Abstract
This study aimed to improve the safety and economy of cement production and to investigate the gasification performance and tar properties of wheat straw in a small electrically heated bubbling fluidized bed by varying three factors, namely, gasification reaction conditions, fuel quality and type, and the natural environment, so as to promote the application of the low-temperature gasification of biomass in the cement industry. The gasification experiment was carried out at temperatures of 550–700 °C, air equivalence ratios of 0.1–0.2, moisture contents of 5.25–24%, blended rubber ratios of 0–100%, and furnace vacuums of 0–0.03 within the parameter ranges, and the component analyses of the produced gases and tars were carried out by gas chromatography (GC) and gas chromatography–mass spectrometry (GC-MS). The experimental findings revealed that the optimal operating conditions for gasification were attained at a temperature of 650 °C, an equivalence ratio of 0.15, a moisture content of 5.25%, a rubber blending ratio of 0, and a vacuum degree of 0. Under these conditions, the concentrations of combustible components (H2, CH4, and CO) in the produced gas were 4.01%, 4.60%, and 21.05%, respectively. The carbon conversion rate was 62.40%, with the cold gas efficiency of 39.37%. The lower heating value of the produced gas was 5.915 MJ/Nm3, accompanied by a tar yield of 118.15 g/Nm3 and lower heating value of 3.385 MJ/Nm3.
1. Introduction
In recent years, spurred by rapid economic development, nations have embarked on a new phase of industrialization. Simultaneously, the escalating release of greenhouse gases (GHGs), primarily CO2, has given rise to frequent climate-related hazards, including global temperature elevation, extreme precipitation events, and glacier melting. Effectively managing and mitigating carbon emissions to establish a global governance framework has emerged as a shared challenge confronting all nations [1]. Simultaneously, the reliance on coal-based energy consumption has contributed to progressive environmental degradation, undermining the sustainable development of national economies. Consequently, reducing dependence on carbon-based fossil fuels is imperative through supply-side structural reform and the increased adoption of clean and renewable energy sources. In order to solve one of the challenges, unutilized agricultural wastes, such as wheat straw and rice straw, play an important role as one of the most stable sources of energy with a short growth cycle in meeting energy needs of countries to achieve long-term development in a sustainable manner [2]. In addition, due to the modernization of industry, the number of automobiles has increased dramatically, and how to dispose of end-of-life tires in an environmentally sound manner has become an urgent problem. Traditional treatment methods, such as open-air decomposition, landfill disposal, or direct incineration, produce large quantities of harmful gases and are not in line with the concept of sustainable development [3]. Tires are mainly composed of rubber, and the reason for treating rubber by gasification is that, on the one hand, rubber itself has high carbon content and high calorific value, and the gas produced by gasification has high calorific value. On the other hand, rubber can effectively reduce the emission of harmful gases, such as NOx and SO2, and promote the achievement of the goal of carbon neutrality through gasification.
As the world’s most abundant renewable energy source, biomass, which is derived directly or indirectly from organic matter produced by photosynthesis in various plants, is dispersed and has a low energy density compared to fossil fuels. In order to improve the utilization rate of biomass energy, it is necessary to improve its energy density through the pretreatment of biomass and corresponding conversion and utilization technologies. Currently, there are two main utilization methods as follows: biochemical conversion and thermochemical conversion [4,5].
Gasification is the more common utilization of biomass thermochemical conversions, through the air, carbon dioxide, water vapor, oxygen, and other gasification agents, and biomass at higher temperatures to partially oxidize the process of generating renewable gases (mainly CO, H2, CH4), which can be divided into the four stages of drying, pyrolysis, combustion, and reduction [6,7].
The gasifier, as the main component of the gasification plant, needs to maintain the stability of syngas production as much as possible when coping with the varying degrees of the mixing of biomass and gasifying agents. According to the difference between the way of contacting reagents, biomass, and the gasification agent with the gasifier, gasifiers are mainly classified into three categories: moving bed (fixed bed), gas flow bed, and fluidized bed [8]. In comparison to fixed-bed reactors, fluidized bed reactors have good fuel adaptability and temperature uniformity within the bed, which is conducive to the systematic control and scale-up application of strong exothermic reactions. In recent years, fluidized bed reactors have been favored by many scholars, and related research work has been widely carried out. In addressing the current challenges of fluidized bed biomass gasification technology, including high impurity content, low heating value of the produced gas, low cold gas efficiency, and the absence of a complete industrial chain, current research primarily focuses on gasification parameters, tar removal, and related aspects [9,10]. Gu et al. [11] concluded that higher reaction temperatures can significantly improve the gas quality and gasification efficiency. Rasmussen, N. et al. [12], through the examination of gasification characteristics in agricultural biomass straw, found that due to the high content of ash and alkali metals in straw-based biomass, the ash melting point temperature is low, and gasification at high temperatures is prone to form an agglomeration of ash and bed material, causing slagging, thus affecting the gasification effect. Tar, as the main impurity in the gaseous product, has a high dew point temperature (400 °C), which can easily lead to various problems such as the clogging of the fuel pipeline and obstruction of system operation [13]. The catalytic cracking and thermal cracking of tar are the most abundant research results on tar removal. Corella et al. [14] utilized olivine and dolomite as bed materials in a bubbling bed gasifier, revealing the catalytic properties of these materials and resulting in a reduction in tar content to 5 g/Nm3 and 1.6 g/Nm3, respectively. A study by M. Virginie et al. [15] found that iron-loaded olivine materials exhibit a dual catalytic effect on tar cracking. The catalytic cracking of tar faces specific challenges, including susceptibility to agglomeration at high temperatures leading to pipeline blockage, low mechanical strength that complicates recovery after fracture, vulnerability to poisoning and deactivation due to carbon buildup and sintering, and high preparation costs with poor economic feasibility. These factors create difficulties for the extensive promotion of industrial applications on a large scale [16]. The thermal cracking method uses the high-temperature heating of pyrolysis and gasification of syngas to crack tar into gases with low molecular weight. Under high temperature conditions, the tar cracking capacity is high and the yield is relatively low. Brandt et al. [17] conducted the plasma thermal cracking of tar at 1290 °C, resulting in a reduction in tar content to 12 mg/Nm3. Zhai et al. [18] carried out the thermal cracking of tar generated from the pyrolysis of rice husks at temperatures ranging from 900 to 1200 °C. The comparative tests revealed a gradual decrease in tar content from 105 g/Nm3 to 0.018 g/Nm3, achieving a tar cracking rate of 99.9%, demonstrating its effectiveness.
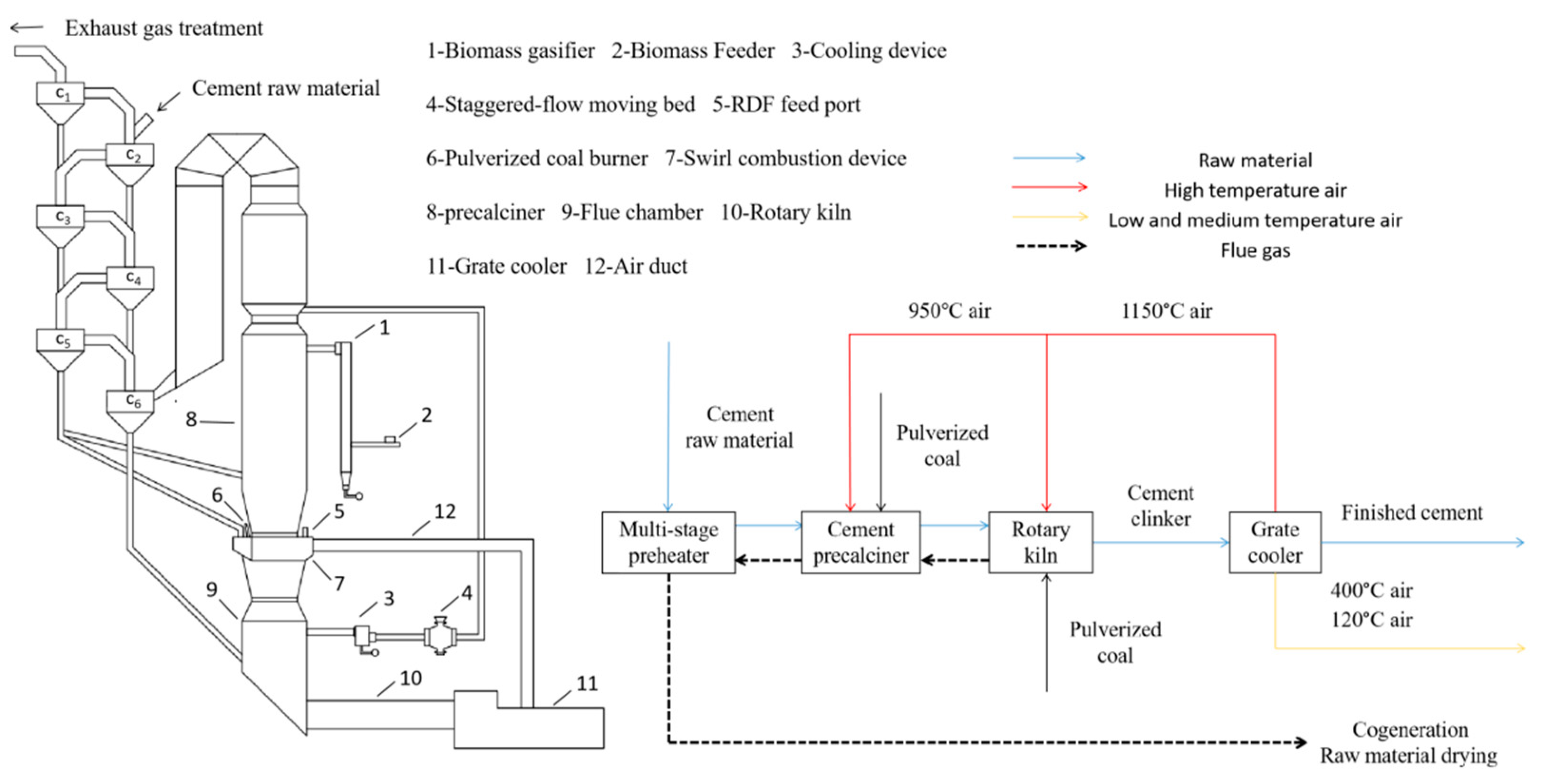
In 2020, China’s cement production exceeded 50% of the global production, and CO2 emissions from the cement industry amounted to 1.23 billion tons, accounting for 12% of the total national CO2 emissions of 10.251 billion tons, and so the country enormous pressure to reduce carbon emissions [19]. The primary technological pathways for reducing carbon emissions in the cement industry encompass several strategies: utilizing alternative fuels, adopting low-carbon cement, and implementing carbon capture, utilization, and storage. Among these, the use of “alternative fuels” has become the preferred carbon emission reduction process in the current stage of the global cement industry due to its significant impact on reducing carbon emissions, relatively high technological maturity, cost-effectiveness in CO2 emission reduction, and minimal impact on the original production system [20,21]. In order to control the generation of CO2 in cement production from the source, it is imperative to enhance the kiln combustion process under the premise of keeping the existing new dry cement production process basically unchanged. This involves incorporating a fluidized bed gasifier outside the decomposition furnace. Through low-temperature gasification, biomass generates combustible gas and carbonaceous fly ash. These byproducts are introduced directly into the middle of the decomposition furnace for combustion, thereby substituting a portion of the coal fuel. This establishes a low-temperature gasification coupled with a high-temperature combustion system. This process separates the gasification and tar removal steps into two distinct stages. Gasification in the low-temperature section effectively mitigates issues such as bed material sintering and furnace slagging. Meanwhile, combustion in the high-temperature section promotes comprehensive tar and residual carbon cracking, maximizing the utilization of heat from the available gas and tar. Consequently, this approach offers a significant improvement over the current high total carbon emission scenario in China’s cement production. The specific process schematic is illustrated in Figure 1.
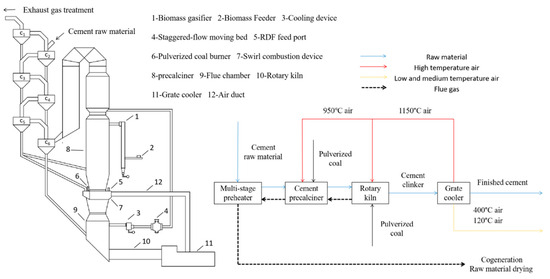
Figure 1.
Equipment and flow chart of cement production system.
In this paper, the impact of various alternative fuel types, qualities, gasification conditions, and environmental factors on gas production characteristics was examined by means of small-scale bubbling fluidized bed tests. Based on the background of the cement production process, it was found that low temperatures (550–700 °C) and low equivalence ratios (0.1–0.2) of the gasification conditions can enhance the safety and economic efficiency of system operation. Notably, targeted research in this domain is currently limited. Furthermore, this study investigated the adaptability of different fuels by incorporating rubber blending. It explored the impact of natural conditions on gasification characteristics by simulating variations in material moisture content (5.25–24%) through the introduction of steam. Additionally, innovative alterations in vacuum levels (0–0.03) were implemented to simulate changes in altitude.
2. Materials and Experimental Methods
2.1. Alternative Fuels and Bed Materials
In this experiment, wheat straw and rubber were employed as alternative fuels. The wheat straw was sourced from an agricultural production and processing base in Lianyungang City, Jiangsu Province, while the rubber originated from a solid waste treatment company in Dujiangyan City, Sichuan Province. Prior to the experiment, the sample powder with a particle size ranging from 0.18 to 0.25 mm was obtained through a pre-treatment process involving crushing, grinding, sieving, and drying, as illustrated in Figure 2. The samples were industrially analyzed according to the Chinese standard for biomass fuel analysis (GB/T 212-2008) [22]. The elemental analysis and determination of the higher heating value were conducted using the organic elemental analyzer (Elementar Vario EL, Elementar Analysensysteme GmbH Ltd., Hanover, Baden-Württemberg, Germany) and oxygen bomb calorimeter (Sundy SDC712, Sundy Science and Technology Inc., Jinan, Shandong Province, China), respectively. Given that industrial combustion typically transpires under constant pressure, which is essential for the subsequent calculations of product properties in gasification tests, it is imperative to convert the higher heating value into the lower heating value. This conversion is conducted in accordance with Formula (1) stipulated in the national standard (GB/T 213-2008) [23]. The results are presented in Table 1.

Figure 2.
Preprocessed fuel powder.

Table 1.
Ultimate analysis, proximate analysis, and calorific value of wheat straw and rubber.
Currently, olivine, dolomite, and quartz sand are the more widely used bed materials in biomass fluidized bed gasification. Among these materials, olivine and dolomite exhibit a promotional effect on the cracking of tar in the gasification products, leading to a reduction in tar concentration. However, in the context of the cement production process upon which this experimental study is based, the primary consideration is not the treatment of tar but rather the necessity for sufficient mechanical strength and resistance to slagging in the bed material, since the tar is transported to the decomposition oven after the gasifier to be effectively treated by high-temperature combustion [24]. As such, screened quartz sand with a particle size ranging from 0.18 to 0.25 mm was employed as the bed material in this experiment.
2.2. Experimental Facility
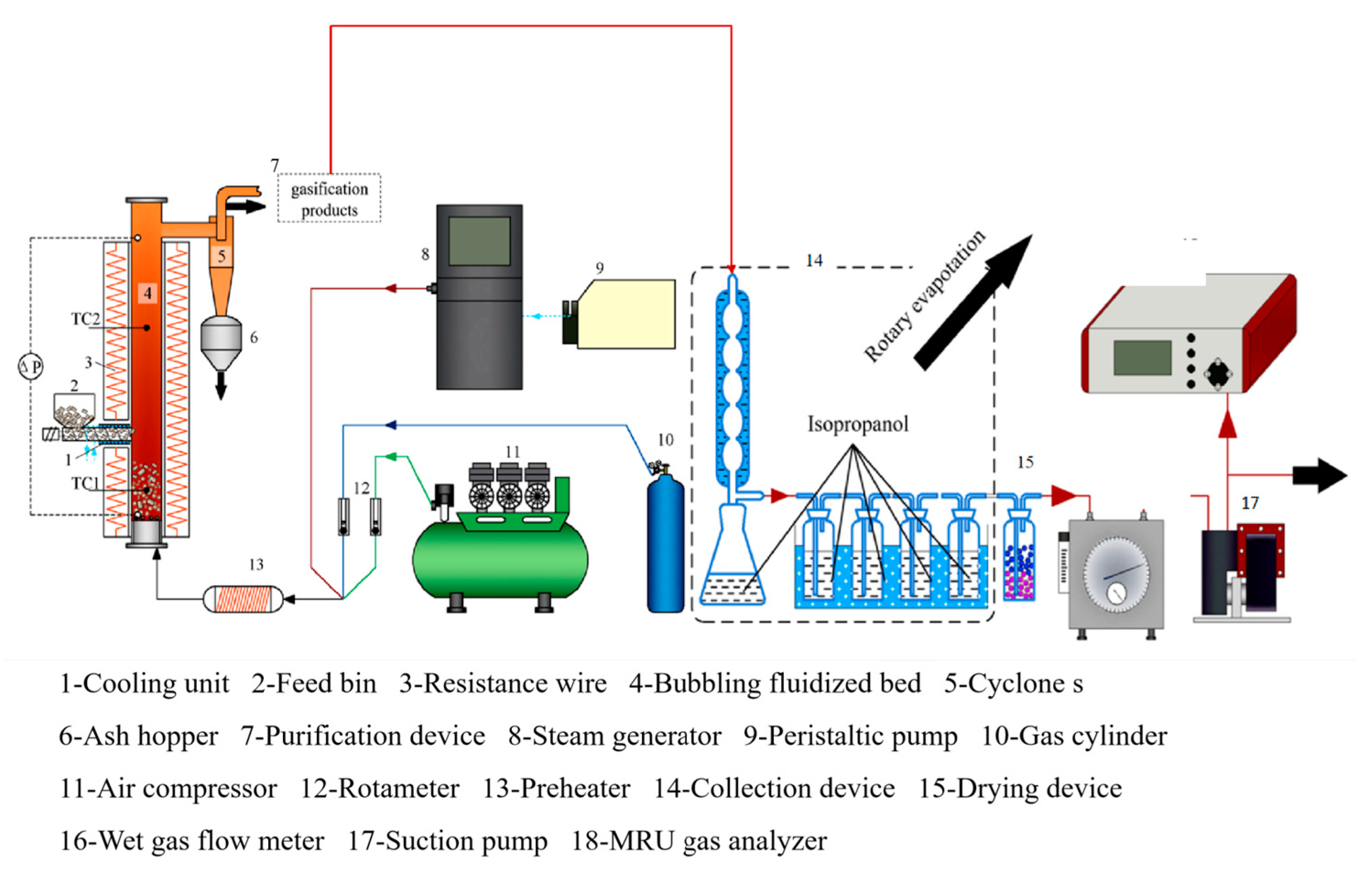
This experiment utilizes an electrically heated bubbling fluidized bed gasifier as the reactor, and its experimental setup is illustrated in Figure 3. The apparatus primarily comprises four components: the main body of the gasification reactor, the feeding system, the gasification medium supply system, and the product collection system.
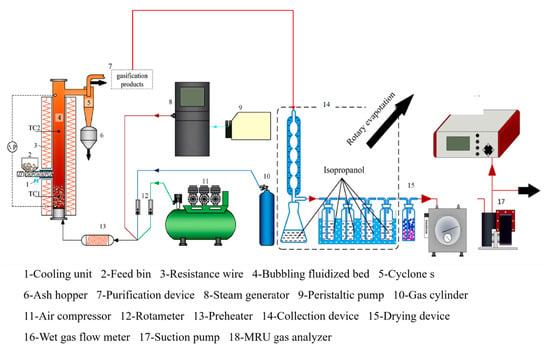
Figure 3.
Schematic diagram of bubbling fluidized bed gasification and collection system.
The gasification reactor’s main body is made of 2520 stainless steel, featuring an inner diameter of 32 mm and a height of 1000 mm. The bottom of the gasifier is equipped with a sieve with an aperture of 0.013 mm as an air distributor to prevent the leakage of material and ensure stable fluidization in the furnace. High-temperature resistance furnaces are positioned on the upper and lower exterior of the reactor, employing a PID temperature controller to regulate the heating amount. These furnaces serve as an external heat source to compensate for the heat loss in the bed. Simultaneously, thermal insulation materials are applied for effective heat insulation, ultimately achieving stabilized control of the gasification temperature. The dense-phase and dilute-phase zones are equipped with thermocouples and pressure sensors to monitor the fluidization status in the gasification reactor in real time. Gasification products are discharged from the upper part of the gasifier through a cyclone separator.
The feeding system comprises a hopper and a screw feeder, with the feeding rate controlled by varying the motor speed through frequency conversion. To prevent overheating, a circulating water pump is employed to facilitate water cooling and protect the feed screw. Additionally, a stirrer is installed in the hopper to mitigate the risk of blockages or uneven feeding during the feeding process.
The gasification media supply system utilizes air and water vapor as gasification media. The high-pressure air flow rate generated by the air compressor is regulated by a rotor flow meter, thereby adjusting the equivalence ratio (ER) and residence time during the gasification reaction. The water vapor flow rate is controlled by a peristaltic pump (LEADFLUID BT101L, LEADFLUID, Hangzhou, Zhejiang Province, China) to manage the system’s humidity, facilitating the study of how variations in material water content affect gasification characteristics without changing the feed rate. The gasification medium is blended and heated to 360 °C by a preheater before being uniformly introduced into the reactor from the bottom of the air distributor. To reduce heat loss during the conveying process and prevent water vapor condensation, it is essential to minimize the length of the connecting pipeline and simultaneously apply a tracer tape on the outside of the pipeline to maintain a temperature above 200 °C. The gasification medium is mixed and heated up to 360 °C in the preheater.
The product collection device primarily comprises three components: a cyclone separator and ash hopper, a condensable component absorption device, and a gas product collection device. These components are responsible for collecting gasification residual carbon, tar, and syngas, respectively. Most of the residual carbon from gasification is separated by a cyclone separator and stored in an ash hopper. To prevent combustion reactions resulting from high temperatures upon contact with air, it is necessary to cool the residual carbon down to room temperature before collection. The condensable component collection unit consists of a condenser tube, a two-port distillation flask, a gas washing cylinder containing isopropanol, and a gas dryer, in which the gas washing cylinder is placed in ice water to lower the temperature and further increase the absorption rate of tar. The gas product collection section regulates the flow rate of the gas into the gas analyzer mainly by means of a rotameter, which is used to monitor the stability of the gas production online. The remaining gas is pumped to a remote environment and collected in gas bags to prevent interference with the gas analyzer.
2.3. Test Procedure
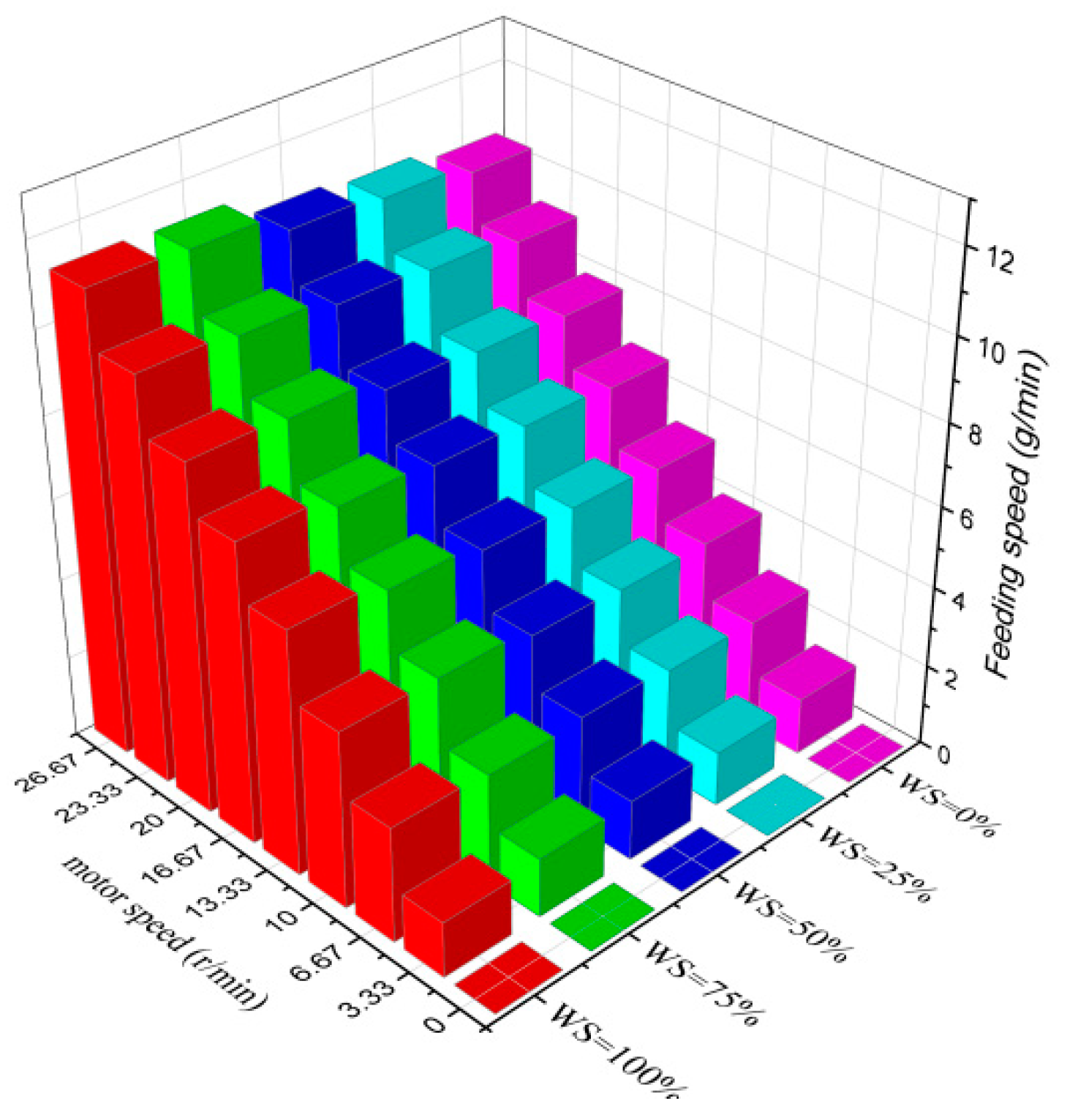
Before carrying out the gasification test, it is essential to ascertain the feeding rate of the screw feeder and the critical fluidization speed of the bed material. Due to the large differences in the physical properties of wheat straw and rubber, it is necessary to measure the feeding rate curves of the two and the samples mixed with each other at different ratios by controlling the motor frequency, and the specific results are shown in Figure 4.
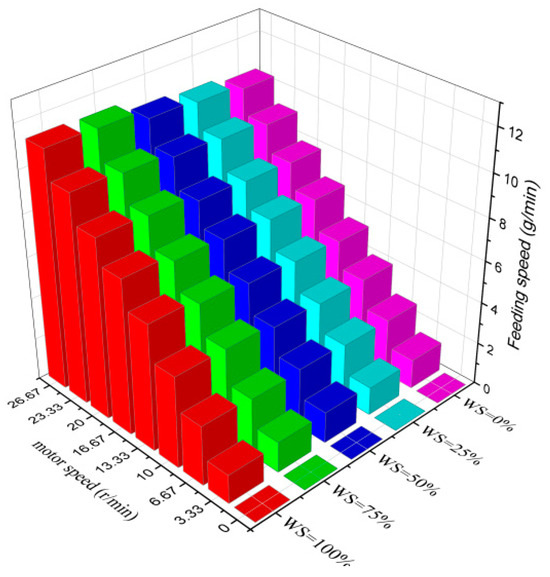
Figure 4.
Feeding rate of wheat straw and rubber with different blending ratios.
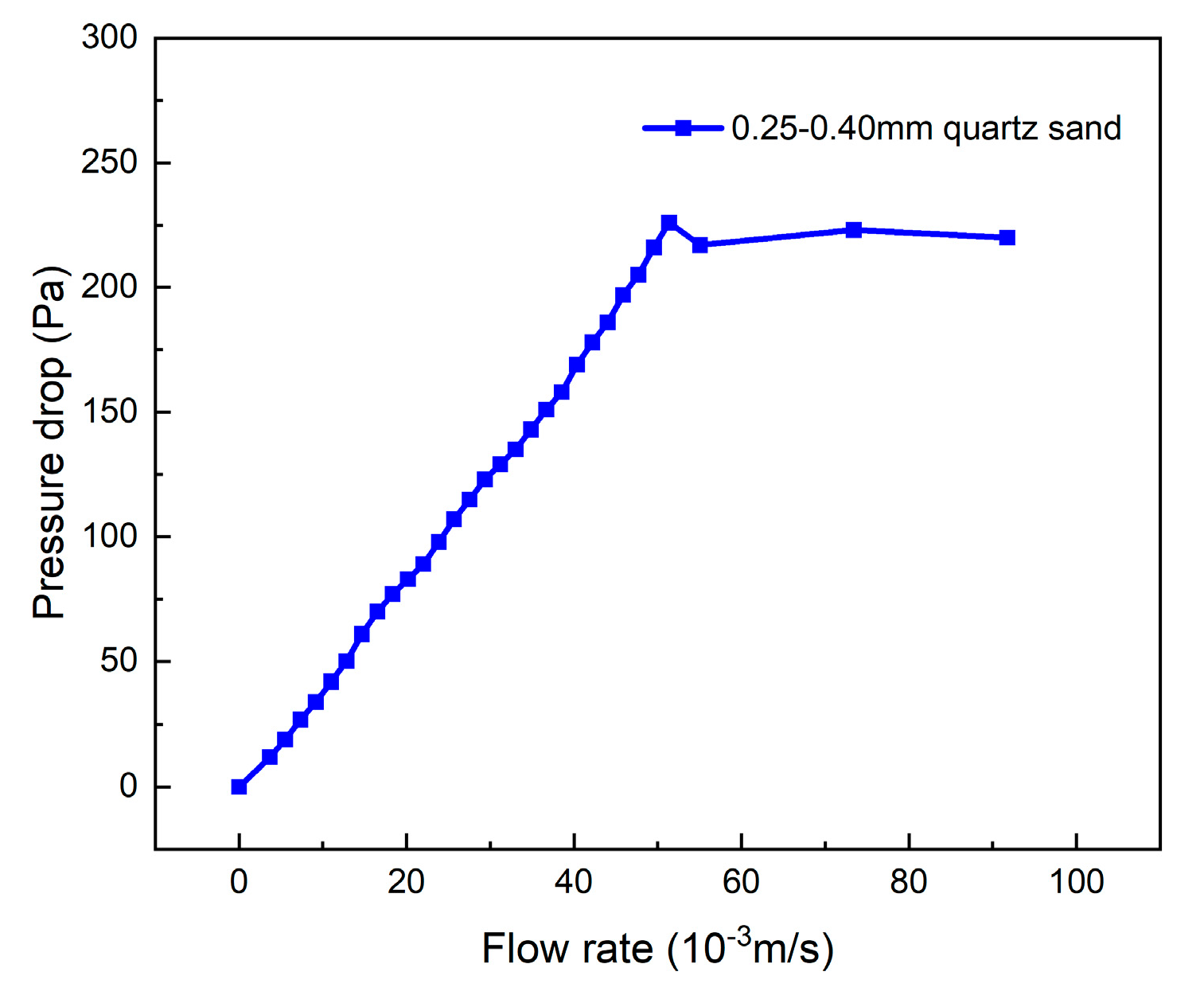
The air distributor has a significant effect on the pressure drop in the bed, and its pressure loss curve needs to be determined in advance. Under the premise that the gasification temperature is 650 °C and the airtightness of the experimental device is good, the air flow rate into the bed is controlled by a rotor flow meter, and the flow rate is gradually increased from 0 to 8 L/min at intervals of 0.5 L/min. Pressure sensors are positioned at the upper part of the reactor and beneath the air distributor. The difference between their values represents the pressure drop of the air distributor corresponding to different fluidization air velocities, denoted as ΔP1. For the determination of the critical fluidization velocity of the bed material, due to the “hysteresis effect” in the pressure drop curve obtained by the “speed-up method”, the “speed-down method” is generally adopted for the fluidization test [25]. The experimental bed height measured 40 mm, and the bottom diameter of the reactor was 32 mm. The corresponding volume of bed material was added from the top of the reactor using a beaker. The inlet flow rate was adjusted to 10 L/min via a rotameter, and the flow rate was gradually decreased to 0 at increments of 0.5 L/min. The pressure drop under different inlet flow rates was recorded as ΔP2, the difference ΔP2-ΔP1 represents the pressure drop of the bed material. By plotting the flow rate–pressure drop curve for 0.25–0.40 mm quartz sand, as depicted in Figure 5, the critical fluidization velocity (umf) is determined to be 0.048 m/s. The fluidization number (N) of the bubbling fluidized bed exhibits optimal performance within the fluidization range of 2.5–3.5. Therefore, the controlled air flow rate in the experiment is set at 0.12 Nm3/h, and at this point, the fluidization number is approximately 3.
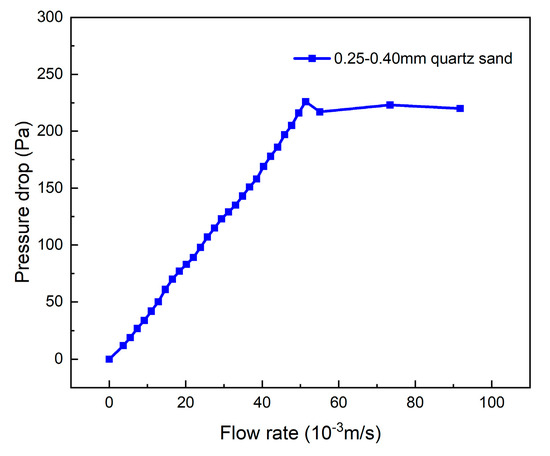
Figure 5.
Fluidization characteristics of quartz sand bed material with diameter of 0.25–0.40 mm and static bed height 40 mm.
The gasification experiments were divided into a preparatory stage and a formal stage.
During the preparation stage, the gasifier and cyclone separator were cleaned of ash before each test, and the entire exhaust pipe underwent cleaning and smoothing. Approximately 0.045 kg of the bed material was introduced to the top of the reactor, forming a fixed bed with a height of 40 mm. The air inlet was regulated using the rotor flow meter and peristaltic pump to ensure uniform subsequent preheating temperatures. The air preheater was set at 360 °C, and the electric furnaces in the dense-phase and dilute-phase zones were heated and adjusted to the required working condition temperature, with a heating rate of 20 °C/min. In this study, the air flow rate was controlled to be unchanged at 0.12 Nm3/h at all times, and the equivalence ratio was varied by adjusting the feed rate of the spiral feeder so as to prevent the change in reaction residence time due to the change in air velocity.
Once the set temperature is reached, the screw feeder is adjusted to a predetermined charging frequency. The pump is activated approximately 30 min later, and the gasification stability is monitored using a gas analyzer. When the temperature distribution within the gasification reactor stabilizes, and given the fluidized state of bubbling in the gasifier, it can be inferred that the gasification reaction has reached a stable state when the temperatures at the upper and lower measurement points fluctuate within a 10 °C range. After the gasification state is stabilized, sampling is conducted at 5 min intervals, with each working condition sampled three times. The average of these three test results is calculated to mitigate the unavoidable random errors in the experiment. For each working condition, the amount of fuel used and the readings of the membrane gas meter before and after the experiment are recorded, which are used to calculate the relevant characteristics of the gasification products.
2.4. Analytical Methods
The gas products collected through aluminum foil bags were analyzed for components using an MRU gas analyzer (equipped with a TCD detector, York Instruments Ltd., Stuttgart, Baden-Württemberg, Germany) as well as a GC-6890N gas chromatograph (equipped with an FID detector, Agilent Ltd., Santa Clara, CA, USA), with the chromatographic conditions of an Agilent HP-5 column (30 m × 0.320 mm, 0.25 μm). A rotary evaporator (RE-52AA) was employed for the spin evaporation of the mixture at 55 °C. The concentration of tar per unit volume of the produced gas, ρ(tar), was calculated by weighing the collected tar mass. The constant pressure low-level heat generation of tar followed the same procedure as obtaining the fuel’s lower heating value. The tar composition was characterized by GC/MS (Agilent 7890B/5977A), and the component contents were determined by semi-quantitative methods.
The composition of the produced gas was quantified by the standard gas correction method, and the lower heating value of the produced gas per unit volume Qv (kJ/Nm3) was calculated by Equation (2).
The volume fraction of each component in the produced gas is denoted by (where i = H2, O2, N2, CO, CH4, CO2, C2H4, C2H6, C3H6, and C3H8). The lower heating value of each component was obtained by referencing the National Institute of Standards and Technology (NIST).
Additional commonly used metrics for calculating the properties of gasification products, including gas yield, carbon conversion, and gasification efficiency, were determined using Equations (3)–(5).
In the given equations, Yg denotes the gas yield of the biomass (Nm3/kg), qv denotes the volumetric flow rate of syngas at standard conditions (m3/h), qm denotes the feed rate of the biomass (kg/h), CCE denotes the carbon conversion efficiency of the alternative fuels (%), Mar denotes the received base moisture of the alternative fuels (%), Cd denotes the corresponding dry base carbon content (%), Qar denotes the lower heating value (kJ/Nm3), abbreviated as LHV, and CGE denotes the cold gas efficiency (%).
In addition, experimental results require quantitative analysis and model validation, which helps to ensure the credibility, reproducibility, and verifiability of the study while providing insights into the data and models to guide decision making and future research directions. For the subsequent work, the simulation calculations were carried out through the ANSYS Fluent platform with the addition of UDF. The model is solved by the SIMPLE algorithm for pressure–velocity coupling, and the structure-based tracer model describes the gas–solid multiscale kinematic properties during the biomass gasification process in the bubbling fluidized bed, coupled with the structure-based mass transfer, heat transfer, and a variety of homogeneous and non-homogeneous chemical reactions within the bubbling fluidized bed to simulate the biomass gasification process in the bubbling fluidized bed. The simulation results of fluidized bed gasification will be published in the future, and this paper mainly focuses on the experimental part of the analysis.
3. Results and Discussion
3.1. Study on the Influence of Gasification Conditions
In this section, the impact of gasification conditions on the gasification characteristics of wheat straw was explored by adjusting different gasification temperatures and equivalence ratios. All values were measured at the reaction steady state, with lower concentrations of C2H4 and C2H6 combined as C2 and C3H6 and C3H8 combined as C3.
3.1.1. Effect of Gasification Temperature
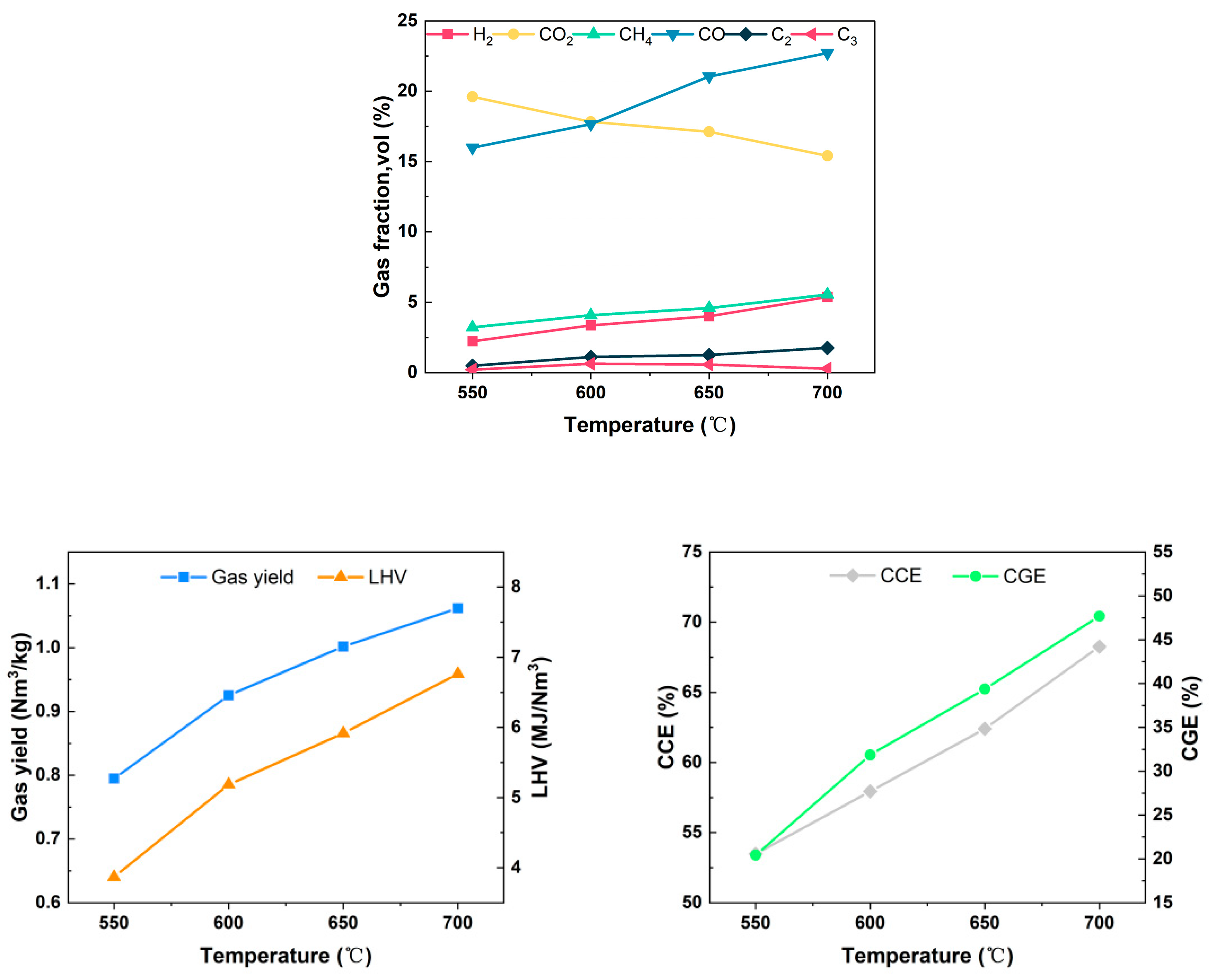
Gasification temperature is a key factor that significantly influences the chemical reactions during the gasification process. The gasification temperature distribution in the actual gasifier is related to many influencing factors such as feedstock characteristics, gasifier type, equivalence ratio, and heat exchange between the reactor and the external environment. In the experiment, the entire gasification reactor was maintained in self-equilibrium by controlling the PID temperature controller on the exterior of the furnace. The air flow rate was set at 0.12 Nm3/h, the equivalence ratio at 0.15, and the moisture content of the feedstock at 5.25%. The investigation focused on the gasification characteristics of wheat straw at temperatures ranging from 550 °C to 700 °C under these specified conditions. Figure 6 illustrates the trend of gas production components at different temperatures.
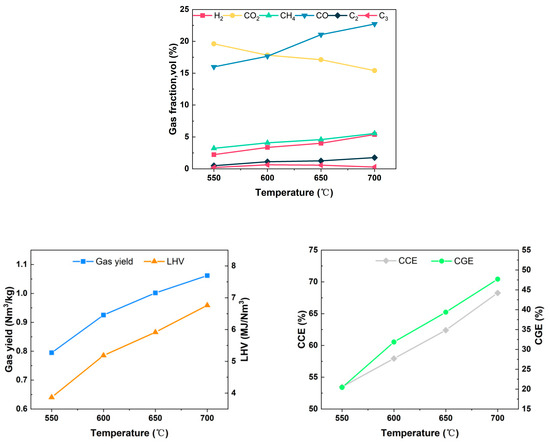
Figure 6.
Effect of temperature on gasification characteristics.
The concentrations and absolute yields of the combustible components increased dramatically as the gasification temperature elevated from 550 °C to 700 °C. The concentration of H2 increased from 2.23% to 5.39%, the concentration of CH4 increased from 3.22% to 5.56%, and the concentration of CO increased from 15.98% to 22.72%, while the concentration of CO2 decreased from 19.60% to 15.40%. Similar conclusions were obtained by Rodrigues et al. [26] in their study. The intensification of pyrolysis and gasification reforming reactions at elevated temperatures may constitute the primary reason for the observed changes in gas production components.
On one hand, the temperature increment facilitated volatile analysis in wheat straw and the secondary cracking of large molecule tars, thereby enhancing the production of small-molecule gases such as H2, CO, and CH4; on the other hand, according to Le Chatelier’s principle, the rate of the heat-absorbing Boudouard reaction and the water–coal gas reaction increased exponentially with the increase in temperature. This resulted in the formation of CO and H2 through the conversion of more C and CO2, consequently leading to a reduction in the concentration of CO2. Despite the temperature increase promoting a rise in the reverse reaction rate of the methanation reaction, the tar cracking reaction was enhanced, leading to a slight increase in the CH4 concentration under the combined effect. Due to the increase in the concentration of combustible components, the LHV of the produced gas exhibited an upward trend with the gasification temperature, escalating from 3.87 MJ/Nm3 at 550 °C to 6.76 MJ/Nm3 at 700 °C. Concurrently, Yg increased from 0.795 Nm3/kg to 1.062 Nm3/kg, CCE surged from 53.48% to 68.26%, and CGE rose from 20.42% to 47.69%. It is evident that the increase in temperature significantly improved the gas production quality.
Based on the cement production process, the tar generated during gasification is not collected by condensation but will be sent to the decomposition oven for combustion along with the produced gas, and it exists in gaseous form at high temperatures. Consequently, the components and LHV of the tar in the product are considered in this study, and their collection and analysis methods have been detailed above. The impact of gasification temperature on the concentration and LHV of tar is presented in Table 2. Yt denotes the tar yield (g/Nm3), Qmt denotes the LHV of tar per unit mass (MJ/kg), and Qvt denotes the LHV of tar per unit volume of the gas produced (MJ/Nm3). The variation in gasification temperature had a minimal impact on the Qmt, which fluctuated between 27.95 and 29.43 MJ/kg. The tar content produced in the gasification process was closely related to the rate of tar generation in the gasifier and the rate of the secondary cracking of tar. With the increase in gasification temperature, the concentration of tar showed an upward and then downward trend. At 550 °C, the gasification temperature was relatively low, which proved insufficient for the complete decomposition and conversion of the volatile components in the feedstock into tar and syngas. Consequently, the tar yield was low during this period. As the temperature exceeded 600 °C, the temperature increase facilitated the decomposition of long-chain tar compounds into small-molecule gases such as H2, CO, and CH4, resulting in a decrease in Yt and Qvt from 130.94 g/Nm3 and 3.683 MJ/Nm3 to 111.02 g/Nm3 and 3.267 MJ/Nm3, respectively.

Table 2.
Effect of gasification temperature on tar properties.
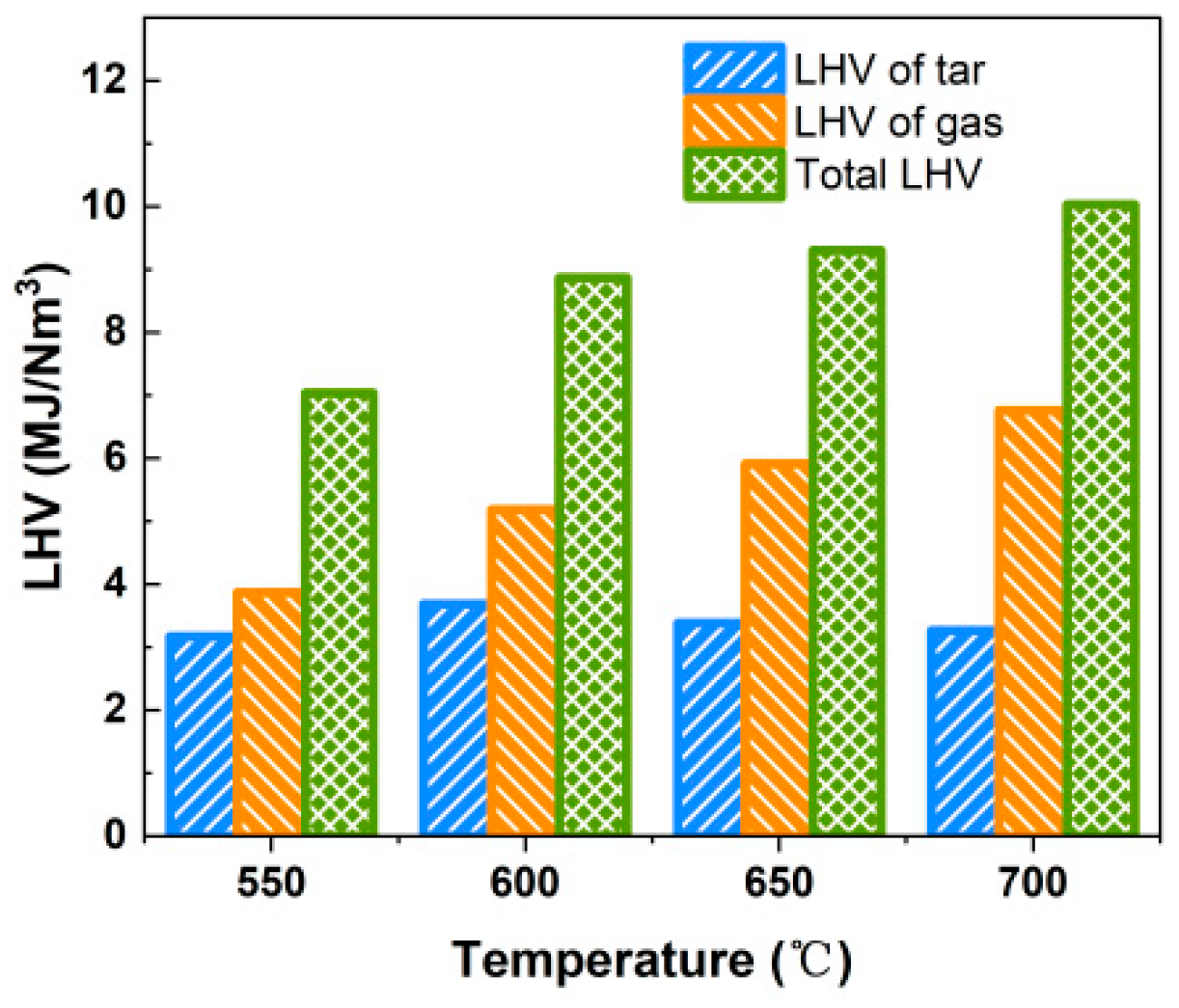
On the premise of avoiding slagging and clogging, fluidized bed gasification can appropriately increase the reaction temperature in order to improve the energy conversion rate of combustible solid waste and the quality of the gas produced. However, the gasification performance of the bubbling fluidized bed is not critical as the gasifier will pre-treat the alternative fuels, and all products generated will be fed to the decomposer. Considering both safety and the economics of cement production, selecting 650 °C as the gasification temperature is a more appropriate choice, provided that the proposed criterion for the LHV of the produced gas is met. The total LHV of the produced gas and tar is above 7.038 MJ/Nm3 in the studied range of gasification temperature, as shown in Figure 7.
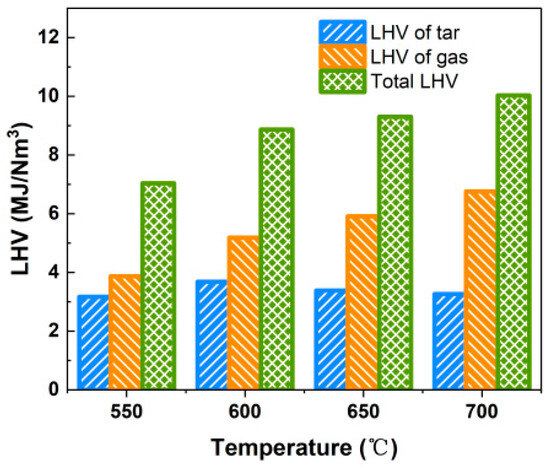
Figure 7.
Effect of gasification temperature on LHV of gas and tar.
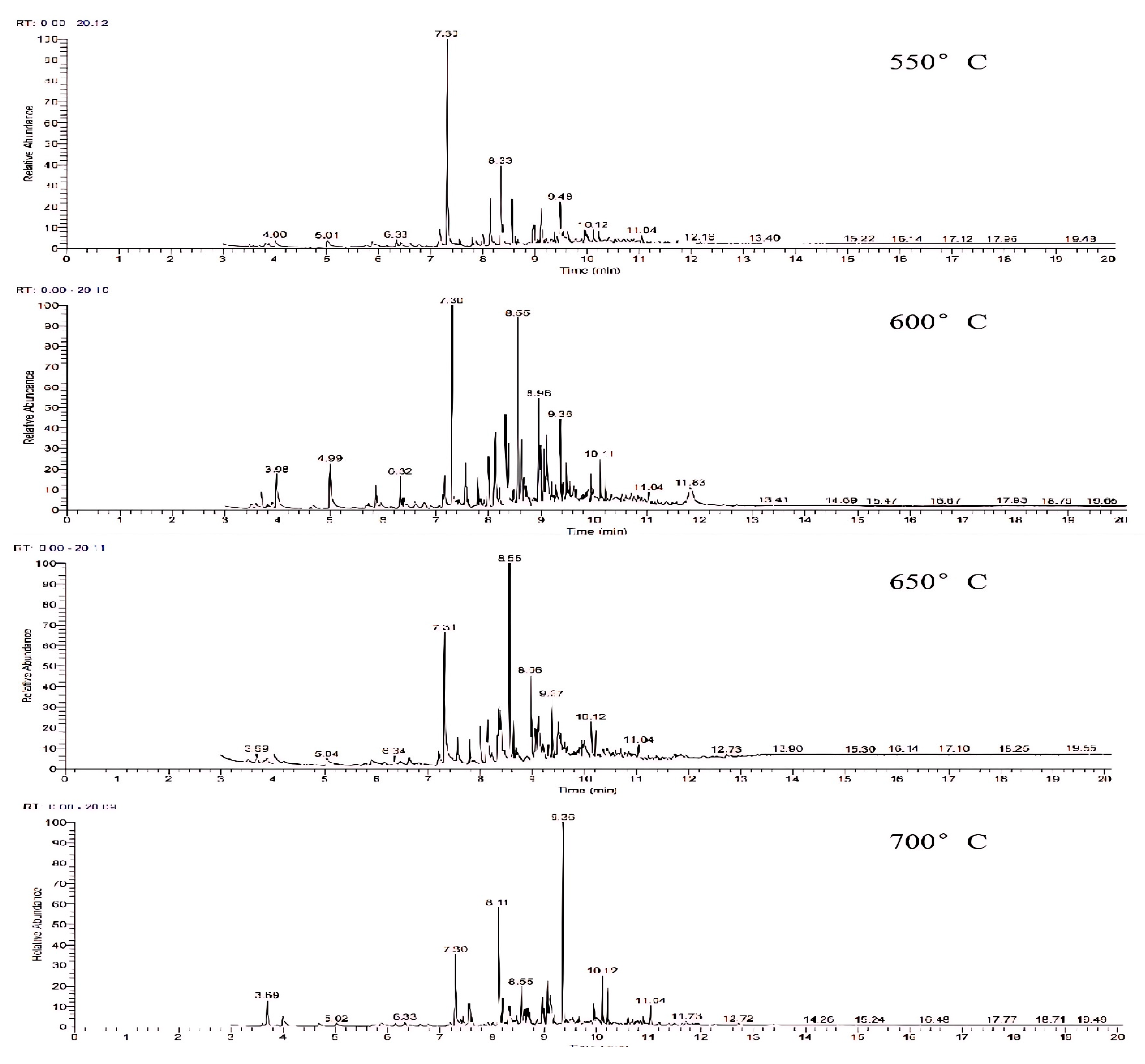
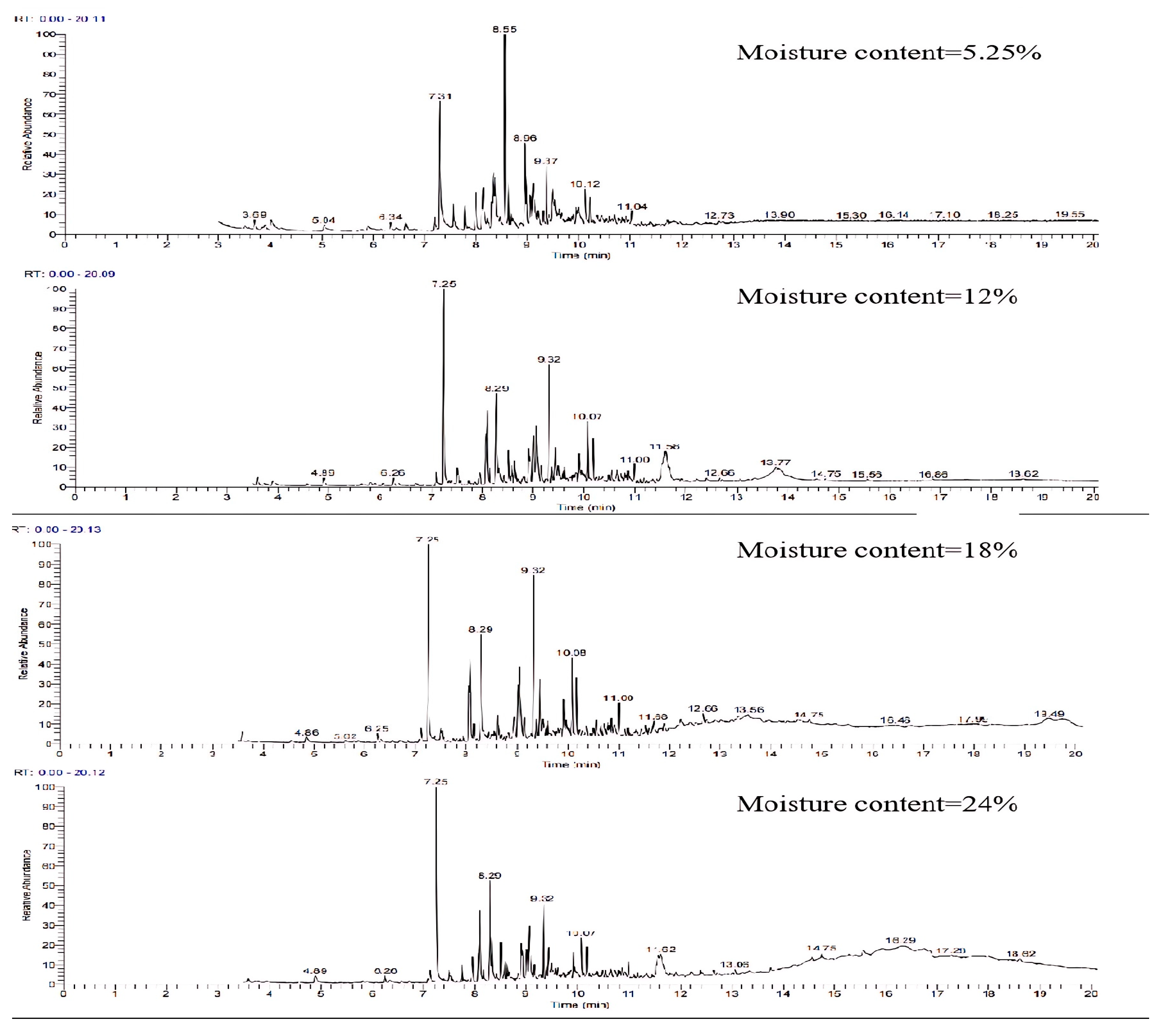
The gasification temperature plays a pivotal role in controlling the reaction rate and chemical equilibrium throughout the reaction process, influencing both the gas-producing components and the composition of the tar. Figure 8 displays the GC-MS ion flow diagrams of tar derived from wheat straw at various gasification temperatures. Observing the range from 550 °C to 700 °C, it is evident that the number of peaks in the ion flow diagram exhibits a pattern of increasing and then decreasing with the rise in reaction temperature. This indicates that the secondary cracking process of tar is more dominant in low-temperature gasification compared to the tar generation process.
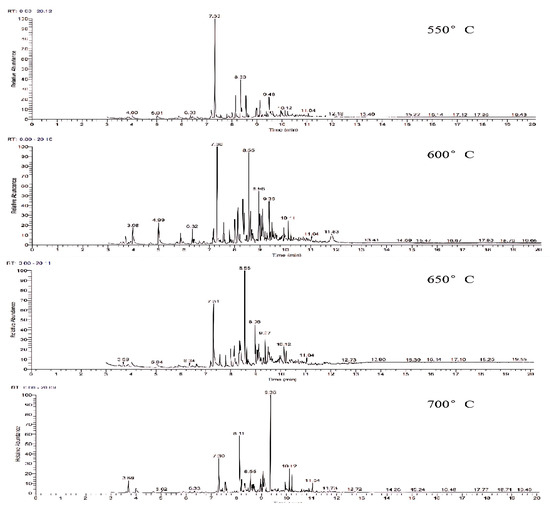
Figure 8.
Ion flow diagram of tar GC-MS of wheat straw at different gasification temperatures.
Due to the large number of components in tar detected by GC-MS, considering the chromatographic separation and mass spectral resolution issues, a comparative analysis was conducted to explore the specific effect of temperature on tar components. This analysis focused on components with a matching degree exceeding 75% and a relative content surpassing 3% in tar samples at temperatures ranging from 550 °C to 700 °C. The results are presented in Table 3. According to the literature [27], tar can be categorized into five groups: aliphatic compounds, heterocyclic compounds, light aromatic compounds (1 ring), light polyaromatic hydrocarbon (PAH) compounds (2–3 rings), and heavy cycloaromatic compounds (4–7 rings). From the table, it can be seen that with the increase in gasification temperature, the content of the tar components exceeding 3% changed significantly, in which the content of light cyclo-aromatics such as phenol, cresol isomers, methyl phenethyl ether, and O-Toluylaldehyde decreased, while P-Divinylbenzene, naphthalene, 1-Methylnaphthalene, and other heavy aromatic hydrocarbons increased. This shift can be attributed to the higher gasification temperature causing the breakdown of chains in light aromatic hydrocarbons, promoting the increase in combustible components such as H2, CO, and CH4. After bond breakage, unsaturated hydrocarbons tend to polymerize with each other. This process results in a decrease in light aromatic hydrocarbons and an increase in heavy aromatic hydrocarbons. Additionally, the higher thermal stability of molecules at elevated temperatures further promotes the formation of heavy aromatic compounds [28].

Table 3.
Analysis of tar GC-MS components at different temperatures.
3.1.2. Effect of Equivalent Ratio
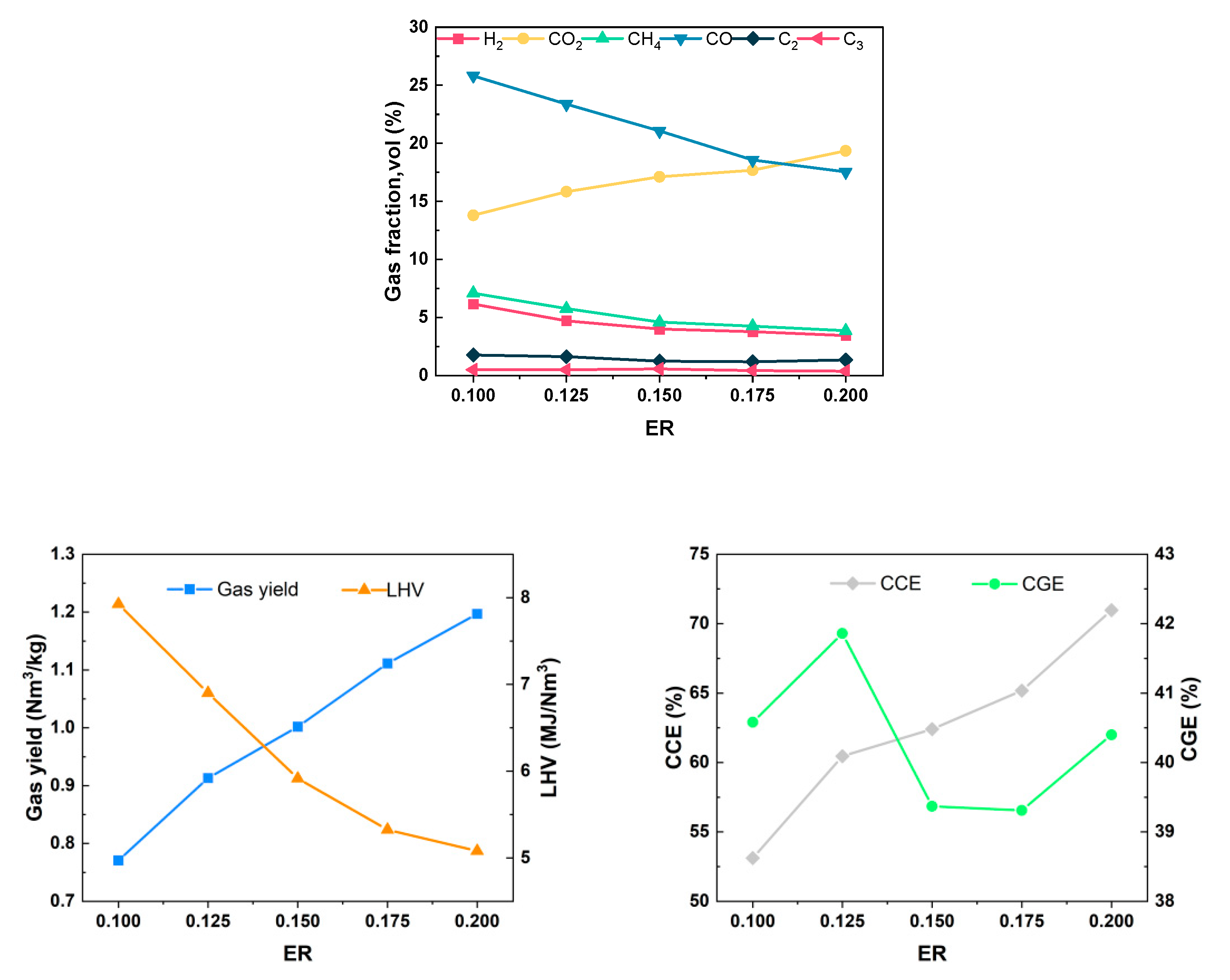
The equivalence ratio indicates the ratio of the actual amount of air introduced in the gasification process to the theoretical amount of air required for complete combustion, expressed as ER. The equivalence ratio has a large impact on the gasification products, including the gasification gas components and LHV of the gas, and it is one of the key influential parameters in the gasification process. To curtail the energy consumption arising from the cement production process, it becomes imperative to decrease the volume of air introduced into the system and incorporate a portion of medium-temperature air at 400 °C. Throughout the experiment, the air flow rate remained constant at 0.12 Nm3/h, the gasification temperature was set at 650 °C, and the water content was 5.25%. The gasification characteristics were investigated at an equivalence ratio of 0.10–0.20.
As the ER increased from 0.1 to 0.2, the content of H2 decreased from 6.16% to 3.45%, the content of CH4 decreased from 7.09% to 3.86%, the content of CO decreased from 25.81% to 17.53%, the content of CO2 increased from 13.80% to 19.35%, the Yg increased from 0.771 Nm3/kg to 1.197 Nm3/kg, the LHV decreased from 7.925 MJ/Nm3 to 5.080 MJ/Nm3, LHV of gas decreased from 7.925 MJ/Nm3 to 5.080 MJ/Nm3, CGE fluctuated around 40%, and CCE increased from 53.11% to 70.98%, as shown in Figure 9. The analysis results may be attributed to the following reasons: At a lower ER, insufficient oxygen in the furnace promotes the dominance of the biomass pyrolysis reaction, resulting in gasification products with higher concentrations of combustible gases. As the ER increases, the increased air volume in the furnace intensifies the combustion reactions of H2, CH4, and CO during pyrolysis. This leads to the consumption of some combustible components, an increase in CO2 content due to combustion, and the release of a significant amount of heat. These conditions are favorable for the cracking of tar molecules and reforming reactions, resulting in a gradual increase in gas yield and a decrease in the LHV of gas. It was calculated that the absolute yields of each component except CO2 changed very little with the increasing ER, which was consistent with the trend obtained in the study by H. Chen et al. [29]. The result suggests that the increased air will selectively and preferentially react with the solid residues in an oxidative manner to produce CO2. The reason for the small overall change in the CGE may be a combined effect due to the change in the ER: when the ER is small, most of the heat required for the gasification reaction is supplied by the electric heating wires on the outside of the chamber, and less heat is generated inside the fluidized bed itself, resulting in an insufficient gasification process for the wheat straw, which is not conducive to the reforming of hydrocarbons and the cracking of tar, so the CGE and CCE are lower. With the increase in the ER, the internal conditions for heat production is improved. However, the surplus combustible gas generated from the pyrolysis and gasification of wheat straw becomes more engaged in the combustion reaction. As a result, CCE gradually increases, while the change in CGE is not pronounced. In future industrial applications, it is essential to reduce the introduction of cold air into the system to enhance economic efficiency while ensuring sufficient heat in the gasifier. Therefore, ER = 0.15 appears to be a more appropriate choice.
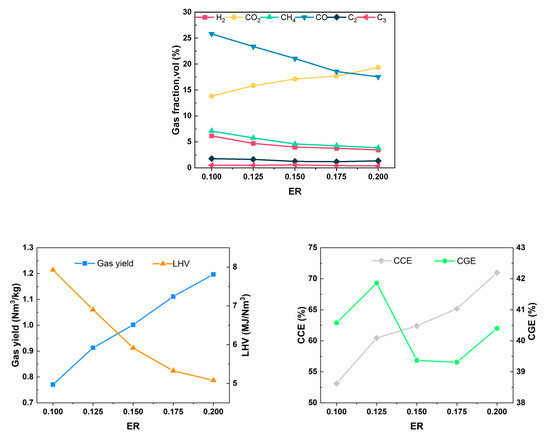
Figure 9.
Effect of ER on gasification characteristics.
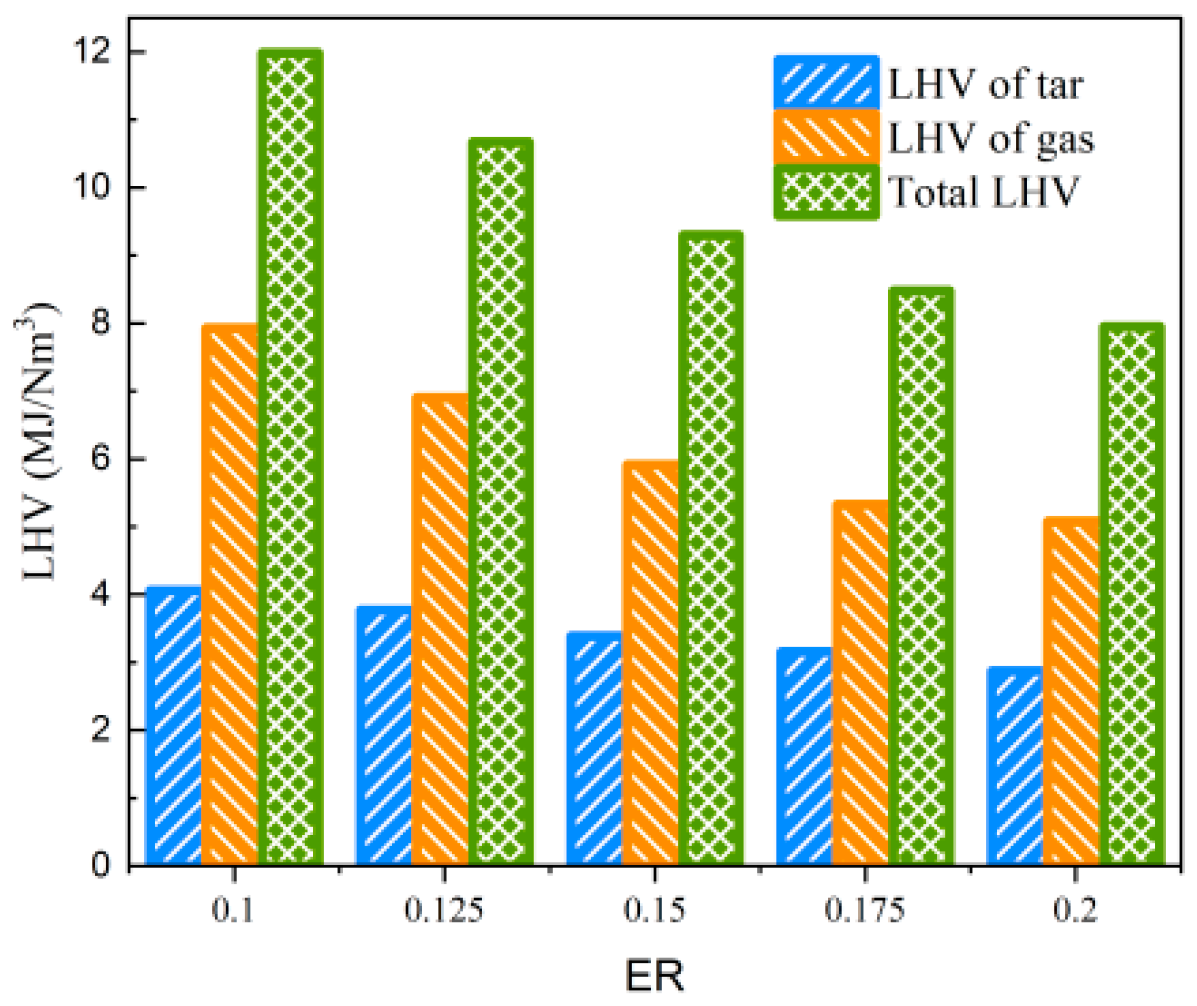
As shown in Table 4, the impact of ER changes on tar properties is primarily manifested in Yt, while it has a lesser effect on Qmt. This is due to the increasing dilution effect of N2 with the rise in ER, and the intensified combustion reaction of biomass feedstock releases a substantial amount of heat, strengthening both the degree of gasification reaction and the secondary cracking of tar. In the ER range of 0.1 to 0.2, the tar concentration decreases from 137.60 g/Nm3 to 102.26 g/Nm3, and the low-level calorific value of tar per unit of produced gas also decreases from 4.063 MJ/Nm3 to 2.869 MJ/Nm3. When the LHV of tar and the produced gas are considered simultaneously, the total LHV of the produced gas and tar is above 7.949 MJ/Nm3, within the range of equivalence ratios studied, as shown in Figure 10.

Table 4.
Effect of ER on tar yield and LHV.
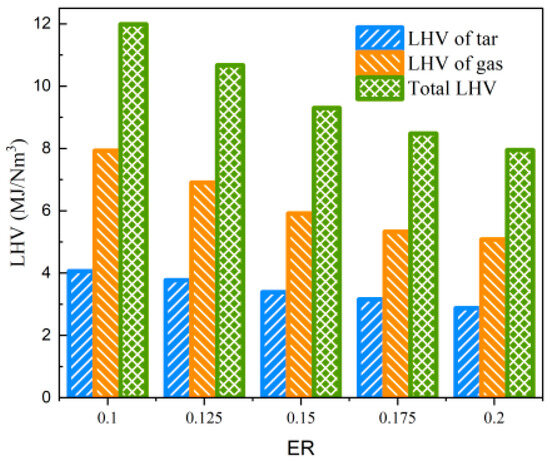
Figure 10.
Effect of ER on LHV of gas and tar.
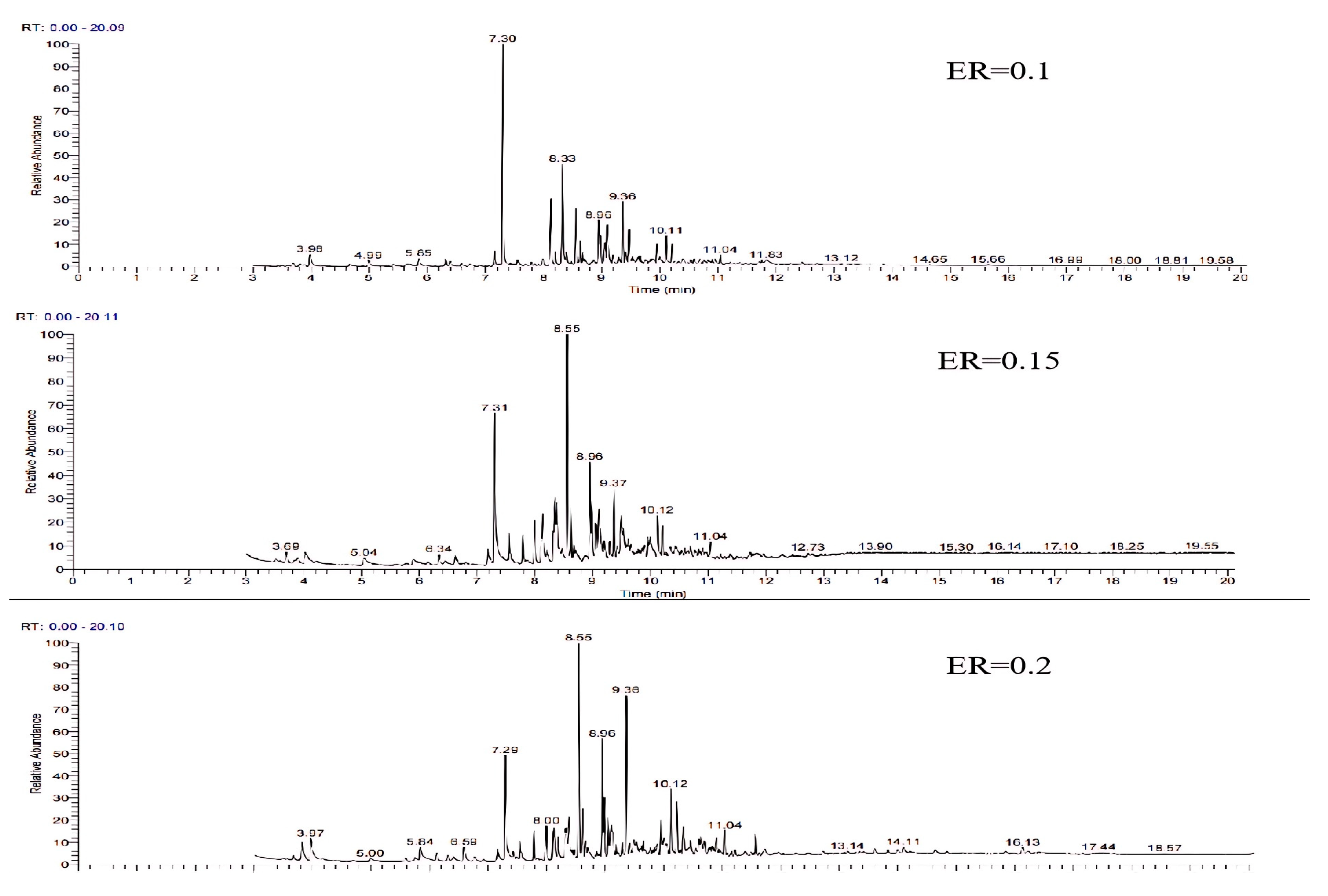
The GC-MS ion flow diagrams and component analyses of tar derived from wheat straw at different equivalence ratios are shown in Figure 11 and Table 5. With the rise in ER, there is a significant reduction in the percentage of aromatic compounds (one ring) such as phenol and cresol isomers and aliphatic compounds like cyclooctyne and undecatriene, while the percentage of light PAHs such as undecatriene and naphthalene increases, and the trend is consistent with the results of the study by T. Phuphuakrat [30]. The analysis may be attributed to following reasons: the increase in the ER, the increase in O2 in the gasifier, the transition of the reaction state from gasification to combustion, the oxidation reaction of part of the tar, and the breakage of C-H and C-O bonds, which leads to the increase in the concentration of free radicals, and the promotion of the dimerization reaction of the aromatic compounds (one ring) to the transformation of the light PAHs and heavy PANs [31].
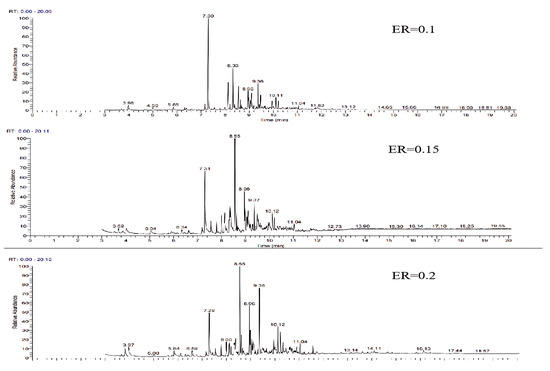
Figure 11.
Ion flow diagram of tar GC-MS of wheat straw at different ERs.

Table 5.
Analysis of tar GC-MS components at different ERs.
3.2. Study on the Influence of Biomass Fuel Quality and Type
The quality and type of biomass fuel can exert a substantial influence on gasification characteristics, encompassing factors such as chemical composition, ash and impurity concentration, and moisture content. The aim of this study is to utilize a bubbling fluidized bed to process different qualities and types of biomass fuels, the applicability of which to the feedstock is debatable.
3.2.1. Effect of Biomass Fuel Moisture Content
Biomass fuels are susceptible to variations in moisture content due to seasonal, climatic, and other factors. In the case of wheat straw, for biomass fuel processing enterprises, the moisture content of incoming materials is generally in the range of 20% to 30%, which is further reduced after crushing, milling, and sieving processes. The moisture content of the feedstock has a large impact on the gasification products, affecting aspects such as the fraction of gas, the LHV of gas, and Yt. It stands out as one of the key influencing parameters in the gasification process. Since this fluidized bed is screw-fed, changes in the moisture content of wheat straw powder may result in variations in downstream speed or, in extreme cases, agglomeration leading to blockages, so the moisture content of the carrier air is adjusted by a peristaltic pump to simulate the changes in the moisture content of the raw material. In the test, the temperature was controlled at 650 °C, the air flow rate was maintained at 0.12 Nm3/h, ER = 0.15, and the air preheater was 360 °C.
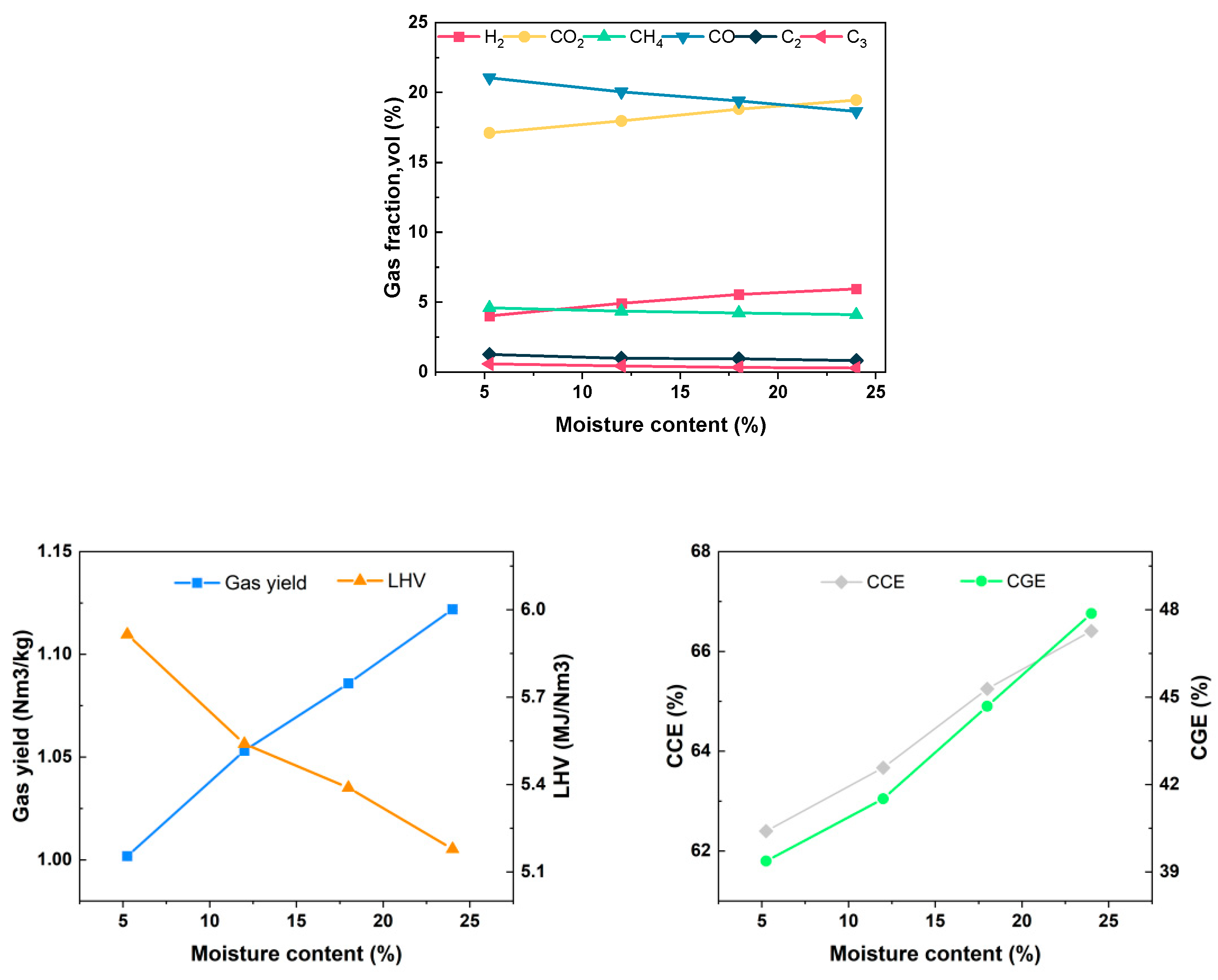
As the moisture content increases from 5.25% to 24%, the concentration of H2 increases from 4.01% to 5.95%, the concentration of CH4 decreases from 4.60% to 4.10%, the concentration of CO decreases from 21.05% to 18.65%, the concentration of CO2 increases from 17.11% to 19.46%, the Yg increases from 1.002 Nm3/kg to 1.122 Nm3/kg, the LHV of gas decreases from 5.915 MJ/Nm3 to 5.178 MJ/Nm3, CGE increases from 39.37% to 47.87%, and CCE increases from 62.40% to 66.41%, as shown in Figure 12. The introduction of water vapor mainly promotes the water–gas reaction, water–gas conversion reaction and water vapor reforming reaction in the positive direction, resulting in an increased production of H2 and CO2. An increase in water vapor concentration can enhance the tar reforming reaction and accelerate tar cracking during the gasification process. However, it also intensifies heat absorption. In situations where there is a delay in the signal feedback from the external electric heating wire, the gasification temperature decreases, leading to a reduced reaction rate and a decline in the gaseous component. Ultimately, this results in a decrease in the LHV of the gas. Compared to the water vapor reforming reaction of methane with tar, the increase in water vapor significantly promotes the water–gas shift reaction. Consequently, the water–gas shift reaction plays a dominant role in the alterations observed in gas production components.
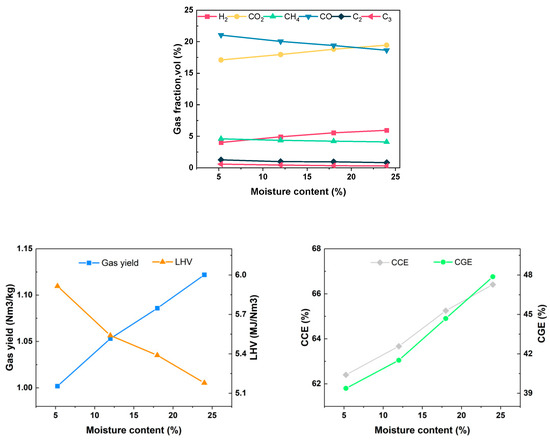
Figure 12.
Effect of water content on gasification characteristics.
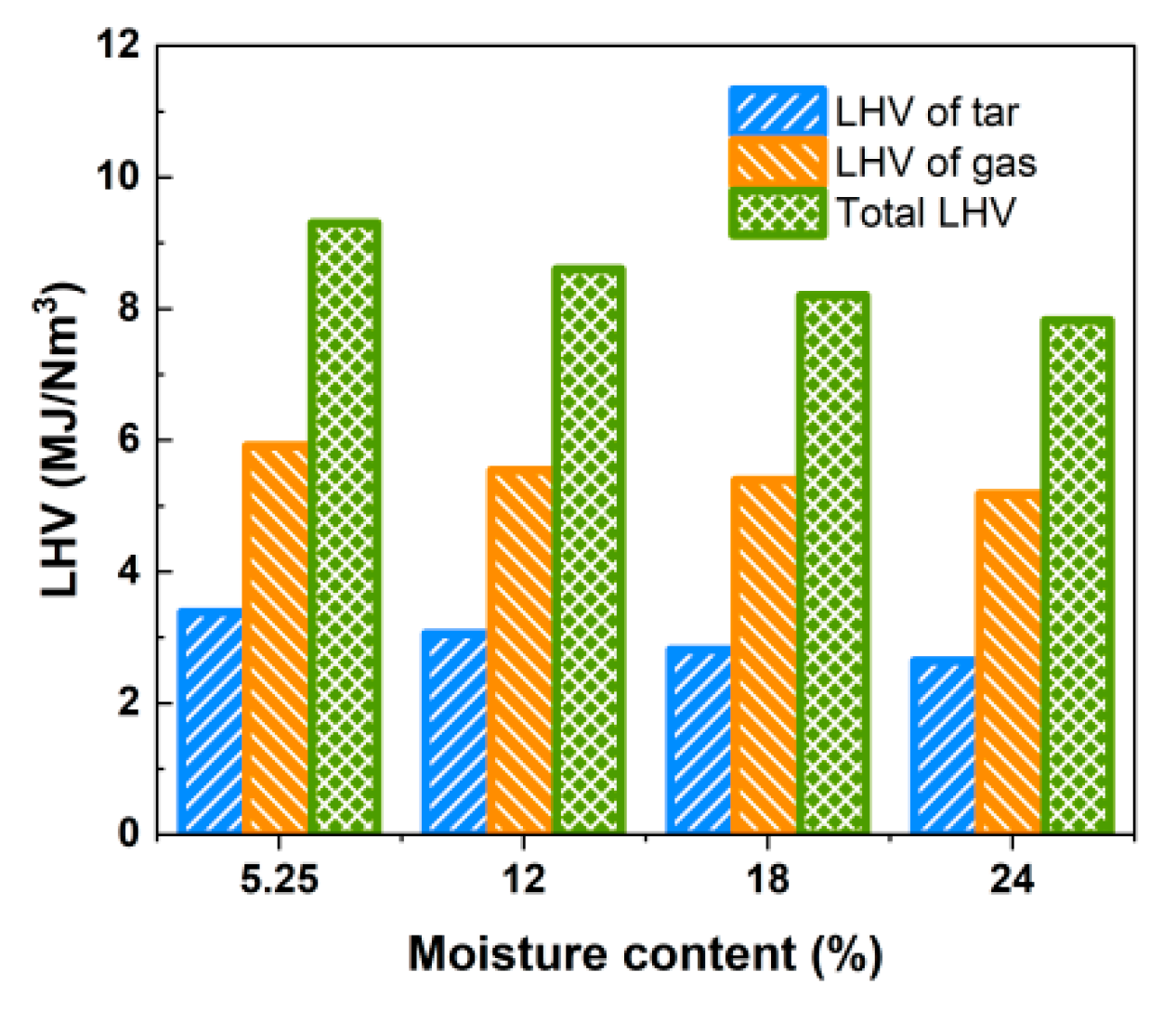
As can be seen from Table 6, Yt is more significantly affected by moisture content, and its content is reduced from 118.15 g/Nm3 with 5.25% moisture content to 88.79 g/Nm3 with 24% water content, which is a decrease of 24.8%, and the low calorific value of tar per unit of gas produced also decreases from 3.385 MJ/Nm3 to 2.643 MJ/Nm3. However, the reduction in Yt gradually slows down. These results suggest that the relative content of water vapor inside the gasifier gradually increases with the rise in moisture content, further enhancing the reforming reaction between tar and coke [32], resulting in the generation of more light gas components from tar cracking. When the LHV of tar and the produced gas are considered simultaneously, the total LHV of produced gas and tar is above 7.821 MJ/Nm3 within the studied range of water content, as depicted in Figure 13.

Table 6.
Effect of biomass moisture content on tar properties.
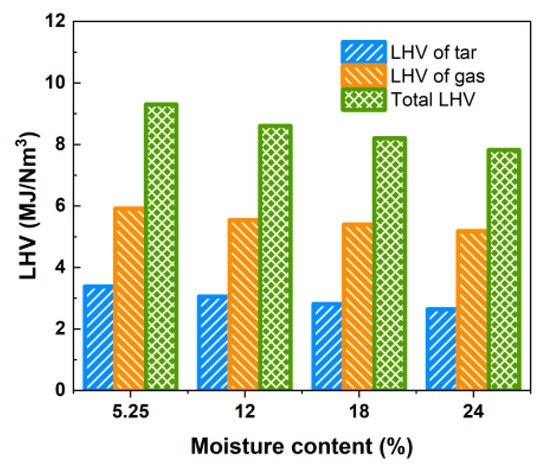
Figure 13.
Effect of moisture content on LHV of gas and tar.
The relative content of water vapor, acting as the flow medium in the reaction process, has a certain impact on the components of tar while influencing the gas production components. The GC-MS ion flow diagrams and component analyses of the tars obtained from the gasification of wheat straw at different water contents are presented in Figure 14 and Table 7. The figure reveals that with the increase in water content and the relative content of water vapor in the inverse gasifier, the number of peaks in the ion flow diagram decreases. Particularly, the number of peaks with precipitation time between 0 and 10 min decreases rapidly, while the number of peaks after 11 min increases. This indicates that the continuous increase in water vapor effectively reduces the number of tar components, facilitating the transformation of many condensable macromolecular gaseous products into a stable structure. The corresponding components in aromatic compounds (1 ring) show a more noticeable reduction in proportion, while the proportion of light PAHs (2–3 rings) increases, and the proportion of heavy PAHs (4–7 rings) remains almost unchanged, as depicted in Table 7. This phenomenon may be attributed to water vapor promoting the reforming reaction of tar, enhancing the formation of free radicals, and intensifying the polymerization reaction between unsaturated hydrocarbons. Simultaneously, it encourages the generation of reactive hydrogen intermediates, preventing the combination of carbonaceous compounds to form heavy PAHs [33].
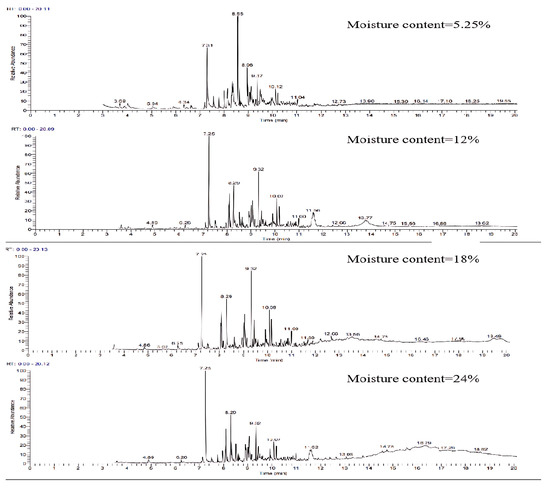
Figure 14.
Ion flow diagram of tar GC-MS of wheat straw at different water contents.

Table 7.
Analysis of tar GC-MS components at different water contents.
3.2.2. Effect of Alternative Fuel Blending Ratio
Since rubber is quite different from wheat straw in terms of elemental composition and industrial analysis results, in this subsection, wheat straw, rubber, and mixtures of the two were selected as raw materials to explore the effect of the introduction of multi-carbon raw materials on the gasification characteristics of wheat straw, and the co-gasification characteristics of wheat straw and rubber were investigated under different mass mixing ratios. In the experiments, the gasification temperature was maintained at 650 °C, the air flow rate was 0.12 Nm3/h, the equivalence ratio was 0.15, the moisture content of wheat straw was 5.25%, and the moisture content of rubber was 3.78%.
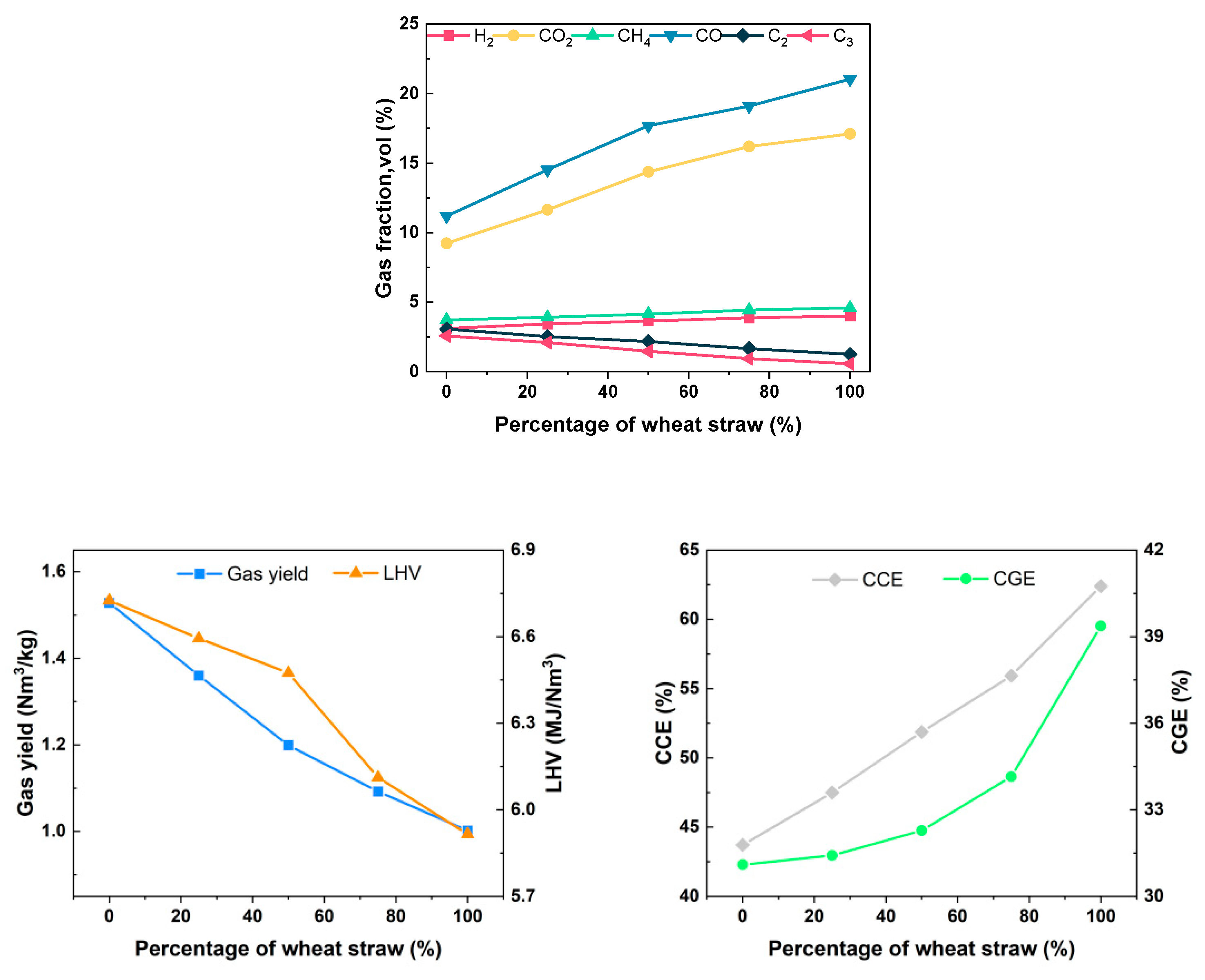
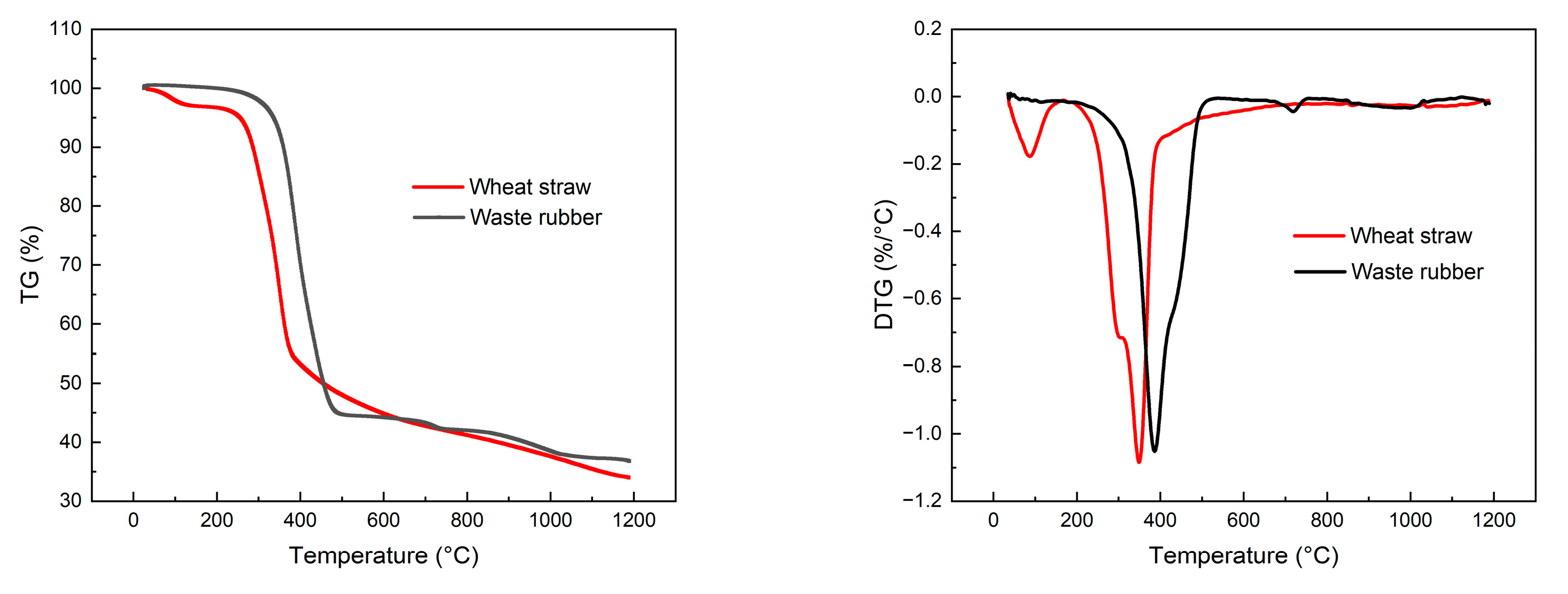
As depicted in Figure 15, the synthesis gas concentration derived from the exclusive gasification of rubber consists of 3.12% H2, 3.73% CH4, 11.19% CO, 9.24% CO2, 3.07% C2, and 2.58% C3, with a Yg of 1.528 Nm3/kg. The LHV of the produced gas is 6.725 MJ/Nm3, resulting in a CGE of 31.10% and a CCE of 43.71%. As the proportion of wheat straw in the feedstock gradually increases, there is a significant rise in the concentration of small-molecule gases such as H2, CH4, CO, and CO2 in the syngas, while the concentration of C2 and C3 decreases. The analysis of the reasons are as follows: From the perspective of elemental and industrial analyses, rubber has a higher content of carbon and volatile matter compared to wheat straw, and its homogeneous blending facilitates heat transfer and promotes the gasification reaction. However, from a chemical composition standpoint, rubber is composed of high-molecular-weight compounds, and its specific heat capacity is greater than that of wheat straw. Therefore, under the same gasification conditions, the temperature of rubber particles is lower, inhibiting the rate of the gasification reaction. To investigate this, thermogravimetric experiments were conducted on wheat straw and rubber under a nitrogen atmosphere using a thermogravimetric analyzer, with a heating rate of 20 °C/min, as shown in Figure 16. The relevant reaction parameters are presented in Table 8. In this table, Ts represents the temperature corresponding to a weight loss of 5%, Tp represents the temperature corresponding to a weight loss of 10%, Tm represents the temperature corresponding to a weight loss of 50%, (dω/dt)max represents the maximum weight loss rate, Tmax represents the peak temperature of the maximum weight loss rate, (dω/dt)mean represents the average weight loss rate, V∞ represents the weight loss percentage at the end of the reaction, and Te represents the termination temperature of the reaction. According to Table 8, the Ts values for wheat straw and rubber are 255.6 °C and 335.3 °C, respectively; the (dω/dt)max values are 1.084 (%/°C) and 1.052 (%/°C), respectively; the (dω/dt)mean values are 0.088 (%/°C) and 0.074 (%/°C), respectively; and the V∞ values are 66.01% and 63.23%, respectively. It can be observed that under the same gasification temperature, the thermal decomposition process of rubber is slower. This finding is consistent with the conclusions drawn by S. Singh [34] for wheat straw through thermogravimetric analysis and by C. Li [35] for rubber. The results indicate that under the experimental gasification temperature conditions, some rubber powder exhibits incomplete gasification, further leading to lower gasification efficiency and carbon conversion rates.
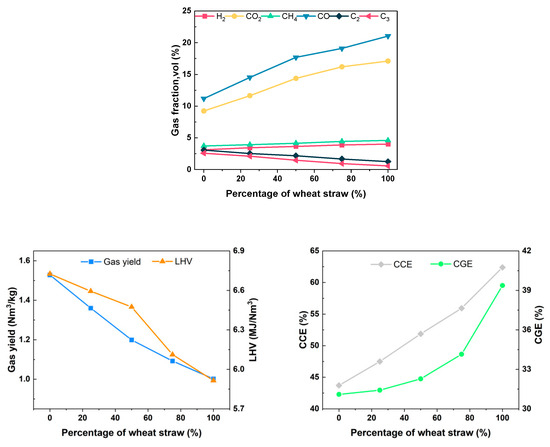
Figure 15.
Effect of different mixing ratios on gasification characteristics.
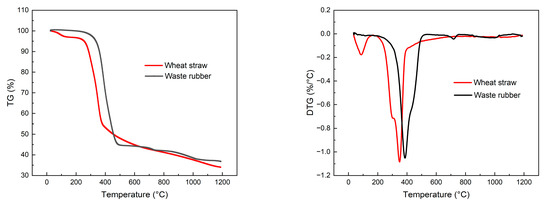
Figure 16.
TG and DTG curves for wheat straw and waste rubber.

Table 8.
The pyrolysis parameters of wheat straw and waste rubber.
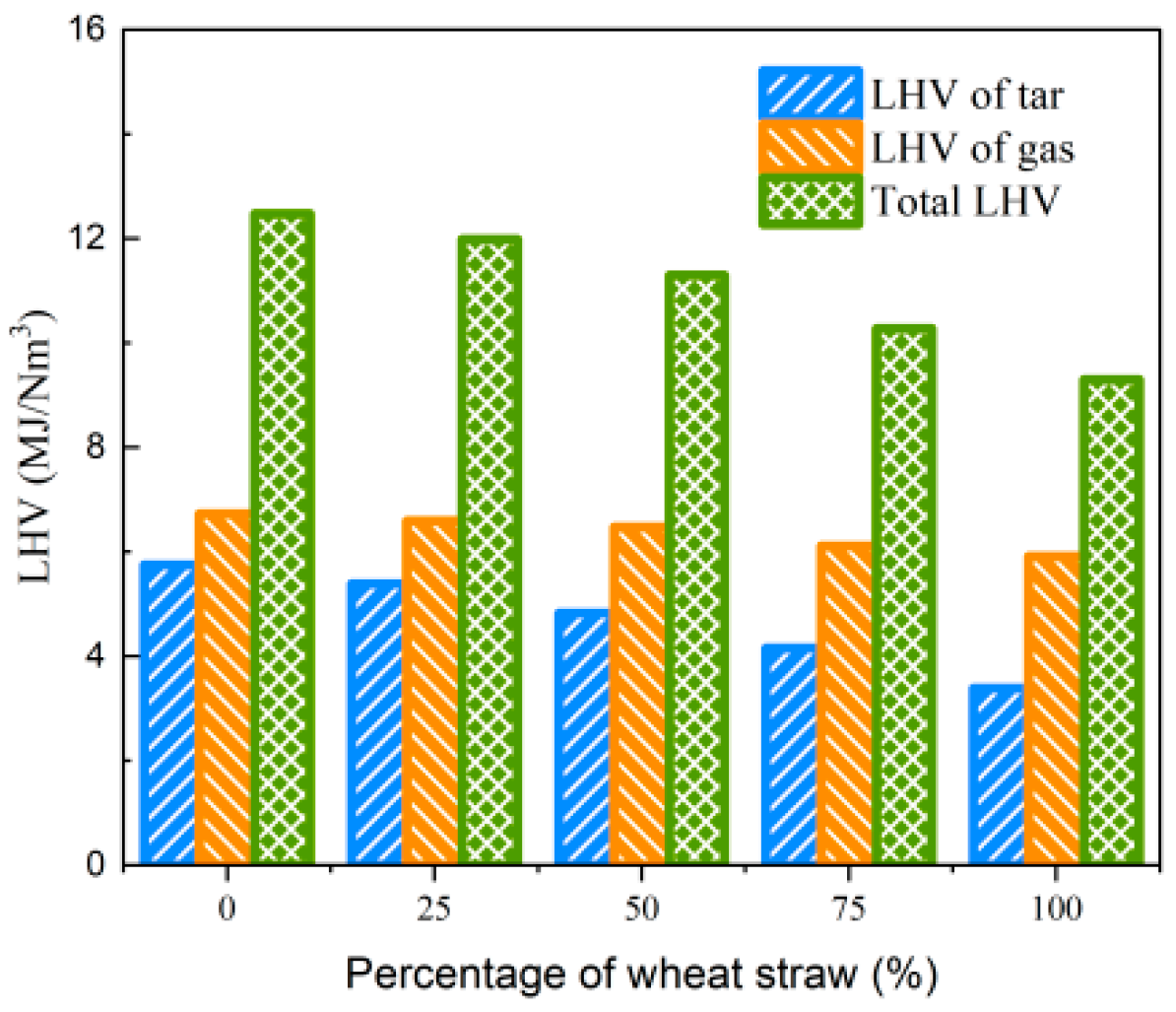
In the experimental process of biomass blending gasification, the observations of the condensable component absorption device revealed a substantial increase in white smoke in the gas washing cylinder compared to gasification using wheat straw alone. The results of collecting and analyzing tar are presented in Table 9. It is evident that as the rubber blending ratio increases from 0% to 100%, and Yt increases from 118.15 g/Nm3 to 190.03 g/Nm3, indicating a 60.8% increase. Furthermore, Qvt also increases from 3.385 MJ/Nm3 to 5.747 MJ/Nm3. When considering the LHV of both tar and the produced gas simultaneously, the total LHV of the produced gas and tar remains above 9.3 MJ/Nm3 within the studied range of blending ratios, as depicted in Figure 17. These findings indicate that, under low-temperature gasification conditions, the introduction of high-carbon feedstock (e.g., rubber) contributes to an increase in the LHV of gas and Yg. However, it also results in incomplete gasification reactions, leading to lower CGE and CCE. In cement production systems, additional equipment for tar decomposition is necessary to enhance comprehensive energy utilization.

Table 9.
Effect of different blending ratios on tar properties.
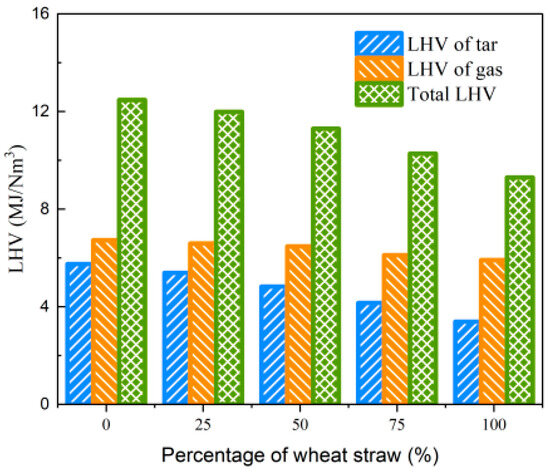
Figure 17.
Effect of biomass blending ratio on LHV of gas and tar.
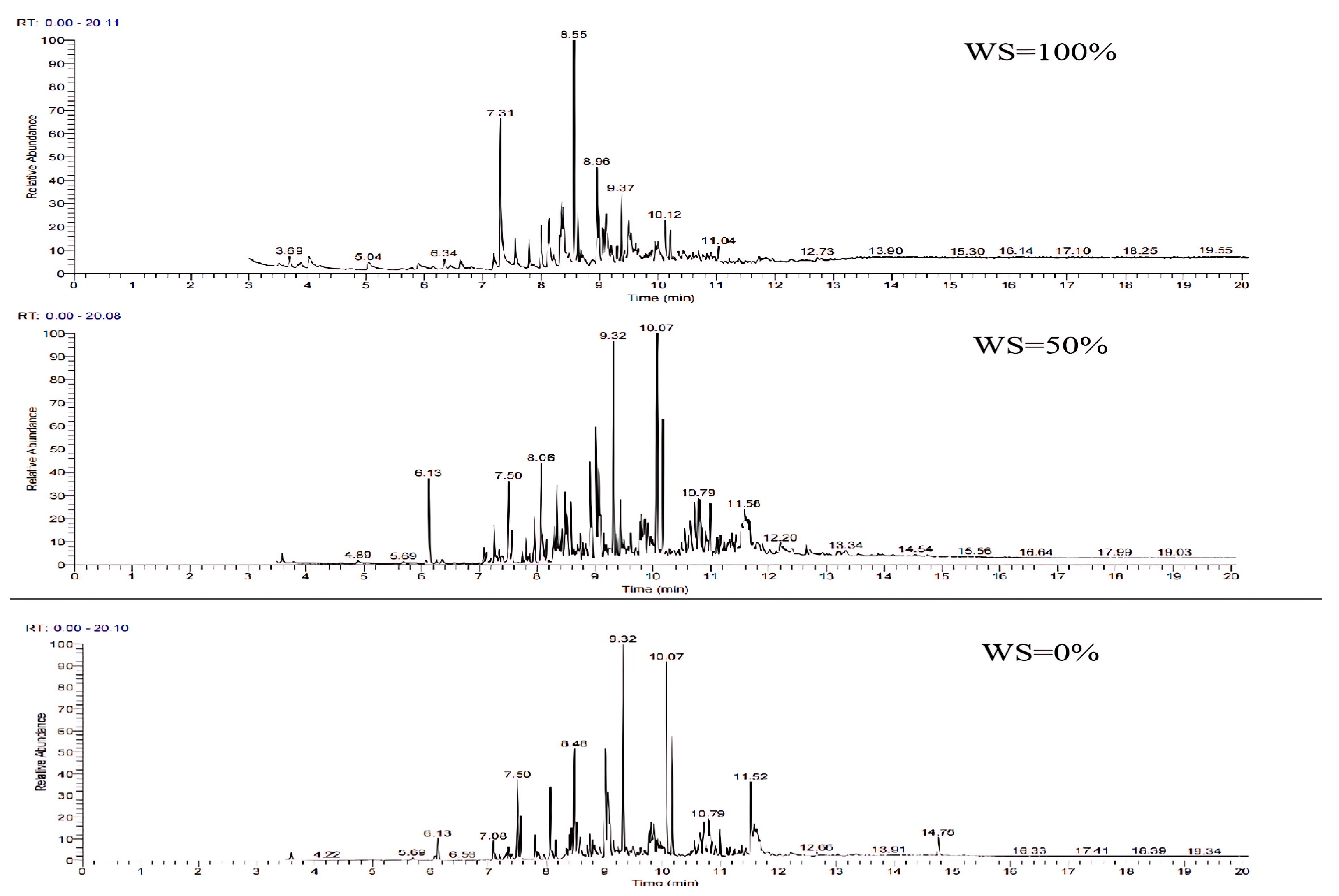
The GC-MS ion flow diagrams and component analyses of the tars obtained from the gasification of wheat straw and rubber at different blending ratios are shown in Figure 18 and Table 10. In comparison with the gasification of wheat straw and rubber alone, the ion flow diagrams exhibits a significant increase in the number of peaks after blending. This effect is primarily attributed to the high carbon and hydrogen content and low oxygen content in the elemental composition of rubber. Consequently, there was a higher presence of polycyclic aromatic hydrocarbons (PAHs) and a lower presence of monocyclic aromatic hydrocarbons (PAHs) containing oxygen in the gasified tar. With the increasing proportion of rubber blending, the ratio of light PAH and heavy PAH aromatic compounds gradually increases. This phenomenon may be attributed to the breakage of chemical bonds, which promotes the polymerization of unsaturated hydrocarbons or their incorporation into aromatic rings, leading to the formation of polycyclic structures. Consequently, this results in an increase in the number of macromolecule tars and an elevation in the LHV of the tar.
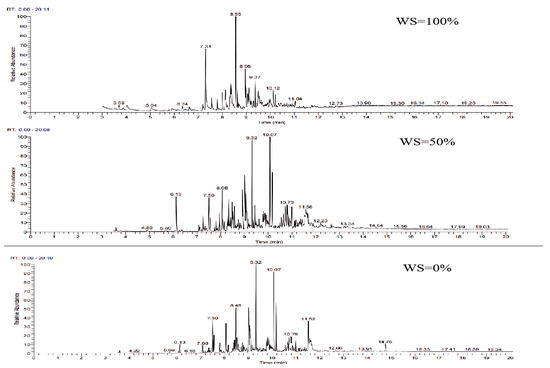
Figure 18.
Ion flow diagram of tar GC-MS of wheat straw and rubber at different blending ratios.

Table 10.
Analysis of tar GC-MS components at different blending ratios.
3.3. Study on the Influence of Natural Environment
The influence of the natural environment on biomass fluidized bed gasification encompasses various factors, including altitude, rainfall, and humidity. Given that the effects of rainfall and humidity are akin to those discussed in the previous section regarding the moisture content of the feedstock, they will not be reiterated here. However, it is worth noting that in practical production applications, fluidized bed gasification equipment may be deployed at different altitudes. Therefore, it becomes crucial to take into account the influence of altitude on gasification characteristics. At altitudes higher than 1500 m, the reduction in air density leads to a decrease in the partial pressure of oxygen within the gasifier. This diminished oxygen pressure falls below the theoretical oxygen demand required for the gasification of alternative fuels. Consequently, it may result in issues such as a prolonged combustion time and diminished CGE. In this section, the vacuum of the system is adjusted to simulate the gasification experiments at high altitude, and the vacuum used for the experiments is 0–0.03, corresponding to an approximate altitude of 0–3000 m. Throughout the experiment, the gasification temperature is maintained at 650 °C, the air flow rate is sustained at 0.12 Nm3/h, with an equivalence ratio of 0.15 and a moisture content of 5.25%. These conditions are chosen to investigate the gasification characteristics of wheat straw under varying vacuum levels.
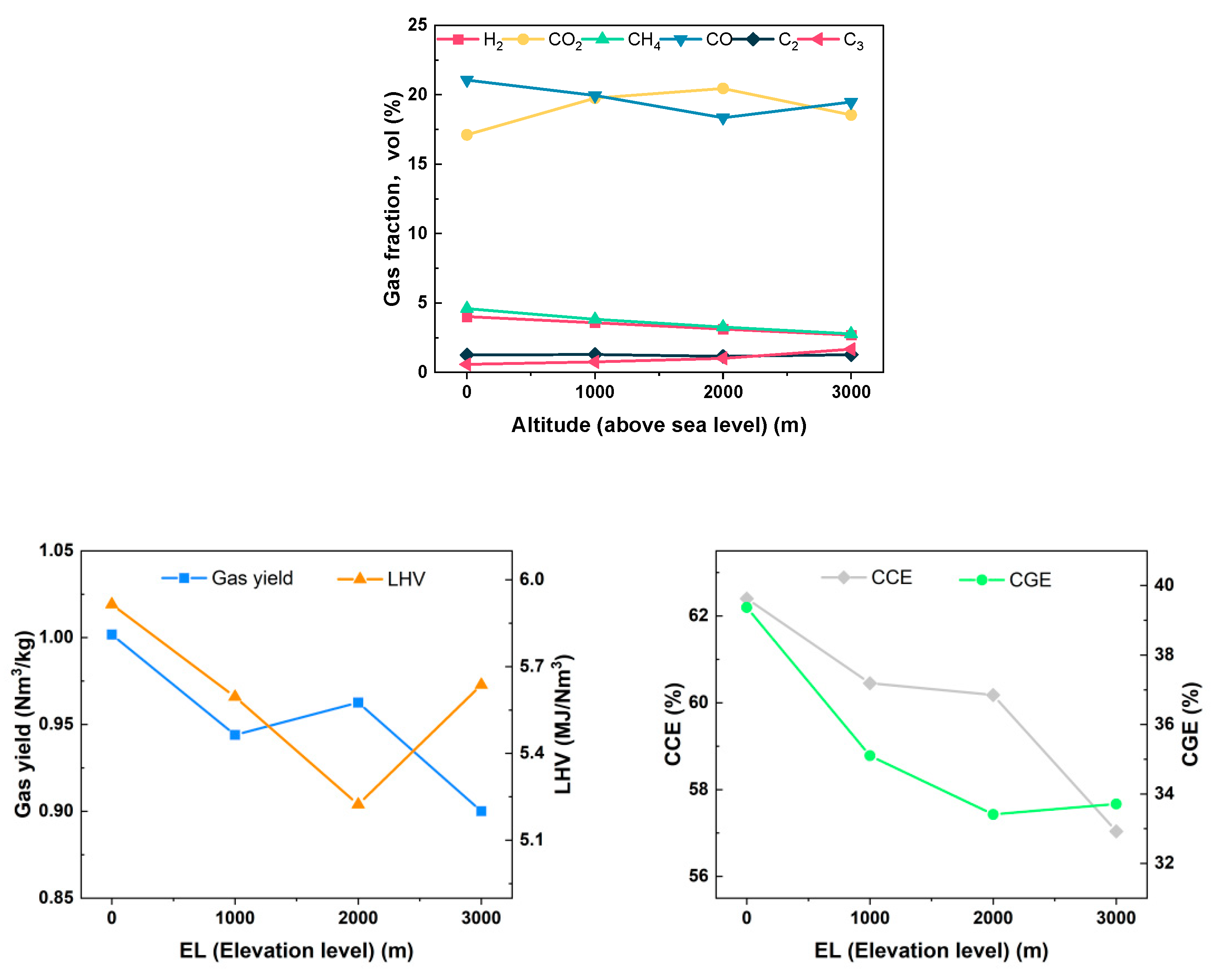
The data presented in Figure 19 indicate that as the altitude increases from 0 to 3000 m, and various gasification performance indicators, including gas components, the LHV of gas, Yg, CGE, CCE, etc., exhibit fluctuations. However, when compared to the low-altitude areas, the gasification effect shows a downward trend, reaching an inflection point at an altitude of 2000 m. At this point, the concentration of CO is minimized at 18.34%, while that of CO2 is maximized at 20.45%. Concurrently, CGE is at its lowest, recorded at 33.41%. The LHV of the gas also reaches its minimum value at 5.223 MJ/Nm3. The reason for this may be analyzed as follows: limited by the experimental conditions, the increase in altitude was simulated by changing the vacuum level. Although the decrease in pressure will induce each gasification reaction toward the direction of the gas volume increase, the gas production rate of the fuel under the same working condition decreases after converting the state yield to the standard gas yield. Simultaneously, due to the reduction in gas partial pressure, from a microscopic point of view, for the biomass particles themselves, the volatile components in them are more easily precipitated for reaction, so the gasification reaction conditions improve, the reaction is sufficient, the CO2 content rises, and the gasable fraction decreases. On a macroscopic scale, as the vacuum level increases, the reaction residence time for biomass decreases. This phenomenon may result in the premature exit of certain particles from the reactor before the reaction reaches completion. Consequently, this can inhibit the tar cracking reaction and ultimately lead to a reduction in gas yield. Moreover, with a further increase in vacuum, the molecular weight of gas per unit volume decreases, reducing the overall gasification medium and inhibiting the subsequent combustion reaction of biomass fuel. Under the combined effect of the two reasons, the combustible components of CO, C2, and C3 increase, while the contents of CH4, H2, and CO2 decrease.
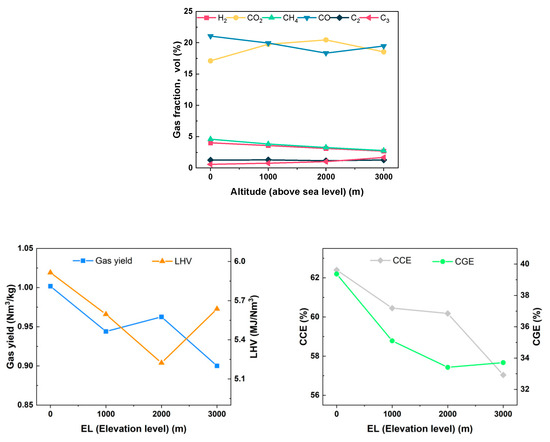
Figure 19.
Effect of elevation level on gasification characteristics.
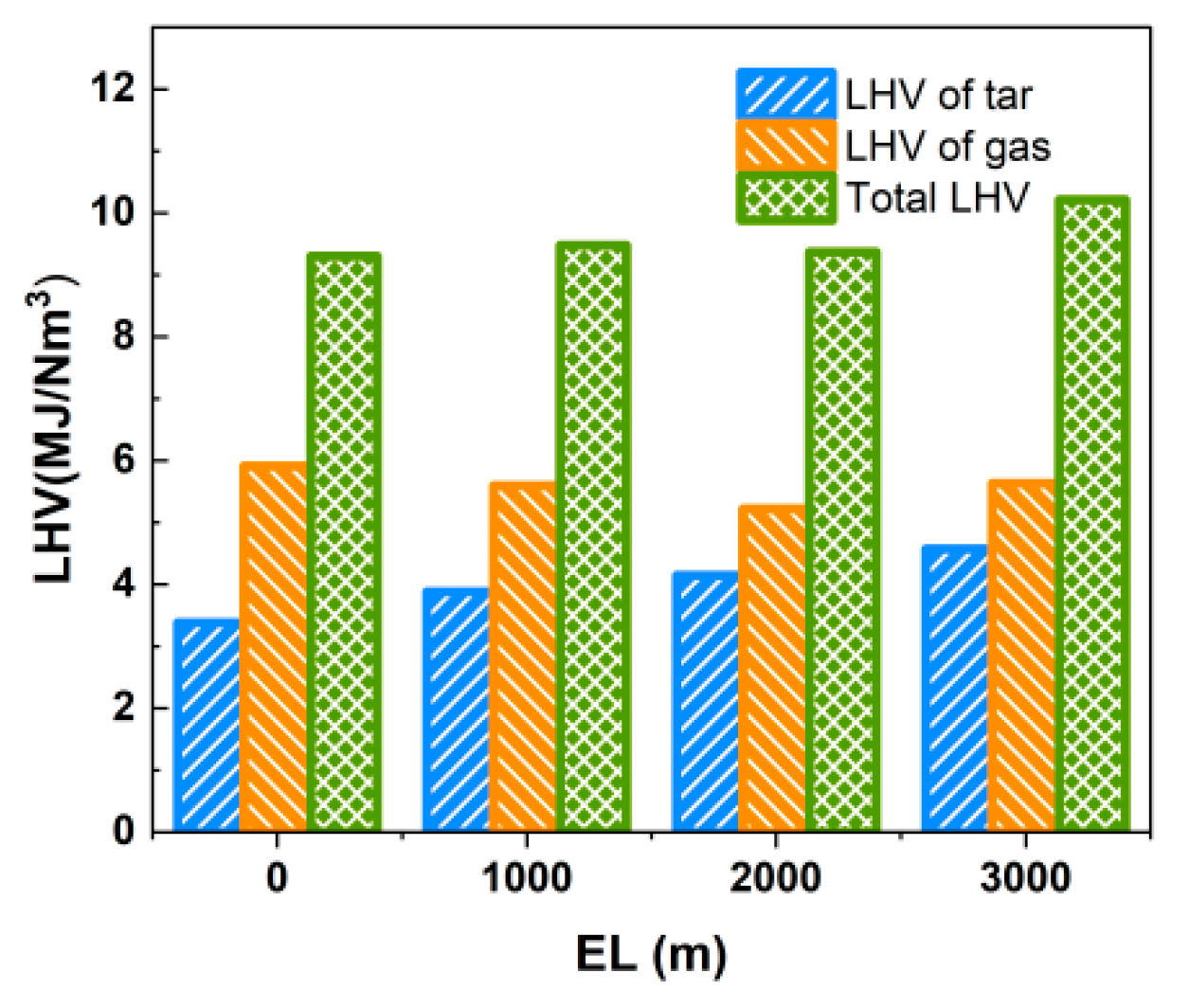
As shown in Table 11, Yt was greatly affected by the elevation level (vacuum degree), which increased from 118.15 g/Nm3 at EL = 0 m to 151.05 g/Nm3 at EL = 3000 m, with an increase of 27.8%, and Qvt also increased from 3.385 MJ/Nm3 to 4.575 MJ/Nm3. The results show that as the elevation level increases, the partial pressure of the gas gradually decreases. While the volatile components in the biomass fuel are more easily precipitated, the decrease in reaction residence time leads to the premature exit of a substantial amount of macromolecular tars from the gasifier before complete cracking, resulting in the decrease in the gasable component in the syngas and the increase in Yt. When the LHV of tar and the produced gas are considered simultaneously, the total LHV of the produced gas and tar is above 9.3 MJ/Nm3 in the range of the equivalence ratios studied, as shown in Figure 20.

Table 11.
Effect of elevation level on tar properties.
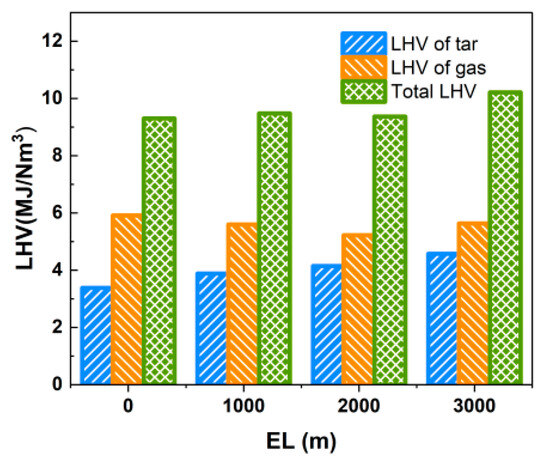
Figure 20.
Effect of elevation level on LHV of gas and tar.
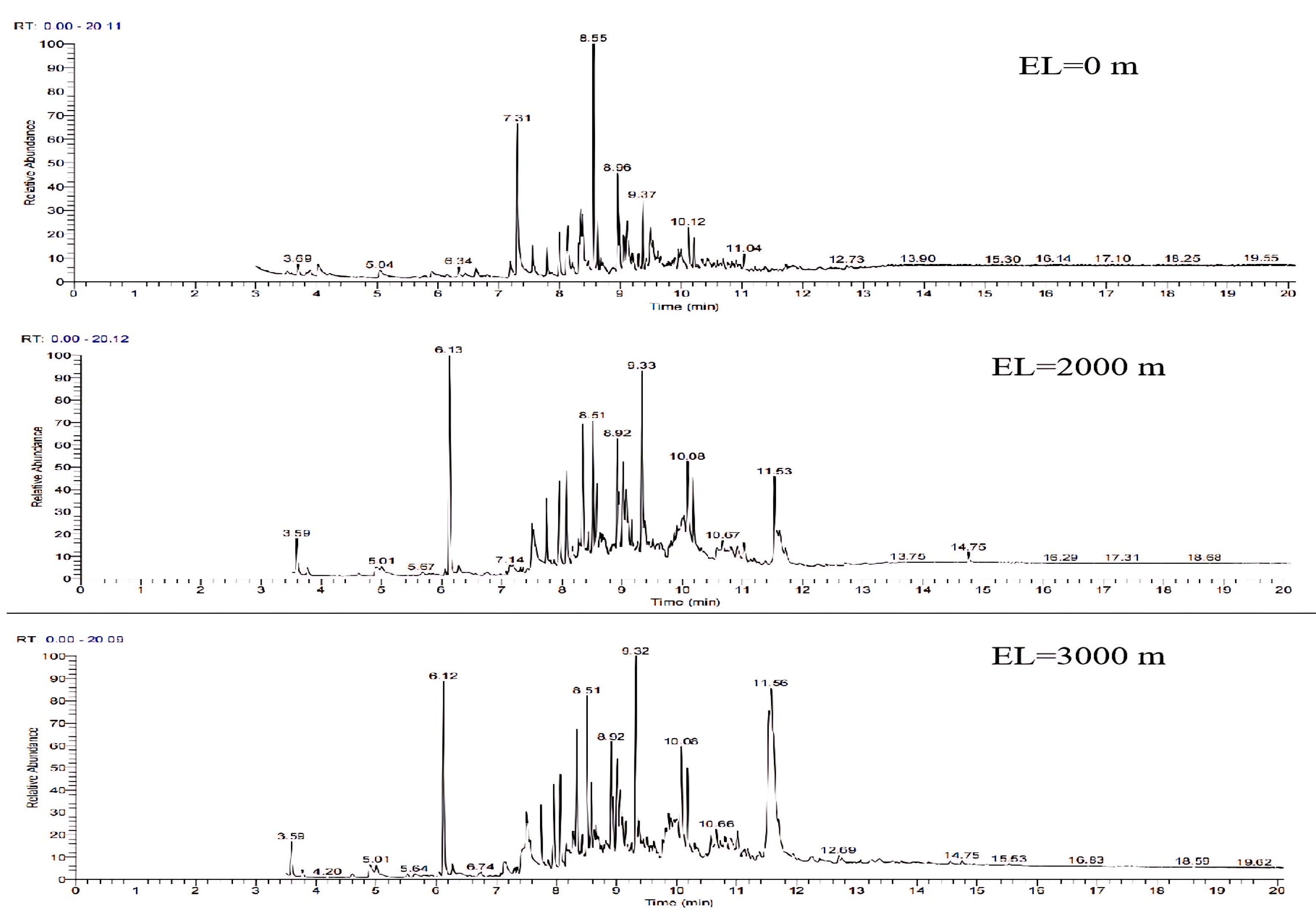
The GC-MS ion flow diagrams and component analyses of the tars obtained from the gasification of wheat straw at different elevation levels are shown in Figure 21 and Table 12. It can be seen that compared with the gasification conditions under atmospheric pressure, the experiment simulated a significant increase in the number of peaks in the ion flow diagrams of the tars collected at a high elevation level by varying the vacuum, and the percentage of aromatic compounds (1 ring) content is obviously reduced, whereas the percentage of light PAH (2–3-ring) and heavy PAH (4–7-ring) content increases significantly. The findings reveals that despite the facilitation of volatile component precipitation due to the decrease in gas partial pressure from wheat straw, the dominant factor is the reduction in the residence time of the biomass gasification reaction caused by the action of the vacuum pump. This inhibits the thermal decomposition of numerous tar molecules, leading to a further reduction in the LHV of the gas and CGE, indicating deteriorating gasification conditions at high elevation levels. Although the total LHV of the gasification products increases with elevation, the elevated levels of polycyclic aromatic hydrocarbons (PAHs) in the tar contribute to an increase in the dew point temperature. This in turn makes the tar more susceptible to condensation in high-temperature environments, presenting significant challenges for the transportation of the produced gas.
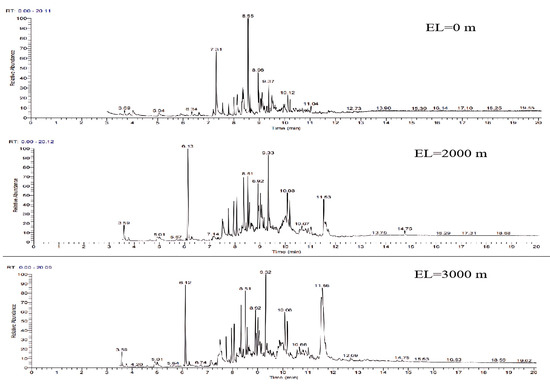
Figure 21.
Ion flow diagram of tar GC-MS of wheat straw at different elevation levels.

Table 12.
Analysis of tar GC-MS components at different elevation levels.
Based on the above analysis, it is evident that the LHV of gas produced at high elevation levels is primarily attributed to an oxygen-poor environment, resulting in a prolonged combustion time and reduced CGE. Introducing oxygen-enriched gas can mitigate these issues by reducing the nitrogen content in the gas, minimizing the conversion of waste chemical energy to gas sensible heat, and enhancing LHV and CGE in fluidized bed gasification. Oxygen-enriched gasification offers notable advantages, including maintaining optimal fluidization in the gasifier, ensuring an even air distribution, and lowering the risk of slagging. Additionally, the cost of oxygen production is considerably lower than that of oxygen gasification, making it a promising solution for improving gasification conditions in high-elevation-level areas.
4. Conclusions
This paper focuses on utilizing wheat straw as the primary raw material and employs a small electrically heated bubbling fluidized bed as the gasification reaction device. The study systematically examines the characteristics of products obtained from gasification experiments, with a comprehensive exploration of various factors, including altering the reaction conditions, varying the quality and type of fuel, and considering the impact of the natural environment. The key findings derived from these experiments are summarized below:
- (1)
- As the gasification temperature increased from 550 °C to 700 °C, the concentrations of H2, CH4, and CO in the produced gas increased from 2.23%, 3.22%, and 15.98% to 5.39%, 5.56%, and 22.72%, respectively, and the LHV of gas increased from 3.866 MJ/Nm3 to 6.761 MJ/Nm3, Yg increased from 0.795 Nm3/kg to 1.062 Nm3/kg, CCE increased from 53.48% to 68.26%, and CGE increased from 20.42% to 40.69%. Qvt and the number of peaks in the GC-MS ionograms showed an increasing and then a decreasing trend, with a significant decrease in the percentage of aromatic compounds (1 ring) in the fractions and an increase in the percentage of light PAHs (2–3 rings) as well as heavy PAHs (4–7 rings).
- (2)
- The variation in the ER exerted a significant impact on gas fraction. With the ER increasing from 0.1 to 0.2, there was a surge in CO2 content from 13.80% to 19.35%. Simultaneously, the concentrations of H2, CH4, and CO witnessed a decline from 6.16%, 7.09%, and 25.81% to 3.45%, 3.86%, and 17.53%, respectively, and the LHV of gas decreased from 7.925 MJ/Nm3 to 5.080 MJ/Nm3 and increased from 0.771 Nm3/kg to 1.197 Nm3/kg, and CCE increased from 53.11% to 70.98%, while CGE was maintained at around 40%. Qvt decreased from 4.063 MJ/Nm3 to 2.869 MJ/Nm3, accompanied by a decline in the percentage of monocyclic aromatic compounds and aliphatic compounds in the fractions and an increase in the percentage of polycyclic aromatic compounds.
- (3)
- As the moisture content escalated from 5.25% to 24%, there was an elevation in H2 and CO2 concentrations in the produced gas, rising from 4.01% and 17.11% to 5.95% and 19.46%, respectively. Concurrently, the concentrations of CH4 and CO witnessed a decline from 4.60% and 21.05% to 4.10% and 18.45%, respectively. This led to an increase in Yg from 1.002 Nm3/kg to 1.122 Nm3/kg, while the LHV of gas decreased from 5.915 MJ/Nm3 to 5.178 MJ/Nm3. CCE increased from 62.40% to 66.41%, and CGE increased from 39.37% to 47.87%. Yt experienced a decrease from 118.15 g/Nm3 to 88.79 g/Nm3, marking a reduction of 24.8%, and Qvt decreased from 3.385 MJ/Nm3 to 2.643 MJ/Nm3. The proportion of monocyclic aromatic hydrocarbons in the tar component decreased, while the proportion of polycyclic aromatic hydrocarbons increased.
- (4)
- Regarding rubber, characterized as a high-carbon fuel, under the reaction conditions of T = 650 °C and ER = 0.15, as the proportion in the blended fuel was increased from 0 to 100%, there was a gradual reduction in the concentrations of H2, CH4, CO, and CO2 in the produced gas. For the gasification of rubber alone, the concentrations were 3.12%, 3.73%, 11.19%, and 9.24%, respectively. Simultaneously, the concentrations of C2 and C3 increased to 3.07% and 2.58%, and the LHV of the gas was 6.725 MJ/Nm3, with a Yg of 1.528 m3/kg. CCE was 43.71%, and CGE is 33.71%. Yt increased to 190.03 g/Nm3, representing a 60.8% increase, and rose to 5.747 MJ/Nm3. Notably, there was a significant increase in the proportion of polycyclic aromatic hydrocarbons (PAHs) in the components, coupled with a notable decrease in oxygenated compounds.
- (5)
- The increase in elevation level induced a decrease in gas partial pressure. As the vacuum level rose from 0 to 0.03, the gasification characteristics generally exhibited a declining trend. At an altitude of 3000 m, the concentrations of H2, CH4, CO, and CO2 were 2.69%, 2.77%, 19.47%, and 18.54%, respectively. The LHV of the gas was 5.637 MJ/Nm3, with a Yg of 0.900 m3/kg. CCE was 57.04%, and CGE was 33.71%. Yt increased to 151.05 g/Nm3, and Qvt was 4.575 MJ/Nm3. The variety of tar components increased, and the proportion of macromolecular tars rose rapidly, featuring a high proportion of heavy polycyclic aromatic hydrocarbons (PAHs) like pyrene, accounting for 15.68%.
- (6)
- Syngas and tar are the primary products of gasification. The former primarily consists of lightweight, small-molecule gases, while the latter is composed of organic polymer compounds and some impurities. The calorific value of syngas and tar in the gasification products varies depending on factors such as the type of fuel, reaction conditions, and collection methods. Generally, the calorific value of the produced gas is higher than that of the tar. Additionally, the better the gasification reaction conditions and efficiency, the greater the difference in calorific value between the produced gas and the tar.
According to the experimental results and analysis, wheat straw, as a typical biomass alternative fuel with good gasification characteristics, has a broad prospect for application and promotion in cement production. Under the premise of ensuring safety and improving economy, T = 650 °C, ER = 0.15, M = 5.25%, a normal pressure, and a low altitude are the optimal reaction condition, at which time the concentration of combustible components H2, CH4, and CO in the produced gas is 4.01%, 4.60%, and 21.05%, Yg = 1.002 Nm3/kg, CCE = 62.40%, CGE = 39.37%, LHV = 5.915 MJ/Nm3, Yt = 118.15 g/Nm3, and Qvt = 3.385 MJ/Nm3.
This paper exclusively centers on the experimental facet of the study. The corresponding gasification simulation results will be forthcoming in a subsequent publication. The objective of the forthcoming work is to employ simulation techniques to corroborate the experimental findings and dissect the underlying reasons for any disparities between the two datasets.
Author Contributions
Conceptualization, B.J.; data curation, T.H.; formal analysis, C.D.; investigation, C.D., Q.G., S.C. and Y.C. (Yu Cai); methodology, T.H.; project administration, Y.C. (Yi Chen); resources, Q.G.; supervision, B.J. and Y.C. (Yi Chen); visualization, C.D.; writing—original draft, C.D.; writing—review and editing, B.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Carbon Peak and Carbon Neutrality Science Technology Innovation Special Funds (Major Scientific and Technological Achievements Transformation) Project of Jiangsu Province (No. BA2022103).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author Yi Chen was employed by the Sinoma International Engineering Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Yin, X.; Wu, C.; Xu, B.; Chen, Y. The effect of biomass gasification on reducing CO2 emission. Acta Energiae Solaris Sin. 2000, 21, 40–44. [Google Scholar]
- Grisolia, G.; Fino, D.; Lucia, U. Biomethanation of Rice Straw: A Sustainable Perspective for the Valorisation of a Field Residue in the Energy Sector. Sustainability 2022, 14, 5679. [Google Scholar] [CrossRef]
- Wang, D.; Jin, B.; Jin, Z.; Wu, W.; Zhou, Z. High hydrogen syngas production from chemical looping co-gasificationof sawdust and waste tires. Chem. Ind. Eng. Prog. 2020, 39, 956. [Google Scholar]
- Darmawan, A.; Aziz, M. Chapter 2–Process and products of biomass conversion technology. In Innovative Energy Conversion from Biomass Waste; Elsevier: Amsterdam, The Netherlands, 2022; pp. 25–60. [Google Scholar]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Gao, Y.; Xie, H.; Yu, Z.; Qin, M.; Wu, Z.; Wang, P.; Zhao, X.; Zhang, S. Two-stage dry reforming process for biomass gasification: Product characteristics and energy analysis. Energies 2023, 16, 4783. [Google Scholar] [CrossRef]
- Gröbl, T.; Walter, H.; Haider, M. Biomass steam gasification for production of SNG—Process design and sensitivity analysis. Appl. Energy 2012, 97, 451–461. [Google Scholar] [CrossRef]
- Samadi, S.H.; Ghobadian, B.; Nosrati, M. Prediction and estimation of biomass energy from agricultural residues using air gasification technology in Iran. Renew. Energy 2020, 149, 1077–1091. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, K.; Yao, X.; Li, J.; Zhao, Z.; Chen, S. Syngas composition and ash characteristics of corn straw under a CO2 atmosphere. Biomass Bioenergy 2022, 166, 106630. [Google Scholar] [CrossRef]
- Gu, H.; Tang, Y.; Yao, J.; Chen, F. Study on biomass gasification under various operating conditions. J. Energy Inst. 2019, 92, 1329–1336. [Google Scholar] [CrossRef]
- Rasmussen, N.B.; Aryal, N. Syngas production using straw pellet gasification in fluidized bed allothermal reactor under different temperature conditions. Fuel 2020, 263, 116706. [Google Scholar] [CrossRef]
- Artetxe, M.; Alvarez, J.; Nahil, M.A.; Olazar, M.; Williams, P.T. Steam reforming of different biomass tar model compounds over Ni/Al2O3 catalysts. Energy Convers. Manag. 2017, 136, 119–126. [Google Scholar] [CrossRef]
- Corella, J.; Sanz, A. Modeling circulating fluidized bed biomass gasifiers. A pseudo-rigorous model for stationary state. Fuel Process. Technol. 2005, 86, 1021–1053. [Google Scholar] [CrossRef]
- Virginie, M.; Adánez, J.; Courson, C.; de Diego, L.; García-Labiano, F.; Niznansky, D.; Kiennemann, A.; Gayán, P.; Abad, A. Effect of Fe–olivine on the tar content during biomass gasification in a dual fluidized bed. Appl. Catal. B Environ. 2012, 121–122, 214–222. [Google Scholar] [CrossRef]
- Narnaware, S.L.; Panwar, N.L. Catalysts and their role in biomass gasification and tar abetment: A review. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Brandt, P.; Henriksen, U. Decomposition of tar in pyrolysis gas by partial oxidation and thermal cracking. Part 2. Presented at the Biomass for Energy and Industry. In Proceedings of the 10th European Conference and Technology Exibition, Wurzburg, Germany, 8–11 June 1998. [Google Scholar]
- Zhai, M.; Wang, X.; Zhang, Y.; Dong, P.; Qi, G.; Huang, Y. Characteristics of rice husk tar secondary thermal cracking. Energy 2015, 93, 1321–1327. [Google Scholar] [CrossRef]
- He, J.Y.; He, J.; Wang, Y.; Fan, Y.; Shi, H.; Cai, B.; Yan, G. Pathway of carbon emissions peak for cement industry in China. Res. Environ. Sci. 2022, 35, 347–355. [Google Scholar]
- Barbhuiya, S.; Kanavaris, F.; Das, B.B.; Idrees, M. Decarbonising cement and concrete production: Strategies, challenges and pathways for sustainable development. J. Build. Eng. 2024, 86, 108861. [Google Scholar] [CrossRef]
- Kukreja, K.; Soni, M.K.; Mohapatra, B.; Panda, D.K. Impact assessment of alternative fuels on production Cost, plant operation and Environment—Case study of Indian cement industry. Sustain. Energy Technol. Assess. 2023, 57, 103300. [Google Scholar]
- GB/T 212-2008; Proximate Analysis of Coal. Standardization Administration of China: Beijing, China, 2008.
- GB/T 213-2008; Determination of Calorific Value of Coal. National Standards of the People’s Republic of China: Beijing, China, 2008.
- Niu, M.; Huang, Y.; Jin, B.; Wang, Y.; Dong, X. Enriched-air gasification of refuse derived fuel in bubbling fluidized bed. CIESC J. 2014, 65, 4971–4977. [Google Scholar]
- Jin, Y. Principles of Fluidization Engineering; Tsinghua University Press: Beijing, China, 2002; Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=abc3ca087cb2502d3108483c76db7156 (accessed on 14 April 2024).
- Rodrigues, S.; Almeida, A.; Ribeiro, A. Influence of temperature on the gasification of cork wastes. Energy Procedia 2017, 136, 127–132. [Google Scholar]
- Hu, S.; Jiang, L.; Wang, Y.; Su, S.; Sun, L.; Xu, B.; He, L.; Xiang, J. Effects of inherent alkali and alkaline earth metallic species on biomass pyrolysis at different temperatures. Bioresour. Technol. 2015, 192, 23–30. [Google Scholar] [CrossRef]
- Qin, Y.; Campen, A.; Wiltowski, T.; Feng, J.; Li, W. The influence of different chemical compositions in biomass on gasification tar formation. Biomass Bioenergy 2015, 83, 77–84. [Google Scholar]
- Chen, H.; Li, B.; Yang, H.; Yang, G.; Zhang, S. Experimental investigation of biomass gasification in a fluidized bed reactor. Energy Fuels 2008, 22, 3493–3498. [Google Scholar]
- Phuphuakrat, T.; Namioka, T.; Yoshikawa, K. Tar removal from biomass pyrolysis gas in two-step function of decomposition and adsorption. Appl. Energy 2010, 87, 2203–2211. [Google Scholar] [CrossRef]
- Shukla, B.; Koshi, M. A novel route for PAH growth in HACA based mechanisms. Combust. Flame 2012, 159, 3589–3596. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, S. Experimental investigation of tar formation and producer gas composition in biomass steam gasification in a 100 kW dual fluidised bed gasifier. Renew. Energy 2019, 132, 416–424. [Google Scholar] [CrossRef]
- Cortazar, M.; Alvarez, J.; Lopez, G.; Amutio, M.; Santamaria, L.; Bilbao, J.; Olazar, M. Role of temperature on gasification performance and tar composition in a fountain enhanced conical spouted bed reactor. Energy Convers. Manag. 2018, 171, 1589–1597. [Google Scholar] [CrossRef]
- Singh, S.; Tagade, A.; Verma, A.; Sharma, A.; Tekade, S.P.; Sawarkar, A.N. Insights into kinetic and thermodynamic analyses of co-pyrolysis of wheat straw and plastic waste via thermogravimetric analysis. Bioresour. Technol. 2022, 356, 127332. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Yellezuome, D.; Cai, J.; Liu, R.; Hu, J. Physicochemical investigation and thermogravimetric analysis of bamboo and poplar wood residues and tire rubber waste: Kinetic and thermodynamic analyses. Ind. Crop. Prod. 2023, 206, 117715. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).