Advancements and Challenges in Direct Air Capture Technologies: Energy Intensity, Novel Methods, Economics, and Location Strategies
Abstract
:1. Introduction
1.1. Sorption Technologies in DAC Processes: L-DAC and S-DAC
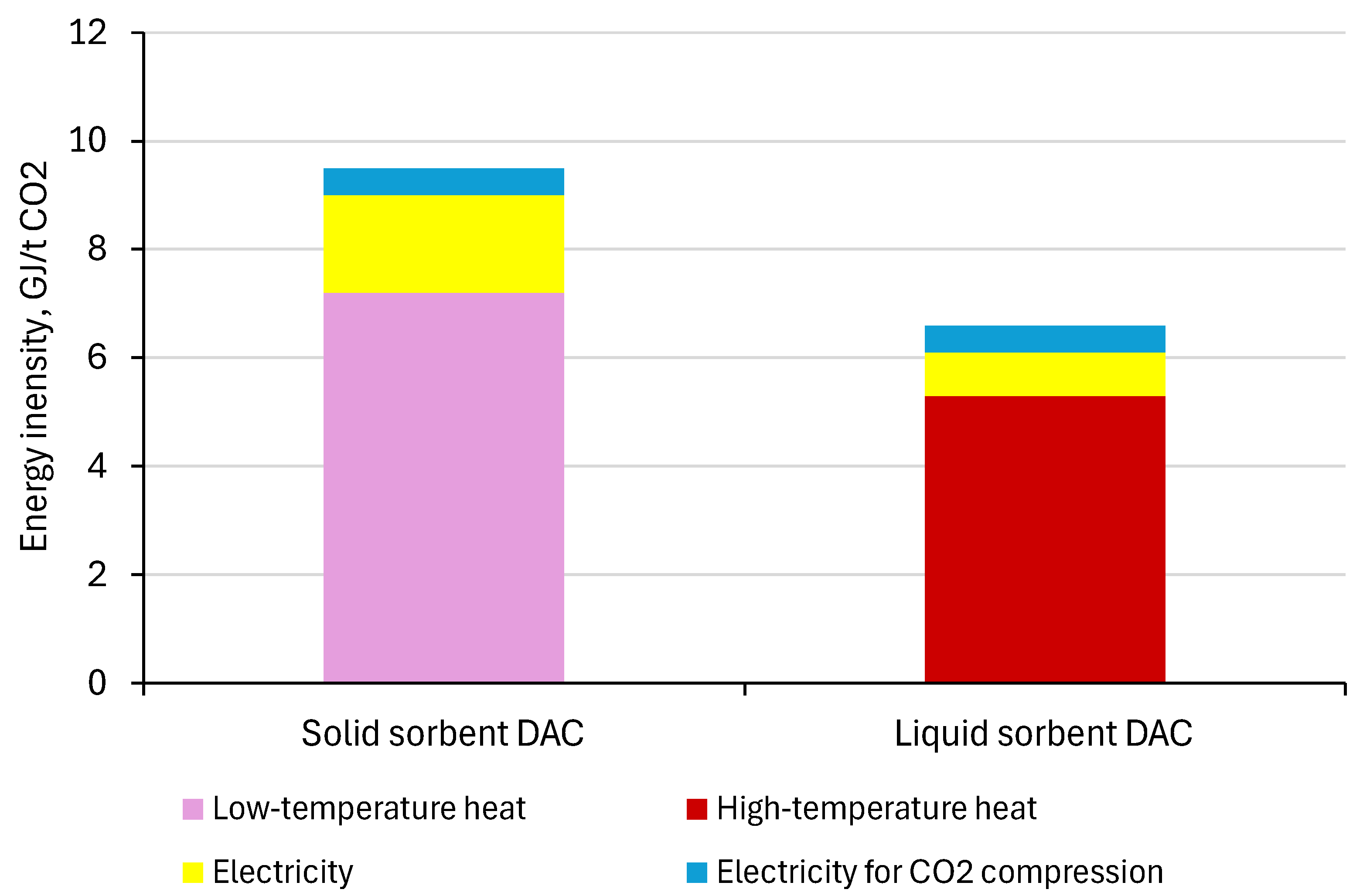
1.2. Energy Intensity of DAC Process
2. Research on New Sorbents
3. Alternative Direct Air Capture Technologies
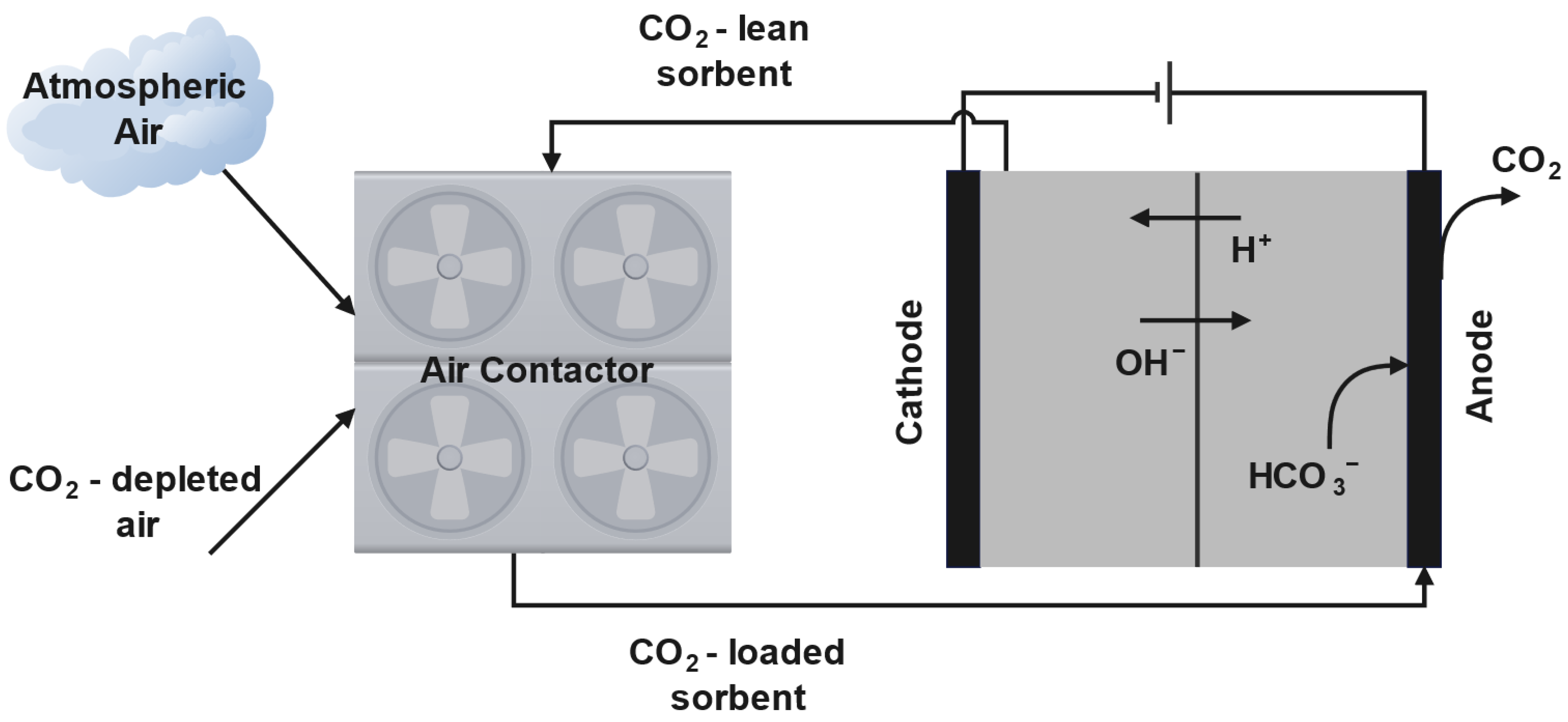
3.1. Electric-Swing Adsorption
3.2. Electrochemical Methods
3.3. Membrane Separation
4. Economic Aspects
5. Location of Installations
6. Perspective
7. Conclusions
- (a)
- Research on developing inexpensive and highly efficient sorbents should continue to reduce regeneration energy demand and process costs. For liquid sorbents, significant issues remain, such as thermal degradation, oxidation, toxicity, and corrosivity. Solid sorbents, on the other hand, should be characterized by high durability and the ability to maintain their properties over many process cycles.
- (b)
- The development of alternative capture technologies could significantly increase the profitability of DAC technology in the future. Both electrochemical and membrane processes still require further research. Electrochemical processes are particularly sensitive to the presence of oxygen, while the efficiency of membrane-based DAC is limited by the current separation capabilities of available membranes. Among the available solutions, ESA technology, developed by the American company Verdox, seems the most promising.
- (c)
- The cost of CO2 capture in DAC technology remains uncertain, with current estimates subject to significant uncertainty. Currently, DAC projects are primarily financed by the private sector; however, widespread implementation of this technology will require government support, including appropriate financial and regulatory instruments. The literature contains few techno-economic analyses based on actual data from pilot or demonstration units [98]. A significant problem is the considerable discrepancy between academic estimates and those declared by startups. Therefore, the need for more detailed and reliable economic analyses is emphasized, which will better assess the potential of DAC technology in the long term.
- (d)
- The greatest advantage of DAC systems is their independence from the location of CO2 emission sources. Nevertheless, the location of installations is not entirely free from limitations. Key factors include energy availability and the possibility of storing or utilizing captured CO2. Local climatic conditions also play a significant role. Air temperature and humidity can significantly impact the efficiency and energy consumption of the capture process, depending on the sorbent used. Therefore, the construction of installations should be preceded by a comprehensive location assessment, considering both the selection of appropriate capture technology and the suitable type of sorbent.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedlingstein, P.; O’sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C.; Luijkx, I.T.; Olsen, A.; Peters, G.P.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Direct Air Capture—Energy System—IEA. Available online: https://www.iea.org/energy-system/carbon-capture-utilisation-and-storage/direct-air-capture (accessed on 25 March 2024).
- Okonkwo, E.C.; AlNouss, A.; Shahbaz, M.; Al-Ansari, T. Developing Integrated Direct Air Capture and Bioenergy with Carbon Capture and Storage Systems: Progress towards 2 °C and 1.5 °C Climate Goals. Energy Convers. Manag. 2023, 296, 117687. [Google Scholar] [CrossRef]
- Bui, M.; Fajardy, M.; Mac Dowell, N. Bio-Energy with CCS (BECCS) Performance Evaluation: Efficiency Enhancement and Emissions Reduction. Appl. Energy 2017, 195, 289–302. [Google Scholar] [CrossRef]
- Collodi, G.; Azzaro, G.; Ferrari, N.; Santos, S. Demonstrating Large Scale Industrial CCS through CCU—A Case Study for Methanol Production. Energy Procedia 2017, 114, 122–138. [Google Scholar] [CrossRef]
- Greig, C.; Uden, S. The Value of CCUS in Transitions to Net-Zero Emissions. Electr. J. 2021, 34, 107004. [Google Scholar] [CrossRef]
- Popielak, P.; Majchrzak-Kucęba, I.; Wawrzyńczak, D. Climate Change Mitigation with CCUS—A Case Study with Benchmarking for Selected Countries in Adapting the European Union’s Green Deal. Int. J. Greenh. Gas Control 2024, 132, 104057. [Google Scholar] [CrossRef]
- Niesporek, K.; Kotowicz, J.; Baszczeńska, O.; Maj, I. Performance Characteristics and Optimization of a Single-Stage Direct Air Capture Membrane System in Terms of Process Energy Intensity. Energies 2024, 17, 2046. [Google Scholar] [CrossRef]
- Bouaboula, H.; Chaouki, J.; Belmabkhout, Y.; Zaabout, A. Comparative Review of Direct Air Capture Technologies: From Technical, Commercial, Economic, and Environmental Aspects. Chem. Eng. J. 2024, 484, 149411. [Google Scholar] [CrossRef]
- Joppa, L.; Luers, A.; Willmott, E.; Friedmann, S.J.; Hamburg, S.P.; Broze, R. Microsoft’s Million-Tonne CO2-Removal Purchase—Lessons for Net Zero. Nature 2021, 597, 629–632. [Google Scholar] [CrossRef]
- Microsoft Inks Another Deal to Capture and Store Its Carbon Emissions Underground—The Verge. Available online: https://www.theverge.com/2023/3/22/23651587/microsoft-climate-tech-startup-carboncapture-wyoming (accessed on 25 March 2024).
- Heirloom Opens First, U.S. Direct Air Capture Plant—The New York Times. Available online: https://www.nytimes.com/2023/11/09/climate/direct-air-capture-carbon.html (accessed on 25 March 2024).
- World’s First Large-Scale Direct Air Capture Plant to Use Siemens Energy Equipment. Available online: https://www.siemens-energy.com/global/en/home/press-releases/worlds-first-large-scale-direct-air-capture-plant-use-siemens-energy-equipment.html (accessed on 21 December 2024).
- Amazon Supports World’s Largest Deployment of DAC Tech. Available online: https://www.aboutamazon.com/news/sustainability/amazon-direct-air-capture-investment-fights-climate-change (accessed on 21 December 2024).
- Aramco, Siemens Energy Launch Direct Air Capture Project. Available online: https://www.cnbc.com/2023/10/09/aramco-siemens-energy-launch-direct-air-capture-project-.html (accessed on 21 December 2024).
- DAC (Direct Air Capture) in 2024: Where Do We Stand?—Klima. Available online: https://klima.com/blog/dac-direct-air-capture-2024/ (accessed on 21 December 2024).
- Ozkan, M.; Nayak, S.P.; Ruiz, A.D.; Jiang, W. Current Status and Pillars of Direct Air Capture Technologies. iScience 2022, 25, 103990. [Google Scholar] [CrossRef]
- Custelcean, R. Direct Air Capture of CO2Using Solvents. Annu. Rev. Chem. Biomol. Eng. 2022, 13, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Keith, D.W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef]
- Bianchi, S. Process Modelling of a Direct Air Capture (DAC) System Based on the Kraft Process. Ph.D. Thesis, Politecnico di Torino, Torino, Italy, 2018. [Google Scholar]
- Li, G.; Yao, J. Direct Air Capture (DAC) for Achieving Net-Zero CO2 Emissions: Advances, Applications, and Challenges. Eng 2024, 5, 1298–1336. [Google Scholar] [CrossRef]
- Zolfaghari, Z.; Aslani, A.; Zahedi, R.; Kazzazi, S. Simulation of Carbon Dioxide Direct Air Capture Plant Using Potassium Hydroxide Aqueous Solution: Energy Optimization and CO2 Purity Enhancement. Energy Convers. Manag. X 2024, 21, 100489. [Google Scholar] [CrossRef]
- Direct Air Capture Technology|Carbon Engineering. Available online: https://carbonengineering.com/our-technology/ (accessed on 13 December 2024).
- Ector County DAC—STRATOS|1PointFive. Available online: https://www.1pointfive.com/projects/ector-county-tx (accessed on 21 December 2024).
- Carbon Removal|1PointFive. Available online: https://www.1pointfive.com/carbon-removal (accessed on 21 December 2024).
- Kua, H.W.; Pedapati, C.; Lee, R.V.; Kawi, S. Effect of Indoor Contamination on Carbon Dioxide Adsorption of Wood-Based Biochar—Lessons for Direct Air Capture. J. Clean. Prod. 2019, 210, 860–871. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; Alonso, M.; Stoica, A.I. Investigation of Effectiveness of KOH-Activated Olive Pomace Biochar for Efficient Direct Air Capture of CO2. Sep. Purif. Technol. 2025, 352, 127997. [Google Scholar] [CrossRef]
- Findley, J.M.; Sholl, D.S. Computational Screening of MOFs and Zeolites for Direct Air Capture of Carbon Dioxide under Humid Conditions. J. Phys. Chem. C 2021, 125, 24630–24639. [Google Scholar] [CrossRef]
- Wilson, S.M.W. High Purity CO2 from Direct Air Capture Using a Single TVSA Cycle with Na-X Zeolites. Sep. Purif. Technol. 2022, 294, 121186. [Google Scholar] [CrossRef]
- Wilson, S.M.W.; Tezel, F.H. Direct Dry Air Capture of CO2 Using VTSA with Faujasite Zeolites. Ind. Eng. Chem. Res. 2020, 59, 8783–8794. [Google Scholar] [CrossRef]
- Ayeleru, O.O.; Modekwe, H.U.; Onisuru, O.R.; Ohoro, C.R.; Akinnawo, C.A.; Olubambi, P.A. Adsorbent Technologies and Applications for Carbon Capture, and Direct Air Capture in Environmental Perspective and Sustainable Climate Action. Sustain. Chem. Clim. Action 2023, 3, 100029. [Google Scholar] [CrossRef]
- Beuttler, C.; Charles, L.; Wurzbacher, J. The Role of Direct Air Capture in Mitigation of Anthropogenic Greenhouse Gas Emissions. Front. Clim. 2019, 1, 469555. [Google Scholar] [CrossRef]
- Shi, W.K.; Zhang, X.J.; Liu, X.; Wei, S.; Shi, X.; Wu, C.; Jiang, L. Temperature-Vacuum Swing Adsorption for Direct Air Capture by Using Low-Grade Heat. J. Clean. Prod. 2023, 414, 137731. [Google Scholar] [CrossRef]
- Davis, M.M.; LeVan, M.D. Experiments on Optimization of Thermal Swing Adsorption. Ind. Eng. Chem. Res. 1989, 28, 778–785. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Grande, C.A.; Rodrigues, A.E. Electric Swing Adsorption for Gas Separation and Purification: A Review. Sep. Sci. Technol. 2014, 49, 1985–2002. [Google Scholar] [CrossRef]
- Zero Carbon Systems. Available online: https://www.zerocarbonsystems.com/ (accessed on 13 December 2024).
- Wurzbacher, J.A.; Gebald, C.; Brunner, S.; Steinfeld, A. Heat and Mass Transfer of Temperature–Vacuum Swing Desorption for CO2 Capture from Air. Chem. Eng. J. 2016, 283, 1329–1338. [Google Scholar] [CrossRef]
- Direct Air Capture Technology: Innovations in CO2 Removal. Available online: https://climeworks.com/direct-air-capture (accessed on 13 December 2024).
- Li, S.; Chen, R.; Wang, J.; Deng, S.; Zhou, H.; Fang, M.; Zhang, H.; Yuan, X. Solar Thermal Energy-Assisted Direct Capture of CO2 from Ambient Air for Methanol Synthesis. npj Mater. Sustain. 2024, 2, 11. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, W.; Yong, J.Y.; Zhang, X.J.; Jiang, L. Solar-Assisted Temperature Vacuum Swing Adsorption for Direct Air Capture: Effect of Relative Humidity. Appl. Energy 2023, 348, 121493. [Google Scholar] [CrossRef]
- Krull, L.F.; Baum, C.M.; Sovacool, B.K. A Geographic Analysis and Techno-Economic Assessment of Renewable Heat Sources for Low-Temperature Direct Air Capture in Europe. Energy Convers. Manag. 2025, 323, 119186. [Google Scholar] [CrossRef]
- Kuru, T.; Khaleghi, K.; Livescu, S. Solid Sorbent Direct Air Capture Using Geothermal Energy Resources (S-DAC-GT)—Region Specific Analysis. Geoenergy Sci. Eng. 2023, 224, 211645. [Google Scholar] [CrossRef]
- Kuru, T.; Khaleghi, K.; Livescu, S. Region Specific Economic Model for Solid Sorbent Direct Air Capture Using Geothermal Energy Resources (S-DAC-GT). Geoenergy Sci. Eng. 2024, 243, 213346. [Google Scholar] [CrossRef]
- Barzagli, F.; Peruzzini, M.; Zhang, R. Direct CO2 Capture from Air with Aqueous and Nonaqueous Diamine Solutions: A Comparative Investigation Based on 13C NMR Analysis. Carbon Capture Sci. Technol. 2022, 3, 100049. [Google Scholar] [CrossRef]
- Custelcean, R.; Williams, N.J.; Garrabrant, K.A.; Agullo, P.; Brethomé, F.M.; Martin, H.J.; Kidder, M.K. Direct Air Capture of CO2 with Aqueous Amino Acids and Solid Bis-Iminoguanidines (BIGs). Ind. Eng. Chem. Res. 2019, 58, 23338–23346. [Google Scholar] [CrossRef]
- Mokhtari-Nori, N.; Qiu, L.; Song, Y.; He, L.; Ganesan, A.; Ivanov, A.S.; Wang, Q.; Wang, T.; Yang, Z.; Dai, S. Unveiling the Porosity Effect of Superbase Ionic Liquid-Modified Carbon Sorbents in CO2 Capture from Air. Mater. Today Energy 2024, 45, 101693. [Google Scholar] [CrossRef]
- Samari, M.; Ridha, F.; Manovic, V.; Macchi, A.; Anthony, E.J. Direct Capture of Carbon Dioxide from Air via Lime-Based Sorbents. Mitig. Adapt. Strat. Glob. Change 2020, 25, 25–41. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhang, C.; Guo, S.; Li, Y. High Gravity-Enhanced Direct Air Capture: A Leap Forward in CO2 Adsorption Technology. Atmosphere 2024, 15, 238. [Google Scholar] [CrossRef]
- Wang, Y.; Anyanwu, J.T.; Hu, Z.; Yang, R.T. Significantly Enhancing CO2 Adsorption on Amine-Grafted SBA-15 by Boron Doping and Acid Treatment for Direct Air Capture. Sep. Purif. Technol. 2023, 309, 123030. [Google Scholar] [CrossRef]
- Xiang, X.; Guo, T.; Yin, Y.; Gao, Z.; Wang, Y.; Wang, R.; An, M.; Guo, Q.; Hu, X. High Adsorption Capacity Fe@13X Zeolite for Direct Air CO2 Capture. Ind. Eng. Chem. Res. 2023, 62, 5420–5429. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Liang, C.; Fang, K.; Li, S.; Guo, X.; Wang, T.; Fang, M. Direct Air Capture of CO2 Using Biochar Prepared from Sewage Sludge: Adsorption Capacity and Kinetics. Sci. Total Environ. 2024, 948, 174887. [Google Scholar] [CrossRef]
- Recker, E.A.; Green, M.; Soltani, M.; Paull, D.H.; Mcmanus, G.J.; Davis, J.H.; Mirjafari, A. Direct Air Capture of CO2via Ionic Liquids Derived from “Waste” Amino Acids. ACS Sustain. Chem. Eng. 2022, 10, 11885–11890. [Google Scholar] [CrossRef]
- Qiu, L.; Peng, L.; Moitra, D.; Liu, H.; Fu, Y.; Dong, Z.; Hu, W.; Lei, M.; Jiang, D.; Lin, H.; et al. Harnessing the Hybridization of a Metal-Organic Framework and Superbase-Derived Ionic Liquid for High-Performance Direct Air Capture of CO2. Small 2023, 19, 2302708. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Moya, C.; Gazzani, M.; Palomar, J. Direct Air Capture Based on Ionic Liquids: From Molecular Design to Process Assessment. Chem. Eng. J. 2023, 468, 143630. [Google Scholar] [CrossRef]
- Dorado-Alfaro, S.; Hospital-Benito, D.; Moya, C.; Navarro, P.; Lemus, J.; Palomar, J. Exploiting Process Thermodynamics in Carbon Capture from Direct Air to Industrial Sources: The Paradigmatic Case of Ionic Liquids. Carbon Capture Sci. Technol. 2024, 13, 100320. [Google Scholar] [CrossRef]
- Krachuamram, S.; Chanapattharapol, K.C.; Kamonsutthipaijit, N. Synthesis and Characterization of NaX-Type Zeolites Prepared by Different Silica and Alumina Sources and Their CO2 Adsorption Properties. Microporous Mesoporous Mater. 2021, 310, 110632. [Google Scholar] [CrossRef]
- Tao, Z.; Tian, Y.; Wu, W.; Liu, Z.; Fu, W.; Kung, C.-W.; Shang, J. Development of Zeolite Adsorbents for CO2 Separation in Achieving Carbon Neutrality. npj Mater. Sustain. 2024, 2, 20. [Google Scholar] [CrossRef]
- Removr. Available online: https://www.removr.com/#Projects (accessed on 16 December 2024).
- Kumar, R.; Ohtani, S.; Tsunoji, N. Direct Air Capture on Amine-Impregnated FAU Zeolites: Exploring for High Adsorption Capacity and Low-Temperature Regeneration. Microporous Mesoporous Mater. 2023, 360, 112714. [Google Scholar] [CrossRef]
- Fu, D.; Davis, M.E. Carbon Dioxide Capture with Zeotype Materials. Chem. Soc. Rev. 2022, 51, 9340–9370. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Choi, S.W.; Igalavithana, A.D.; Yang, X.; Tsang, D.C.W.; Wang, C.H.; Kua, H.W.; Lee, K.B.; Ok, Y.S. Sustainable Gasification Biochar as a High Efficiency Adsorbent for CO2 Capture: A Facile Method to Designer Biochar Fabrication. Renew. Sustain. Energy Rev. 2020, 124, 109785. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for the Direct Capture of CO2 from Ambient Air. Angew. Chem. Int. Ed. 2020, 59, 6984–7006. [Google Scholar] [CrossRef]
- Panda, D.; Kulkarni, V.; Singh, S.K. Evaluation of Amine-Based Solid Adsorbents for Direct Air Capture: A Critical Review. React. Chem. Eng. 2022, 8, 10–40. [Google Scholar] [CrossRef]
- Low, M.Y.; Barton, L.V.; Pini, R.; Petit, C. Analytical Review of the Current State of Knowledge of Adsorption Materials and Processes for Direct Air Capture. Chem. Eng. Res. Des. 2023, 189, 745–767. [Google Scholar] [CrossRef]
- Grande, C.A.; Ribeiro, R.P.L.; Oliveira, E.L.G.; Rodrigues, A.E. Electric Swing Adsorption as Emerging CO2 Capture Technique. Energy Procedia 2009, 1, 1219–1225. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, L.; Zhao, L.; Deng, S.; Li, H. Thermodynamic Analysis on Carbon Dioxide Capture by Electric Swing Adsorption (ESA) Technology. J. CO2 Util. 2018, 26, 388–396. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, W.; Wang, R.Q.; Gonzalez-Diaz, A.; Rojas-Michaga, M.F.; Michailos, S.; Pourkashanian, M.; Zhang, X.J.; Font-Palma, C. Sorption Direct Air Capture with CO2 Utilization. Prog. Energy Combust. Sci. 2023, 95, 101069. [Google Scholar] [CrossRef]
- Lee, T.S.; Cho, J.H.; Chi, S.H. Carbon Dioxide Removal Using Carbon Monolith as Electric Swing Adsorption to Improve Indoor Air Quality. Build. Environ. 2015, 92, 209–221. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, F.; Xie, K.; Singh, R.; Zhao, J.; Xiao, P.; Webley, P.A. Synthesis of a Novel Hybrid Adsorbent Which Combines Activated Carbon and Zeolite NaUSY for CO2 Capture by Electric Swing Adsorption (ESA). Chem. Eng. J. 2018, 336, 659–668. [Google Scholar] [CrossRef]
- Lee, W.H.; Zhang, X.; Banerjee, S.; Jones, C.W.; Realff, M.J.; Lively, R.P. Sorbent-Coated Carbon Fibers for Direct Air Capture Using Electrically Driven Temperature Swing Adsorption. Joule 2023, 7, 1241–1259. [Google Scholar] [CrossRef]
- Liu, G.; Yang, A.; Darton, R.C. Numerical Modeling and Comparative Analysis of Electrolysis and Electrodialysis Systems for Direct Air Capture. ACS Sustain Chem Eng 2024, 12, 3951–3965. [Google Scholar] [CrossRef]
- Liu, L.; Gong, F.; Xiao, R. Direct Air Carbon Capture and Recovery Utilizing Alkaline Solution Circulation. Energy Fuels 2023, 37, 9339–9346. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, S.; Edwards, J.P.; Xiao, Y.C.; Zhao, Y.; Miao, R.K.; Fan, M.; Chen, Y.; Huang, J.E.; Sargent, E.H.; et al. Regeneration of Direct Air CO2 Capture Liquid via Alternating Electrocatalysis. Joule 2023, 7, 2107–2117. [Google Scholar] [CrossRef]
- Rosen, N.; Welter, A.; Schwankl, M.; Plumeré, N.; Staudt, J.; Burger, J. Assessment of the Potential of Electrochemical Steps in Direct Air Capture through Techno-Economic Analysis. Energy Fuels 2024, 38, 15469–15481. [Google Scholar] [CrossRef]
- Ohiomoba, E.; Omosebi, A.; Kharel, P.; Gao, X.; Liu, K. Electrochemical Stripping of CO2 from Potassium-Based Salts to Facilitate Direct Air Capture. Electrochim. Acta 2024, 512, 145521. [Google Scholar] [CrossRef]
- Shu, Q.; Legrand, L.; Kuntke, P.; Tedesco, M.; Hamelers, H.V.M. Electrochemical Regeneration of Spent Alkaline Absorbent from Direct Air Capture. Environ. Sci. Technol. 2020, 54, 8990–8998. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, R.; Wagterveld, R.M.; Digdaya, I.A.; Xiang, C.; Vermaas, D.A. Electrochemical Carbon Dioxide Capture to Close the Carbon Cycle. Energy Environ. Sci. 2021, 14, 781–814. [Google Scholar] [CrossRef]
- Sabatino, F.; Gazzani, M.; Gallucci, F.; Van Sint Annaland, M. Modeling, Optimization, and Techno-Economic Analysis of Bipolar Membrane Electrodialysis for Direct Air Capture Processes. Ind. Eng. Chem. Res. 2022, 61, 12668–12679. [Google Scholar] [CrossRef]
- Renfrew, S.E.; Starr, D.E.; Strasser, P. Electrochemical Approaches toward CO2Capture and Concentration. ACS Catal 2020, 10, 13058–13074. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, H.Z.; Diederichsen, K.M.; Van Voorhis, T.; Hatton, T.A. Electrochemically Mediated Carbon Dioxide Separation with Quinone Chemistry in Salt-Concentrated Aqueous Media. Nat. Commun. 2020, 11, 2278. [Google Scholar] [CrossRef]
- Abdinejad, M.; Seo, H.; Lev Massen-Hane, M.E.; Hatton, T.A. Oxygen-Stable Electrochemical CO2 Capture Using Redox-Active Heterocyclic Benzodithiophene Quinone. Angew. Chem. Int. Ed. 2024, 63, e202412229. [Google Scholar] [CrossRef]
- Voskian, S.; Hatton, T.A. Faradaic Electro-Swing Reactive Adsorption for CO2 Capture. Energy Env. Sci 2019, 12, 3530–3547. [Google Scholar] [CrossRef]
- Shaw, R.A.; Hatton, T.A. Electrochemical CO2 Capture Thermodynamics. Int. J. Greenh. Gas Control 2020, 95, 102878. [Google Scholar] [CrossRef]
- Choi, G.H.; Song, H.J.; Lee, S.; Kim, J.Y.; Moon, M.W.; Yoo, P.J. Electrochemical Direct CO2 Capture Technology Using Redox-Active Organic Molecules to Achieve Carbon-Neutrality. Nano Energy 2023, 112, 108512. [Google Scholar] [CrossRef]
- Seo, H.; Hatton, T.A. Electrochemical Direct Air Capture of CO2 Using Neutral Red as Reversible Redox-Active Material. Nat. Commun. 2023, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Technology—Verdox. Available online: https://verdox.com/technology (accessed on 16 December 2024).
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-Combustion Carbon Capture by Membrane Separation, Review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Kotowicz, J.; Janusz-Szymańska, K.; Wiciak, G. Technologie Membranowe Wychwytu Dwutlenku Węgla Ze Spalin Dla Nadkrytycznego Bloku Węglowego; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2015. [Google Scholar]
- Gkotsis, P.; Peleka, E.; Zouboulis, A. Membrane-Based Technologies for Post-Combustion CO2 Capture from Flue Gases: Recent Progress in Commonly Employed Membrane Materials. Membranes 2023, 13, 898. [Google Scholar] [CrossRef]
- Carapellucci, R.; Giordano, L.; Vaccarelli, M. Study of a Natural Gas Combined Cycle with Multi-Stage Membrane Systems for CO2 Post-Combustion Capture. Energy Procedia 2015, 81, 412–421. [Google Scholar] [CrossRef]
- Kotowicz, J.; Chmielniak, T.; Janusz-Szymańska, K. The Influence of Membrane CO2 Separation on the Efficiency of a Coal-Fired Power Plant. Energy 2010, 35, 841–850. [Google Scholar] [CrossRef]
- Ignatusha, P.; Lin, H.; Kapuscinsky, N.; Scoles, L.; Ma, W.; Patarachao, B.; Du, N. Membrane Separation Technology in Direct Air Capture. Membranes 2024, 14, 30. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Zamidi Ahmad, M.; Malankowska, M.; Coronas, J. A New Relevant Membrane Application: CO2 Direct Air Capture (DAC). Chem. Eng. J. 2022, 446, 137047. [Google Scholar] [CrossRef]
- Fujikawa, S.; Selyanchyn, R. Direct Air Capture by Membranes. MRS Bull. 2022, 47, 416–423. [Google Scholar] [CrossRef]
- Fujikawa, S.; Selyanchyn, R.; Kunitake, T. A New Strategy for Membrane-Based Direct Air Capture. Polym. J. 2020, 53, 111–119. [Google Scholar] [CrossRef]
- Fujikawa, S.; Ariyoshi, M.; Selyanchyn, R.; Kunitake, T. Ultra-Fast, Selective CO2 Permeation by Free-Standing Siloxane Nanomembranes. Chem. Lett. 2019, 48, 1351–1354. [Google Scholar] [CrossRef]
- Niesporek, K.; Kotowicz, J.; Janusz-Szymańska, K.; Baszczeńska, O. Wpływ Zmiany Selektywności Membrany Na Charakterystyki Pracy Membranowej Jednostki Bezpośredniego Wychwytu Dwutlenku Węgla z Powietrza. Rynek Energii 2024, 173, 58–63. [Google Scholar]
- Castel, C.; Bounaceur, R.; Favre, E. Membrane Processes for Direct Carbon Dioxide Capture From Air: Possibilities and Limitations. Front. Chem. Eng. 2021, 3, 668867. [Google Scholar] [CrossRef]
- Broehm, M.; Strefler, J.; Bauer, N. Techno-Economic Review of Direct Air Capture Systems for Large Scale Mitigation of Atmospheric CO2. SSRN Electron. J. 2015, 1–28. [Google Scholar] [CrossRef]
- House, K.Z.; Baclig, A.C.; Ranjan, M.; Van Nierop, E.A.; Wilcox, J.; Herzog, H.J. Economic and Energetic Analysis of Capturing CO2from Ambient Air. Proc. Natl. Acad. Sci. USA 2011, 108, 20428–20433. [Google Scholar] [CrossRef] [PubMed]
- Socolow, R.; Desmond, M.; Aines, R.; Blackstock, J.; Bolland, O.; Kaarsberg, T.; Lewis, N.; Mazzotti, M.; Pfeffer, A.; Sawyer, K.; et al. Direct Air Capture of CO2 with Chemicals: A Technology Assessment for the APS Panel on Public Affairs; APS Physics: College Park, MD, USA, 2011. [Google Scholar]
- Ishimoto, Y.; Sugiyama, M.; Kato, E.; Moriyama, R.; Tsuzuki, K.; Kurosawa, A. Putting Costs of Direct Air Capture in Context; FCEA Working Paper No. 2; Forum for Climate Engineering Assessment: Washington, DC, USA, 2017. [Google Scholar]
- Executive Summary—Direct Air Capture 2022—Analysis—IEA. Available online: https://www.iea.org/reports/direct-air-capture-2022/executive-summary (accessed on 19 December 2024).
- Panmao, Z. Climate Change 2022—Mitigation of Climate Change; Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- How to Get Direct Air Capture Costs to under $150 per Ton|World Economic Forum. Available online: https://www.weforum.org/stories/2023/08/how-to-get-direct-air-capture-under-150-per-ton-to-meet-net-zero-goals/ (accessed on 20 December 2024).
- Secretary Granholm Launches Carbon Negative Earthshots to Remove Gigatons of Carbon Pollution from the Air by 2050|Department of Energy. Available online: https://www.energy.gov/articles/secretary-granholm-launches-carbon-negative-earthshots-remove-gigatons-carbon-pollution (accessed on 13 January 2025).
- Sievert, K.; Schmidt, T.S.; Steffen, B. Considering Technology Characteristics to Project Future Costs of Direct Air Capture. Joule 2024, 8, 979–999. [Google Scholar] [CrossRef]
- Erans, M.; Sanz-Pérez, E.S.; Hanak, D.P.; Clulow, Z.; Reiner, D.M.; Mutch, G.A. Direct Air Capture: Process Technology, Techno-Economic and Socio-Political Challenges. Energy Environ. Sci 2022, 15, 1360–1405. [Google Scholar] [CrossRef]
- Meckling, J.; Biber, E. A Policy Roadmap for Negative Emissions Using Direct Air Capture. Nat. Commun. 2021, 12, 2051. [Google Scholar] [CrossRef]
- Ozkan, M. Atmospheric Alchemy: The Energy and Cost Dynamics of Direct Air Carbon Capture. MRS Energy Sustain. 2024, 1–16. [Google Scholar] [CrossRef]
- Sabatino, F.; Grimm, A.; Gallucci, F.; van Sint Annaland, M.; Kramer, G.J.; Gazzani, M. A Comparative Energy and Costs Assessment and Optimization for Direct Air Capture Technologies. Joule 2021, 5, 2047–2076. [Google Scholar] [CrossRef]
- Fasihi, M.; Efimova, O.; Breyer, C. Techno-Economic Assessment of CO2 Direct Air Capture Plants. J. Clean. Prod. 2019, 224, 957–980. [Google Scholar] [CrossRef]
- Azarabadi, H.; Lackner, K.S. A Sorbent-Focused Techno-Economic Analysis of Direct Air Capture. Appl Energy 2019, 250, 959–975. [Google Scholar] [CrossRef]
- Boerst, J.; Pena Cabra, I.; Sharma, S.; Zaremsky, C.; Iyengar, A.K.S. Strategic Siting of Direct Air Capture Facilities in the United States. Energies 2024, 17, 3755. [Google Scholar] [CrossRef]
- Brooks, B.G.J.; Geissler, C.H.; An, K.; McCoy, S.T.; Middleton, R.S.; Ogland-Hand, J.D. The Performance of Solvent-Based Direct Air Capture across Geospatial and Temporal Climate Regimes. Front. Clim. 2024, 6, 1394728. [Google Scholar] [CrossRef]
- An, K.; Farooqui, A.; McCoy, S.T. The Impact of Climate on Solvent-Based Direct Air Capture Systems. Appl Energy 2022, 325, 119895. [Google Scholar] [CrossRef]
- Cai, X.; Coletti, M.A.; Sholl, D.S.; Allen-Dumas, M.R. Assessing Impacts of Atmospheric Conditions on Efficiency and Siting of Large-Scale Direct Air Capture Facilities. JACS Au 2024, 4, 1883–1891. [Google Scholar] [CrossRef]
- Schellevis, H.M.; de la Combé, J.D.; Brilman, D.W.F. Optimizing Direct Air Capture under Varying Weather Conditions. Energy Adv. 2024, 3, 1678–1687. [Google Scholar] [CrossRef]
- Leonzio, G.; Fennell, P.S.; Shah, N. Modelling and Analysis of Direct Air Capture Systems in Different Locations. Chem Eng Trans 2022, 96, 1–6. [Google Scholar] [CrossRef]
- Sendi, M.; Bui, M.; Mac Dowell, N.; Fennell, P. Geospatial Analysis of Regional Climate Impacts to Accelerate Cost-Efficient Direct Air Capture Deployment. One Earth 2022, 5, 1153–1164. [Google Scholar] [CrossRef]
- Remiorz, L.; Wiciak, G.; Grzywnowicz, K. Novel Concept of Supporting the Membrane Separation of CO2 in Power Plants by Thermoacoustic Dehumidification. Energy 2019, 189, 116191. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Ferrari, M.C.; Brandani, S. Humidity Impact on the Gas Permeability of PIM-1 Membrane for Post-Combustion Application. Energy Procedia 2014, 63, 194–201. [Google Scholar] [CrossRef]




| Section | Reaction | Enthalpy of Reaction |
|---|---|---|
| Air Contactor | Or | Or |
| Pellet Reactor | Or | Or |
| Calciner | ||
| Slaker |
| DAC Process Type | Sorbent | Reference |
|---|---|---|
| L-DAC | Diamines | [44] |
| L-DAC | Aqueous amino acid salts | [45] |
| L-DAC | Ionic liquids | [46] |
| S-DAC | Lime-based sorbents | [47] |
| S-DAC | TEPA-Al2O3 | [48] |
| S-DAC | Amine-grafted SBA-15 by boron doping and acid treatment | [49] |
| S-DAC | Zeolites | [29,50] |
| S-DAC | KOH-activated olive pomace biochar | [27] |
| S-DAC | NaOH-activated biochar prepared from sewage sludge | [51] |
| S-DAC | Ionic liquid-modified carbon | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotowicz, J.; Niesporek, K.; Baszczeńska, O. Advancements and Challenges in Direct Air Capture Technologies: Energy Intensity, Novel Methods, Economics, and Location Strategies. Energies 2025, 18, 496. https://doi.org/10.3390/en18030496
Kotowicz J, Niesporek K, Baszczeńska O. Advancements and Challenges in Direct Air Capture Technologies: Energy Intensity, Novel Methods, Economics, and Location Strategies. Energies. 2025; 18(3):496. https://doi.org/10.3390/en18030496
Chicago/Turabian StyleKotowicz, Janusz, Kamil Niesporek, and Oliwia Baszczeńska. 2025. "Advancements and Challenges in Direct Air Capture Technologies: Energy Intensity, Novel Methods, Economics, and Location Strategies" Energies 18, no. 3: 496. https://doi.org/10.3390/en18030496
APA StyleKotowicz, J., Niesporek, K., & Baszczeńska, O. (2025). Advancements and Challenges in Direct Air Capture Technologies: Energy Intensity, Novel Methods, Economics, and Location Strategies. Energies, 18(3), 496. https://doi.org/10.3390/en18030496







