Recovered Ammonia as a Sustainable Energy Carrier: Innovations in Recovery, Combustion, and Fuel Cells
Abstract
1. Introduction
2. Main Sources of Ammonia Recovery from Waste
- (A)
- Concentration of ammoniacal nitrogen (N-NH3) < 100 mg/L
- (B)
- Concentrations of N-NH3 between 100 and 500 mg/L
- (C)
- Concentrations of N-NH3 between 500 and 2000 mg/L
- (D)
- Concentrations of N-NH3 > 2000 mg/L
2.1. Landfill Leachate
2.2. Biogas Digestate Liquid Fraction
3. Ammonia Recovery Technologies and Circular Solutions
3.1. Liquid-Phase Ammonia Recovery
3.2. Gas-Phase Ammonia Recovery
3.3. Technical Comparison of Liquid-Phase and Gas-Phase Ammonia Recovery Technologies
- High COD levels can significantly impact the performance of membrane technologies. Traditional polymer membranes, such as cellulose acetate, often experience fouling under elevated COD conditions, leading to recovery efficiencies below 60%. Advanced membranes, such as graphene oxide-enhanced nanofiltration systems, demonstrate superior fouling resistance and maintain ammonia retention rates of up to 89% even in wastewaters with COD concentrations exceeding 1000 mg/L [103].
- Adsorbent technologies are highly sensitive to pH variability. For example, natural zeolites experience a 40% reduction in ammonium uptake capacity when the pH shifts from neutral to acidic (<6), as their ion-exchange capabilities are diminished. Similarly, biochars optimized for neutral conditions show reduced adsorption capacities in alkaline environments where ammonium is converted to ammonia gas, decreasing their efficiency [13,96].
- Ammonia stripping remains a robust technology for liquid-phase recovery, with its performance less influenced by COD levels. By elevating pH above 10 and operating at temperatures exceeding 60 °C, stripping can achieve recovery efficiencies above 90%, even in waste streams with high organic loads. Advanced configurations, such as rotating packed beds, further enhance gas–liquid interactions and mass transfer. However, streams with low ammonia concentrations (<200 mg/L TAN) can exhibit inefficiencies, requiring additional energy input to maintain effective volatilization [85].
- Gas-phase technologies, while effective, are hindered by high humidity levels. For instance, the ammonia adsorption capacity of activated carbons drops from 73 mg NH3/g in dry conditions to 22 mg NH3/g under relative humidity exceeding 70%. Even advanced materials such as metal–organic frameworks (MOFs) face capacity reductions of 15–20% under similar conditions [116].
3.4. Advancing Ammonia Recovery Within a Circular Economy Framework
4. Recovered Ammonia Uses
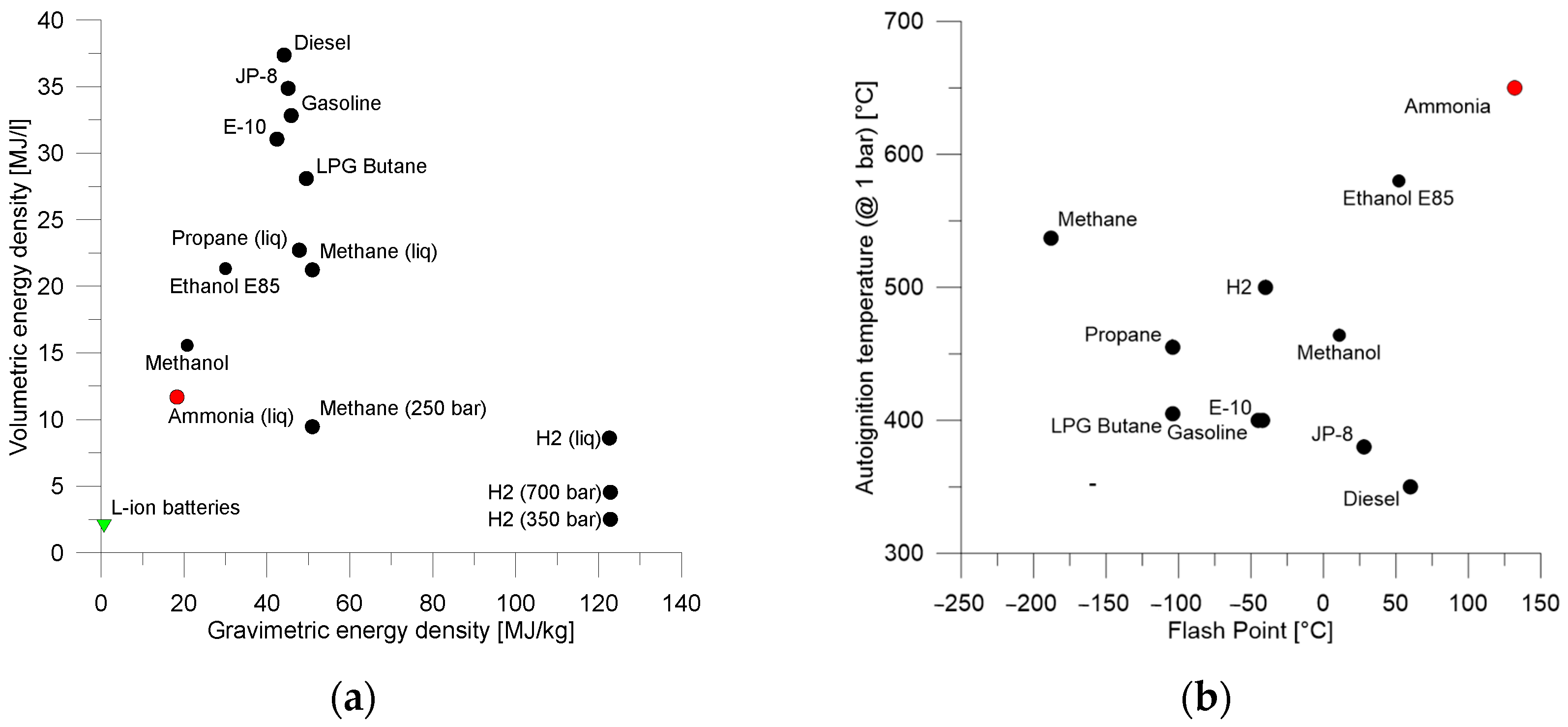
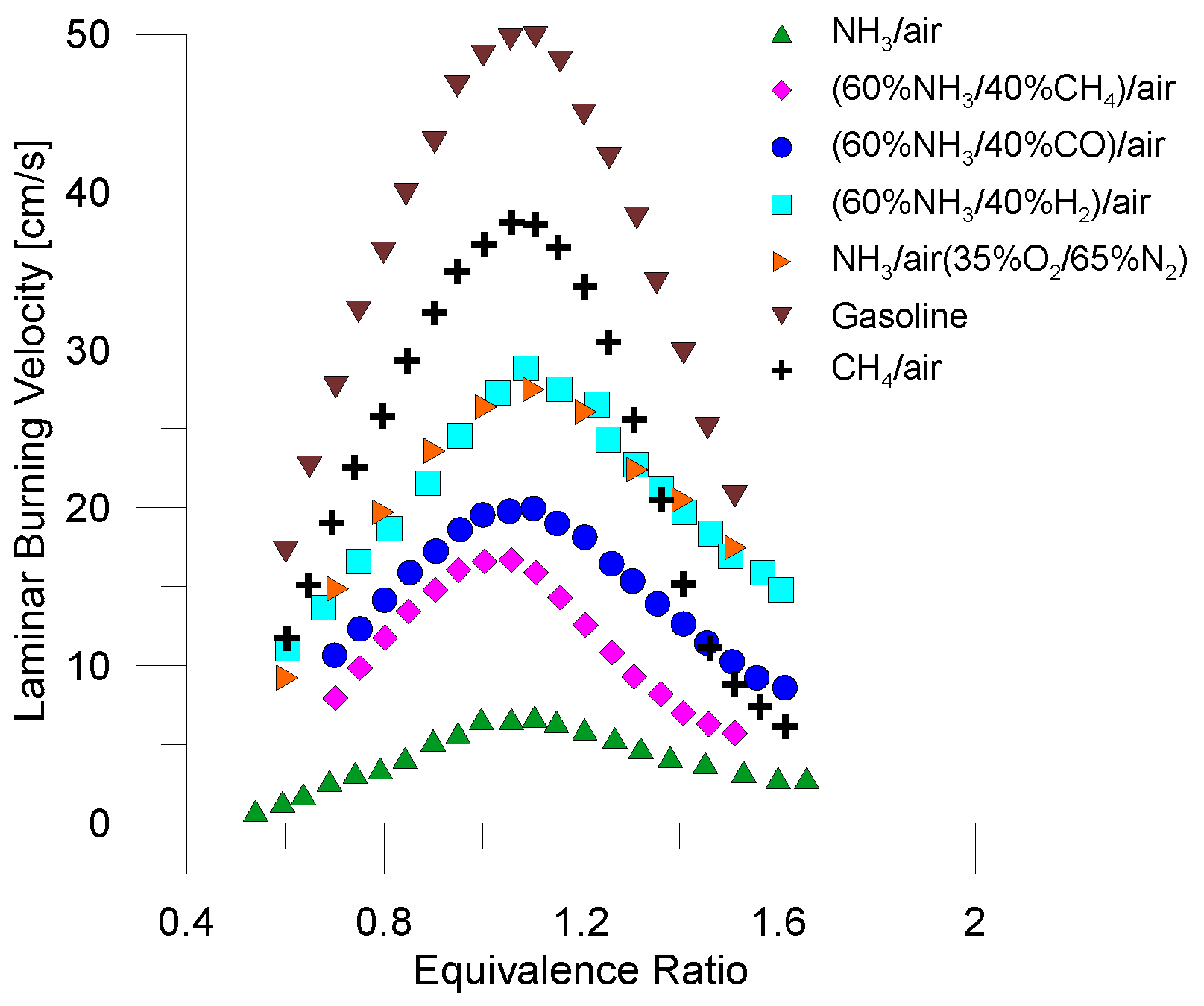
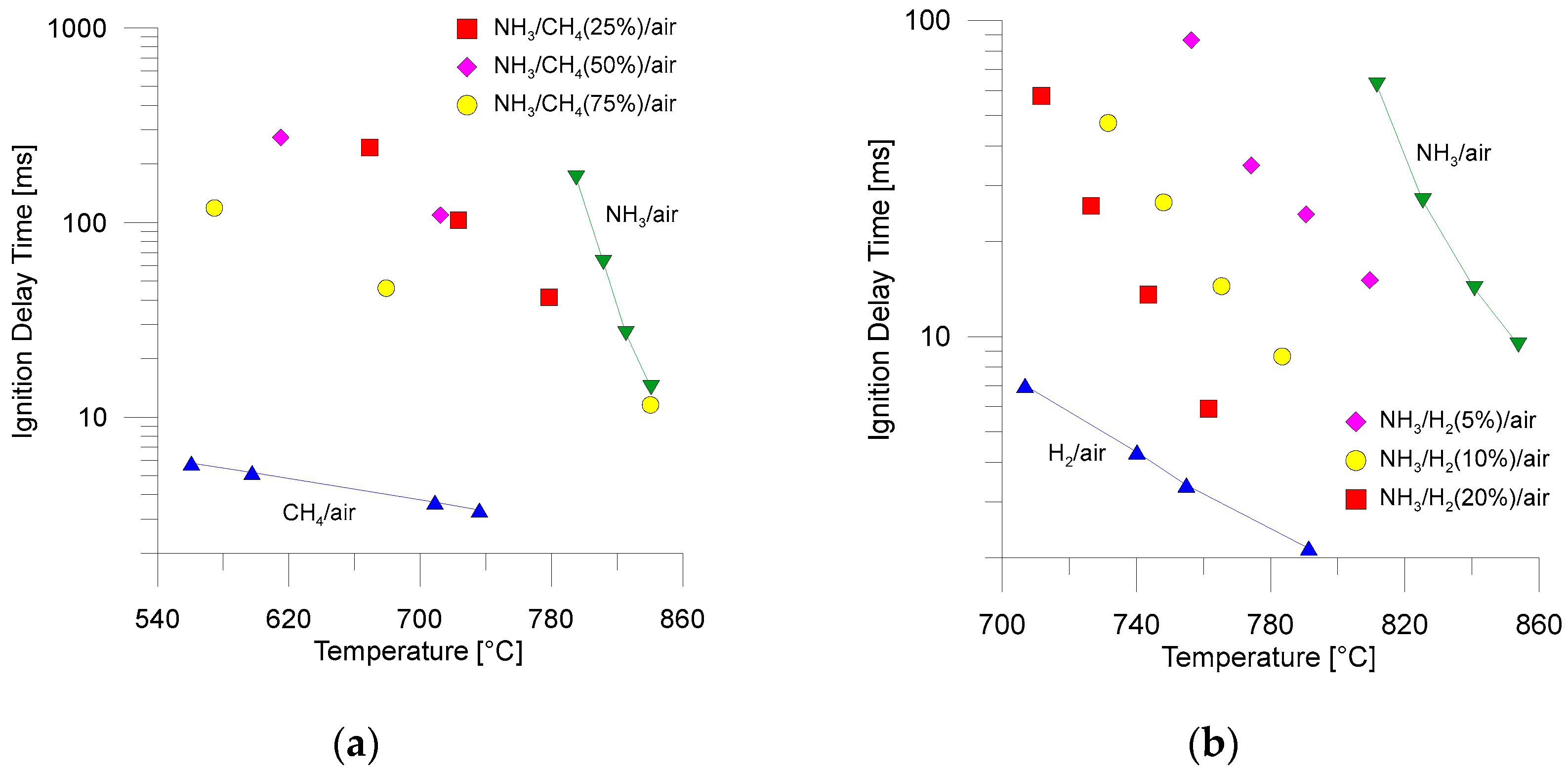
4.1. Direct Combustion
- AEGL-1 is the airborne concentration causing notable discomfort to the general population, set at 30 ppm regardless of exposure duration. All effects are fully reversible after exposure ceases.
- AEGL-2 represents concentrations at varying exposure times that may cause irreversible or serious, long-lasting adverse health effects, or impair the ability to escape.
- AEGL-3 represents the airborne concentrations above which the general population, including susceptible individuals, could experience life-threatening health effects or death.
4.2. Ammonia Cracking
4.2.1. Ruthenium-Based Catalysts
4.2.2. Nickel-Based and Alternative Metal Catalysts
4.2.3. Advanced Composite Catalysts
4.2.4. Reactor Technology
4.2.5. Purity and Operational Considerations in Ammonia Cracking
4.2.6. Environmental and Economic Impacts
4.2.7. Integrated Energy Use of Recovered Ammonia from Waste
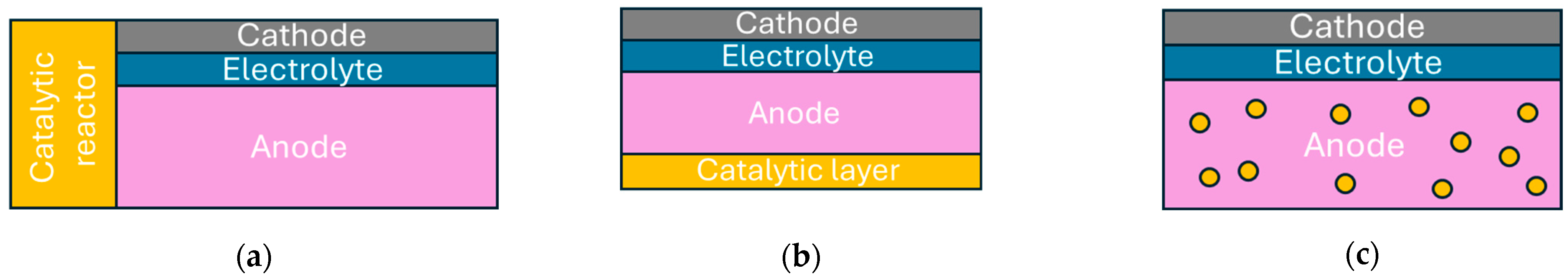
4.3. Direct Ammonia Fuel Cells
4.3.1. Alkaline Electrolyte Fuel Cells
4.3.2. Solid Electrolyte Fuel Cells
Solid-Oxide Fuel Cells (SOFCs)
Proton-Conducting Fuel Cells (PCFCs)
4.3.3. Semiconductor Fuel Cells
4.3.4. Microbial Fuel Cells
4.4. Case Studies on the Experiment of Recovered Ammonia Reuses
4.4.1. Direct Combustion and Direct Reuse of Ammonia
4.4.2. Fuel Cells
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shanghai Merchant Ship Design & Research Institute. 2019. Available online: https://www.linkedin.com/posts/shanghai-merchant-ship-design-%26-researchinstitute_180k-dwt-bc-of-carbon-free-issued-and-obtained-activity-6609776461731717120-oZtk/ (accessed on 20 December 2019).
- van Linden, N.; Wang, Y.; Sudhölter, E.; Spanjers, H.; van Lier, J.B. Selectivity of vacuum ammonia stripping using porous gas-permeable and dense pervaporation membranes under various hydraulic conditions and feed water compositions. J. Memb. Sci. 2022, 642, 120005. [Google Scholar] [CrossRef]
- Iskander, S.M.; Brazil, B.; Novak, J.T.; He, Z. Resource recovery from landfill leachate using bioelectrochemical systems: Opportunities, challenges, and perspectives. Bioresour. Technol. 2016, 201, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, H. Treatment and recovery methods for leachate concentrate from landfill and incineration: A state-of-the-art review. J. Clean. Prod. 2021, 329, 129720. [Google Scholar] [CrossRef]
- Haslina, H.; NorRuwaida, J.; Dewika, M.; Rashid, M.; Md Ali, A.H.; Khairunnisa, M.P.; Afiq Daniel Azmi, M. Landfill Leachate Treatment Methods and Its Potential for Ammonia Removal and Recovery—A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012064. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Acute Exposure Guideline Levels. Acute Exposure Guideline Levels for Selected Chemicals: Volume 6; National Academies Press (US): Washington, DC, USA, 2008. [Google Scholar]
- Nghiem, L.D.; Hai, F.I.; Listowski, A. Water reclamation and nitrogen extraction from municipal solid waste landfill leachate. Desalination Water Treat. 2016, 57, 29220–29227. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Pilot plant for the capture of ammonia from the atmosphere of pig and poultry farms using gas-permeable membrane technology. Membranes 2021, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Serdarevic, A. Landfill Leachate Management—Control and Treatment. In Advanced Technologies, Systems, and Applications II. IAT 2017; Lecture Notes in Networks and Systems; Springer: Cham, Switzerland, 2018; Volume 28, pp. 618–632. [Google Scholar] [CrossRef]
- Abbas, A.A.; Jingsong, G.; Ping, L.Z.; Ya, P.Y.; Al-Rekabi, W.S. Review on Landfill Leachate Treatments. Am. J. Appl. Sci. 2009, 6, 672–684. [Google Scholar] [CrossRef]
- Available online: https://www.eea.europa.eu/highlights/eu-met-air-pollution-limits (accessed on 10 December 2024).
- Available online: https://www.clean-air-farming.eu/en/background/eu-policies (accessed on 10 December 2024).
- Gu, N.; Liu, J.; Ye, J.; Chang, N.; Li, Y.Y. Bioenergy, ammonia and humic substances recovery from municipal solid waste leachate: A review and process integration. Bioresour. Technol. 2019, 293, 122159. [Google Scholar] [CrossRef] [PubMed]
- Karmann, C.; Mágrová, A.; Jeníček, P.; Bartáček, J.; Kouba, V. Advances in nitrogen removal and recovery technologies from reject water: Economic and environmental perspectives. Bioresour. Technol. 2024, 391, 129888. [Google Scholar] [CrossRef]
- Ikäheimo, J.; Kiviluoma, J.; Weiss, R.; Holttinen, H. Power-to-ammonia in future North European 100% renewable power and heat system. Int. J. Hydrogen Energy 2018, 43, 17295–17308. [Google Scholar] [CrossRef]
- Grasham, O.; Dupont, V.; Camargo-Valero, M.A.; García-Gutiérrez, P.; Cockerill, T. Combined ammonia recovery and solid oxide fuel cell use at wastewater treatment plants for energy and greenhouse gas emission improvements. Appl. Energy 2019, 240, 698–708. [Google Scholar] [CrossRef]
- Grasham, O.; Dupont, V.; Cockerill, T.; Camargo-Valero, M.A.; Twigg, M.V. Hydrogen: Via reforming aqueous ammonia and biomethane co-products of wastewater treatment: Environmental and economic sustainability. Sustain. Energy Fuels 2020, 4, 5835–5850. [Google Scholar] [CrossRef]
- Powders, M.T.; Luqmani, B.A.; Pidou, M.; Zhu, M.; McAdam, E.J. The use of ammonia recovered from wastewater as a zero-carbon energy vector to decarbonise heat, power and transport—A review. Water Res. 2025, 268, 122649. [Google Scholar] [CrossRef] [PubMed]
- Moosazadeh, M.; Mansourimarand, A.; Ajori, S.; Taghikhani, V.; Yoo, C.K. Waste-to-Ammonia: A sustainable pathway for energy transition. Renew. Sustain. Energy Rev. 2025, 208, 115012. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Dincer, I.; Erdemir, D.; Aydin, M.I.; Karasu, H.; Vezina, G. Ammonia Energy Technologies; Springer International Publishing: Cham, Switzerland, 2022; Volume 91. [Google Scholar] [CrossRef]
- Gill, S.S.; Chatha, G.S.; Tsolakis, A.; Golunski, S.E.; York, A.P.E. Assessing the effects of partially decarbonising a diesel engine by co-fuelling with dissociated ammonia. Int. J. Hydrogen Energy 2012, 37, 6074–6083. [Google Scholar] [CrossRef]
- Cross-ministerial Strategic Innovation Promotion Program (SIP). 2015 Energy Carriers. Available online: http://www.jst.go.jp/sip/pdf/SIP_energycarriers2015_en.pdf (accessed on 14 October 2019).
- Renewable Energy to Fuels Through Utilization of Energy-Dense Liquids (REFUEL) Program Overview B. PROGRAM OVERVIEW. Available online: https://arpa-e.energy.gov/sites/default/files/documents/files/REFUEL_ProgramOverview.pdf (accessed on 10 December 2024).
- Inrirai, P.; Keogh, J.; Centeno-Pedrazo, A.; Artioli, N.; Manyar, H. Recent advances in processes and catalysts for glycerol carbonate production via direct and indirect use of CO2. J. CO2 Util. 2024, 80, 102693. [Google Scholar] [CrossRef]
- International Energy Agency I. International Energy Agency. 2017 Renewable Energy for Industry. Available online: https://www.iea.org/publications/insights/insightpublications/Renewable_Energy_for_Industry.pdf (accessed on 24 October 2019).
- Tiwari, S.; Pekris, M.J.; Doherty, J.J. A review of liquid hydrogen aircraft and propulsion technologies. Int. J. Hydrogen Energy 2024, 57, 1174–1196. [Google Scholar] [CrossRef]
- Beler-Baykal, B.; Allar, A.D. Upgrading fertilizer production wastewater effluent quality for ammonium discharges through ion exchange with clinoptilolite. Environ. Technol. 2008, 29, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Ibrahim, A.M.; Al-Sulaiman, A.M.; Okasha, R.A. Landfill leachate: Sources, nature, organic composition, and treatment: An environmental overview. Ain Shams Eng. J. 2024, 15, 102293. [Google Scholar] [CrossRef]
- Cerrillo, M.; Riau, V.; Bonmatí, A. Recent Advances in Bioelectrochemical Systems for Nitrogen and Phosphorus Recovery Using Membranes. Membranes 2023, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.; Porto, R.F.; Quintaes, B.R.; Bila, D.M.; Lavagnolo, M.C.; Campos, J.C. A review on membrane concentrate management from landfill leachate treatment plants: The relevance of resource recovery to close the leachate treatment loop. Waste Manag. Res. 2023, 41, 264–284. [Google Scholar] [CrossRef]
- Ersahin, M.E.; Cicekalan, B.; Cengiz, A.I.; Zhang, X.; Ozgun, H. Nutrient recovery from municipal solid waste leachate in the scope of circular economy: Recent developments and future perspectives. J. Environ. Manag. 2023, 335, 117518. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Wang, J.; Cui, D.; Dong, X.; Tang, C.; Zhang, L.; Yue, D. Recent Advances of Landfill Leachate Treatment. J. Indian. Inst. Sci. 2021, 101, 685–724. [Google Scholar] [CrossRef]
- Ilmasari, D.; Sahabudin, E.; Riyadi, F.A.; Abdullah, N.; Yuzir, A. Future trends and patterns in leachate biological treatment research from a bibliometric perspective. J. Environ. Manag. 2022, 318, 115594. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, A.; Said, M.I.M.; Azman, S.B.; Dandajeh, A.A.; Lemar, G.S.; Jagun, Z.T. Utilization of microbial fuel cells as a dual approach for landfill leachate treatment and power production: A review. Environ. Sci. Pollut. Res. 2024, 31, 41683–41733. [Google Scholar] [CrossRef]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour. Conserv. Recycl. 2016, 107, 142–156. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Dey, B.; Unpaprom, Y.; Ramaraj, R.; Maniam, G.P.; Govindan, N.; Jayaraman, S.; Arunachalam, T.; Paramasivan, B. Engineering principles and process designs for phosphorus recovery as struvite: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 105579. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Vyas, S.; Koul, Y.; Prajapati, P.; Varjani, S.; Chang, J.S.; Bilal, M.; Moustakas, K.; Show, P.L.; Vithanage, M. Tricks and tracks in waste management with a special focus on municipal landfill leachate: Leads and obstacles. Sci. Total Environ. 2023, 860, 160377. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Asakura, H. Treatment of landfill leachate with different techniques: An overview. J. Water Reuse Desalination 2021, 11, 66–96. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Cornejo-Ponce, L.; Sagade, A.A.; Mani, M.; Aroulmoji, V.; Femilaa Rajan, V.; Manavalan, K. A concise review of recent biohydrogen production technologies. Sustain. Energy Technol. Assess. 2024, 62, 103606. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Goh, H.H.; Chew, K.W. From liquid waste to mineral fertilizer: Recovery, recycle and reuse of high-value macro-nutrients from landfill leachate to contribute to circular economy, food security, and carbon neutrality. Process Saf. Environ. Prot. 2023, 170, 791–807. [Google Scholar] [CrossRef]
- Abbà, A.; Domini, M.; Baldi, M.; Pedrazzani, R.; Bertanza, G. Ammonia Recovery from Livestock Manure Digestate through an Air-Bubble Stripping Reactor: Evaluation of Performance and Energy Balance. Energies 2023, 16, 1643. [Google Scholar] [CrossRef]
- Abbà, A.; Domini, M.; Baldi, M.; Collivignarelli, M.C.; Bertanza, G. Investigation of the Main Parameters Influencing the Kinetics of an Ammonia Stripping Plant Treating Swine Digestate. Sustainability 2023, 15, 10494. [Google Scholar] [CrossRef]
- Baldi, M.; Collivignarelli, M.C.; Abbà, A.; Benigna, I. The valorization of ammonia in manure digestate by means of alternative stripping reactors. Sustainability 2018, 10, 3073. [Google Scholar] [CrossRef]
- Bao, Y.; Fu, Y.; Wang, C.; Wang, H. An effective integrated system used in separating for anaerobic digestate and concentrating for biogas slurry. Environ. Technol. 2021, 42, 4434–4443. [Google Scholar] [CrossRef] [PubMed]
- Bonmatí, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Finzi, A.; Ferrari, O.; Riva, E.; Provolo, G. Nitrogen recovery from intensive livestock farms using a simplified ammonia stripping process. Front. Sustain. Food Syst. 2024, 8, 1406962. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Magrí, A.; Béline, F.; Dabert, P. Feasibility and interest of the anammox process as treatment alternative for anaerobic digester supernatants in manure processing—An overview. J. Environ. Manag. 2013, 131, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Uppuluri, N.S.T.; Deng, Y.; Zheng, Y.; Dong, R.; Müller, J.; Oechsner, H.; Li, B.; Guo, J. Comparison of phosphorus species in livestock manure and digestate by different detection techniques. Sci. Total Environ. 2023, 874, 162547. [Google Scholar] [CrossRef] [PubMed]
- Rizzioli, F.; Bertasini, D.; Bolzonella, D.; Frison, N.; Battista, F. A critical review on the techno-economic feasibility of nutrients recovery from anaerobic digestate in the agricultural sector. Sep. Purif. Technol. 2023, 306, 122690. [Google Scholar] [CrossRef]
- Scotto di Perta, E.; Grieco, R.; Papirio, S.; Esposito, G.; Cervelli, E.; Bovo, M.; Pindozzi, S. Ammonia Air Stripping from Different Livestock Effluents Prior to and after Anaerobic Digestion. Sustainability 2023, 15, 9402. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, L.; Ye, Q.; Wang, Q.; Liao, D.; Chang, X.; Xi, S.; He, R. Chemical and microbiological characterization of pig manures and digestates. Environ. Technol. 2023, 44, 1916–1925. [Google Scholar] [CrossRef]
- Trotta, S.; Adani, F.; Fedele, M.; Salvatori, M. Nitrogen and phosphorus recovery from cow digestate by struvite precipitation: Process optimization to maximize phosphorus recovery. Results Eng. 2023, 20, 101478. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Z.; Zheng, Y.; Chen, Y.; Yin, F.; Zhang, W.; Dong, H.; Xin, H. Characterization of volatile organic compound (VOC) emissions from swine manure biogas digestate storage. Atmosphere 2019, 10, 411. [Google Scholar] [CrossRef]
- Laureni, M.; Palatsi, J.; Llovera, M.; Bonmatí, A. Influence of pig slurry characteristics on ammonia stripping efficiencies and quality of the recovered ammonium-sulfate solution. J. Chem. Technol. Biotechnol. 2013, 88, 1654–1662. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Kiran Kumar Reddy, G. Aerobic granular sludge technology: Mechanisms of granulation and biotechnological applications. Bioresour. Technol. 2018, 247, 1128–1143. [Google Scholar] [CrossRef]
- Kuglarz, M.; Grübel, K.; Bohdziewicz, J. Post-digestion liquor treatment in the method combining chemical precipitation with reverse osmosis. Arch. Environ. Prot. 2014, 40, 29–42. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, X.; Li, X.; Yuan, Y. Performance of aerobic granular sludge in a sequencing batch bioreactor for slaughterhouse wastewater treatment. Bioresour. Technol. 2015, 190, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Valorisation of food waste via fungal hydrolysis and lactic acid fermentation with Lactobacillus casei Shirota. Bioresour. Technol. 2016, 217, 129–136. [Google Scholar] [CrossRef]
- Shi, X.; Zuo, J.; Zhang, M.; Wang, Y.; Yu, H.; Li, B. Enhanced biogas production and in situ ammonia recovery from food waste using a gas-membrane absorption anaerobic reactor. Bioresour. Technol. 2019, 292, 121864. [Google Scholar] [CrossRef] [PubMed]
- Amanidaz, N.; Gholizadeh, A.; Alavi, N.; Majlessi, M.; Rafiee, M.; Zamanzadeh, M.; Rashidi, M.; Mirzaee, S.A. Volatile fatty acids and ammonia recovery, simultaneously cathodic hydrogen production and increasing thermophilic dark fermentation of food waste efficiency. Int. J. Hydrogen Energy 2023, 48, 15026–15036. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, Z.; Hua, X.; Dong, D.; Liang, D.; Sun, Y. Ammonia nitrogen removal from chlor-alkali chemical industry wastewater by magnesium ammonium phosphate precipitation method. Adv. Mat. Res. 2012, 573–574, 1096–1100. [Google Scholar] [CrossRef]
- Zhang, T.; Narbaitz, R.M.; Sartaj, M.; Downey, J. Chlorine vs. Sodium Chloride Regeneration of Zeolite Column for Ammonium Removal from an Explosives Impacted Mining Wastewater. Water 2022, 14, 3094. [Google Scholar] [CrossRef]
- Kuokkanen, E.; Repo, E.; Warchoł, J.K.; Sillanpää, M. Ammonium adsorption from synthetic and real mining wastewaters by eight-clay based adsorbents. Desalination Water Treat. 2016, 57, 8289–8301. [Google Scholar] [CrossRef]
- Ho, K.C.; Chan, M.K.; Chen, Y.M.; Subhramaniyun, P. Treatment of rubber industry wastewater review: Recent advances and future prospects. J. Water Process Eng. 2023, 52, 103559. [Google Scholar] [CrossRef]
- Dhameliya, K.B.; Ambasana, C. Assessment of Wastewater Contaminants Caused by Textile Industries. J. Pure Appl. Microbiol. 2023, 17, 1477–1485. [Google Scholar] [CrossRef]
- Javed, F.; Rehman, F.; Khan, A.U.; Fazal, T.; Hafeez, A.; Rashid, N. Real textile industrial wastewater treatment and biodiesel production using microalgae. Biomass Bioenergy 2022, 165, 106559. [Google Scholar] [CrossRef]
- Alvan, S.; Aji, A.D.S.; Mahendra, I.; Al Arif, Z.; Prayogo, W.; Azizah, R.N.; Awfa, D.; Qadafi, M.; Suryawan, I.W.K.; Ratnaningsih, W. Leather Tannery Wastewater Treatment Using Electro-Fenton Process—Effects on Ammonia, Chromium, Total Suspended Solid, Biological Oxygen Demand and Chemical Oxygen Demand Removal. J. Ecol. Eng. 2024, 25, 331–338. [Google Scholar] [CrossRef]
- Sawalha, H.; Alsharabaty, R.; Sarsour, S.; Al-Jabari, M. Wastewater from leather tanning and processing in Palestine: Characterization and management aspects. J. Environ. Manag. 2019, 251, 109596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, Y.; Chai, X.; Liao, X.; He, Q.; Shi, B. Ammonia nitrogen in tannery wastewater: Distribution, origin and prevention. J. Am. Leather Chem. Assoc. 2012, 107, 40–50. [Google Scholar]
- Atsbha, M.W.; Liu, R.; Nir, O. Circular hybrid membrane process treating high-salinity ammonium-rich pharmaceutical wastewater. Chem. Eng. J. 2024, 497, 154690. [Google Scholar] [CrossRef]
- Zuo, L.; Yao, H.; Li, H.; Fan, L.; Jia, F. Nitrogen removal efficiency for pharmaceutical wastewater with a single-stage anaerobic ammonium oxidation process. Int. J. Environ. Res. Public. Health 2020, 17, 7972. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Fauzi, M.A.H.M.; Razali, M.A.; Hafiz, M.F.U.M.; Noor, A. Organic and nutrient removal from pulp and paper industry wastewater by extended aeration activated sludge system. IOP Conf. Ser. Earth Environ. Sci. 2021, 842, 012021. [Google Scholar] [CrossRef]
- Liang, X.; Xu, Y.; Yin, L.; Wang, R.; Li, P.; Wang, J.; Liu, K. Sustainable Utilization of Pulp and Paper Wastewater. Water 2023, 15, 4135. [Google Scholar] [CrossRef]
- Ospina-Betancourth, C.; Acharya, K.; Allen, B.; Head, I.M.; Sanabria, J.; Curtis, T.P. Valorization of pulp and paper industry wastewater using sludge enriched with nitrogen-fixing bacteria. Water Environ. Res. 2021, 93, 1734–1747. [Google Scholar] [CrossRef]
- Elmobarak, W.F.; Hameed, B.H.; Almomani, F.; Abdullah, A.Z. A Review on the Treatment of Petroleum Refinery Wastewater Using Advanced Oxidation Processes. Catalysts 2021, 11, 782. [Google Scholar] [CrossRef]
- Fang, H.-Y.; Chou, M.-S.; Huang, C.-W. Nitrification of ammonia-nitrogen in refinery wastewater. Water Res. 1993, 27, 1761–1765. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Kumoro, A.C.; Aryanti, N.; Utomo, D.P. Removal of organic pollutants from rubber wastewater using hydrophilic nanocomposite rGO-ZnO/PES hybrid membranes. J. Environ. Chem. Eng. 2021, 9, 106421. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, S.; Li, B.; Li, H.; Ling, Z.; Zhang, G.; Liu, M. Current Status of Zero Liquid Discharge Technology for Desulfurization Wastewater. Water 2024, 16, 900. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, X.; Yan, B. Complex treatment of the ammonium nitrogen wastewater from rare-earth separation plant. Desalination Water Treat. 2009, 8, 109–117. [Google Scholar] [CrossRef]
- Ren, S.; Huang, S.; Liu, B. Enhanced removal of ammonia nitrogen from rare earth wastewater by NaCl modified vermiculite: Performance and mechanism. Chemosphere 2022, 302, 134742. [Google Scholar] [CrossRef]
- Remus, R.; Aguado-Monsonet, M.A.; Roudier, S.; Delgado Sanc, L. Best Available Techniques (BAT) Reference Document for Iron and Steel Production. European Commission, Joint Research Centre. 2013. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2019-11/IS_Adopted_03_2012.pdf (accessed on 15 December 2024).
- Kinidi, L.; Tan, I.A.W.; Abdul Wahab, N.B.; Tamrin, K.F.; Bin Hipolito, C.N.; Salleh, S.F. Recent Development in Ammonia Stripping Process for Industrial Wastewater Treatment. Int. J. Chem. Eng. 2018, 2018, 181087. [Google Scholar] [CrossRef]
- Chen, T.L.; Chen, L.H.; Lin, Y.J.; Yu, C.P.; Ma, H.W.; Chiang, P.C. Advanced ammonia nitrogen removal and recovery technology using electrokinetic and stripping process towards a sustainable nitrogen cycle: A review. J. Clean. Prod. 2021, 309, 127369. [Google Scholar] [CrossRef]
- Zhao, Q.B.; Ma, J.; Zeb, I.; Yu, L.; Chen, S.; Zheng, Y.M.; Frear, C. Ammonia recovery from anaerobic digester effluent through direct aeration. Chem. Eng. J. 2015, 279, 31–37. [Google Scholar] [CrossRef]
- Lima, S.; Cosenza, A.; Caputo, G.; Grisafi, F.; Scargiali, F. Utilization of native Chlorella strain in laboratory-scale raceway reactor for synthetic wastewater treatment: A study in batch and continuous modes with multi-substrate modeling. J. Water Process Eng. 2024, 60, 105145. [Google Scholar] [CrossRef]
- Risberg, K.; Cederlund, H.; Pell, M.; Arthurson, V.; Schnürer, A. Comparative characterization of digestate versus pig slurry and cow manure—Chemical composition and effects on soil microbial activity. Waste Manag. 2017, 61, 529–538. [Google Scholar] [CrossRef]
- European Union. Council Directive 1999/31/EC of 26 April 1999 on the landfill of waste. Off. J. Eur. Communities 1999, L182, 1–19. [Google Scholar]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2000, L327, 1–73. [Google Scholar]
- Cosenza, A.; Lima, S.; Gurreri, L.; Mancini, G.; Scargiali, F. Microalgae in the Mediterranean area: A geographical survey outlining the diversity and technological potential. Algal Res. 2024, 82, 103669. [Google Scholar] [CrossRef]
- European Commission. A New Circular Economy Action Plan: For a Cleaner and More Competitive Europe. Brussels. 2020. Available online: https://op.europa.eu/en/publication-detail/-/publication/45cc30f6-cd57-11ea-adf7-01aa75ed71a1/language-en (accessed on 15 December 2024).
- Collivignarelli, M.C.; Bertanza, G.; Abbà, A.; Sordi, M.; Pedrazzani, R. Synergy between anaerobic digestion and a post-treatment based on Thermophilic Aerobic Membrane Reactor (TAMR). Environ. Prog. Sustain. Energy 2017, 36, 1802–1809. [Google Scholar] [CrossRef]
- Pandey, B.; Chen, L. Technologies to recover nitrogen from livestock manure—A review. Sci. Total Environ. 2021, 784, 147098. [Google Scholar] [CrossRef]
- Kang, S.; Chun, J.; Park, N.; Lee, S.M.; Kim, H.J.; Son, S.U. Hydrophobic zeolites coated with microporous organic polymers: Adsorption behavior of ammonia under humid conditions. Chem. Commun. 2015, 51, 11814–11817. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Butterly, C.; Zhang, W.; He, J.-Z.; Chen, D. Adsorbent materials for ammonium and ammonia removal: A review. J. Clean. Prod. 2021, 283, 124611. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, M.; Tong, X.; Liu, S.; Qu, D.; Xiao, S. Recovery of ammonium nitrogen from human urine by an open-loop hollow fiber membrane contactor. Sep. Purif. Technol. 2020, 239, 116579. [Google Scholar] [CrossRef]
- Bao, X.; Wu, Q.; Shi, W.; Wang, W.; Yu, H.; Zhu, Z.; Zhang, X.; Zhang, Z.; Zhang, R.; Cui, F. Polyamidoamine dendrimer grafted forward osmosis membrane with superior ammonia selectivity and robust antifouling capacity for domestic wastewater concentration. Water Res. 2019, 153, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Darestani, M.; Haigh, V.; Couperthwaite, S.J.; Millar, G.J.; Nghiem, L.D. Hollow fibre membrane contactors for ammonia recovery: Current status and future developments. J. Environ. Chem. Eng. 2017, 5, 1349–1359. [Google Scholar] [CrossRef]
- Osman, A.I.; Chen, Z.; Elgarahy, A.M.; Farghali, M.; Mohamed, I.M.A.; Priya, A.K.; Hawash, H.B.; Yap, P.S. Membrane Technology for Energy Saving: Principles, Techniques, Applications, Challenges, and Prospects. Adv. Energy Sustain. Res. 2024, 5, 2400011. [Google Scholar] [CrossRef]
- Sert, G.; Bunani, S.; Yörükoğlu, E.; Kabay, N.; Egemen, Ö.; Arda, M.; Yüksel, M. Performances of some NF and RO membranes for desalination of MBR treated wastewater. J. Water Process Eng. 2017, 16, 193–198. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Vanotti, M.B.; Martín-Ramos, P. Effect of acid flow rate, membrane surface area, and capture solution on the effectiveness of suspended gpm systems to recover ammonia. Membranes 2021, 11, 538. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.; Boo, C.; Song, I.H.; Park, C. Investigating the potential of ammonium retention by graphene oxide ceramic nanofiltration membranes for the treatment of semiconductor wastewater. Chemosphere 2022, 286, 131745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, L.; Ma, Y.; Cai, Z.; Cao, Y.; Huang, K.; Jiang, L. A Mini-Review on NH3Separation Technologies: Recent Advances and Future Directions. Energy Fuels 2022, 36, 14516–14533. [Google Scholar] [CrossRef]
- Saleem, M.; Spagni, A.; Alibardi, L.; Bertucco, A.; Lavagnolo, M.C. Assessment of dynamic membrane filtration for biological treatment of old landfill leachate. J. Environ. Manag. 2018, 213, 27–35. [Google Scholar] [CrossRef]
- He, Q.; Xi, J.; Shi, M.; Feng, L.; Yan, S.; Deng, L. Developing a Vacuum-Assisted Gas-Permeable Membrane Process for Rapid Ammonia Recovery and CO2 Capture from Biogas Slurry. ACS Sustain. Chem. Eng. 2020, 8, 154–162. [Google Scholar] [CrossRef]
- Hu, M.; Zheng, S.; Mi, B. Organic Fouling of Graphene Oxide Membranes and Its Implications for Membrane Fouling Control in Engineered Osmosis. Environ. Sci. Technol. 2016, 50, 685–693. [Google Scholar] [CrossRef]
- Farghali, M.; Chen, Z.; Osman, A.I.; Ali, I.M.; Hassan, D.; Ihara, I.; Rooney, D.W.; Yap, P.S. Strategies for ammonia recovery from wastewater: A review. Environ. Chem. Lett. 2024, 22, 2699–2751. [Google Scholar] [CrossRef]
- Serra-Toro, A.; Vinardell, S.; Astals, S.; Madurga, S.; Llorens, J.; Mata-Álvarez, J.; Mas, F.; Dosta, J. Ammonia recovery from acidogenic fermentation effluents using a gas-permeable membrane contactor. Bioresour. Technol. 2022, 356, 127273. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; An, S.; Choi, Y. Ammonia harvesting via membrane gas extraction at moderately alkaline pH: A step toward net-profitable nitrogen recovery from domestic wastewater. Chem. Eng. J. 2021, 405, 126662. [Google Scholar] [CrossRef]
- Ulu, F.; Kobya, M. Ammonia removal from wastewater by air stripping and recovery struvite and calcium sulphate precipitations from anesthetic gases manufacturing wastewater. J. Water Process Eng. 2020, 38, 101641. [Google Scholar] [CrossRef]
- Li, W.; Shi, X.; Zhang, S.; Qi, G. Modelling of ammonia recovery from wastewater by air stripping in rotating packed beds. Sci. Total Environ. 2020, 702, 134971. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Chu, H.; Dong, B. Pretreatment and membrane hydrophilic modification to reduce membrane fouling. Membranes 2013, 3, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, L.; Masoomi, M.Y.; Garcia, H. Regeneration and reconstruction of metal-organic frameworks: Opportunities for industrial usage. Coord. Chem. Rev. 2022, 472, 214776. [Google Scholar] [CrossRef]
- Lee, J.W.; Barin, G.; Peterson, G.W.; Xu, J.; Colwell, K.A.; Long, J.R. A microporous amic acid polymer for enhanced ammonia capture. ACS Appl. Mater. Interfaces 2017, 9, 33504–33510. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Kumar, V.; Kim, K.H.; Kukkar, D. Metal-organic frameworks (MOFs): Potential and challenges for capture and abatement of ammonia. J. Mater. Chem. A Mater. 2017, 5, 22877–22896. [Google Scholar] [CrossRef]
- Vellingiri, K.; Deep, A.; Kim, K.H. Metal-Organic Frameworks as a Potential Platform for Selective Treatment of Gaseous Sulfur Compounds. ACS Appl. Mater. Interfaces 2016, 8, 29835–29857. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Züttel, A.; Kim, S.; Ko, Y.; Kim, W. Effect of Boron Doping On Graphene Oxide for Ammonia Adsorption. ChemNanoMat 2017, 3, 794–797. [Google Scholar] [CrossRef]
- Jasuja, H.; Peterson, G.W.; Decoste, J.B.; Browe, M.A.; Walton, K.S. Evaluation of MOFs for air purification and air quality control applications: Ammonia removal from air. Chem. Eng. Sci. 2015, 124, 118–124. [Google Scholar] [CrossRef]
- Gonzalez-Salgado, I.; Guigui, C.; Sperandio, M. Transmembrane chemical absorption technology for ammonia recovery from wastewater: A critical review. Chem. Eng. J. 2022, 444, 136491. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Liu, R.; Song, L.; Zhang, Y.; Dai, X. Ammonia recovery from anaerobic digestate: State of the art, challenges and prospects. Bioresour. Technol. 2022, 363, 127957. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, C.; He, P.; He, J.; Dai, X.; Li, W.; Yang, X.; Li, X.; Huang, X.; Feng, J. Nitrogen resource recovery from mature leachate via heat extraction technology: An engineering project application. Water Sci. Technol. 2022, 85, 549–561. [Google Scholar] [CrossRef]
- Morelli, B.; Cashman, S.; Cissy Ma, X.; Garland, J.; Turgeon, J.; Fillmore, L.; Bless, D.; Nye, M. Effect of nutrient removal and resource recovery on life cycle cost and environmental impacts of a small scale water resource recovery facility. Sustainability 2018, 10, 3546. [Google Scholar] [CrossRef]
- Lin, Y.; Guo, M.; Shah, N.; Stuckey, D.C. Economic and environmental evaluation of nitrogen removal and recovery methods from wastewater. Bioresour. Technol. 2016, 215, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Sena, M.; Hicks, A. Life cycle assessment review of struvite precipitation in wastewater treatment. Resour. Conserv. Recycl. 2018, 139, 194–204. [Google Scholar] [CrossRef]
- van Zelm, R.; Seroa da Motta, R.D.P.; Lam, W.Y.; Menkveld, W.; Broeders, E. Life cycle assessment of side stream removal and recovery of nitrogen from wastewater treatment plants. J. Ind. Ecol. 2020, 24, 913–922. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, Y.; Kong, S.; Deng, Q.; Qin, S.; Yao, L.; Zhao, T.; Qi, S. Benefits of refined NH3 emission controls on PM2.5 mitigation in Central China. Sci. Total Environ. 2022, 814, 151957. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Bai, Z.H.; Winiwarter, W.; Kiesewetter, G.; Heyes, C.; Ma, L. Mitigating ammonia emission from agriculture reduces PM2.5 pollution in the Hai River Basin in China. Sci. Total Environ. 2017, 609, 1152–1160. [Google Scholar] [CrossRef]
- Xu, L.; Dong, F.; Zhuang, H.; He, W.; Ni, M.; Feng, S.P.; Lee, P.H. Energy upcycle in anaerobic treatment: Ammonium, methane, and carbon dioxide reformation through a hybrid electrodeionization–solid oxide fuel cell system. Energy Convers. Manag. 2017, 140, 157–166. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Patel, H.; Woudstra, T.; Aravind, P.V. Thermodynamic Analysis of Solid Oxide Fuel Cell Integrated System Fuelled by Ammonia from Struvite Precipitation Process. Fuel Cells 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, Y.; Zhang, G.; Ruan, R.; Wang, Y.; Wu, X.; Zheng, H.; Zhang, Q.; Cao, L. New progress of ammonia recovery during ammonia nitrogen removal from various wastewaters. World J. Microbiol. Biotechnol. 2020, 36, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, M.; Zeng, H.; Min, R.; Wang, J.; Zhang, G. Application of physicochemical techniques to the removal of ammonia nitrogen from water: A systematic review. Environ. Geochem. Health 2024, 46, 344. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, X.; Bao, X.; Lam, K.L. Life cycle assessment of ammonium sulfate recovery from urban wastewater. Blue-Green. Syst. 2024, 6, 90–99. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Cheng, X.; Wang, Z.; Guo, F.; Zhang, J. An efficient molten steel slag gas quenching process: Integrating carbon solidification and waste heat recovery. Waste Manag. 2024, 186, 249–258. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Wang, S.; Guiberti, T.F.; Roberts, W.L. Review on the recent advances on ammonia combustion from the fundamentals to the applications. Fuel Commun. 2022, 10, 100053. [Google Scholar] [CrossRef]
- Labovský, J.; Jelemenský, L. Verification of CFD pollution dispersion modelling based on experimental data. J. Loss Prev. Process Ind. 2011, 24, 166–177. [Google Scholar] [CrossRef]
- Wang, Y.; Wright, L.A. A Comparative Review of Alternative Fuels for the Maritime Sector: Economic, Technology, and Policy Challenges for Clean Energy Implementation. World 2021, 2, 456–481. [Google Scholar] [CrossRef]
- Belvoir, F.; Bull, M.G. Development of an Ammonia-Burning Gas Turbine Engine; Defense Technical Information Center: Fort Belvoir, VA, USA, 1968. [Google Scholar]
- He, X.; Shu, B.; Nascimento, D.; Moshammer, K.; Costa, M.; Fernandes, R.X. Auto-ignition kinetics of ammonia and ammonia/hydrogen mixtures at intermediate temperatures and high pressures. Combust. Flame 2019, 206, 189–200. [Google Scholar] [CrossRef]
- Liao, W.; Wang, Y.; Chu, Z.; Yang, B. Investigating auto-ignition characteristics and kinetic modeling of NH3/CH4 mixtures using an RCM. Combust. Flame 2024, 260, 113257. [Google Scholar] [CrossRef]
- Hussein, N.A.; Valera-Medina, A.; Alsaegh, A.S. Ammonia- hydrogen combustion in a swirl burner with reduction of NOx emissions. Energy Procedia 2019, 158, 2305–2310. [Google Scholar] [CrossRef]
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Lietti, L.; Righini, L.; Castoldi, L.; Artioli, N.; Forzatti, P. Labeled 15NO study on N2 and N2O formation over Pt-Ba/Al2O3 NSR catalysts. Top. Catal. 2013, 56, 7–13. [Google Scholar] [CrossRef]
- Hewlett, S.; Hewlett, S. Investigating the Potential of Industrial Waste Stream Ammonia as a Fuel for Low Carbon, Gas Turbine Power Generation. Doctoral Dissertation, Cardiff University, Cardiff, UK, 2021. [Google Scholar]
- Hewlett, S.G.; Valera-Medina, A.; Pugh, D.G.; Bowen, P.J. Gas turbine co-firing of steelworks ammonia with coke oven gas or methane: A fundamental and cycle analysis. In Proceedings of the ASME Turbo Expo, Phoenix, AZ, USA, 17–21 June 2019; Volume 3. [Google Scholar] [CrossRef]
- Hewlett, S.G.; Pugh, D.G.; Valera-Medina, A.; Giles, A.; Runyon, J.; Goktepe, B.; Bowen, P.J. Industrial wastewater as an enabler of green ammonia to power via gas turbine technology. In Proceedings of the ASME Turbo Expo, Online, 21–25 September 2020; Volume 3. [Google Scholar] [CrossRef]
- Lucentini, I.; Garcia, X.; Vendrell, X.; Llorca, J. Review of the Decomposition of Ammonia to Generate Hydrogen. Ind. Eng. Chem. Res. 2021, 60, 18560–18611. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhou, X.P.; Au, C.T. A mini-review on ammonia decomposition catalysts for on-site generation of hydrogen for fuel cell applications. Appl. Catal. A Gen. 2004, 277, 1–9. [Google Scholar] [CrossRef]
- Hossein Ali, Y.R.; Shin, D. Green Hydrogen Production Technologies from Ammonia Cracking. Energies 2022, 15, 8246. [Google Scholar] [CrossRef]
- Vielstich Wolf. Handbook of Fuel Cells: Fundamentals, Technology, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Maddaloni, M.; Centeno-Pedrazo, A.; Avanzi, S.; Mazumdar, N.J.; Manyar, H.; Artioli, N. Novel Ionic Liquid Synthesis of Bimetallic Fe–Ru Catalysts for the Direct Hydrogenation of CO2 to Short Chain Hydrocarbons. Catalysts 2023, 13, 1499. [Google Scholar] [CrossRef]
- Cechetto, V.; Di Felice, L.; Medrano, J.A.; Makhloufi, C.; Zuniga, J.; Gallucci, F. H2 production via ammonia decomposition in a catalytic membrane reactor. Fuel Process. Technol. 2021, 216, 106772. [Google Scholar] [CrossRef]
- Dilshani, A.; Wijayananda, A.; Rathnayake, M. Life cycle net energy and global warming impact assessment for hydrogen production via decomposition of ammonia recovered from source-separated human urine. Int. J. Hydrogen Energy 2022, 47, 24093–24106. [Google Scholar] [CrossRef]
- Coney, C.; Hardacre, C.; Morgan, K.; Artioli, N.; York, A.P.E.; Millington, P.; Kolpin, A.; Goguet, A. Investigation of the oxygen storage capacity behaviour of three way catalysts using spatio-temporal analysis. Appl. Catal. B 2019, 258, 117918. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, D.; Lei, Y.; Zhu, T.; Hu, J.; Tang, Y.; Chen, Z.; Huang, J.; Lai, Y.; Lin, Z. Markedly enhanced hydrogen production in wastewater via ammonia-mediated metal oxyhydroxides active sites on bifunctional electrocatalysts. Nano Energy 2023, 117, 108896. [Google Scholar] [CrossRef]
- Tian, Y.; Mao, Z.; Wang, L.; Liang, J. Green Chemistry: Advanced Electrocatalysts and System Design for Ammonia Oxidation. Small Struct. 2023, 4, 2200266. [Google Scholar] [CrossRef]
- Raza, T.; Yang, J.; Wang, R.; Xia, C.; Raza, R.; Zhu, B.; Yun, S. Recent advance in physical description and material development for single component SOFC: A mini-review. Chem. Eng. J. 2022, 444, 136533. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, K.; He, F.; Zhu, F.; Zhou, Y.; Yuan, W.; Liu, Y.; Liu, M.; Choi, Y.M.; Chen, Y. Challenges and Advancements in the Electrochemical Utilization of Ammonia Using Solid Oxide Fuel Cells. Adv. Mater. 2024, 36, e2313966. [Google Scholar] [CrossRef] [PubMed]
- Pandya, R.S.; Kaur, T.; Bhattacharya, R.; Bose, D.; Saraf, D. Harnessing microorganisms for bioenergy with Microbial Fuel Cells: Powering the future. Water-Energy Nexus 2024, 7, 1–12. [Google Scholar] [CrossRef]
- Zhu, B.; Raza, R.; Abbas, G.; Singh, M. An electrolyte-free fuel cell constructed from one homogenous layer with mixed conductivity. Adv. Funct. Mater. 2011, 21, 2465–2469. [Google Scholar] [CrossRef]
- Qian, J.; Zhou, X.; Liu, L.; Liu, J.; Zhao, L.; Shen, H.; Hu, X.; Qian, X.; Chen, H.; Zhou, X.; et al. Direct ammonia low-temperature symmetrical solid oxide fuel cells with composite semiconductor electrolyte. Electrochem. Commun. 2022, 135, 107216. [Google Scholar] [CrossRef]
- Nowicki, K.M.; Carins, G.; Bayne, J.; Tupberg, C.; Irvine, G.J.; Irvine, J.T.S. Characterisation of direct ammonia proton conducting tubular ceramic fuel cells for maritime applications. J. Mater. Chem. A Mater. 2022, 11, 352–363. [Google Scholar] [CrossRef]
- Wang, B.; Li, T.; Gong, F.; Othman, M.H.D.; Xiao, R. Ammonia as a green energy carrier: Electrochemical synthesis and direct ammonia fuel cell—A comprehensive review. Fuel Process. Technol. 2022, 235, 107380. [Google Scholar] [CrossRef]
- Rathore, S.S.; Biswas, S.; Fini, D.; Kulkarni, A.P.; Giddey, S. Direct ammonia solid-oxide fuel cells: A review of progress and prospects. Int. J. Hydrogen Energy 2021, 46, 35365–35384. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, Z.; An, L. Carbon-free sustainable energy technology: Direct ammonia fuel cells. J. Power Sources 2020, 476, 228454. [Google Scholar] [CrossRef]
- Li, D.; Shi, Y.; Gao, F.; Yang, L.; Kehoe, D.K.; Romeral, L.; Gun’ko, Y.K.; Lyons, M.G.; Wang, J.J.; Mullarkey, D.; et al. Characterising and control of ammonia emission in microbial fuel cells. Chem. Eng. J. 2020, 389, 124462. [Google Scholar] [CrossRef]
- Rossi, R.; Yang, W.; Zikmund, E.; Pant, D.; Logan, B.E. In situ biofilm removal from air cathodes in microbial fuel cells treating domestic wastewater. Bioresour. Technol. 2018, 265, 200–206. [Google Scholar] [CrossRef]
- Yan, C.; Liu, L. Sn-doped V2O5 nanoparticles as catalyst for fast removal of ammonia in air via PEC and PEC-MFC. Chem. Eng. J. 2020, 392, 123738. [Google Scholar] [CrossRef]
- Yildirim, Ö.; Nölker, K.; Büker, K.; Kleinschmidt, R. Chemical Conversion of Steel Mill Gases to Urea: An Analysis of Plant Capacity. Chem. Ing. Tech. 2018, 90, 1529–1535. [Google Scholar] [CrossRef]
- Ma, Y.; Bae, J.W.; Kim, S.H.; Jovičević-Klug, M.; Li, K.; Vogel, D.; Ponge, D.; Rohwerder, M.; Gault, B.; Raabe, D. Reducing Iron Oxide with Ammonia: A Sustainable Path to Green Steel. Adv. Sci. 2023, 10, 2300111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zou, P.; Jeerh, G.; Chen, S.; Shields, J.; Wang, H.; Tao, S. Electricity Generation from Ammonia in Landfill Leachate by an Alkaline Membrane Fuel Cell Based on Precious-Metal-Free Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12817–12824. [Google Scholar] [CrossRef]
- Davey, C.J.; Luqmani, B.; Thomas, N.; McAdam, E.J. Transforming wastewater ammonia to carbon free energy: Integrating fuel cell technology with ammonia stripping for direct power production. Sep. Purif. Technol. 2022, 289, 120755. [Google Scholar] [CrossRef]
- Yi, X.; Lu, T.; Li, Y.; Ai, Q.; Hao, R. Collaborative planning of multi-energy systems integrating complete hydrogen energy chain. Renew. Sustain. Energy Rev. 2025, 210, 115147. [Google Scholar] [CrossRef]


| Sector (Industrial Activity) | Waste Material (Flow) Containing Ammonia | N-NH3 (mg/L) | pH | COD (mg/L) | Ref. |
|---|---|---|---|---|---|
| Landfills (waste management) | Leachate | <30–>4000 | <6.5–9 | <1000–70,000 | [3,4,13,29,30,31,32,33,34,35,36,37,38,39,40,41] |
| Biogas production | Livestock digestate liquid phase | 834–3795 | 5.9–9.1 | 3814–46,300 | [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] |
| Wastewater treatment | Municipal sewage wastewater | 10–68 | 6.5–7.5 | 95–506 | [57] |
| Wastewater treatment | Digested sludge | 1510–1679 | 7.4–7.8 | 1684–2398 | [58] |
| Agriculture | Energy crops digestate | 1300–2400 | 7.5–8.4 | [51] | |
| Animal byproduct processing | Slaughterhouse wastewater | 100–140 | 1100–1400 | [59] | |
| Food waste | Leachate from food waste | 30–140 | 4.3 | 1100–199,500 | [60,61,62] |
| Chemical industry | Spent catalysts, process effluents | 177 | 11.6 | 188 | [63] |
| Fertilizer production | Process effluents | 50–200 | 6.0–9.0 | 500–1500 | [28] |
| Mining and mineral processing | Ammonium-laden effluents from ore leaching | 20–80 | 6.0–8.5 | - | [64,65] |
| Plastic and rubber production | Wastewater containing ammonia from polymerization | 5–300 | 3.7–9.4 | 2834–26,914 | [66] |
| Textile industry | Dye bath effluents, process wastewater | 34–49 | 6.3–13.1 | 635–4459 | [67,68] |
| Tanning and leather industry | Effluents from ammonia-based deliming processes | 123–150 | 3.3–12.4 | 1670–11,413 | [69,70,71] |
| Pharmaceuticals | Fermentation by-products, process effluents | 5000–6000 | 8.0–12.0 | 500–3000 | [72,73] |
| Pulp and paper | Black liquor, bleaching effluents | 42–200 | 6.3–9.0 | 1195–20,000 | [74,75,76] |
| Petroleum refining | Ammonia-rich sour water from refining processes | 20–335 | 4.3–10 | 1200–3134 | [77,78,79] |
| Energy production (coal-fired power plants) | Flue gas desulfurization (FGD) wastewater | 1–10 | 6.0–9.0 | 50–500 | [80] |
| Steel and metallurgical sector (rare) | Ammonia-containing wastewater | 50–200 | 8.5–9.5 | 200–6500 | [81,82,83] |
| Gas emission: | Off-gas from incineration plants, metallurgical furnaces, etc. | Gas emissions 6–8 g/Nm3 (raw coke oven gas); 10–40 mg/Nm3 (BF off-gas) Note: For each tonne of coke produced, approximately 3 kg of ammonia is generated. | [83] | ||
| Parameter | Traditional Membranes | Advanced Membranes | Ref. |
|---|---|---|---|
| Efficiency | 60–75% | Up to 89% | [100,101,102,103,104,105,106] |
| Fouling resistance | Moderate | High | |
| Operational lifespan | 2–3 years | >5 years | |
| Cost | Lower initial investment | Higher initial, lower long-term costs | |
| Applications | Low-COD effluents | High-COD or variable composition streams |
| Technology | Pretreatment Processes | Key Benefits | References |
|---|---|---|---|
| Stripping technologies | 1. pH adjustment: use lime or NaOH to raise pH above 9, converting NH4⁺ to NH3. 2. Coagulation: Lime helps precipitate CaCO3, reducing organic and suspended solids. 3. Thermal pretreatment: heating the influent to enhance ammonia volatilization and mass transfer. | Enhances NH3 volatilization, reduces clogging, and improves overall stripping efficiency | [42,108] |
| Membrane-based technologies | 1. Filtration and sedimentation: removal of large suspended solids to reduce fouling. 2. Coagulation and flocculation: alum or ferric chloride help reduce organic fouling. 3. pH control: maintains membrane efficiency by optimizing ammonia selectivity. | Minimizes fouling, maintains selectivity, and prolongs membrane lifespan | [99,108] |
| Adsorption-based technologies | 1. Coagulation or flocculation: alum or ferric chloride reduce competing organic/inorganic contaminants. 2. pH adjustment: optimizes ammonia capture within a neutral to slightly alkaline range. 3. Surface modifications: use iron-loaded activated carbon or nanocomposites. | Improves adsorption capacity, reduces organic fouling, and enhances ammonia capture | [96] |
| Parameter | Liquid-Phase Technologies | Gas-Phase Technologies | Ref. |
|---|---|---|---|
| Efficiency | 90–95% (e.g., membranes, adsorption, stripping) | 70–92% (e.g., GPMs, MOFs) | [8,13,14,116] |
| Operational requirements | High pretreatment demand; temperature > 60 °C, pH > 10 | Moderate pretreatment; adaptability to humid conditions | |
| Environmental outcomes | Enables nutrient recovery (e.g., ammonium sulfate) | Reduces NH3 emissions; produces fertilizers (e.g., NH4HSO4) | |
| Challenges | Fouling, organic load variability | Humidity sensitivity, fouling in agricultural streams |
| Ammonia Concentrations Expressed in ppm | |||||
|---|---|---|---|---|---|
| Exposure Time | 10 min | 30 min | 1 h | 4 h | 8 h |
| AEGL-1 | 30 | 30 | 30 | 30 | 30 |
| AEGL-2 | 220 | 220 | 160 | 110 | 110 |
| AEGL-3 | 2700 | 1600 | 1100 | 550 | 390 |
| Compound | Lower Flammability Limit (%) | Upper Flammability Limit (%) |
|---|---|---|
| Ammonia | 15 | 28 |
| Methanol | 6.7 | 36 |
| Ethanol (E85) | 1.4 | 19 |
| Methane | 4.4 | 17 |
| Propane | 4.4 | 17 |
| LPG butane | 2.1 | 9.5 |
| Gasoline (E10) | 1.9 | 8.5 |
| Jet fuel (JP8) | 0.6 | 8 |
| Diesel | 0.6 | 6.5 |
| Hydrogen | 4 | 75 |
| Marine Diesel Oil (MDO) | 0.6 | 6.5 |
| Dimethyl ether (DME) | 3.4 | 28 |
| Compound | Fuel Cost | Emissions (Well-to-Tank) | ||||
|---|---|---|---|---|---|---|
| CO2 | CO | SOx | NOx | |||
| USD/kgeq * | (g/kgfuel) | |||||
| Marine Fuels (Source: International Maritime Organization IMO) | ||||||
| Marine Gas Oil (MGO) | 0.68 | 575 | N/A | <0.005 | 0.01 | |
| Marine bio-diesel | 1.53 | 19 + 67 ** | N/A | 0.0400 | 0.06 | |
| Ammonia | 1.32 (2.87) | 64.9–84.4 (18.6–29.8) | 0.004 (-) | 0.0004 (-) | 0.04 (0.04) | |
| Methanol | 0.98 (2.30) | 20.0 (17.0 + 120.0 **) | 0.006 (0.025) | 0.0020 (0.0480) | 0.05 (0.06) | |
| Hydrogen | 1.50 (2.93) | 78.0–84.2 (7.9–9.7) | <0.074 (<0.007) | <0.0700 (<0.0640) | <0.10 (<0.04) | |
| Liquified Natural Gas (LNG) | 0.66 | 8.3–26.7 | 0.003 | <0.0230 | <0.09 | |
| Liquified biogas (LBG) | 2.29 | 27.0 | 0.010 | 0.0730 | 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Corte, D.; Maddaloni, M.; Vahidzadeh, R.; Domini, M.; Bertanza, G.; Ansari, S.U.; Marchionni, M.; Tola, V.; Artioli, N. Recovered Ammonia as a Sustainable Energy Carrier: Innovations in Recovery, Combustion, and Fuel Cells. Energies 2025, 18, 508. https://doi.org/10.3390/en18030508
La Corte D, Maddaloni M, Vahidzadeh R, Domini M, Bertanza G, Ansari SU, Marchionni M, Tola V, Artioli N. Recovered Ammonia as a Sustainable Energy Carrier: Innovations in Recovery, Combustion, and Fuel Cells. Energies. 2025; 18(3):508. https://doi.org/10.3390/en18030508
Chicago/Turabian StyleLa Corte, Daniele, Marina Maddaloni, Reza Vahidzadeh, Marta Domini, Giorgio Bertanza, Samee Ullah Ansari, Matteo Marchionni, Vittorio Tola, and Nancy Artioli. 2025. "Recovered Ammonia as a Sustainable Energy Carrier: Innovations in Recovery, Combustion, and Fuel Cells" Energies 18, no. 3: 508. https://doi.org/10.3390/en18030508
APA StyleLa Corte, D., Maddaloni, M., Vahidzadeh, R., Domini, M., Bertanza, G., Ansari, S. U., Marchionni, M., Tola, V., & Artioli, N. (2025). Recovered Ammonia as a Sustainable Energy Carrier: Innovations in Recovery, Combustion, and Fuel Cells. Energies, 18(3), 508. https://doi.org/10.3390/en18030508










