The Formation–Structure–Functionality Relationship of Catalyst Layers in Proton Exchange Membrane Fuel Cells
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Sample Preparation
2.2. Particle Characterization
2.3. Rheological Measurements
2.4. Catalyst Layer Characterization
2.5. Fuel Cell Operation
2.6. Fuel Cell Diagnosis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Yuan, S.; Liang, Y.; Li, H.; Xu, Z.; Xu, Q.; Yin, J.; Shen, S.; Yan, X.; Zhang, J. Engineering the Catalyst Layers towards Enhanced Local Oxygen Transport of Low-Pt Proton Exchange Membrane Fuel Cells: Materials, Designs, and Methods. Int. J. Hydrogen Energy 2023, 48, 4389–4417. [Google Scholar] [CrossRef]
- Schneider, P.; Batool, M.; Godoy, A.O.; Singh, R.; Gerteisen, D.; Jankovic, J.; Zamel, N. Impact of Platinum Loading and Layer Thickness on Cathode Catalyst Degradation in PEM Fuel Cells. J. Electrochem. Soc. 2023, 170, 024506. [Google Scholar] [CrossRef]
- Lee, C.H.; Kort-Kamp, W.J.M.; Yu, H.; Cullen, D.A.; Patterson, B.M.; Arman, T.A.; Komini Babu, S.; Mukundan, R.; Borup, R.L.; Spendelow, J.S. Grooved Electrodes for High-Power-Density Fuel Cells. Nat. Energy 2023, 8, 685–694. [Google Scholar] [CrossRef]
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Cetinbas, F.C.; Wang, X.; Ahluwalia, R.K.; Kariuki, N.N.; Winarski, R.P.; Yang, Z.; Sharman, J.; Myers, D.J. Microstructural Analysis and Transport Resistances of Low-Platinum-Loaded PEFC Electrodes. J. Electrochem. Soc. 2017, 164, F1596–F1607. [Google Scholar] [CrossRef]
- Muzaffar, T.; Kadyk, T.; Eikerling, M. Tipping Water Balance and the Pt Loading Effect in Polymer Electrolyte Fuel Cells: A Model-Based Analysis. Sustain. Energy Fuels 2018, 2, 1189–1196. [Google Scholar] [CrossRef]
- Schuler, T.; Chowdhury, A.; Freiberg, A.T.; Sneed, B.; Spingler, F.B.; Tucker, M.C.; More, K.L.; Radke, C.J.; Weber, A.Z. Fuel-Cell Catalyst-Layer Resistance via Hydrogen Limiting-Current Measurements. J. Electrochem. Soc. 2019, 166, F3020–F3031. [Google Scholar] [CrossRef]
- Mu, Y.T.; Weber, A.Z.; Gu, Z.L.; Schuler, T.; Tao, W.Q. Mesoscopic Analyses of the Impact of Morphology and Operating Conditions on the Transport Resistances in a Proton-Exchange-Membrane Fuel-Cell Catalyst Layer. Sustain. Energy Fuels 2020, 4, 3623–3639. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A. Boosting Fuel Cell Performance with Accessible Carbon Mesopores. ACS Energy Lett. 2018, 3, 618–621. [Google Scholar] [CrossRef]
- Banham, D.; Choi, J.Y.; Kishimoto, T.; Ye, S. Integrating PGM-Free Catalysts into Catalyst Layers and Proton Exchange Membrane Fuel Cell Devices. Adv. Mater. 2019, 31, 1804846. [Google Scholar] [CrossRef]
- Ott, S.; Orfanidi, A.; Schmies, H.; Anke, B.; Nong, H.N.; Hübner, J.; Gernert, U.; Gliech, M.; Lerch, M.; Strasser, P. Ionomer Distribution Control in Porous Carbon-Supported Catalyst Layers for High-Power and Low Pt-Loaded Proton Exchange Membrane Fuel Cells. Nat. Mater. 2020, 19, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, S.; Mehrazi, S.; Zhou, F.; Pan, M.; Zhang, R.; Chuang, P.-Y.A.; Sui, P.-C. Effect of Dispersion Method and Catalyst on the Crack Morphology and Performance of Catalyst Layer of PEMFC. J. Electrochem. Soc. 2021, 168, 114506. [Google Scholar] [CrossRef]
- Xie, J.; Xu, F.; Wood, D.L.; More, K.L.; Zawodzinski, T.A.; Smith, W.H. Influence of Ionomer Content on the Structure and Performance of PEFC Membrane Electrode Assemblies. Electrochim. Acta 2010, 55, 7404–7412. [Google Scholar] [CrossRef]
- Cetinbas, F.C.; Ahluwalia, R.K. Agglomerates in Polymer Electrolyte Fuel Cell Electrodes: Part II. Transport Characterization. J. Electrochem. Soc. 2018, 165, F1059–F1066. [Google Scholar] [CrossRef]
- Inoue, G.; Ohnishi, T.; So, M.; Park, K.; Ono, M.; Tsuge, Y. Simulation of Carbon Black Aggregate and Evaluation of Ionomer Structure on Carbon in Catalyst Layer of Polymer Electrolyte Fuel Cell. J. Power Sources 2019, 439, 227060. [Google Scholar] [CrossRef]
- Mu, Y.T.; Weber, A.Z.; Gu, Z.L.; Tao, W.Q. Mesoscopic Modeling of Transport Resistances in a Polymer-Electrolyte Fuel-Cell Catalyst Layer: Analysis of Hydrogen Limiting Currents. Appl. Energy 2019, 255, 113895. [Google Scholar] [CrossRef]
- Mehrazi, S.; Homayouni, T.; Kakati, N.; Sarker, M.; Rolfe, P.; Chuang, P.Y.A. A Rheo-Impedance Investigation on the Interparticle Interactions in the Catalyst Ink and Its Impact on Electrode Network Formation in a Proton Exchange Membrane Fuel Cell. Appl. Energy 2024, 359, 122680. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Sun, H.; He, W.; Fan, Y.; Wang, S. Investigation of Dry Ionomer Volume Fraction in Cathode Catalyst Layer under Different Relative Humilities and Nonuniform Ionomer-Gradient Distributions for PEM Fuel Cells. Electrochim. Acta 2020, 353, 136491. [Google Scholar] [CrossRef]
- Hosokawa, M.; Nogi, K.; Naito, M.; Yokoyama, T. Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780444531223. [Google Scholar]
- Zaccarelli, E. Colloidal Gels: Equilibrium and Non-Equilibrium Routes. J. Phys. Condens. Matter 2007, 19, 323101. [Google Scholar] [CrossRef]
- Johnson, L.C.; Zia, R.N.; Moghimi, E.; Petekidis, G. Influence of Structure on the Linear Response Rheology of Colloidal Gels. J. Rheol. 2019, 63, 583–608. [Google Scholar] [CrossRef]
- Geier, O.; Vasenkov, S.; Kärger, J. Pulsed Field Gradient Nuclear Magnetic Resonance Study of Long-Range Diffusion in Beds of NaX Zeolite: Evidence for Different Apparent Tortuosity Factors in the Knudsen and Bulk Regimes. J. Chem. Phys. 2002, 117, 1935–1938. [Google Scholar] [CrossRef]
- Malek, K.; Coppens, M.O. Knudsen Self- and Fickian Diffusion in Rough Nanoporous Media. J. Chem. Phys. 2003, 119, 2801–2811. [Google Scholar] [CrossRef]
- Inoue, G.; Kawase, M. Understanding Formation Mechanism of Heterogeneous Porous Structure of Catalyst Layer in Polymer Electrolyte Fuel Cell. Int. J. Hydrogen Energy 2016, 41, 21352–21365. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Kudo, K.; Kitano, N.; Morimoto, Y. Molecular Dynamics Simulations on O2 Permeation through Nafion Ionomer on Platinum Surface. Electrochim. Acta 2016, 188, 767–776. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Zhang, J. Review of Characterization and Modeling of Polymer Electrolyte Fuel Cell Catalyst Layer: The Blessing and Curse of Ionomer. Front. Energy 2017, 11, 334–364. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Kumaraguru, S.; Koestner, R.; Fuller, T.; Gu, W.; Kariuki, N.; Myers, D.; Dudenas, P.J.; Kusoglu, A. Editors’ Choice—Ionomer Side Chain Length and Equivalent Weight Impact on High Current Density Transport Resistances in PEMFC Cathodes. J. Electrochem. Soc. 2021, 168, 024518. [Google Scholar] [CrossRef]
- Normile, S.J.; Sabarirajan, D.C.; Calzada, O.; De Andrade, V.; Xiao, X.; Mandal, P.; Parkinson, D.Y.; Serov, A.; Atanassov, P.; Zenyuk, I.V. Direct Observations of Liquid Water Formation at Nano- and Micro-Scale in Platinum Group Metal-Free Electrodes by Operando X-ray Computed Tomography. Mater. Today Energy 2018, 9, 187–197. [Google Scholar] [CrossRef]
- Malek, K.; Mashio, T.; Eikerling, M. Microstructure of Catalyst Layers in PEM Fuel Cells Redefined: A Computational Approach. Electrocatalysis 2011, 2, 141–157. [Google Scholar] [CrossRef]
- Mojica, F.; Rahman, M.A.; Sarker, M.; Hussey, D.S.; Jacobson, D.L.; LaManna, J.M.; Chuang, P.Y.A. Study of Converging-Diverging Channel Induced Convective Mass Transport in a Proton Exchange Membrane Fuel Cell. Energy Convers. Manag. 2021, 237, 114095. [Google Scholar] [CrossRef]
- Novitski, D.; Holdcroft, S. Determination of O2 Mass Transport at the Pt|PFSA Ionomer Interface under Reduced Relative Humidity. ACS Appl. Mater. Interfaces 2015, 7, 27314–27323. [Google Scholar] [CrossRef]
- Orfanidi, A.; Madkikar, P.; El-Sayed, H.A.; Harzer, G.S.; Kratky, T.; Gasteiger, H.A. The Key to High Performance Low Pt Loaded Electrodes. J. Electrochem. Soc. 2017, 164, F418–F426. [Google Scholar] [CrossRef]
- Baker, D.R.; Caulk, D.A.; Neyerlin, K.C.; Murphy, M.W. Measurement of Oxygen Transport Resistance in PEM Fuel Cells by Limiting Current Methods. J. Electrochem. Soc. 2009, 156, B991. [Google Scholar] [CrossRef]
- Harzer, G.S.; Schwämmlein, J.N.; Damjanović, A.M.; Ghosh, S.; Gasteiger, H.A. Cathode Loading Impact on Voltage Cycling Induced PEMFC Degradation: A Voltage Loss Analysis. J. Electrochem. Soc. 2018, 165, F3118–F3131. [Google Scholar] [CrossRef]
- Liu, Y.; Murphy, M.W.; Baker, D.R.; Gu, W.; Ji, C.; Jorne, J.; Gasteiger, H.A. Proton Conduction and Oxygen Reduction Kinetics in PEM Fuel Cell Cathodes: Effects of Ionomer-to-Carbon Ratio and Relative Humidity. J. Electrochem. Soc. 2009, 156, B970. [Google Scholar] [CrossRef]
- Cetinbas, F.C.; Ahluwalia, R.K.; Kariuki, N.N.; De Andrade, V.; Myers, D.J. Effects of Porous Carbon Morphology, Agglomerate Structure and Relative Humidity on Local Oxygen Transport Resistance. J. Electrochem. Soc. 2020, 167, 013508. [Google Scholar] [CrossRef]
- Shimoaka, T.; Wakai, C.; Sakabe, T.; Yamazaki, S.; Hasegawa, T. Hydration Structure of Strongly Bound Water on the Sulfonic Acid Group in a Nafion Membrane Studied by Infrared Spectroscopy and Quantum Chemical Calculation. Phys. Chem. Chem. Phys. 2015, 17, 8843–8849. [Google Scholar] [CrossRef] [PubMed]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, S.; Nicoud, L.; Jaquet, B.; Lattuada, M.; Morbidelli, M. Fractal-like Structures in Colloid Science. Adv. Colloid Interface Sci. 2016, 235, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Berlinger, S.A.; Dudenas, P.J.; Bird, A.; Chen, X.; Freychet, G.; McCloskey, B.D.; Kusoglu, A.; Weber, A.Z. Impact of Dispersion Solvent on Ionomer Thin Films and Membranes. ACS Appl. Polym. Mater. 2020, 2, 5824–5834. [Google Scholar] [CrossRef]
- Lee, S.J.; Yu, T.L.; Lin, H.L.; Liu, W.H.; Lai, C.L. Solution Properties of Nafion in Methanol/Water Mixture Solvent. Polymer 2004, 45, 2853–2862. [Google Scholar] [CrossRef]
- Mashio, T.; Ohma, A.; Tokumasu, T. Molecular Dynamics Study of Ionomer Adsorption at a Carbon Surface in Catalyst Ink. Electrochim. Acta 2016, 202, 14–23. [Google Scholar] [CrossRef]
- Kusano, T.; Hiroi, T.; Amemiya, K.; Ando, M.; Takahashi, T.; Shibayama, M. Structural Evolution of a Catalyst Ink for Fuel Cells during the Drying Process Investigated by CV-SANS. Polym. J. 2015, 47, 546–555. [Google Scholar] [CrossRef]
- Ma, S.; Chen, Q.; Jøgensen, F.H.; Stein, P.C.; Skou, E.M. 19F NMR Studies of NafionTM Ionomer Adsorption on PEMFC Catalysts and Supporting Carbons. Solid State Ion. 2007, 178, 1568–1575. [Google Scholar] [CrossRef]
- Andersen, S.M. Nano Carbon Supported Platinum Catalyst Interaction Behavior with Perfluorosulfonic Acid Ionomer and Their Interface Structures. Appl. Catal. B Environ. 2016, 181, 146–155. [Google Scholar] [CrossRef]
- Conchuir, B.O.; Harshe, Y.M.; Lattuada, M.; Zaccone, A. Analytical Model of Fractal Aggregate Stability and Restructuring in Shear Flows. Ind. Eng. Chem. Res. 2014, 53, 9109–9119. [Google Scholar] [CrossRef]
- Yakovlev, Y.V.; Lobko, Y.V.; Vorokhta, M.; Nováková, J.; Mazur, M.; Matolínová, I.; Matolín, V. Ionomer Content Effect on Charge and Gas Transport in the Cathode Catalyst Layer of Proton-Exchange Membrane Fuel Cells. J. Power Sources 2021, 490, 229531. [Google Scholar] [CrossRef]
- Nakauchi, M.; Mabuchi, T.; Hori, T.; Yoshimoto, Y.; Kinefuchi, I.; Takeuchi, H.; Tokumasu, T. Gas-Surface Interaction Study of Oxygen Molecules on Ionomer Surface in Catalyst Layer. ECS Trans. 2017, 80, 197. [Google Scholar] [CrossRef]
- Kinefuchi, I.; Oyama, J.; Yokoyama, K.; Kubo, N.; Tokumasu, T.; Matsumoto, Y. Direct Simulation Monte Carlo Analysis of Gas Transport in Microporous Structure Based on X-ray Computed Tomography. ECS Trans. 2013, 58, 71. [Google Scholar] [CrossRef]
- Sarker, M.; Rahman, M.A.; Mojica, F.; Mehrazi, S.; Kort-Kamp, W.; Chuang, P.A. Experimental and computational study of the microporous layer and hydrophobic treatment in the gas diffusion layer of a proton exchange membrane fuel cell. J. Power Sources 2021, 509, 230350. [Google Scholar] [CrossRef]
- Rahman, M.A.; Sarker, M.; Mojica, F.; Chuang, P.A. A physics-based 1-D PEMFC model for simulating two-phase water transport in the electrode and gas diffusion media. Appl. Energy 2022, 316, 119101. [Google Scholar] [CrossRef]
- Kaneko, T.; Yoshimoto, Y.; Hori, T.; Takagi, S.; Ooyama, J.; Terao, T.; Kinefuchi, I. Relation between Oxygen Gas Diffusivity and Porous Characteristics under Capillary Condensation of Water in Cathode Catalyst Layers of Polymer Electrolyte Membrane Fuel Cells. Int. J. Heat Mass Transf. 2020, 150, 119277. [Google Scholar] [CrossRef]

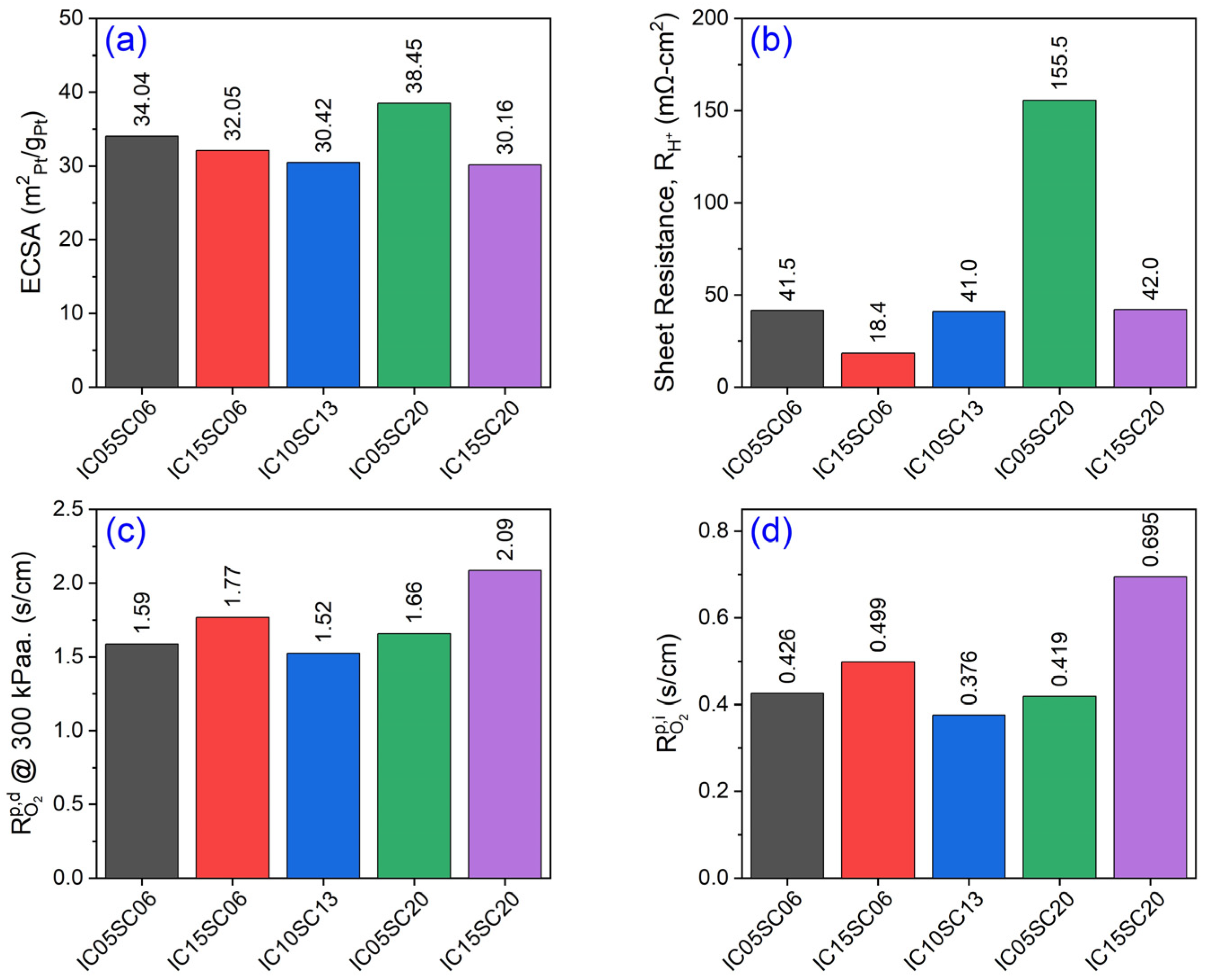
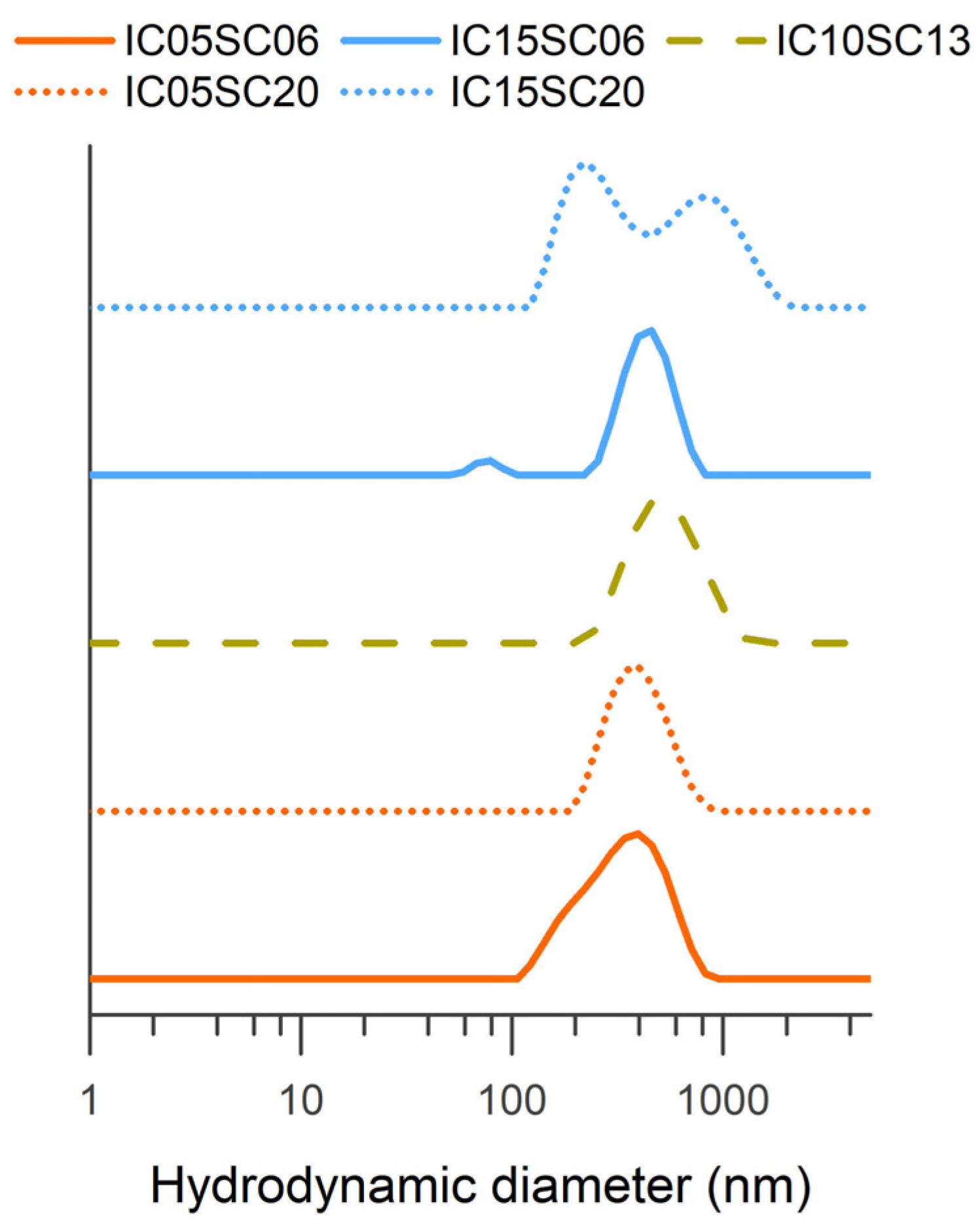


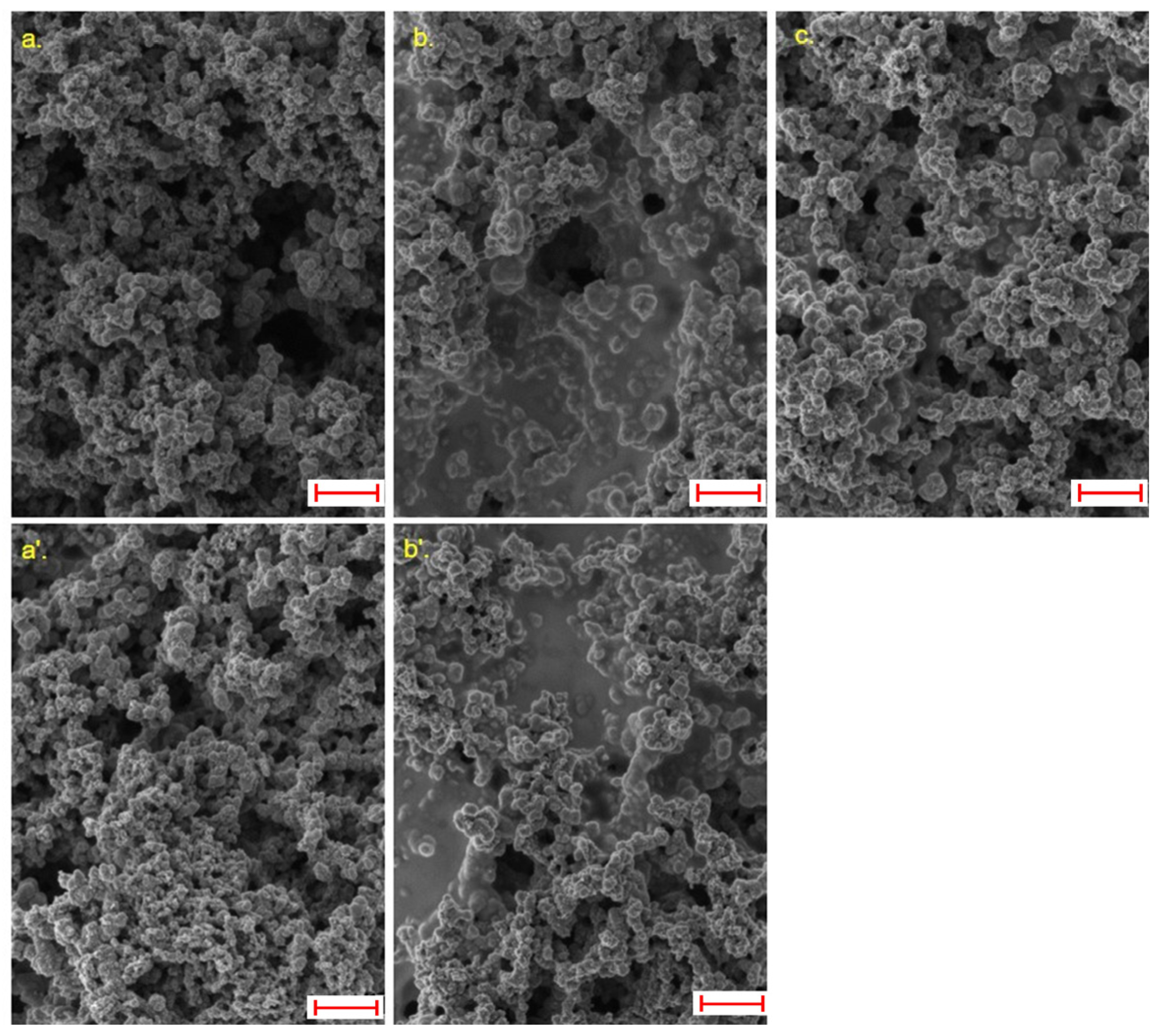
| Sample | I/C Ratio | Solid Content (%) | (%) | (%) |
|---|---|---|---|---|
| IC05SC06 | 0.5 | 6.25 | 1.5 | 0.96 |
| IC05SC20 | 0.5 | 20.0 | 5.1 | 3.95 |
| IC15SC06 | 1.5 | 6.25 | 1.1 | 1.96 |
| IC15SC20 | 1.5 | 20.0 | 3.6 | 6.84 |
| IC10SC13 | 1.0 | 13.5 | 2.8 | 3.60 |
| Test | Temp. (°C) | Inlet | Load Control | Step Hold Time (min) | |||
|---|---|---|---|---|---|---|---|
| Gas (an/ca) | Pressure (kPa) | RH (%) | Flow Rate (an/ca, L/min) | ||||
| Dry polarization | 70 | H2/Air | 100 | 60 | 0.4/0.2 | OCV-0.2 | 10 |
| Wet polarization | 70 | H2/Air | 300 | 100 | 0.4/0.2 | OCV-0.2 | 10 |
| CV | 30 | H2/N2 | 100 | 100 | 0.02/0.04 | 1.2–0.1 V | N/A |
| EIS | 60 | H2/N2 | 300 | 100 | 0.1/0.1 | 0.2 V DC w/10 mV AC | N/A |
| Dry limiting current | 80 | H2/Air&N2 | 100, 150, 200, 300 | 64 | 0.4/0.2 | 0.3–0.09 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Kakati, N.; Sarker, M.; Mojica, F.; Chuang, P.-Y.A. The Formation–Structure–Functionality Relationship of Catalyst Layers in Proton Exchange Membrane Fuel Cells. Energies 2024, 17, 2093. https://doi.org/10.3390/en17092093
Yang D, Kakati N, Sarker M, Mojica F, Chuang P-YA. The Formation–Structure–Functionality Relationship of Catalyst Layers in Proton Exchange Membrane Fuel Cells. Energies. 2024; 17(9):2093. https://doi.org/10.3390/en17092093
Chicago/Turabian StyleYang, Donglei, Nitul Kakati, Mrittunjoy Sarker, Felipe Mojica, and Po-Ya Abel Chuang. 2024. "The Formation–Structure–Functionality Relationship of Catalyst Layers in Proton Exchange Membrane Fuel Cells" Energies 17, no. 9: 2093. https://doi.org/10.3390/en17092093
APA StyleYang, D., Kakati, N., Sarker, M., Mojica, F., & Chuang, P. -Y. A. (2024). The Formation–Structure–Functionality Relationship of Catalyst Layers in Proton Exchange Membrane Fuel Cells. Energies, 17(9), 2093. https://doi.org/10.3390/en17092093







