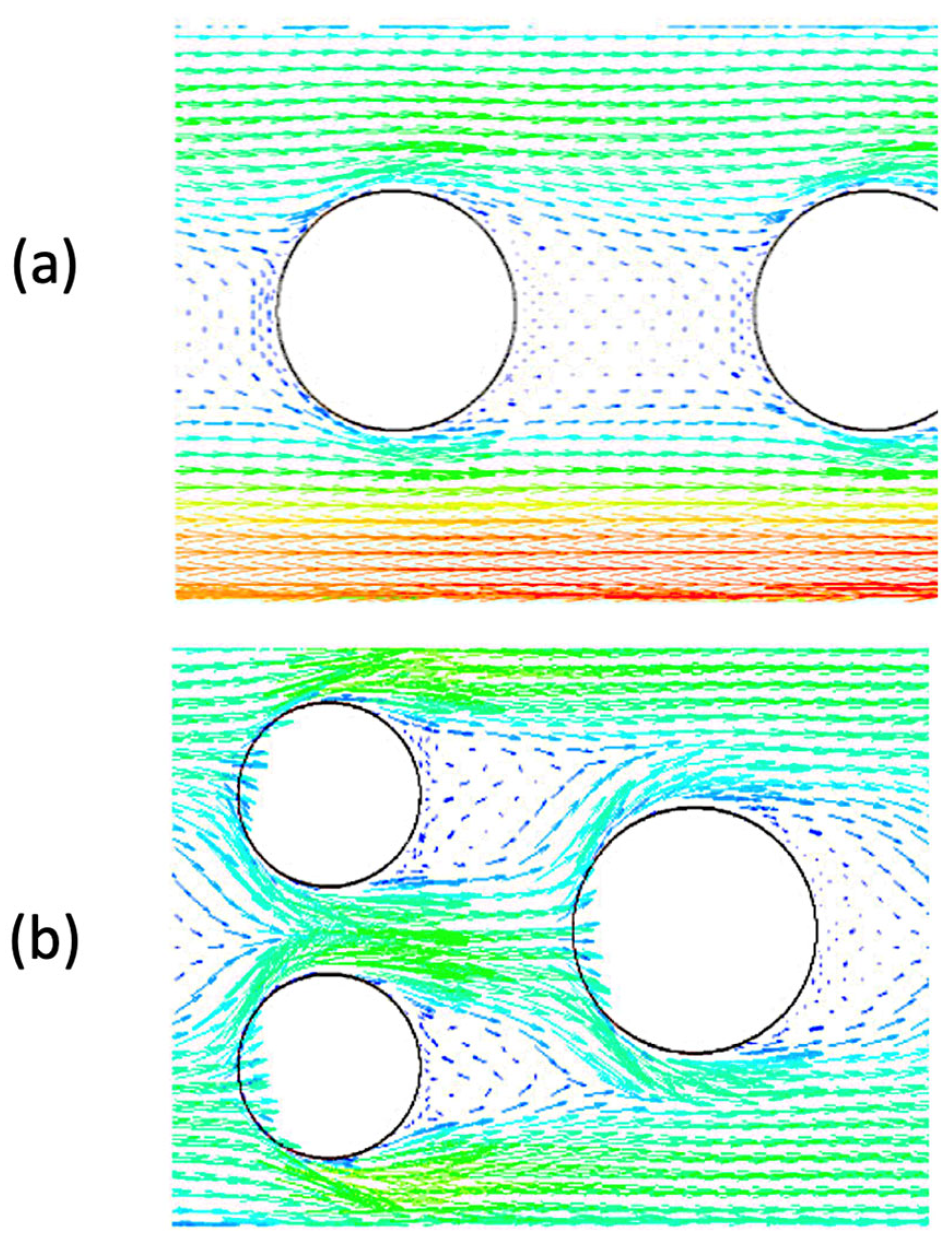

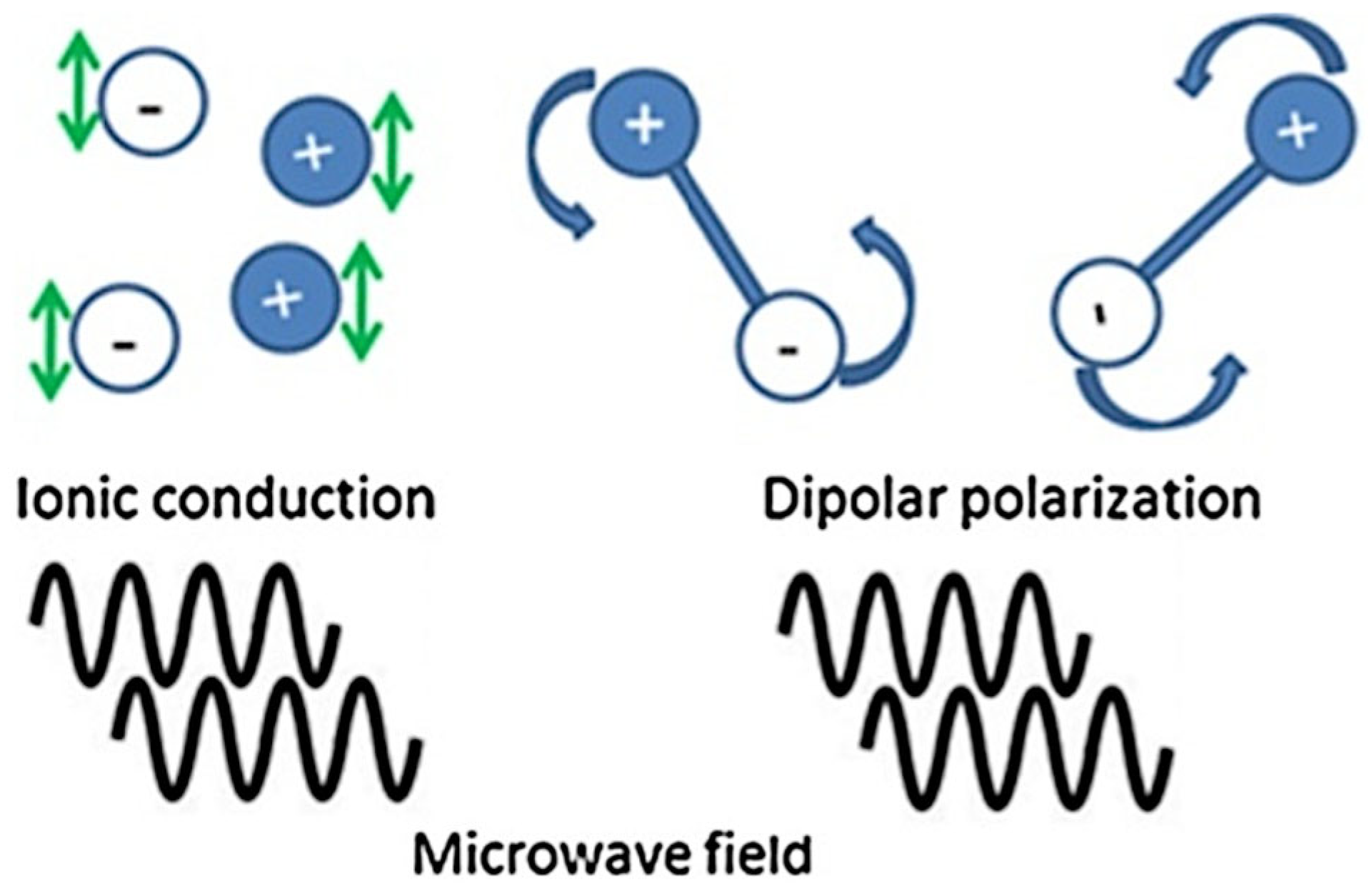
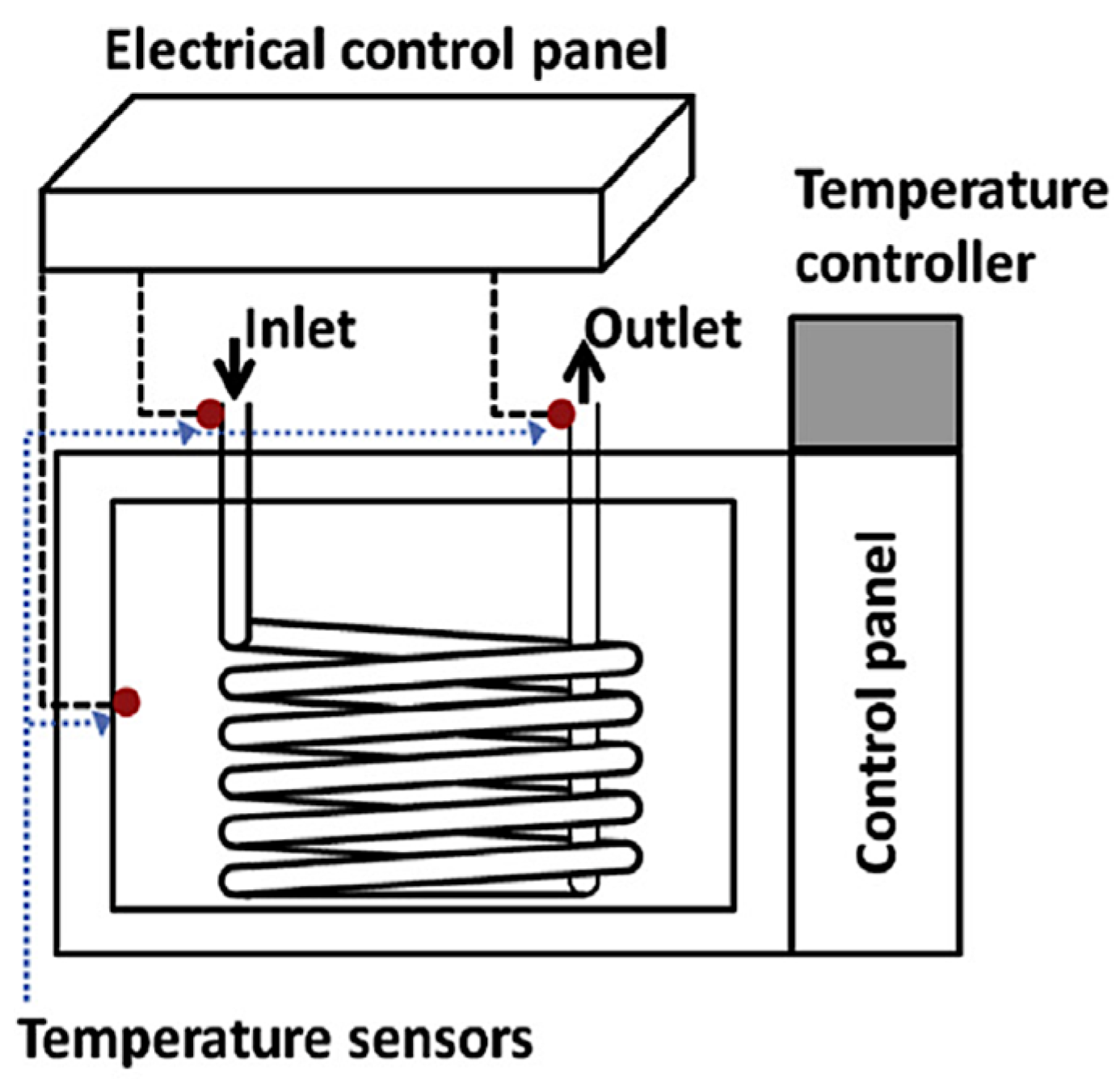
| Microchannel Reactor Features | Microchannel Reactor Material | Feedstock | Alcohol | Catalyst | Reaction Conditions (Temp, Time, Oil/Alcohol Ratio *) | Yield | Year | Reference |
|---|---|---|---|---|---|---|---|---|
| ID: 0.9 mm, T-mixer | - | rice bran oil fatty acid distillate | ethanol | 0.4 wt.% sulphuric acid | 280 °C, 19 min, 1:7 | 93.00% | 2023 | [32] |
| ID: 0.8 mm, T-mixer | stainless steel | waste cooking oil | methanol | 3.9 wt.% GO@MgO | 63 °C, 174.2 s, 2.67:1 (vol ratio) | 99.23% | 2022 | [33] |
| ID: 0.8 mm, T-mixer | stainless steel | waste cooking oil | methanol | 4.7 wt.% MgO | 63 °C, 176.39 s, 2.46:1(vol ratio) | 93.84% | 2022 | [33] |
| 0.4 mm (W) × 0.4 mm (D), Tesla-shaped | polymethyl methacrylate | vegetable oil | methanol | 1.0 wt.% NaOH | 60 °C, 4.85 s, 1:9 | 97.90% | 2022 | [34] |
| - | - | rice bran oil fatty acid distillate | - | catalyst free | 360 °C, 35 min, 1:11 | 97.10% | 2021 | [35] |
| ID: 0.8 mm, T-mixer | stainless steel | waste cooking oil | methanol | 8.5 wt.% calcined cow bone | 63.1 °C, 60 s, 2.25:1 (vol ratio) | 99.24% | 2021 | [36] |
| ID: 1 mm, T-mixer | polytetrafluoroethylene | sunflower oil | methanol | lipase enzyme | 40 °C, 20 min, 1:90 | 94.00% | 2021 | [37] |
| Vol: 1 cm3, T-mixer | - | waste cooking oil | methanol | 8.5 wt.% calcined cow bone-supported KOH | 63.53 °C, 85 s, 2:1 (vol ratio) | 97.21% | 2021 | [38] |
| ID: 0.8 mm, T-mixer | Teflon | sunflower oil | methanol | 0.1 g g−1 CaO (waste chicken eggshell) | 60 °C, 10 min, 3:1 (vol ratio) | 51.20% | 2021 | [39] |
| ID: 0.8 mm, T-mixer | - | waste cooking oil | methanol | 7.87 wt.% kettle limescale deposit | 60 °C, 12.5 min, 5:2.15 (vol ratio) | 96.58% | 2020 | [40] |
| ID: 0.8 mm, T-mixer | - | rice bran oil fatty acid distillate | ethanol | catalyst free | 300 °C, 24 min, 1:5 | 75.00% | 2020 | [41] |
| ID: 0.3 mm, Ultrasonic mixing | copper coils | Aegle Marmelos Correa seed oil | methanol | 1.3 wt.% sodium methoxide | 48 °C, 15 s, 1:9 | 98.00% | 2020 | [42] |
| ID: 0.8 mm, Ultrasonic mixing | copper coils | Aegle Marmelos Correa seed oil | methanol | 1.3 wt.% sodium methoxide | 48 °C, 25 s, 1:9 | 91.80% | 2020 | [42] |
| ID: 0.8 mm, T-mixer | - | waste cooking oil | methanol | 8.1 wt.% clinoptilolite supported KOH | 65 °C, 13.4 min, 1:2.25 | 97.40% | 2020 | [18] |
| ID: 0.69 mm, T-mixer | fluorinated ethylene propylene | palm oil | methanol | 5 wt.% KOH | 25 °C, 40 s, 1:7.6 | 98.60% | 2020 | [1] |
| 0.4 mm (W) × 0.78 mm (D), T-mixer | polymethyl methacrylate | sunflower oil | methanol | 4 wt.% lipase | 30 °C, 50 µL/min (flow rate), 1:2.5 | 68.90% | 2020 | [43] |
| Micromixer Type | Main Findings/Mixing Method of Fluid Flow | Year | Reference |
|---|---|---|---|
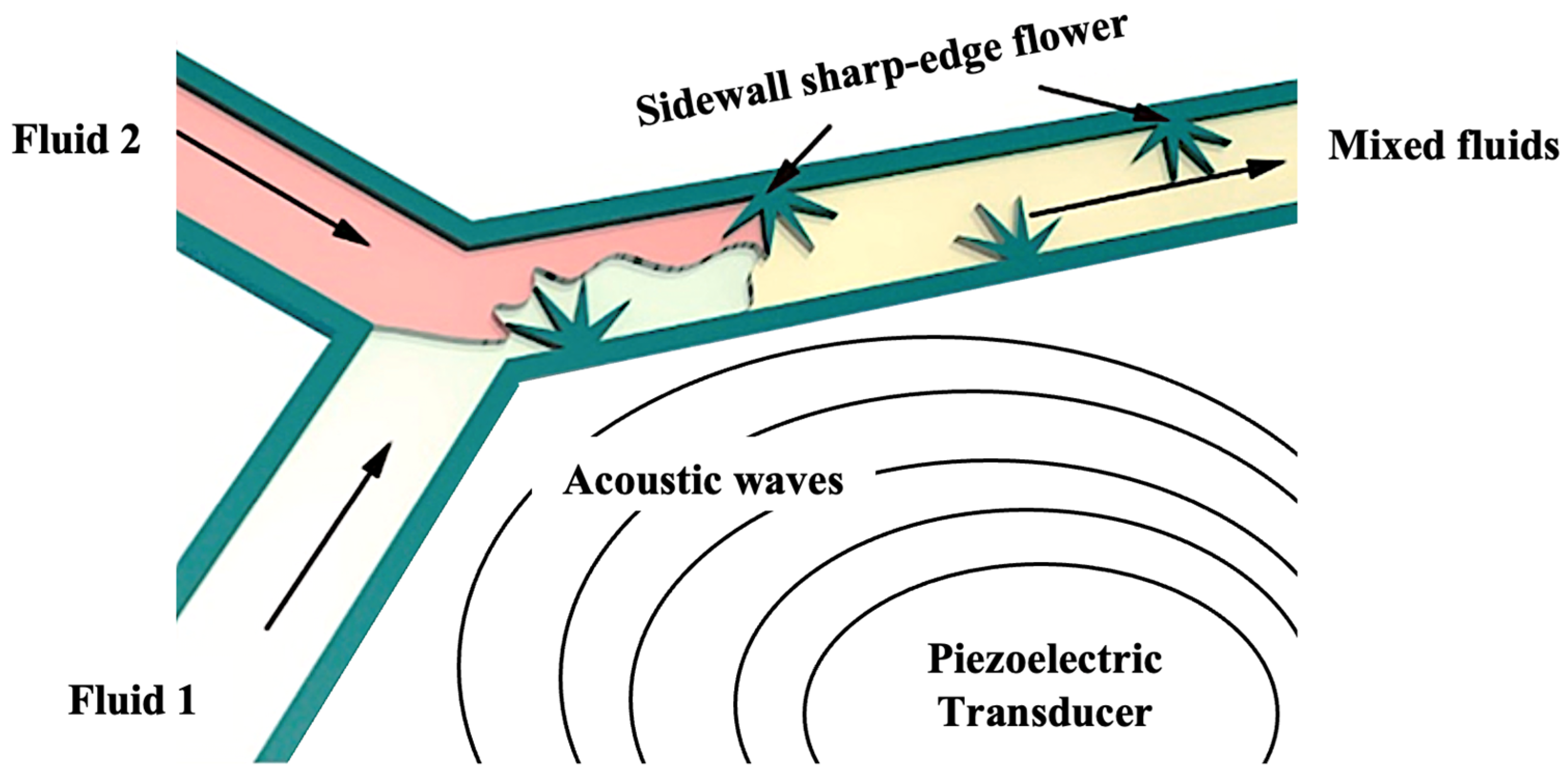
| Flower-like sharp-edged acoustic micromixer | Counter-rotating vortices produce waves and vortices upon acoustic actuation to mix fluid flow | 2022 | [45] |
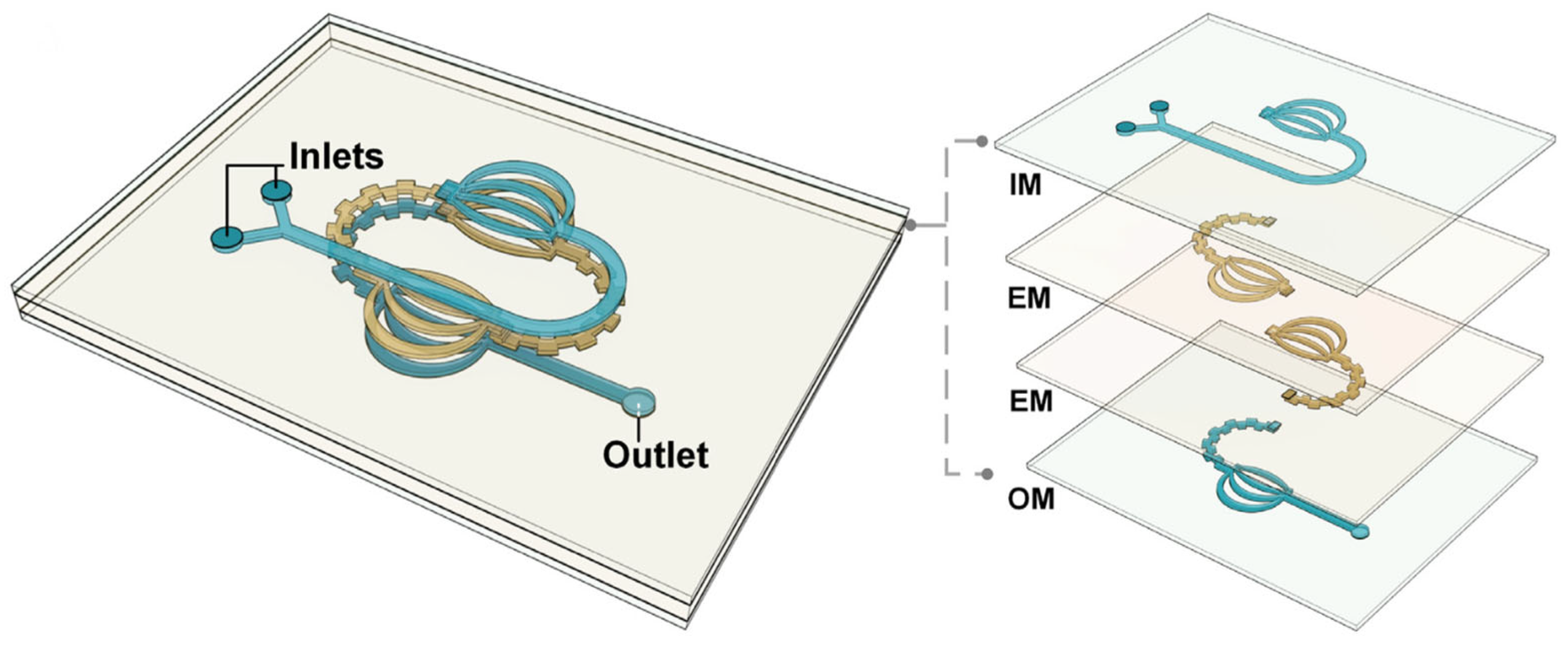
| Lamination-based split-and-recombine micromixer | Multiple stackable layers of enhancement modules within the reactor enhance the mixing of fluid flow | 2022 | [58] |
| Space chaotic micromixer | Splitting and re-channelling the flow from within the reactor, throughout the channel | 2022 | [49] |
| Plane chaotic micromixer | Structural designs within the microchannels inducing fluid mixing via turbulent flow | 2022 | [49] |
| Plane chaotic micromixer | Dividing the direction of flow via double symmetrical V-shaped baffle configuration split structures throughout the channel | 2022 | [44] |
| Microchannel Reactor Type | Main Findings | Year | Reference |
|---|---|---|---|
| Microchannel reactor with triangular baffles and circular obstructions |
| 2019 | [46] |
| Microchannel reactor with flower-like sharp-edged acoustic micromixer |
| 2022 | [45] |
| Microchannel reactor with split-and-recombine lamination-based passive micromixer |
| 2022 | [58] |
| T-junction microchannel reactor |
| 2020 | [1] |
| Microchannel reactor with a micromixer with static elements |
| 2017 | [90] |
| Microchannel reactor with single-phase turbulence |
| 2015 | [91] |
| Microchannel reactor with circular obstructions |
| 2015 | [92] |
| Split-and-recombine microchannel reactor |
| 2020 | [93] |
| Reference | Challenges/Limitations of Microchannel Reactors | Potential Solutions |
|---|---|---|
| [96] | Low throughput, as the output from a single microchannel module is typically between 10 mL/h and 200 mL/h. | Adopt the numbering-up approach by simultaneously operating multiple microchannel reactors. |
| [97] | Maintenance of equal reactant delivery, pressure and flow rate at inlet points, in the case of operating multiple microchannel reactors. | Set up an automated system to monitor the operation of multiple microchannel reactors, including reactant flow properties and inlet and outlet pressure. |
| [98] | Monitoring of multiple reactors, in the case of operating multiple microchannel reactors. | |
| [99] | Leakage from common points of failure such as inlets, outlets and joints combining various segments of the reactor. | During the fabrication of microchannel reactors, emphasise materials that are inert towards the relevant reactants and chemicals. |
| [100] | Micro cracks at curvature points and joints upon prolonged exposure to reactants and chemicals. | |
| [14] | Separation of by-products and the purification of products in a continuous flow process. | Set up a membrane separation for continuous flow biodiesel production or integrate with existing biodiesel production infrastructure for the processing of post-reaction products. |
| [101] | Fouling causes discrepancies in volumetric flow rate, product contamination and clogging of microchannels. | Ensure a smooth internal surface of microchannels to prevent fouling. Additionally, check on the output of microchannel reactors periodically to look out for signs of fouling through differences in the flow rate, volume and mass of product output. |
| [53] | Clogging was caused by the dislodging of heterogeneous catalyst over time, which were embedded onto the microchannel reactor. | |
| [102,103] | Clogging due to saponification, as a result of using high-free fatty acid (FFA) feedstocks for biodiesel production. | Identify and pre-treat high-FFA feedstocks through acid-catalysed esterification reaction prior to transesterification. |
| [104] | Precise and accurate fabrication of microchannel reactors, since a minute offset in dimension may cause a substantial effect on the results of reactions within. | Ensure periodic calibration of fabrication equipment (e.g., laser cutter, 3D printer) to maintain accurate and precise fabrication of microchannel reactors. |
| Microwave Condition | Feedstock | Alcohol | Catalyst | Reaction Conditions (Temp, Time, Oil/Alcohol Ratio *) | Yield | Year | Reference |
|---|---|---|---|---|---|---|---|
| - | soybean oil | methanol | 5 wt.% SrO–ZnO/MOF | 80 °C, 5 min, 1:11 | 99.5% | 2023 | [118] |
| - | waste cooking oil | methanol | 5 wt.% SrO–ZnO/MOF | 80 °C, 30 min, 1:11 | 90.0% | 2023 | [118] |
| 180 W | waste cotton seed cooking oil | methanol | 1.34 wt.% calcium oxide | 50 °C, 9.87 min, 1:9.63 | 99.83% | 2022 | [119] |
| 800 W | jatropha oil | methanol | 5 wt.% waste oyster-derived calcium oxide | 65 °C, 180 min, 1:9 | 91.1% | 2022 | [120] |
| 48 W | canola oil | methanol | 5 wt.% dolomite | 65 °C, 120 min, 1:9 | 99.1% | 2022 | [121] |
| 50 W | oleic acid | methanol | 8 wt.% sulphonated UiO-66-SO3H | 100 °C 1 h, 1:20 | 98.3% | 2022 | [106] |
| 300 W | catfish oil | methanol | 1 wt.% KOH | 53.2 °C, 94.4 s, 1:5.6 | 98.9% | 2022 | [122] |
| 1000 W | waste cooking oil | methanol | 5 wt.% sugarcane bagasse biochar | 60 °C, 15 min, 1:15 | 92.12% | 2022 | [123] |
| 800 W | camelina oil | methanol | 1.26 wt.% KOH | -, 5.85 min, 1:6.91 | 95.31% | 2022 | [105] |
| 500 W | chicken feather meal oil | methanol | 1 wt.% CaO | 40–60 °C, 5 min, 1:8 | 95.0% | 2022 | [124] |
| 600 W | waste cooking oil | methanol | 0.8 wt.% NaOH | 65 °C, 2 min, 1:12 | 98.2% | 2021 | [125] |
| 600 W | dairy scum oil | methanol | 1 wt.% KOH | 60 °C, 5 min, 1:7 | 93.47% | 2021 | [126] |
| 300 W | waste cooking oil | methanol | 4 wt.% CaO | 65 °C, 75 min, 1:8 | 98.2% | 2020 | [127] |
| 300 W | sunflower oil | methanol | 0.09:1 molar ratio or 4-dodecyl benzene sulphonic acid to oil | 76 °C, 30 min, 1:9 | 100% | 2020 | [128] |
| 850 W | crude ceiba pentandra oil | methanol | 0.84 wt.% KOH | -, 388 s, 60% | 96.19% | 2020 | [129] |
| 595 W | waste lard | methanol | 8 wt.% zeolite-supported CaO | -, 1.25 h, 1:30 | 90.89% | 2020 | [130] |
| 900 W | waste cooking oil | methanol | 5.47 wt.% activated limestone-based catalyst | 65 °C, 55.26 min, 1:12.21 | 96.65% | 2020 | [131] |
| 800 W | palm oil | methanol | 6 wt.% choline hydroxide (ChOH) | 68 °C, 5 min, 1:13.24, 20 mL/min flow rate | 89.72% | 2020 | [132] |
| Parameter | Batch | Semi-Batch | Continuous |
|---|---|---|---|
| Space requirement | High | Medium | Low |
| Capital requirement | High | Medium | Low |
| Operating cost | High | Medium | Low |
| Product quality | Batch-to-batch variation | Uniform | Uniform |
| Running time | Until chemical equilibrium | Until chemical equilibrium | Until catalyst inactivation or process maintenance |
| Production rate | Low | High | Highest |
| Selectivity | Low | High | High |
| Versatility | High | Low | Low |
| Operational challenges | Low | Low | Medium |
| Heat transfer | Inferior | Superior | Superior |
| Reaction time | Slow | Fast | Quick |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramaniam, K.; Wong, K.Y.; Wong, K.H.; Chong, C.T.; Ng, J.-H. Enhancing Biodiesel Production: A Review of Microchannel Reactor Technologies. Energies 2024, 17, 1652. https://doi.org/10.3390/en17071652
Subramaniam K, Wong KY, Wong KH, Chong CT, Ng J-H. Enhancing Biodiesel Production: A Review of Microchannel Reactor Technologies. Energies. 2024; 17(7):1652. https://doi.org/10.3390/en17071652
Chicago/Turabian StyleSubramaniam, Koguleshun, Kang Yao Wong, Kok Hoe Wong, Cheng Tung Chong, and Jo-Han Ng. 2024. "Enhancing Biodiesel Production: A Review of Microchannel Reactor Technologies" Energies 17, no. 7: 1652. https://doi.org/10.3390/en17071652









