Green Hydrogen for Energy Transition: A Critical Perspective
Abstract
1. Introduction
1.1. High Energy Content per Unit Mass
1.2. Energy per Unit Volume (Consideration of Challenges)
1.3. Fuel Cell Efficiency
1.4. Potential for High Energy Release
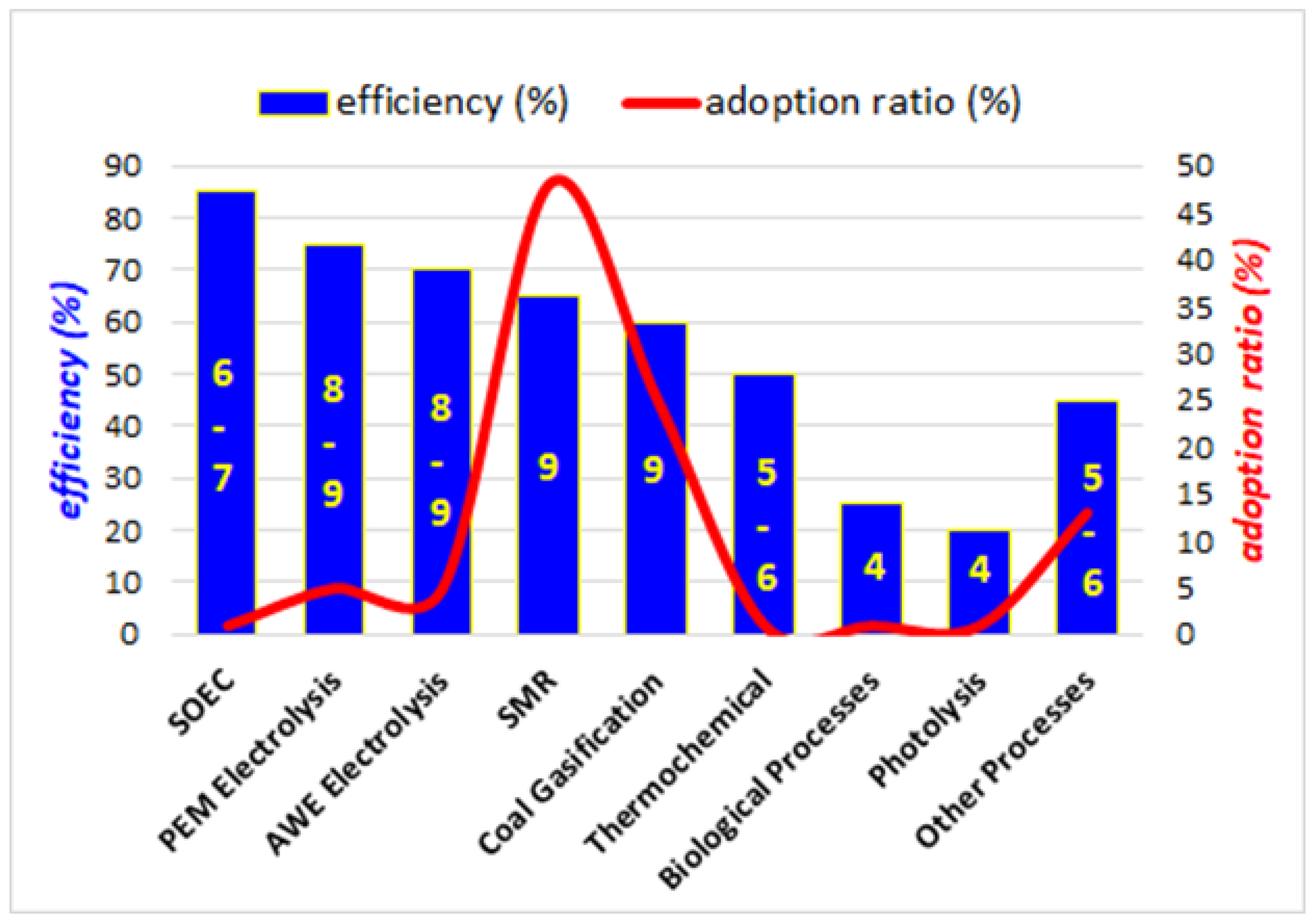
2. The Chromatic Scale of Hydrogen Production Technologies
3. Current Methods of Production of Green Hydrogen
3.1. Electrolysis
- -
- Proton Exchange Membrane (PEM) Electrolysis. This method uses a solid polymer electrolyte that conducts protons, separates hydrogen and oxygen, and insulates the electrodes. The main advantages include high efficiency (~70–80%), quick response times, and compact design. Hydrogen purity is >99.99%, which is suitable for fuel cells. Challenges include the use of expensive materials such as platinum (Pt) and iridium (Ir), with Pt loading at 0.05–0.3 mg/cm2. The energy consumption ranges from 50 to 60 kWh per kg of hydrogen, with operating temperatures between 50 °C and 80 °C. Under optimal conditions, this allows for a lifespan of over 5 × 104 operational hours [33,34,35,36,37].
- -
- Alkaline Water Electrolysis (AWE). In this process, water is split using an alkaline electrolyte (usually potassium hydroxide) between the two electrodes. The advantages are that it is a mature technology with lower costs than PEM and can operate for long periods. However, AWE has a lower efficiency (~60–70%), slower response times, and requires a larger physical footprint. Hydrogen purity is ~99.5%, making it less suitable for applications requiring ultra-high purity. Energy use is ~55–65 kWh per kg of hydrogen, with current densities of 0.2–0.4 A/cm2 and a lifespan of 6 × 104–9 × 104 h. Details on the AWE working principle, together with recent developments in used electrocatalysts can be found in refs. [29,38].
- -
- Solid Oxide Electrolysis Cell (SOEC). This method uses a solid ceramic electrolyte and operates at high temperatures (typically 700–1000 °C). Advantages: Higher efficiency (~80–85) due to the use of waste heat, which reduces electricity requirements to as low as ~40–50 kWh per kg of hydrogen. Several papers report studies showcasing how SOEC technology can efficiently produce hydrogen with minimal energy loss, often by utilizing waste heat from industrial processes to enhance the overall efficiency [39,40,41].
3.2. Advanced Membranes for Electrolysis
- -
- Dynamic load management: Electrolyzers can be designed to operate flexibly, ramping up hydrogen production when there is excess renewable energy and scaling back when energy is scarce. This helps absorb surplus energy and prevent curtailment, effectively acting as a controllable load that can stabilize the grid.
- -
- Demand Response: Electrolyzers can participate in demand response programs, where they reduce or increase power consumption in response to grid signals, thereby helping maintain grid stability.
3.3. Photolysis
- -
- Photoelectrochemical (PEC) Water Splitting [46,47,48]. This method involves using sunlight directly to split water molecules in a photoelectrochemical cell. The principal advantage is the direct use of solar energy, potentially achieving solar-to-hydrogen (STH) efficiencies of 2–5%, which is far below the commercial viability target of >15%. Challenges include low efficiency, high cost, and stability of photoelectrodes.
- -
- Photocatalytic Water Splitting [49,50,51,52]. This technique uses semiconductor materials that absorb sunlight and generate electrons to split water into hydrogen and oxygen. A very recent review by Kafadi et al. [53] discusses advancements in utilizing two-dimensional transition metal carbides, nitrides, or carbonitrides, also known as MXene-based materials, for hydrogen production through photocatalysis. MXenes exhibit exceptional electrical conductivity, tunable surface chemistry, and structural flexibility, making them excellent candidates for photocatalytic applications. Indeed, composites such as Ti3C2 with other semiconductors like Zn2In2S5 or g-C3N4 significantly enhance photocatalytic performance, achieving hydrogen evolution rates (HER) of ~50–100 µmol/h under visible light. These materials improve charge separation, electron transport, and visible-light absorption, leading to higher hydrogen evolution rates under specific conditions. This review highlights various preparation methods, such as hydrothermal and hydrofluoric acid (HF) etching techniques, which influence the physiochemical properties of MXenes and their photocatalytic efficiency. In addition, replacing noble metals like platinum with cost-effective MXene-based materials (e.g., RuNi composites) is proposed to reduce production costs while maintaining high catalytic activity. This research emphasizes the need for further optimization of MXene-based heterostructures and scalable, sustainable production techniques to achieve practical solar-to-hydrogen conversion efficiencies.
- Broad solar spectrum absorption
- Charge separation and transport
- Photocatalysis efficiency
- Selective permeability and gas separation
- Cost-effectiveness and scalability
3.4. Biological Processes
- Microbial Electrolysis Cells (MECs).
- Photobiological Water Splitting.
3.5. Thermodynamical Water Splitting
4. Green Hydrogen Storage Methods and Grid Integration
- -
- Direct use in industry: As feedstock for various industrial processes, particularly in sectors that are difficult to decarbonize, such as steel production and chemical manufacturing.
- -
- Conversion to Fuels: Through processes like Hydrogen-to-Fuel (HtF), hydrogen can be converted into synthetic fuels, which can be used in transportation or stored for later use.
- -
- Energy Storage: Hydrogen can be stored in large quantities over long periods, providing a reliable means to balance the grid and manage fluctuations in the renewable energy supply.
4.1. Green Hydrogen Storage
4.1.1. Large-Scale Storage
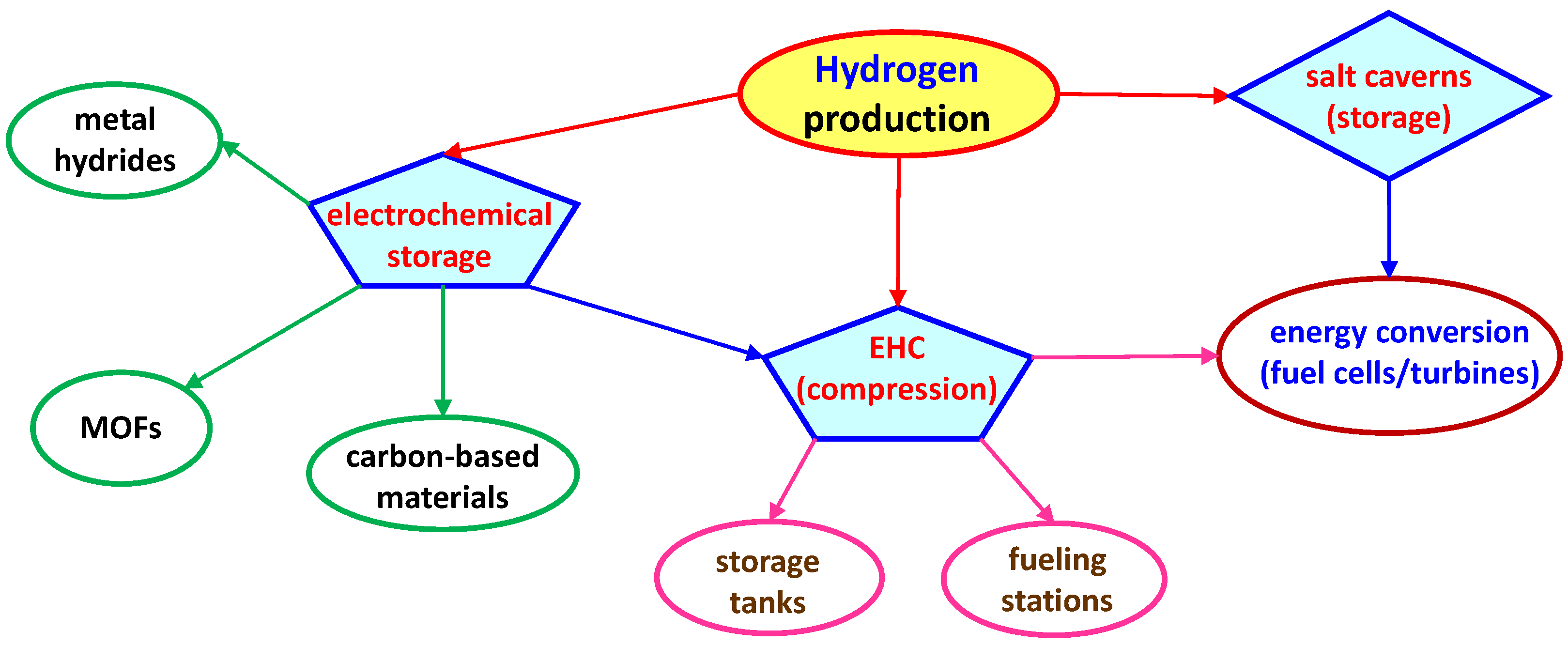
4.1.2. Electrochemical-Based Storage Methods
- -
- Metal hydrides. These compounds, such as magnesium hydride (MgH2), are formed when hydrogen gas reacts with metals. They can store hydrogen in solid form [85], which can be released by applying heat. Metal hydrides allow for reversible hydrogen storage and release, making them highly efficient for electrochemical applications like fuel cells. They offer a high volumetric hydrogen density and can undergo multiple cycles without significant degradation.
- -
- Carbon-based materials. Materials such as carbon nanotubes (CNTs), graphene, and activated carbons (ACs) possess exceptionally high surface areas, allowing them to adsorb hydrogen effectively. These materials are lightweight, exhibit good conductivity, and are ideal for electrochemical cells, where rapid hydrogen uptake and release are required. For example, Reza et al. [86] discussed graphene, CNTS, and ACs for hydrogen storage, highlighting their roles in modern energy storage and electrochemical cell applications. Sdanghi et al. [87] analyzed hydrogen storage in microporous carbon materials, including ACs, CNTs, and nanofibers, and evaluated the adsorption properties and structural characteristics influencing hydrogen storage capacity. Attia et al. [88] have recently reviewed the integration of metal nanoparticles on porous ACs and CNTs for hydrogen storage. Moreover, recent advances in electrochemical hydrogen storage using graphene and its derivatives have been illustrated by Kopak [89], who also evaluated the impact of material modifications on the overall efficiency. Finally, Boateng et al. [90] explored graphene and CNT modifications for improved energy and hydrogen storage and highlighted functionalization techniques for enhanced adsorption properties.
- -
- Metal-Organic Framework (MOFs). MOFs are crystalline materials with extremely high surface areas and tunable pore sizes, enabling hydrogen storage through the physisorption process. They provide high hydrogen storage capacities at low pressures, and their properties can be tailored for specific electrochemical applications to enhance the efficiency of hydrogen storage and release in fuel cells. Hydrogen adsorption in MOFs primarily occurs through two mechanisms: physisorption and chemisorption. Understanding the distinctions between these mechanisms is crucial for optimizing hydrogen storage performance. The former involves weak van der Waals interactions between hydrogen molecules and the MOF surface. These interactions are typically characterized by low enthalpies of adsorption, ranging from 4 to 7 kJ/mol, which means that hydrogen is adsorbed without forming strong chemical bonds. As a result, physisorption is generally reversible and occurs at near-ambient temperatures [91]. In contrast, chemisorption involves the formation of stronger chemical bonds between hydrogen molecules and the MOF, leading to higher enthalpies of adsorption. This process is often irreversible under standard conditions and can alter the electronic structure of the MOF [92]. However, in the context of MOFs, hydrogen storage predominantly occurs via physisorption due to the typically low binding energies involved. Factors influencing hydrogen adsorption in MOFs are: (a) surface area and pore volume, that is, higher surface areas and larger pore volumes in MOFs provide more adsorption sites for hydrogen, enhancing storage capacity [93]; (b) pore size and geometry, i.e., the size and shape of the pores influence the accessibility and diffusion of hydrogen molecules within the MOF structure [94]; and (c) introducing functional groups or metal sites within the MOF can increase the binding affinity for hydrogen, potentially enhancing storage capacity [95]. However, this must be balanced to avoid overly strong interactions that could lead to chemisorption.
- -
- Chemical hydrogen storage. This method involves storing hydrogen in chemical compounds such as ammonia, methanol, or formic acid, which can release hydrogen through catalytic reactions. These compounds allow for stable and safe storage under ambient conditions. Furthermore, the electrochemical oxidation of these compounds in fuel cells directly produces electricity, which is a compact and efficient energy source. For example, Mishra et al. [96] analyzed in their review the application of formic acid and ammonia as hydrogen carriers along with the development of high-efficiency catalysts for hydrogen release, whereas Meng et al. [97] proposed an innovative electrochemical synthesis of formamide, which could be integrated with hydrogen storage and release technologies.
- -
- Electrochemical Hydrogen Compression (EHC). EHC involves using electrochemical reactions to compress hydrogen gas to high pressure, which can then be stored in tanks [98,99]. Compared to mechanical compression, this method is more efficient and has fewer moving parts. In detail, this process utilizes electrochemical reactions facilitated by PEM technology. Hydrogen is oxidized at the anode, passes through the PEM as protons, and is reduced to molecular hydrogen at the cathode under increased pressure. This technology offers advantages in terms of efficiency, scalability, and integration into renewable energy systems. Its ability to provide pure hydrogen at high pressures makes it suitable for various applications, including energy storage and fueling stations [98,99,100]. Indeed, EHC is particularly useful for small-scale, distributed hydrogen storage systems. For example, Zängler et al. [101] have recently proposed the first tubular EHC design, which enhances scalability and operational efficiency. This study highlights the compactness and integration capabilities of this design. In another work, Sdanghi et al. [102] discuss water management solutions for EHC devices to improve system performance at pressures up to 10 MPa. The study integrates drying systems downstream of the compressor. Nordio et al. [103] focus on the electrochemical behavior of hydrogen compressors when dealing with different gas mixtures, analyzing recovery, purity, and electric consumption. Zou et al. [104] examine the electrochemical performance of hydrogen compressors in diverse gas mixtures, analyzing their recovery, purity, and electric consumption. The above references collectively provide a thorough understanding of the EHC technology, covering its theoretical foundations, design innovations, and practical applications.
4.2. Green Hydrogen Integrated into Sector-Coupled Smart Grids
- -
- Grid balancing. Excess renewable energy stored as GH2 can be integrated with smart grid technologies that monitor and predict renewable energy generation and grid demand in real-time. This allows for the optimized operation of electrolyzers, matching hydrogen production with renewable availability and grid needs.
- -
- Power-to-Gas (PtG). Surplus electricity can be used to produce hydrogen via electrolysis, which can then be injected into a natural gas grid (blended with natural gas) or used in industrial processes to provide a large-scale energy storage solution.
- -
- Energy security. Stored hydrogen provides a buffer against supply disruptions and ensures a continuous energy supply.
- -
- Sector flexibility. Hydrogen storage enables its use across different sectors—such as in Hydrogen-to-Mobility (HtM) for transportation or Hydrogen-to-Chemicals (HtC) for industrial applications-depending on demand.
- -
- Advanced forecasting and control systems: Implementing AI and machine learning algorithms to predict renewable energy generation and grid demand can help in the optimal scheduling of hydrogen production, ensuring that it complements the grid’s needs.
4.2.1. Electricity
4.2.2. Heating/Cooling
4.2.3. Transportation
4.2.4. Industry
4.2.5. Decentralization and Digitalization
4.2.6. Energy Storage and Flexibility
4.3. Integration of Green Hydrogen in Various Industrial Sectors
4.3.1. GH2 as an Energy Carrier
- Cement production
- Glass manufacturing
- Textile and pulp and paper industries
- Aluminum production
- Transportation and mobility
4.3.2. GH2 as a Feedstock
- Green steel production
- Green ammonia production
- Chemical production
- Refining and petrochemicals
4.3.3. The Benefits of Green Hydrogen in the Agri-Food Industry
- Decarbonization of energy-intensive processes
- Energy storage and flexibility
- Sustainable packaging and logistics
- Water recycling and waste management
- Reducing carbon footprint
- Regulatory compliance and future-proofing
- Research and innovation opportunities
4.3.4. Key Factors Influencing Water Footprint
4.4. Illustration of Some of the Best-Known Examples of GH2 Integrated into Sector-Coupled Smart Grids
- H2RES Project (Denmark)
- HEAVENN Project (Northern Netherlands)
- NortH2 project (Netherlands)
- REFHYNE Project (Germany)
- Westküste 100 Project (Germany)
- HyDeploy Project (United Kingdom)
- HyNet North West Project (United Kingdom)
- HydrOm Hub Project (Oman)
- Haru Oni Project (Chile)
- ACWA Power Neom Project (Saudi Arabia)
4.5. Benefits of Optimized Integration and System Efficiency
5. Power-to-X Technologies: Current Methodologies for Efficient and Long-Lasting Hydrogen Storage
5.1. Power-to-Heat (PtH)
5.2. Power-to-Gas (PtG), Power-to-Hydrogen (PtH2)
5.3. Power-to-Liquids (PtL)
5.4. Power-to-Chemicals (PtC)
5.5. Power-to-Mobility (PtM)
5.6. Advantages of PtH2 in Producing Green Hydrogen
5.7. Power-to-Hydrogen-to-Power (PtH2tP)
6. Role of H2 Conversion Processes: H2-to-Fuel and H2-to-Gas Technologies
6.1. Hydrogen-to-Fuel (H2tF)
6.1.1. Fuel Cells
6.1.2. Combustion in Gas Turbines
6.2. Hydrogen-to-Gas (H2tG)
7. Key Challenges and Critical Issues of Green Hydrogen-Integrated Smart Grids
7.1. Key Challenges
- -
- -
- Incorporation of seawater. The use of seawater offers a viable alternative to freshwater sources. However, seawater electrolysis requires advanced desalination processes or corrosion-resistant materials to handle impurities, which can increase costs. Moreover, although seawater utilization reduces competition for freshwater, it introduces new challenges, such as the energy intensity of desalination and handling of brine waste [210,211,212,213].
- -
- High production costs. The cost of producing GH2 through electrolysis is currently higher than that of conventional production methods of gray and blue hydrogen (e.g., SMR). This cost disparity is a major barrier to widespread adoption [214].
- -
- -
- Infrastructure development. The existing infrastructure for hydrogen production, storage, transportation, and distribution remains underdeveloped. Establishing and retrofitting the necessary infrastructure requires substantial investment and coordination [217].
- -
- Grid integration and stability. Integrating GH2 into existing smart grids introduces challenges related to grid stability [218,219], particularly when dealing with variable renewable energy sources like wind and solar [220,221]. Balancing supply and demand in real-time while accommodating hydrogen production adds complexity to grid management.
- -
- -
- Public acceptance and safety. The public perception of hydrogen safety, particularly regarding its storage and transportation, poses a significant challenge. Addressing safety concerns and raising awareness of the benefits of hydrogen is crucial.
7.2. Gaps
7.3. Future Prospects and Potential Advancements
7.4. Research Directions
8. Analysis of Possible Strategies to Reduce the Operating Costs of Green Hydrogen
8.1. Cost Reduction in Green Hydrogen Production
- Advancements in electrolysis technology.
- Scaling up renewable energy integration.
8.2. Cost Reduction in Hydrogen Storage
- Advancements in hydrogen storage materials
- Development of hydrogen hubs and pipelines
8.3. Cost Reduction in Hydrogen Distribution
- Optimization of hydrogen transport
8.4. Policy and Market Mechanisms
- Government subsidies and incentives
8.5. Other Factors
8.5.1. Electrolysis Efficiency
- High energy input.
- Material limitations.
8.5.2. Compression and Storage Losses
- Energy-intensive compression.
- Storage challenges.
8.5.3. Fuel Cell Inefficiencies
- Conversion losses.
- Durability and degradation.
8.5.4. Systemic Challenges
- Thermodynamic limits:
- Intermittence of renewable energy.
- Integration and scalability.
8.5.5. Economic Factors
- High costs.
8.5.6. Scale and Integration Challenges
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| AWE | Alkaline Water Electrolysis |
| ATR | Auto-Thermal Reforming |
| BEVs | Battery-Electric Vehicles |
| CCS | Carbon Capture and Storage |
| CHP | Combined Heat and Power |
| DERs | Decentralized Energy Resources |
| EHC | Electrochemical Hydrogen Compression |
| EMS | Energy Management Systems |
| ESTs | Energy Storage Technologies |
| FCVs | Fuel Cell Vehicles |
| GHG | Greenhouse Gas |
| HDR | Hydrogen Direct Reduction |
| HISCSG | Hydrogen-Incorporated Sector-Coupled Smart Grids |
| IoT | Internet of Things |
| LHV | Lower Heating Value |
| LOHC | Liquid Organic Hydrogen Carrier |
| MEC | Microbial Electrolysis Cells |
| MOFs | Metal-Organic Frameworks |
| PEC | Photo Electro-Chemical (water splitting) |
| PEM | Proton Exchange Membrane |
| PtG | Power-to-Gas |
| PtH | Power-to-Heat |
| PtH2 | Power-to-Hydrogen |
| PtX | Power-to-X technologies (energy conversion technologies) |
| PtH2tP | Power-to-Hydrogen-to-Power |
| RESs | Renewable Energy Sources |
| SGD | Smart Grids and Digitalization |
| SMR | Steam Methane Reforming |
| SOEC | Solid Oxide Electrolysis Cells |
| SOFC | Solid Oxide Fuel Cells |
| TRL | Technology Readiness Level |
References
- Maka, A.O.M.; Mehmood, M. Green hydrogen energy production: Current status and potential. Clean Energy 2024, 8, 1–7. [Google Scholar] [CrossRef]
- Fernández-Arias, P.; Antón-Sancho, Á.; Lampropoulos, G.; Vergara, D. On Green Hydrogen Generation Technologies: A Bibliometric Review. Appl. Sci. 2024, 14, 2524. [Google Scholar] [CrossRef]
- Wei, S.; Sacchi, R.; Tukker, A.; Suh, S.; Steubing, B. Future environmental impacts of global hydrogen production. Energy Environ. Sci. 2024, 17, 2157–2172. [Google Scholar] [CrossRef]
- Maestre, V.M.; Ortiz, A.; Ortiz, I. Challenges and prospects of renewable hydrogen-based strategies for full decarbonization of stationary power applications. Renew. Sustain. Energy Rev. 2021, 152, 111628. [Google Scholar] [CrossRef]
- Sørensen, B.; Spazzafumo, G. Hydrogen; Academic Press: Cambridge, MA, USA, 2018; pp. 5–105. [Google Scholar] [CrossRef]
- Shadidi, B.; Najafi, G.; Yusaf, T. A review of hydrogen as a fuel in internal combustion engines. Energies 2021, 14, 6209. [Google Scholar] [CrossRef]
- Sørensen, B.; Spazzafumo, G. Fuel cells. In Hydrogen and Fuel Cells; Academic Press: Cambridge, MA, USA, 2018; pp. 107–220. [Google Scholar] [CrossRef]
- Johnson, K.; Veenstra, M.J.; Gotthold, D.; Simmons, K.; Alvine, K.; Hobein, B.; Houston, D.; Newhouse, N.; Yeggy, B.; Vaipan, A. Advancements and Opportunities for On-Board 700 Bar Compressed Hydrogen Tanks in the Progression Towards the Commercialization of Fuel Cell Vehicles. SAE Int. J. Altern. Powertrains 2017, 6, 201–218. [Google Scholar] [CrossRef]
- Hassan, Q.; Azzawi, I.D.J.; Sameen, A.Z.; Salman, H.M. Hydrogen Fuel Cell Vehicles: Opportunities and Challenges. Sustainability 2023, 15, 11501. [Google Scholar] [CrossRef]
- Durkin, K.; Khanafer, A.; Liseau, P.; Stjernström-Eriksson, A.; Svahn, A.; Tobiasson, L.; Andrade, T.S.; Ehnberg, J. Hydrogen-Powered Vehicles: Comparing the Powertrain Efficiency and Sustainability of Fuel Cell versus Internal Combustion Engine Cars. Energies 2024, 17, 1085. [Google Scholar] [CrossRef]
- Haseli, Y. Maximum conversion efficiency of hydrogen fuel cells. Int. J. Hydrogen Energy 2018, 43, 9015–9021. [Google Scholar] [CrossRef]
- Rolo, I.; Costa, V.A.F.; Brito, F.P. Hydrogen-Based Energy Systems: Current Technology Development Status, Opportunities and Challenges. Energies 2024, 17, 180. [Google Scholar] [CrossRef]
- Massarweh, O.; Al-khuzaei, M.; Al-Shafi, M.; Bicer, Y.; Abushaikha, A.S. Blue hydrogen production from natural gas reservoirs: A review of application and feasibility. J. CO2 Util. 2023, 70, 102438. [Google Scholar] [CrossRef]
- Shen, M.; Hu, Z.; Kong, F.; Tong, L.; Yin, S.; Liu, C.; Zhang, P.; Wang, L.; Ding, Y. Comprehensive technology and economic evaluation based on the promotion of large-scale carbon capture and storage demonstration projects. Rev. Environ. Sci. Bio/Techlol. 2023, 22, 823–885. [Google Scholar] [CrossRef]
- Luo, B.; Hu, H.; Liu, K.; Chong, D.K.; Li, Y. Mini-Review of Opportunities and Challenges of Carbon Capture and Storage (CCS) Technology in Addressing Climate Change. In Environmental Science and Engineering, Proceedings of the 10th International Conference on Energy Engineering and Environmental Engineering, ICEEEE 2023, Singapore, 6–8 August 2023; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Chen, F.; Chen, B.; Ma, Z.; Mehana, M. Economic assessment of clean hydrogen production from fossil fuels in the intermountain-west region, USA. Renew. Sustain. Energy Transit. 2024, 5, 100077. [Google Scholar] [CrossRef]
- Diab, J.; Fulcheri, L.; Hessel, V.; Rohani, V.; Frenklach, M. Why turquoise hydrogen will Be a game changer for the energy transition. Int. J. Hydrogen Energy 2022, 47, 25831–25848. [Google Scholar] [CrossRef]
- The Future of Hydrogen for G20: Seizing Today’s Opportunities; International Energy Agency: Paris, France, 2019.
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Incer-Valverde, J.; Korayem, A.; Tsatsaronis, G.; Morosuk, T. ‘Colors’ of hydrogen: Definitions and carbon intensity. Energy Convers. Manag. 2023, 291, 117294. [Google Scholar] [CrossRef]
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Ajanovic, A.; Sayer, M.; Haas, R. The economics and the environmental benignity of different colors of hydrogen. Int. J. Hydrogen Energy 2022, 47, 24136–24154. [Google Scholar] [CrossRef]
- Lubbe, F.; Rongé, J.; Bosserez, T.; Martens, J.A. Golden hydrogen. Curr. Opin. Green Sustain. Chem. 2023, 39, 100732. [Google Scholar] [CrossRef]
- Saha, P.; Akash, F.A.; Shovon, S.M.; Monir, M.U.; Ahmed, M.T.; Khan, M.F.H.; Sarkar, S.M.; Islam, M.K.; Hasan, M.M.; Vo, D.-V.N.; et al. Grey, blue, and green hydrogen: A comprehensive review of production methods and prospects for zero-emission energy. Int. J. Green Energy 2024, 21, 1383–1397. [Google Scholar] [CrossRef]
- Huang, J.; Balcombe, P.; Feng, Z. Technical and economic analysis of different colours of producing hydrogen in China. Fuel 2023, 337, 127227. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Olapade, O.; Alghoul, M.; Salman, H.M.; Jaszczur, M. Hydrogen fuel as an important element of the energy storage needs for future smart cities. Int. J. Hydrogen Energy 2023, 48, 30247–30262. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M. Green Hydrogen Production by Water Electrolysis; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Panigrahy, B.; Narayan, K.; Rao, B.R. Green hydrogen production by water electrolysis: A renewable energy perspective. Mater. Today Proc. 2022, 67, 1310–1314. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Millet, P. Fundamentals of Water Electrolysis. In Electrochemical Power Sources: Fundamentals, Systems, and Applications Hydrogen Production by Water Electrolysis; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 2; pp. 37–62. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Agrawal, D.; Mahajan, N.; Singh, S.A.; Sreedhar, I. Green hydrogen production pathways for sustainable future with net zero emissions. Fuel 2024, 359, 130131. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Paul, B.; Andrews, J. PEM unitised reversible/regenerative hydrogen fuel cell systems: State of the art and technical challenges. Renew. Sustain. Energy Rev. 2017, 79, 585–599. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. Recent advances in hydrogen production through proton exchange membrane water electrolysis—A review. Sustain. Energy Fuels 2023, 7, 3560–3583. [Google Scholar] [CrossRef]
- Liu, R.T.; Xu, Z.-L.; Li, F.-M.; Chen, F.-Y.; Yu, J.-Y.; Yan, Y.; Chen, Y.; Xia, B.Y. Recent advances in proton exchange membrane water electrolysis. Chem. Soc. Rev. 2023, 52, 5652–5683. [Google Scholar] [CrossRef]
- Li, X.; Yao, Y.; Tian, Y.; Jia, J.; Ma, W.; Yan, X.; Liang, J. Recent advances in key components of proton exchange membrane water electrolysers. Mater. Chem. Front. 2024, 8, 2493–2510. [Google Scholar] [CrossRef]
- Dash, S.; Arjun Singh, K.; Jose, S.; Vincent Herald Wilson, D.; Elangovan, D.; Subbarama Kousik, S.; Sendhil Kumar, N. Advances in green hydrogen production through alkaline water electrolysis: A comprehensive review. Int. J. Hydrogen Energy 2024, 19, 614–629. [Google Scholar] [CrossRef]
- Pandiyan, A.; Uthayakumar, A.; Subrayan, R.; Cha, S.W.; Moorthy, S.B.K. Review of solid oxide electrolysis cells: A clean energy strategy for hydrogen generation. Nanomater. Energy 2019, 8, 2–22. [Google Scholar] [CrossRef]
- Liu, H.; Clausen, L.R.; Wang, L.; Chen, M. Pathway toward cost-effective green hydrogen production by solid oxide electrolyzer. Energy Environ. Sci. 2023, 16, 2090–2111. [Google Scholar] [CrossRef]
- Liu, H.; Yu, M.; Tong, X.; Wang, Q.; Chen, M. High Temperature Solid Oxide Electrolysis for Green Hydrogen Production. Chem. Rev. 2024, 124, 10509–10576. [Google Scholar] [CrossRef] [PubMed]
- Nami, H.; Rizvandi, O.B.; Chatzichristodoulou, C.; Hendriksen, P.V.; Frandsen, H.L. Techno-economic analysis of current and emerging electrolysis technologies for green hydrogen production. Energy Convers. Manag. 2022, 269, 116162. [Google Scholar] [CrossRef]
- Kamaroddin, M.F.A.; Sabli, N.; Tuan Abdullah, T.A.; Siajam, S.I.; Abdullah, L.C.; Abdul Jalil, A.; Ahmad, A. Membrane-Based Electrolysis for Hydrogen Production: A Review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Chand, K.; Paladino, O. Recent developments of membranes and electrocatalysts for the hydrogen production by anion exchange membrane water electrolysers: A review. Arab. J. Chem. 2023, 16, 104451. [Google Scholar] [CrossRef]
- Tang, J.; Su, C.; Shao, Z. Advanced membrane-based electrode engineering toward efficient and durable water electrolysis and cost-effective seawater electrolysis in membrane electrolyzers. Exploration 2024, 4, 20220112. [Google Scholar] [CrossRef] [PubMed]
- Ros, C.; Andreu, T.; Morante, J.R. Photoelectrochemical water splitting: A road from stable metal oxides to protected thin film solar cells. J. Mater. Chem. A 2020, 8, 10625–10669. [Google Scholar] [CrossRef]
- Lee, J.W.; Cho, K.H.; Yoon, J.S.; Kim, Y.M.; Sung, Y.M. Photoelectrochemical water splitting using one-dimensional nanostructures. J. Mater. Chem. A 2020, 8, 10625–10669. [Google Scholar] [CrossRef]
- Vilanova, A.; Dias, P.; Lopes, T.; Mendes, A. The route for commercial photoelectrochemical water splitting: A review of large-area devices and key upscaling challenges. Chem. Soc. Rev. 2024, 53, 2388–2434. [Google Scholar] [CrossRef]
- Villa, K.; Galán-Mascarós, J.R.; López, N.; Palomares, E. Photocatalytic water splitting; challenges. Sustain. Energy Fuels 2021, 5, 4560–4569. [Google Scholar] [CrossRef]
- Tao, X.; Zhao, Y.; Wang, S.; Li, C.; Li, R. Recent advances and perspectives for solar-driven water splitting using particulate photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lin, L.; Takata, T.; Hisatomi, T.; Domen, K. A perspective on two pathways of photocatalytic water splitting and their practical application systems. Phys. Chem. Chem. Phys. 2023, 25, 6586–6601. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.H.; Nguyen, T.A.; Le, M.V.; Nguyen, D.Q.; Wang, Y.H.; Wu, J.C.S. Photocatalytic hydrogen production from seawater splitting: Current status, challenges, strategies and prospective applications. Chem. Eng. J. 2024, 484, 149213. [Google Scholar] [CrossRef]
- Kafadi, A.D.G.; Hafeez, H.Y.; Mohammed, J.; Ndikilar, C.E.; Suleiman, A.B.; Isah, A.T. A recent prospective and progress on MXene-based photocatalysts for efficient solar fuel (hydrogen) generation via photocatalytic water-splitting. Int. J. Hydrogen Energy 2024, 53, 1242–1258. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Higashi, T.; Domen, K. Interfacial Design of Particulate Photocatalyst Materials for Green Hydrogen Production. ChemSusChem 2024, 17, e202400663. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tan, H.L.; Toe, C.Y.; Scott, J.; Wang, L.; Amal, R.; Ng, Y.H. Photocatalytic and Photoelectrochemical Systems: Similarities and Differences. Adv. Mater. 2020, 32, 1904717. [Google Scholar] [CrossRef]
- Pei, J.; Li, H.; Yu, D.; Zhang, D. g-C3N4-Based Heterojunction for Enhanced Photocatalytic Performance: A Review of Fabrications, Applications, and Perspectives. Catalysts 2024, 14, 825. [Google Scholar] [CrossRef]
- Yin, X.; Li, J.; Du, L.; Zhan, F.; Kawashima, K.; Li, W.; Qiu, W.; Liu, Y.; Yang, X.; Wang, K. Boosting Photoelectrochemical Performance of BiVO4through Photoassisted Self-Reduction. ACS Appl. Energy Mater. 2020, 3, 4403–4410. [Google Scholar] [CrossRef]
- Raub, A.A.M.; Bahru, R.; Nashruddin, S.N.A.M.; Yunas, J. Advances of nanostructured metal oxide as photoanode in photoelectrochemical (PEC) water splitting application. Heliyon 2024, 10, e39079. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kim, I.S.; Miyatake, K. Proton-conductive aromatic membranes reinforced with poly(vinylidene fluoride) nanofibers for high-performance durable fuel cells. Sci. Adv. 2023, 9, eadg9057. [Google Scholar] [CrossRef]
- Jaafar, S.N.H.; Minggu, L.J.; Arifin, K.; Kassim, M.B.; Wan, W.R.D. Natural dyes as TiO2 sensitizers with membranes for photoelectrochemical water splitting: An overview. Renew. Sustain. Energy Rev. 2017, 78, 698–709. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Y.X.; Zhou, J.W.; Lin, S.W.; Lu, C.Z. Pollen Carbon-Based Rare-Earth Composite Material for Highly Efficient Photocatalytic Hydrogen Production from Ethanol-Water Mixtures. ACS Omega 2022, 7, 30495–30503. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elrahman, N.K.; Al-Harbi, N.; Al-Hadeethi, Y.; Alruqi, A.B.; Mohammed, H.; Umar, A.; Akbar, S. Influence of Nanomaterials and Other Factors on Biohydrogen Production Rates in Microbial Electrolysis Cells—A Review. Molecules 2022, 27, 8594. [Google Scholar] [CrossRef] [PubMed]
- Dubrovin, I.A.; Hirsch, L.O.; Rozenfeld, S.; Gandu, B.; Menashe, O.; Schechter, A.; Cahan, R. Hydrogen Production in Microbial Electrolysis Cells Based on Bacterial Anodes Encapsulated in a Small Bioreactor Platform. Microorganisms 2022, 10, 1007. [Google Scholar] [CrossRef]
- Hemschemeier, A.; Posewitz, M.C.; Happe, T. Hydrogenases and hydrogen production. In The Chlamydomonas Sourcebook: Organellar and Metabolic Processes; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2, pp. 343–367. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Wang, L.; Fan, C.; Liu, H. Advances in Whole-Cell Photobiological Hydrogen Production. Adv. Nanobiomed. Res. 2021, 1, 2000051. [Google Scholar] [CrossRef]
- Abanades, S. Metal oxides applied to thermochemical water-splitting for hydrogen production using concentrated solar energy. ChemEngineering 2019, 3, 63. [Google Scholar] [CrossRef]
- Oudejans, D.; Offidani, M.; Constantinou, A.; Albonetti, S.; Dimitratos, N.; Bansode, A. Comprehensive Review on Two-Step Thermochemical Water Splitting for Hydrogen Production in a Redox Cycle. Energies 2022, 15, 3044. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ashida, S.; Inubuse, N.; Shimizu, S.; Miura, Y.; Mizutani, T.; Saitow, K.-I. Room-temperature thermochemical water splitting: Efficient mechanocatalytic hydrogen production. J. Mater. Chem. A Mater. 2024, 12, 30906–30918. [Google Scholar] [CrossRef]
- Ji, M.; Wang, J. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Rafa, N.; Musharrat, A.; Lam, S.S.; Boretti, A. Sustainable hydrogen production: Technological advancements and economic analysis. Int. J. Hydrogen Energy 2022, 47, 37227–37255. [Google Scholar] [CrossRef]
- Sikiru, S.; Adedayo, H.B.; Olutoki, J.O.; ur Rehman, Z. Hydrogen integration in power grids, infrastructure demands and techno-economic assessment: A comprehensive review. J. Energy Storage 2024, 104, 114520. [Google Scholar] [CrossRef]
- Kodgire, P. Hydrogen—Imminent clean and green energy: Hydrogen production technologies life cycle assessment review. Process Saf. Environ. Prot. 2025, 193, 483–500. [Google Scholar] [CrossRef]
- Brear, M.J.; Baldick, R.; Cronshaw, I.; Olofsson, M. Sector coupling: Supporting decarbonisation of the global energy system. Electr. J. 2020, 33, 106832. [Google Scholar] [CrossRef]
- Genovese, M.; Schlüter, A.; Scionti, E.; Piraino, F.; Corigliano, O.; Fragiacomo, P. Power-to-hydrogen and hydrogen-to-X energy systems for the industry of the future in Europe. Int. J. Hydrogen Energy 2023, 48, 16545–16568. [Google Scholar] [CrossRef]
- Youssef, A.R.; Mallah, M.; Ali, A.; Mohamed, E.E.M. Advancement of Power-to-Hydrogen and Heat-to-Hydrogen technologies and their applications in renewable-rich power grids. Comput. Electr. Eng. 2024, 120, 109843. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Mansur, M.; Muttaqi, K.M.; Dong, Z.Y.; Blaabjerg, F. Hydrogen energy storage integrated hybrid renewable energy systems: A review analysis for future research directions. Int. J. Hydrogen Energy 2022, 47, 17285–17312. [Google Scholar] [CrossRef]
- Xie, Z.; Jin, Q.; Su, G.; Lu, W. A Review of Hydrogen Storage and Transportation: Progresses and Challenges. Energies 2024, 17, 4070. [Google Scholar] [CrossRef]
- Barison, E.; Donda, F.; Merson, B.; Le Gallo, Y.; Réveillère, A. An Insight into Underground Hydrogen Storage in Italy. Sustainability 2023, 15, 6886. [Google Scholar] [CrossRef]
- Saadat, Z.; Farazmand, M.; Sameti, M. Integration of underground green hydrogen storage in hybrid energy generation. Fuel 2024, 371, 131899. [Google Scholar] [CrossRef]
- Mehr, A.S.; Phillips, A.D.; Brandon, M.P.; Pryce, M.T.; Carton, J.G. Recent challenges and development of technical and technoeconomic aspects for hydrogen storage, insights at different scales; A state of art review. Int. J. Hydrogen Energy 2024, 70, 786–815. [Google Scholar] [CrossRef]
- Gianni, E.; Tyrologou, P.; Couto, N.; Carneiro, J.F.; Scholtzová, E.; Koukouzas, N. Underground hydrogen storage. Open Res. Eur. 2024, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shi, X.; Yang, C.; Bai, W.; Wei, X.; Yang, K.; Li, P.; Li, H.; Li, Y.; Wang, G. Site selection evaluation for salt cavern hydrogen storage in China. Renew. Energy 2024, 224, 120143. [Google Scholar] [CrossRef]
- Kaur, M.; Pal, K. Review on hydrogen storage materials and methods from an electrochemical viewpoint. J. Energy Storage 2019, 23, 234–249. [Google Scholar] [CrossRef]
- Pistidda, C. Solid-State Hydrogen Storage for a Decarbonized Society. Hydrogen 2021, 2, 428–443. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.; Haque, M.N.; Islam, S.N.; Hossain, M.A.; Hassan, M.; Roy, H. Advanced Applications of Carbonaceous Materials in Sustainable Water Treatment, Energy Storage, and CO2 Capture: A Comprehensive Review. Sustainability 2023, 15, 8815. [Google Scholar] [CrossRef]
- Sdanghi, G.; Canevesi, R.L.S.; Celzard, A.; Thommes, M.; Fierro, V. Characterization of Carbon Materials for Hydrogen Storage and Compression. C 2020, 6, 46. [Google Scholar] [CrossRef]
- Attia, N.F.; Elashery, S.E.A.; Nour, M.A.; Policicchio, A.; Agostino, R.G.; Abd-Ellah, M.; Jiang, S.; Oh, H. Recent advances in sustainable and efficient hydrogen storage nanomaterials. J. Energy Storage 2024, 100, 113519. [Google Scholar] [CrossRef]
- Kopac, T. Evaluation of recent studies on electrochemical hydrogen storage by graphene-based materials: Impact of modification on overall effectiveness. Int. J. Hydrogen Energy 2024, 69, 777–803. [Google Scholar] [CrossRef]
- Boateng, E.; Thiruppathi, A.R.; Hung, C.K.; Chow, D.; Sridhar, D.; Chen, A. Functionalization of graphene-based nanomaterials for energy and hydrogen storage. Electrochim. Acta 2023, 452, 142340. [Google Scholar] [CrossRef]
- Sutton, A.L.; Mardel, J.I.; Hill, M.R. Metal-Organic Frameworks (MOFs) As Hydrogen Storage Materials at Near-Ambient Temperature. Chem. A Eur. J. 2024, 30, e202400717. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Ahmed, S.; Haq, M.; Alraih, A.M.; Hidouri, T.; Kamyab, H.; Vo, D.-V.N.; Ibrahim, H. Advancements in sorption-based materials for hydrogen storage and utilization: A comprehensive review. Energy 2024, 309, 132855. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Nák, V.Z.; Saldan, I.; Giannakoudakis, D.; Barczak, M.; Pasán, J. Factors Affecting Hydrogen Adsorption in Metal–Organic Frameworks: A Short Review. Nanomaterials 2021, 11, 1638. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Ghoshal, D. Strategies for the Improvement of Hydrogen Physisorption in Metal-Organic Frameworks and Advantages of Flexibility for the Enhancement. J. Mol. Eng. Mater. 2022, 10, 2240003. [Google Scholar] [CrossRef]
- Mishra, A.; Kim, D.; Altahtamouni, T.; Kasak, P.; Popelka, A.; Park, H.; Han, D.S. A comparative study on carbon neutral hydrogen carrier production: Formic acid from CO2 vs. ammonia. J. CO2 Util. 2024, 82, 102756. [Google Scholar] [CrossRef]
- Meng, N.; Shao, J.; Li, H.; Wang, Y.; Fu, X.; Liu, C.; Yu, Y.; Zhang, B. Electrosynthesis of formamide from methanol and ammonia under ambient conditions. Nat. Commun. 2022, 13, 5452. [Google Scholar] [CrossRef]
- Zou, J.; Han, N.; Yan, J.; Feng, Q.; Wang, Y.; Zhao, Z.; Fan, J.; Zeng, L.; Li, H.; Wang, H. Electrochemical Compression Technologies for High-Pressure Hydrogen: Current Status, Challenges and Perspective. Electrochem. Energy Rev. 2020, 3, 690–729. [Google Scholar] [CrossRef]
- Marciuš, D.; Kovač, A.; Firak, M. Electrochemical hydrogen compressor: Recent progress and challenges. Int. J. Hydrogen Energy 2022, 47, 24179–24193. [Google Scholar] [CrossRef]
- Pineda-Delgado, J.L.; Menchaca-Rivera, J.A.; Pérez-Robles, J.F.; Aviles-Arellano, L.M.; Chávez-Ramirez, A.U.; Gutierrez, B.C.K.; de Jesús Hernández-Cortes, R.; Rivera, J.G.; Rivas, S. Energetic evaluations of an electrochemical hydrogen compressor. J. Energy Storage 2022, 55, 105675. [Google Scholar] [CrossRef]
- Zängler, W.; Mohseni, M.; Keller, R.; Wessling, M. A tubular electrochemical hydrogen compressor. Int. J. Hydrogen Energy 2024, 66, 48–54. [Google Scholar] [CrossRef]
- Sdanghi, G.; Dillet, J.; Branco, M.; Prouvé, T.; Maranzana, G. An innovative water management system for the electrochemical compression of hydrogen up to 10 MPa. Int. J. Hydrogen Energy 2024, 87, 117–129. [Google Scholar] [CrossRef]
- Nordio, M.; Rizzi, F.; Manzolini, G.; Mulder, M.; Raymakers, L.; Van Sint Annaland, M.; Gallucci, F. Experimental and modelling study of an electrochemical hydrogen compressor. Chem. Eng. J. 2019, 369, 432–442. [Google Scholar] [CrossRef]
- Zou, J.; Jin, Y.; Wen, Z.; Xing, S.; Han, N.; Yao, K.; Zhao, Z.; Chen, M.; Fan, J.; Li, H.; et al. Insights into electrochemical hydrogen compressor operating parameters and membrane electrode assembly degradation mechanisms. J. Power Sources 2021, 484, 229249. [Google Scholar] [CrossRef]
- Habibi, M.; Vahidinasab, V.; Mohammadi-Ivatloo, B.; Aghaei, J.; Taylor, P. Exploring Potential Gains of Mobile Sector-Coupling Energy Systems in Heavily Constrained Networks. IEEE Trans. Sustain. Energy 2022, 13, 2092–2105. [Google Scholar] [CrossRef]
- Hayati, M.M.; Safari, A.; Nazari-Heris, M.; Oshnoei, A. Hydrogen-Incorporated Sector-Coupled Smart Grids: A Systematic Review and Future Concepts. Green Energy Technol. Part F 2024, 2414, 25–58. [Google Scholar] [CrossRef]
- Ramsebner, J.; Haas, R.; Ajanovic, A.; Wietschel, M. The sector coupling concept: A critical review. WIREs Energy Environ. 2021, 10, e396. [Google Scholar] [CrossRef]
- Rainer Hinrichs-Rahlwes, R.; Skowron, A.; Renné, D.; Ceglarz, A.; Fell, H.-J.; Tischler, L.; Binz, S.L.; Marquitan, S.; Sen, S.; Gokarn, K. Sector Coupling: A Key Concept for Accelerating the Energy Transformation; IRENA: Abu Dhabi, United Arab Emirates, 2022. [Google Scholar]
- IRENA. Renewable Energy in District Heating and Cooling: A Sector Roadmap for Remap; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2017; pp. 33–35. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2017/Mar/IRENA_REmap_DHC_Report_2017.pdf (accessed on 30 November 2024).
- Bernath, C.; Deac, G.; Sensfuß, F. Influence of heat pumps on renewable electricity integration: Germany in a European context. Energy Strategy Rev. 2019, 26, 100389. [Google Scholar] [CrossRef]
- Pindoriya, R.M.; Ahuja, V.; Sharma, A.; Singh, A.; Jain, S. An Analytical Review on State-of-the-Art of Green Hydrogen Technology for Fuel Cell Electric Vehicles Applications. In Proceedings of the 2023 IEEE 3rd International Conference on Sustainable Energy and Future Electric Transportation, SeFet 2023, Bhubaneswar, India, 9–12 August 2023; IEEE: Piscataway, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Marouani, I.; Guesmi, T.; Alshammari, B.M.; Alqunun, K.; Alzamil, A.; Alturki, M.; Hadj Abdallah, H. Integration of Renewable-Energy-Based Green Hydrogen into the Energy Future. Processes 2023, 11, 2685. [Google Scholar] [CrossRef]
- Tuluhong, A.; Chang, Q.; Xie, L.; Xu, Z.; Song, T. Current Status of Green Hydrogen Production Technology: A Review. Sustainability 2024, 16, 9070. [Google Scholar] [CrossRef]
- Muhtadi, A.; Pandit, D.; Nguyen, N.; Mitra, J. Distributed Energy Resources Based Microgrid: Review of Architecture, Control, and Reliability. IEEE Trans. Ind. Appl. 2021, 57, 2223–2235. [Google Scholar] [CrossRef]
- Panda, S.; Mohanty, S.; Rout, P.K.; Sahu, B.K.; Parida, S.M.; Kotb, H.; Flah, A.; Tostado-Véliz, M.; Abdul Samad, B.; Shouran, M. An Insight into the Integration of Distributed Energy Resources and Energy Storage Systems with Smart Distribution Networks Using Demand-Side Management. Appl. Sci. 2022, 12, 8914. [Google Scholar] [CrossRef]
- Idries, A.; Krogstie, J.; Rajasekharan, J. Challenges in platforming and digitizing decentralized energy services. Energy Inform. 2022, 5, 8. [Google Scholar] [CrossRef]
- Thanapalan, K.; Constant, E. Overview of Energy Storage Technologies for Excess Renewable ENERGY production. In Proceedings of the WMSCI 2020—24th World Multi-Conference on Systemics, Cybernetics and Informatics, Online, 13–16 September 2020. [Google Scholar]
- Wei, P.; Abid, M.; Adun, H.; Kemena Awoh, D.; Cai, D.; Zaini, J.H.; Bamisile, O. Progress in Energy Storage Technologies and Methods for Renewable Energy Systems Application. Appl. Sci. 2023, 13, 5626. [Google Scholar] [CrossRef]
- Kiehbadroudinezhad, M.; Merabet, A.; Hosseinzadeh-Bandbafha, H. Review of Latest Advances and Prospects of Energy Storage Systems: Considering Economic, Reliability, Sizing, and Environmental Impacts Approach. Clean Technol. 2022, 4, 477–501. [Google Scholar] [CrossRef]
- Sahoo, S.; Timmann, P. Energy Storage Technologies for Modern Power Systems: A Detailed Analysis of Functionalities, Potentials, and Impacts. IEEE Access 2023, 11, 49689–49729. [Google Scholar] [CrossRef]
- Wang, S.; Kong, L.; Cai, G.; Yan, H.; Han, Z.; Liu, C.; Wan, Y.; Yang, S.; Wang, X. Current Status, Challenges and Prospects of Key Application Technologies for Hydrogen Storage in Power System. In Proceedings of the Chinese Society of Electrical Engineering 2023, Beijing, China, 15–17 September 2023; Editorial Office of Proceedings of the CSEE: Beijing, China, 2023. [Google Scholar] [CrossRef]
- Dorel, S.; Lucian, M.; Gheorghe, L.; Cristan, L.G. Green Hydrogen, a Solution for Replacing Fossil Fuels to Reduce CO2 Emissions. Processes 2024, 12, 1651. [Google Scholar] [CrossRef]
- Franco, A.; Rocca, M. Renewable Electricity and Green Hydrogen Integration for Decarbonization of ‘Hard-to-Abate’ Industrial Sectors. Electricity 2024, 5, 471–490. [Google Scholar] [CrossRef]
- Bahnasawy, N.; Al Anany, S.; Allam, N.K. Electrochemical catalysis for the production of green cement: Towards decarbonizing the cement industry. Catal. Sci. Technol. 2024, 14, 4087–4105. [Google Scholar] [CrossRef]
- HYIELD: Europe’s First Industrial-Scale Waste-to-Hydrogen Plant. Available online: https://hyield.eu/ (accessed on 9 September 2024).
- Gallagher, M.J.; Vinod, A.; Dewing, R.A. Blue and Green Hydrogen Production, Distribution, and Supply for the Glass Industry and the Potential Impact of Hydrogen Fuel Blending in Glass Furnaces. Ceram. Trans. 2023, 271, 127–135. [Google Scholar] [CrossRef]
- Zier, M.; Stenzel, P.; Kotzur, L.; Stolten, D. A review of decarbonization options for the glass industry. Energy Convers. Manag. X 2021, 10, 100083. [Google Scholar] [CrossRef]
- Del Rio, D.D.F.; Sovacool, B.K.; Foley, A.M.; Griffiths, S.; Bazilian, M.; Kim, J.; Rooney, D. Decarbonizing the glass industry: A critical and systematic review of developments, sociotechnical systems and policy options. Renew. Sustain. Energy Rev. 2022, 155, 111885. [Google Scholar] [CrossRef]
- Mobarakeh, M.R.; Silva, M.S.; Kienberger, T. Pulp and paper industry: Decarbonisation technology assessment to reach CO2 neutral emissions—An austrian case study. Energies 2021, 14, 1161. [Google Scholar] [CrossRef]
- Moya, J.; Pavel, C. Energy Efficiency and GHG Emissions: Prospective Scenarios for the Pulp and Paper Industry; European Comission: Joint Research Centre, Publications office: Luxembourg, 2018. [Google Scholar] [CrossRef]
- Lipiäinen, S.; Apajalahti, E.L.; Vakkilainen, E. Decarbonization Prospects for the European Pulp and Paper Industry: Different Development Pathways and Needed Actions. Energies 2023, 16, 746. [Google Scholar] [CrossRef]
- Skuibida, O. Green Aluminum: Trends and Prospects. Grail Sci. 2022, 18–19, 165–169. [Google Scholar] [CrossRef]
- Reyes-Bozo, L.; Fúnez-Guerra, C.; Luis Salazar, J.; Vyhmeister, E.; Valdés-González, H.; Jaén Caparrós, M.; Clemente-Jul, C.; Carro-de Lorenzo, F.; de Simón-Martín, M. Green hydrogen integration in aluminum recycling: Techno-economic analysis towards sustainability transition in the expanding aluminum market. Energy Convers. Manag. X 2024, 22, 100548. [Google Scholar] [CrossRef]
- Khalid, M.; Ahmad, F.; Panigrahi, B.K.; Al-Fagih, L. A comprehensive review on advanced charging topologies and methodologies for electric vehicle battery. J. Energy Storage 2022, 53, 105084. [Google Scholar] [CrossRef]
- Singh, S.; Saket, R.K.; Khan, B. A comprehensive state-of-the-art review on reliability assessment and charging methodologies of grid-integrated electric vehicles. IET Electr. Syst. Transp. 2023, 13, e12073. [Google Scholar] [CrossRef]
- Mohideen, M.M.; Subramanian, B.; Sun, J.; Ge, J.; Guo, H.; Radhamani, A.V.; Ramakrishna, S.; Liu, Y. Techno-economic analysis of different shades of renewable and non-renewable energy-based hydrogen for fuel cell electric vehicles. Renew. Sustain. Energy Rev. 2023, 174, 113153. [Google Scholar] [CrossRef]
- Miraftabzadeh, S.M.; Saldarini, A.; Cattaneo, L.; El Ajami, S.; Longo, M.; Foiadelli, F. Comparative analysis of decarbonization of local public transportation: A real case study. Heliyon 2024, 10, e25778. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.R.; Choi, H.; Ahn, J. Well-to-wheel analysis of greenhouse gas emissions for electric vehicles based on electricity generation mix: A global perspective. Transp. Res. D Transp. Environ. 2017, 51, 340–350. [Google Scholar] [CrossRef]
- Alstom Coradia iLint. Available online: https://www.alstom.com/solutions/rolling-stock/alstom-coradia-ilint-worlds-1st-hydrogen-powered-passenger-train (accessed on 8 September 2024).
- Ready for a Railvolution. Available online: https://www.db.com/what-next/responsible-growth/climate-technologies--Klimatechnologien/hydrogen-train--Wasserstoffzug/index?language_id=1 (accessed on 26 December 2024).
- Lopez, G.; Farfan, J.; Breyer, C. Trends in the global steel industry: Evolutionary projections and defossilisation pathways through power-to-steel. J. Clean. Prod. 2022, 375, 134182. [Google Scholar] [CrossRef]
- A Fossil-Free Future—Hybrit. Available online: https://www.hybritdevelopment.se/en/a-fossil-free-future/ (accessed on 9 September 2024).
- Rouwenhorst, K.H.R.; Travis, A.S.; Lefferts, L. 1921–2021: A Century of Renewable Ammonia Synthesis. Sustain. Chem. 2022, 3, 149–171. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Yara Clean Ammonia|Enabling the Hydrogen Economy|Yara International. Available online: https://www.yara.com/yara-clean-ammonia/ (accessed on 9 September 2024).
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9, 580808. [Google Scholar] [CrossRef]
- Reddy, V.J.; Hariram, N.P.; Maity, R.; Ghazali, M.F.; Kumarasamy, S. Sustainable E-Fuels: Green Hydrogen, Methanol and Ammonia for Carbon-Neutral Transportation. World Electr. Veh. J. 2023, 14, 349. [Google Scholar] [CrossRef]
- Sollai, S.; Porcu, A.; Tola, V.; Ferrara, F.; Pettinau, A. Renewable methanol production from green hydrogen and captured CO2: A techno-economic assessment. J. CO2 Util. 2023, 68, 102345. [Google Scholar] [CrossRef]
- Goren, A.Y.; Dincer, I.; Gogoi, S.B.; Boral, P.; Patel, D. Recent developments on carbon neutrality through carbon dioxide capture and utilization with clean hydrogen for production of alternative fuels for smart cities. Int. J. Hydrogen Energy 2024, 19, 551–578. [Google Scholar] [CrossRef]
- Du, L.; Yang, Y.; Zhou, L.; Liu, M. Greenhouse Gas Reduction Potential and Economics of Green Hydrogen via Water Electrolysis: A Systematic Review of Value-Chain-Wide Decarbonization. Sustainability 2024, 16, 4602. [Google Scholar] [CrossRef]
- Kontou, V.; Peppas, A.; Kottaridis, S.; Politi, C.; Karellas, S. Transforming CO2 into Synthetic Fuels: Simulation, and Optimization Analysis of Methanol Production from Industrial Wastes. Eng 2024, 5, 1337–1359. [Google Scholar] [CrossRef]
- REFHYNE—Clean Refinery Hydrogen for Europe. Available online: https://www.refhyne.eu/ (accessed on 9 September 2024).
- Fucci, F.; Perone, C.; La Fianza, G.; Brunetti, L.; Giametta, F.; Catalano, P. Study of a prototype of an advanced mechanical ventilation system with heat recovery integrated by heat pump. Energy Build. 2016, 133, 111–121. [Google Scholar] [CrossRef]
- Tamborrino, A.; Squeo, G.; Leone, A.; Paradiso, V.M.; Romaniello, R.; Summo, C.; Pasqualone, A.; Catalano, P.; Bianchi, B.; Caponio, F. Industrial trials on coadjuvants in olive oil extraction process: Effect on rheological properties, energy consumption, oil yield and olive oil characteristics. J. Food Eng. 2017, 205, 34–46. [Google Scholar] [CrossRef]
- Caponio, F.; Squeo, G.; Brunetti, L.; Pasqualone, A.; Summo, C.; Paradiso, V.M.; Catalano, P.; Bianchi, B. Influence of the feed pipe position of an industrial scale two-phase decanter on extraction efficiency and chemical-sensory characteristics of virgin olive oil. J. Sci. Food Agric. 2018, 98, 4279–4286. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Jing, D.; Zhao, L. Continuous hydrogen production characteristics and environmental analysis of a pilot-scale hydrogen production system by photocatalytic water splitting. Energy Convers. Manag. 2022, 268, 115988. [Google Scholar] [CrossRef]
- Vitta, S. Sustainability of hydrogen manufacturing: A review. RSC Sustain. 2024, 2, 3202–3221. [Google Scholar] [CrossRef]
- Jayachandran, M.; Gatla, R.K.; Flah, A.; Milyani, A.H.; Milyani, H.M.; Blazek, V.; Prokop, L.; Kraiem, H. Challenges and Opportunities in Green Hydrogen Adoption for Decarbonizing Hard-to-Abate Industries: A Comprehensive Review. IEEE Access 2024, 12, 23363–23388. [Google Scholar] [CrossRef]
- H2RES: State-of-the-Art-Green Hydrogen Production. Available online: https://stateofgreen.com/en/solutions/h2res-green-hydrogen-production/ (accessed on 6 September 2024).
- Heavenn—H2 Energy Applications in Valley Environments for Nothern Netherlands. Available online: https://heavenn.org/ (accessed on 6 September 2024).
- NortH2|Kickstarting the Green Hydrogen Economy. Available online: https://www.north2.eu/ (accessed on 6 September 2024).
- Westkueste100.de. Available online: https://www.westkueste100.de/en/ (accessed on 6 September 2024).
- Hydrogen is Vital to Tackling Climate Change—HyDeploy. Available online: https://hydeploy.co.uk/ (accessed on 6 September 2024).
- HyNet North West. Available online: https://hynet.co.uk/ (accessed on 6 September 2024).
- Hydrom—Home. Available online: https://hydrom.om/ (accessed on 6 September 2024).
- Haru Oni. Available online: https://hifglobal.com/haru-oni (accessed on 6 September 2024).
- ACWA POWER|NEOM Green Hydrogen Project. Available online: https://acwapower.com/en/projects/neom-green-hydrogen-project/ (accessed on 6 September 2024).
- Kharel, S.; Shabani, B. Hydrogen as a long-term large-scale energy storage solution to support renewables. Energies 2018, 11, 2825. [Google Scholar] [CrossRef]
- Nguyen, E.; Olivier, P.; Pera, M.C.; Pahon, E.; Roche, R. Impacts of intermittency on low-temperature electrolysis technologies: A comprehensive review. Int. J. Hydrogen Energy 2024, 70, 474–492. [Google Scholar] [CrossRef]
- Park, S.; Shin, Y.; Jeong, E.; Han, M. Techno-economic analysis of green and blue hybrid processes for ammonia production. Korean J. Chem. Eng. 2023, 40, 2657–2670. [Google Scholar] [CrossRef]
- Gharibvand, H.; Gharehpetian, G.B.; Anvari-Moghaddam, A. A survey on microgrid flexibility resources, evaluation metrics and energy storage effects. Renew. Sustain. Energy Rev. 2024, 201, 114632. [Google Scholar] [CrossRef]
- Sorrenti, I.; Rasmussen, T.B.H.; You, S.; Wu, Q. The role of power-to-X in hybrid renewable energy systems: A comprehensive review. Renew. Sustain. Energy Rev. 2022, 165, 112380. [Google Scholar] [CrossRef]
- Cholewa, T.; Semmel, M.; Mantei, F.; Güttel, R.; Salem, O. Process Intensification Strategies for Power-to-X Technologies. ChemEngineering 2022, 6, 13. [Google Scholar] [CrossRef]
- Incer-Valverde, J.; Patiño-Arévalo, L.J.; Tsatsaronis, G.; Morosuk, T. Hydrogen-driven Power-to-X: State of the art and multicriteria evaluation of a study case. Energy Convers. Manag. 2022, 266, 115814. [Google Scholar] [CrossRef]
- Bloess, A.; Schill, W.P.; Zerrahn, A. Power-to-heat for renewable energy integration: A review of technologies, modeling approaches, and flexibility potentials. Appl. Energy 2018, 212, 1611–1626. [Google Scholar] [CrossRef]
- Fambri, G.; Mazza, A.; Guelpa, E.; Verda, V.; Badami, M. Power-to-heat plants in district heating and electricity distribution systems: A techno-economic analysis. Energy Convers. Manag. 2023, 276, 116543. [Google Scholar] [CrossRef]
- Kou, X.; Wang, R.; Du, S.; Xu, Z.; Zhu, X. Heat pump assists in energy transition: Challenges and approaches. DeCarbon 2024, 3, 100033. [Google Scholar] [CrossRef]
- Liu, W.; Wen, F.; Xue, Y. Power-to-gas technology in energy systems: Current status and prospects of potential operation strategies. J. Mod. Power Syst. Clean Energy 2017, 5, 439–450. [Google Scholar] [CrossRef]
- Borge-Diez, D.; Rosales-Asensio, E.; Açıkkalp, E.; Alonso-Martínez, D. Analysis of Power to Gas Technologies for Energy Intensive Industries in European Union. Energies 2023, 16, 538. [Google Scholar] [CrossRef]
- Drünert, S.; Neuling, U.; Zitscher, T.; Kaltschmitt, M. Power-to-Liquid fuels for aviation—Processes, resources and supply potential under German conditions. Appl. Energy 2020, 277, 115578. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamaguchi, M. Ammonia as a hydrogen energy carrier. Int. J. Hydrogen Energy 2022, 47, 22832–22839. [Google Scholar] [CrossRef]
- Andika, R.; Nandiyanto, A.B.D.; Putra, Z.A.; Bilad, M.R.; Kim, Y.; Yun, C.M.; Lee, M. Co-electrolysis for power-to-methanol applications. Renew. Sustain. Energy Rev. 2018, 95, 227–241. [Google Scholar] [CrossRef]
- Mbatha, S.; Everson, R.C.; Musyoka, N.M.; Langmi, H.W.; Lanzini, A.; Brilman, W. Power-to-methanol process: A review of electrolysis, methanol catalysts, kinetics, reactor designs and modelling, process integration, optimisation, and techno-economics. Sustain. Energy Fuels 2021, 5, 3490–3569. [Google Scholar] [CrossRef]
- Hank, C.; Gelpke, S.; Schnabl, A.; White, R.J.; Full, J.; Wiebe, N.; Smolinka, T.; Schaadt, A.; Henning, H.-M.; Hebling, C. Economics & carbon dioxide avoidance cost of methanol production based on renewable hydrogen and recycled carbon dioxide—Power-to-methanol. Sustain. Energy Fuels 2018, 2, 1244–1261. [Google Scholar] [CrossRef]
- Lee, B.; Lee, H.; Lim, D.; Brigljević, B.; Cho, W.; Cho, H.-S.; Kim, C.-H.; Lim, H. Renewable methanol synthesis from renewable H2 and captured CO2: How can power-to-liquid technology be economically feasible? Appl. Energy 2020, 279, 115827. [Google Scholar] [CrossRef]
- Madi, H.; Schildhauer, T.; Moioli, E. Comprehensive analysis of renewable energy integration in decarbonised mobility: Leveraging power-to-X storage with biogenic carbon sources. Energy Convers. Manag. 2024, 321, 119081. [Google Scholar] [CrossRef]
- Murray, P.; Carmeliet, J.; Orehounig, K. Multi-Objective Optimisation of Power-to-Mobility in Decentralised Multi-Energy Systems. Energy 2020, 205, 117792. [Google Scholar] [CrossRef]
- Varvoutis, G.; Lampropoulos, A.; Mandela, E.; Konsolakis, M.; Marnellos, G.E. Recent Advances on CO2 Mitigation Technologies: On the Role of Hydrogenation Route via Green H2. Energies 2022, 15, 4790. [Google Scholar] [CrossRef]
- Skordoulias, N.; Koytsoumpa, E.I.; Karellas, S. Techno-economic evaluation of medium scale power to hydrogen to combined heat and power generation systems. Int. J. Hydrogen Energy 2022, 47, 26871–26890. [Google Scholar] [CrossRef]
- Seck, G.S.; Hache, E.; Sabathier, J.; Guedes, F.; Reigstad, G.A.; Straus, J.; Wolfgang, O.; Ouassou, J.A.; Askeland, M.; Hjorth, I.; et al. Hydrogen and the decarbonization of the energy system in europe in 2050: A detailed model-based analysis. Renew. Sustain. Energy Rev. 2022, 167, 112779. [Google Scholar] [CrossRef]
- Risco-Bravo, A.; Varela, C.; Bartels, J.; Zondervan, E. From green hydrogen to electricity: A review on recent advances, challenges, and opportunities on power-to-hydrogen-to-power systems. Renew. Sustain. Energy Rev. 2024, 189, 113930. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Boretti, A. Towards hydrogen gas turbine engines aviation: A review of production, infrastructure, storage, aircraft design and combustion technologies. Int. J. Hydrogen Energy 2024, 88, 279–288. [Google Scholar] [CrossRef]
- Ali, A.; Houda, M.; Waqar, A.; Khan, M.B.; Deifalla, A.; Benjeddou, O. A review on application of hydrogen in gas turbines with intercooler adjustments. Results Eng. 2024, 22, 101979. [Google Scholar] [CrossRef]
- Mohammed, A.G.; Mansyur, N.; Hasini, H.; Elfeky, K.E.; Wang, Q.; Ali, M.H.; Om, N.I. Review on the ammonia-blend as an alternative fuel for micro gas turbine power generation. Int. J. Hydrogen Energy 2024, 82, 428–447. [Google Scholar] [CrossRef]
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582. [Google Scholar] [CrossRef]
- Marin, G.E.; Mingaleeva, G.R.; Novoselova, M.S.; Akhmetshin, A.R. Adding hydrogen fuel to the synthesis gas for the possibility of combustion in a gas turbine. Int. J. Hydrogen Energy 2024, 96, 378–384. [Google Scholar] [CrossRef]
- Ghazal, R.M.; Akroot, A.; Wahhab, H.A.A.; Alhamd, A.E.J.; Hamzah, A.H.; Bdaiwi, M. The Influence of Gas Fuel Enrichment with Hydrogen on the Combustion Characteristics of Combustors: A Review. Sustainability 2024, 16, 9423. [Google Scholar] [CrossRef]
- Gondal, I.A. Hydrogen integration in power-to-gas networks. Int. J. Hydrogen Energy 2019, 44, 1803–1815. [Google Scholar] [CrossRef]
- Klatzer, T.; Bachhiesl, U.; Wogrin, S. State-of-the-art expansion planning of integrated power, natural gas, and hydrogen systems. Int. J. Hydrogen Energy 2022, 47, 20585–20603. [Google Scholar] [CrossRef]
- Neacsa, A.; Eparu, C.N.; Stoica, D.B. Hydrogen–Natural Gas Blending in Distribution Systems—An Energy, Economic, and Environmental Assessment. Energies 2022, 15, 6143. [Google Scholar] [CrossRef]
- Cristello, J.B.; Yang, J.M.; Hugo, R.; Lee, Y.; Park, S.S. Feasibility analysis of blending hydrogen into natural gas networks. Int. J. Hydrogen Energy 2023, 48, 17605–17629. [Google Scholar] [CrossRef]
- Woods, P.; Bustamante, H.; Aguey-Zinsou, K.F. The hydrogen economy—Where is the water? Energy Nexus 2022, 7, 100123. [Google Scholar] [CrossRef]
- Squadrito, G.; Maggio, G.; Nicita, A. The green hydrogen revolution. Renew. Energy 2023, 216, 119041. [Google Scholar] [CrossRef]
- Kabir, M.M.; Akter, M.M.; Huang, Z.; Tijing, L.; Shon, H.K. Hydrogen production from water industries for a circular economy. Desalination 2023, 554, 116448. [Google Scholar] [CrossRef]
- Lokesh, S.; Srivastava, R. Advanced Two-Dimensional Materials for Green Hydrogen Generation: Strategies toward Corrosion Resistance Seawater Electrolysis─Review and Future Perspectives. Energy Fuels 2022, 36, 13417–13450. [Google Scholar] [CrossRef]
- Badea, G.E.; Hora, C.; Maior, I.; Cojocaru, A.; Secui, C.; Filip, S.M.; Dan, F.C. Sustainable Hydrogen Production from Seawater Electrolysis: Through Fundamental Electrochemical Principles to the Most Recent Development. Energies 2022, 15, 8560. [Google Scholar] [CrossRef]
- Mishra, A.; Park, H.; El-Mellouhi, F.; Suk Han, D. Seawater electrolysis for hydrogen production: Technological advancements and future perspectives. Fuel 2024, 361, 130636. [Google Scholar] [CrossRef]
- Varras, G.; Chalaris, M. Critical Review of Hydrogen Production via Seawater Electrolysis and Desalination: Evaluating Current Practices. J. Electrochem. Energy Convers. Storage 2024, 21, 044001. [Google Scholar] [CrossRef]
- Shah, M.; Patel, C.; Patel, K. A Comprehensive Study on Hydrogen Gas Production Using Renewable Energy Sources. In Green Hydrogen for Environmental Sustainability; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2024; pp. 175–197. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A review on the role, cost and value of hydrogen energy systems for deep decarbonisation. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Industrial decarbonization via hydrogen: A critical and systematic review of developments, socio-technical systems and policy options. Energy Res. Soc. Sci. 2021, 80, 102208. [Google Scholar] [CrossRef]
- Khalid, M. Smart grids and renewable energy systems: Perspectives and grid integration challenges. Energy Strategy Rev. 2024, 51, 101299. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J. Revolution in Renewables: Integration of Green Hydrogen for a Sustainable Future. Energies 2024, 17, 4148. [Google Scholar] [CrossRef]
- Alzahrani, A.; Ramu, S.K.; Devarajan, G.; Vairavasundaram, I.; Vairavasundaram, S. A Review on Hydrogen-Based Hybrid Microgrid System: Topologies for Hydrogen Energy Storage, Integration, and Energy Management with Solar and Wind Energy. Energies 2022, 15, 7979. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Quainoo, K.A.; Hamzat, A.K.; Darko, C.K.; Agyemang, C.K. Innovative Strategies for Combining Solar and Wind Energy with Green Hydrogen Systems. Appl. Sci. 2024, 14, 9771. [Google Scholar] [CrossRef]
- Akinsooto, O.; Ogundipe, O.B.; Ikemba, S. Strategic policy initiatives for optimizing hydrogen production and storage in sustainable energy systems. Int. J. Frontline Res. Rev. 2024, 2, 1–21. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Tao, Y.; Qiu, J.; Lai, S.; Zhao, J. Integrated Electricity and Hydrogen Energy Sharing in Coupled Energy Systems. IEEE Trans. Smart Grid 2021, 12, 1149–1162. [Google Scholar] [CrossRef]
- Tao, Y.; Qiu, J.; Lai, S.; Sun, X. Coordinated Planning of Electricity and Hydrogen Networks with Hydrogen Supply Chain for Fuel Cell Electric Vehicles. IEEE Trans. Sustain. Energy 2023, 14, 1010–1023. [Google Scholar] [CrossRef]
- Sun, X.; Cao, X.; Zeng, B.; Zhai, Q.; Guan, X. Multistage Dynamic Planning of Integrated Hydrogen-Electrical Microgrids under Multiscale Uncertainties. IEEE Trans. Smart Grid 2023, 14, 3482–3498. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Hamzat, A.K.; Whidborne, J.; Kuang, B.; Jenkins, K.W. Integration of renewable energy sources in tandem with electrolysis: A technology review for green hydrogen production. Int. J. Hydrogen Energy, 2024; in press. [Google Scholar] [CrossRef]
- Astriani, Y.; Tushar, W.; Nadarajah, M. Optimal planning of renewable energy park for green hydrogen production using detailed cost and efficiency curves of PEM electrolyzer. Int. J. Hydrogen Energy 2024, 79, 1331–1346. [Google Scholar] [CrossRef]
- Abdelsalam, R.A.; Mohamed, M.; Farag, H.E.Z.; El-Saadany, E.F. Green hydrogen production plants: A techno-economic review. Energy Convers. Manag. 2024, 319, 118907. [Google Scholar] [CrossRef]
- Patonia, A.; Poudineh, R. Cost-Competitive Green Hydrogen: How to Lower the Cost of Electrolysers? The Oxford Institute for Energy Studies: Oxford, UK, 2022. [Google Scholar]
- Cheng, C.; Hughes, L. The role for offshore wind power in renewable hydrogen production in Australia. J. Clean. Prod. 2023, 391, 136223. [Google Scholar] [CrossRef]
- Zun, M.T.; McLellan, B.C. Cost Projection of Global Green Hydrogen Production Scenarios. Hydrogen 2023, 4, 932–960. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Sun, H.; Yang, L.; Huang, L. Advancements and Policy Implications of Green Hydrogen Production from Renewable Sources. Energies 2024, 17, 3548. [Google Scholar] [CrossRef]
- Javanshir, N.; Pekkinen, S.; Santasalo-Aarnio, A.; Syri, S. Green hydrogen and wind synergy: Assessing economic benefits and optimal operational strategies. Int. J. Hydrogen Energy 2024, 83, 811–825. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Tashie-Lewis, B.C.; Nnabuife, S.G. Production, Hydrogen Distribution, Storage and Power Conversion in a Hydrogen Economy—A Technology Review. Chem. Eng. J. Adv. 2021, 8, 100172. [Google Scholar] [CrossRef]
- Zhang, T.; Uratani, J.; Huang, Y.; Xu, L.; Griffiths, S.; Ding, Y. Hydrogen liquefaction and storage: Recent progress and perspectives. Chem. Eng. J. Adv. 2023, 8, 100172. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, R.; Shi, Z.; Lin, J. Review of common hydrogen storage tanks and current manufacturing methods for aluminium alloy tank liners. Int. J. Lightweight Mater. Manuf. 2024, 7, 269–284. [Google Scholar] [CrossRef]
- Pawelczyk, E.; Łukasik, N.; Wysocka, I.; Rogala, A.; Gębicki, J. Recent Progress on Hydrogen Storage and Production Using Chemical Hydrogen Carriers. Energies 2022, 15, 4964. [Google Scholar] [CrossRef]
- Lipiäinen, S.; Lipiäinen, K.; Ahola, A.; Vakkilainen, E. Use of existing gas infrastructure in European hydrogen economy. Int. J. Hydrogen Energy 2023, 48, 31317–31329. [Google Scholar] [CrossRef]
- Takach, M.; Sarajlić, M.; Peters, D.; Kroener, M.; Schuldt, F.; Von Maydell, K. Review of Hydrogen Production Techniques from Water Using Renewable Energy Sources and Its Storage in Salt Caverns. Energies 2022, 15, 1415. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Abbasi, G.R.; Lashari, N.; Patil, S.; Abdurrahman, M. A Mini-Review on Underground Hydrogen Storage: Production to Field Studies. Energy Fuels 2023, 37, 8128–8141. [Google Scholar] [CrossRef]
- Patanwar, Y.K.; Kim, H.M.; Deb, D.; Gujjala, Y.K. Underground storage of hydrogen in lined rock caverns: An overview of key components and hydrogen embrittlement challenges. Int. J. Hydrogen Energy 2024, 50, 116–133. [Google Scholar] [CrossRef]
- Belkadi, M.; Smaili, A. Thermal analysis of a multistage active magnetic regenerator cycle for hydrogen liquefaction. Int. J. Hydrogen Energy 2018, 43, 3499–3511. [Google Scholar] [CrossRef]
- Kamiya, K.; Matsumoto, K.; Numazawa, T.; Masuyama, S.; Takeya, H.; Saito, A.T.; Kumazawa, N.; Futatsuka, K.; Matsunaga, K.; Shirai, T. Active magnetic regenerative refrigeration using superconducting solenoid for hydrogen liquefaction. Appl. Phys. Express 2022, 15, 053001. [Google Scholar] [CrossRef]
- Öberg, S.; Odenberger, M.; Johnsson, F. Exploring the competitiveness of hydrogen-fueled gas turbines in future energy systems. Int. J. Hydrogen Energy 2022, 47, 624–644. [Google Scholar] [CrossRef]
- Antweiler, W.; Schlund, D. The emerging international trade in hydrogen: Environmental policies, innovation, and trade dynamics. J. Environ. Econ. Manag. 2024, 127, 103035. [Google Scholar] [CrossRef]
- Jakob, M.; Overland, I. Green industrial policy can strengthen carbon pricing but not replace it. Energy Res. Soc. Sci. 2024, 116, 103669. [Google Scholar] [CrossRef]
- Li, W.; Tian, H.; Ma, L.; Wang, Y.; Liu, X.; Gao, X. Low-temperature water electrolysis: Fundamentals, progress; new strategies. Mater. Adv. 2022, 3, 5598–5644. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Begum, R.A.; Hossain, M.J.; Ker, P.J.; Mahlia, T.M.I. Hydrogen electrolyser technologies and their modelling for sustainable energy production: A comprehensive review and suggestions. Int. J. Hydrogen Energy 2023, 48, 27841–27871. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Fursikov, P.V.; Volodin, A.A.; Bocharnikov, M.S.; Shimkus, Y.Y.; Kashin, A.M.; Yartys, V.A.; Chidziva, S.; Pasupathi, S.; Lototskyy, M.V. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int. J. Hydrogen Energy 2021, 46, 13647–13657. [Google Scholar] [CrossRef]
- Klopčič, N.; Grimmer, I.; Winkler, F.; Sartory, M.; Trattner, A. A review on metal hydride materials for hydrogen storage. J. Energy Storage 2023, 72, 108456. [Google Scholar] [CrossRef]
- Rothmund, E.V.; He, J.; Zhang, Z.; Xiao, S. Revealing the critical pore size for hydrogen storage via simultaneous enclathration and physisorption in activated carbon. J. Mater. Chem. A Mater. 2024, 12, 21830–21844. [Google Scholar] [CrossRef]
- Dornheim, M.; Baetcke, L.; Akiba, E.; Ares, J.-R.; Autrey, T.; Barale, J.; Baricco, M.; Brooks, K.; Chalkiadakis, N.; Charbonnier, V. Research and development of hydrogen carrier based solutions for hydrogen compression and storage. Prog. Energy 2022, 4, 042005. [Google Scholar] [CrossRef]
- Saadat, N.; Dhakal, H.N.; Tjong, J.; Jaffer, S.; Yang, W.; Sain, M. Recent advances and future perspectives of carbon materials for fuel cell. Renew. Sustain. Energy Rev. 2021, 138, 110535. [Google Scholar] [CrossRef]
- Caponi, R.; Bocci, E.; Del Zotto, L. Techno-Economic Model for Scaling Up of Hydrogen Refueling Stations. Energies 2022, 15, 7518. [Google Scholar] [CrossRef]
- Genovese, M.; Fragiacomo, P. Hydrogen refueling station: Overview of the technological status and research enhancement. J. Energy Storage 2023, 61, 106758. [Google Scholar] [CrossRef]
- Amirthan, T.; Perera, M.S.A. The role of storage systems in hydrogen economy: A review. J. Energy Storage 2022, 61, 106758. [Google Scholar] [CrossRef]


| Hydrogen Type | CO2 Emissions (kg CO2/kg H2) | Direct H2O Usage (L/kg H2) 1 | Process H2O Usage (L/kg H2) 2 | Energy Requirement (kWh/kg H2) | Cost ($/kg H2) |
|---|---|---|---|---|---|
| Black/Brown | 19–20 | 2–4 | 150–200 | 30–40 | 1–2 |
| Gray | 9–10 | 4 | 100–150 | 40–50 | 1–2 |
| Blue | 1–2 | 4 | 100–150 | 40–50 | 1.5–3 |
| Turquoise | 0 (solid C) | 2 | 50 | 20–30 | 2–3 |
| Purple | 0 | 9 | 250–300 | 50–55 | 3–5 |
| Green | 0 | 9 | 100–200 | 50–55 | 4–6 |
| Electrolysis Type | Faradaic Efficiency (%) | Hydrogen Purity (%) | Cost ($/kg H2) | Key Advantages | Key Limitations |
|---|---|---|---|---|---|
| Proton Exchange Membrane (PEM) | 85–95 | 99.99 | 3–7 | high purity, compact design | expensive catalysts (Pt, Ir) |
| Alkaline Water Electrolysis (AWE) | 95–99 | 99.5 | 2.5–6.5 | mature, low-cost materials | larger footprint, slower response |
| Solid Oxide Electrolysis (SOEC) | 90–97 | 99.9 | 2.7–6 | high efficiency uses heat energy | high operating temperature (>700 °C) |
| Anion Exchange Membrane (AEM) | 90–96 | 99.8 | 3–7.5 | potential for low-cost catalysts | less mature technology |
| Membrane Type | Material | Conductivity (S/cm) | Durability (hours) | Operating Temp (°C) | Cost ($/m2) | Notes |
|---|---|---|---|---|---|---|
| PEM | perfluorosulfonic acid (PFSA) | 0.1–0.2 | >20,000 | 60–80 | 800–1200 | High efficiency, expensive materials |
| AWE | alkaline polymer | 0.05–0.15 | 10,000–15,000 | 30–60 | 150–300 | Low-cost catalysts, less mature tech. |
| SOE | Yttria-stabilized zirconia | 0.01–0.05 | >30,000 | 700–900 | 1000–1500 | High temperature, excellent durability |
| composite membranes | hybrid organic-inorganic | 0.1–0.15 | ~15,000 | 50–100 | 500–800 | Improved thermal stability and mechanical support |
| Product | Without GH2 (Liters per kg or MJ) | With GH2 (Liters per kg or MJ) |
|---|---|---|
| Gasoline | ~1.5 L/MJ | ~11 L/MJ (including water for GH2) |
| Aluminum | ~3000 L/kg (electricity-intensive) | ~3500 L/kg (if powered with GH2) |
| Cement | ~100 L/kg (mineral processing and cooling) | ~150 L/kg (for high-temp GH2 processes) |
| Steel | ~140 L/kg (blast furnace processes) | ~200 L/kg (if GH2 replaces coke/coal) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelico, R.; Giametta, F.; Bianchi, B.; Catalano, P. Green Hydrogen for Energy Transition: A Critical Perspective. Energies 2025, 18, 404. https://doi.org/10.3390/en18020404
Angelico R, Giametta F, Bianchi B, Catalano P. Green Hydrogen for Energy Transition: A Critical Perspective. Energies. 2025; 18(2):404. https://doi.org/10.3390/en18020404
Chicago/Turabian StyleAngelico, Ruggero, Ferruccio Giametta, Biagio Bianchi, and Pasquale Catalano. 2025. "Green Hydrogen for Energy Transition: A Critical Perspective" Energies 18, no. 2: 404. https://doi.org/10.3390/en18020404
APA StyleAngelico, R., Giametta, F., Bianchi, B., & Catalano, P. (2025). Green Hydrogen for Energy Transition: A Critical Perspective. Energies, 18(2), 404. https://doi.org/10.3390/en18020404







