Closing the Loop of Biowaste Composting by Anaerobically Co-Digesting Leachate, a By-Product from Composting, with Glycerine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Feedstocks for MP Tests
2.2. Inoculum Used in Testing the MP
2.3. System for Measurement of MP
2.4. Organization of the Experiments
2.5. Analytical Methods
2.6. Kinetic Model for BOD Analyses, for MP, VFA and COD Removal
- Ct,MP (NL/kg CODadded) is the cumulative MP at digestion time t (days);
- Cf,MP (NL/kg CODadded) is the maximal MP;
- Ci,MP (NL/kg CODadded) is the initial MP;
- kMP (d−1) is the rate constant of MP.
- Ct,BOD7 (mg/L) is the cumulative BOD7 during 7 days (t) of measurements;
- Cf,BOD7 (mg/L) is the final BOD7;
- Ci,BOD7 (mg/L) is the initial BOD7;
- kBOD7 (d−1) is the rate constant of oxygen consumption for the oxidation of BOD compounds.
- Ct,COD (mg/L) is the COD concentration at digestion time t (days);
- Ci,COD (mg/L) is the initial COD concentration;
- kCOD (mg/(L·d)) is the rate constant of COD removal, which is equal to the rate of COD removal (rCOD, mg/(L·d)).
- Ct,VFA (mg/L) is the VFA concentration at digestion time t (days);
- Ci,VFA (mg/L) is the initial VFA concentration;
- kVFA (mg/(L·d) is the rate constant of the decrease in VFA concentration, which is equal to the rate of VFA removal (rVFA, mg/(L·d)).
3. Results and Discussion
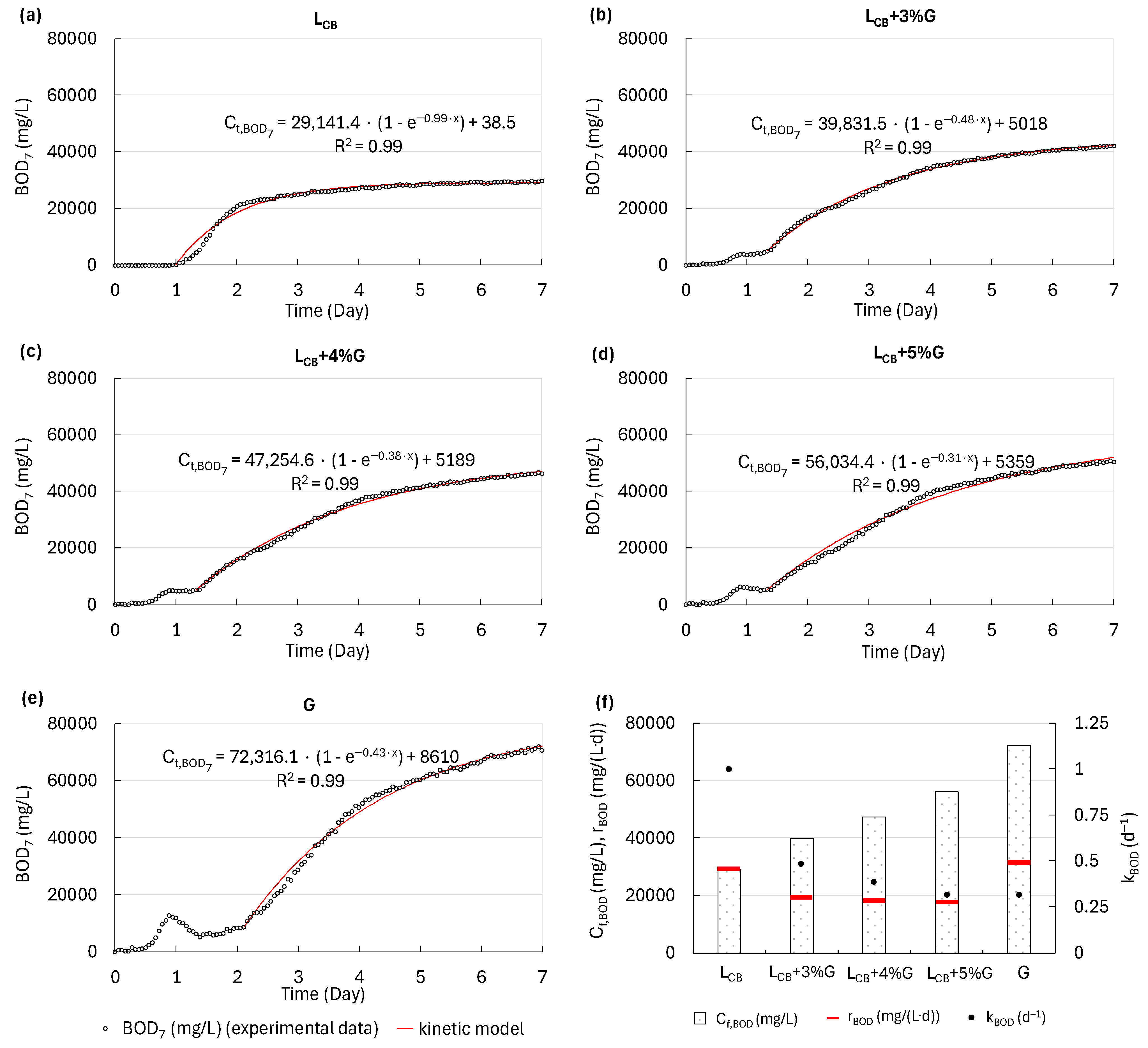
3.1. The Amounts and Biodegradability of Leachate
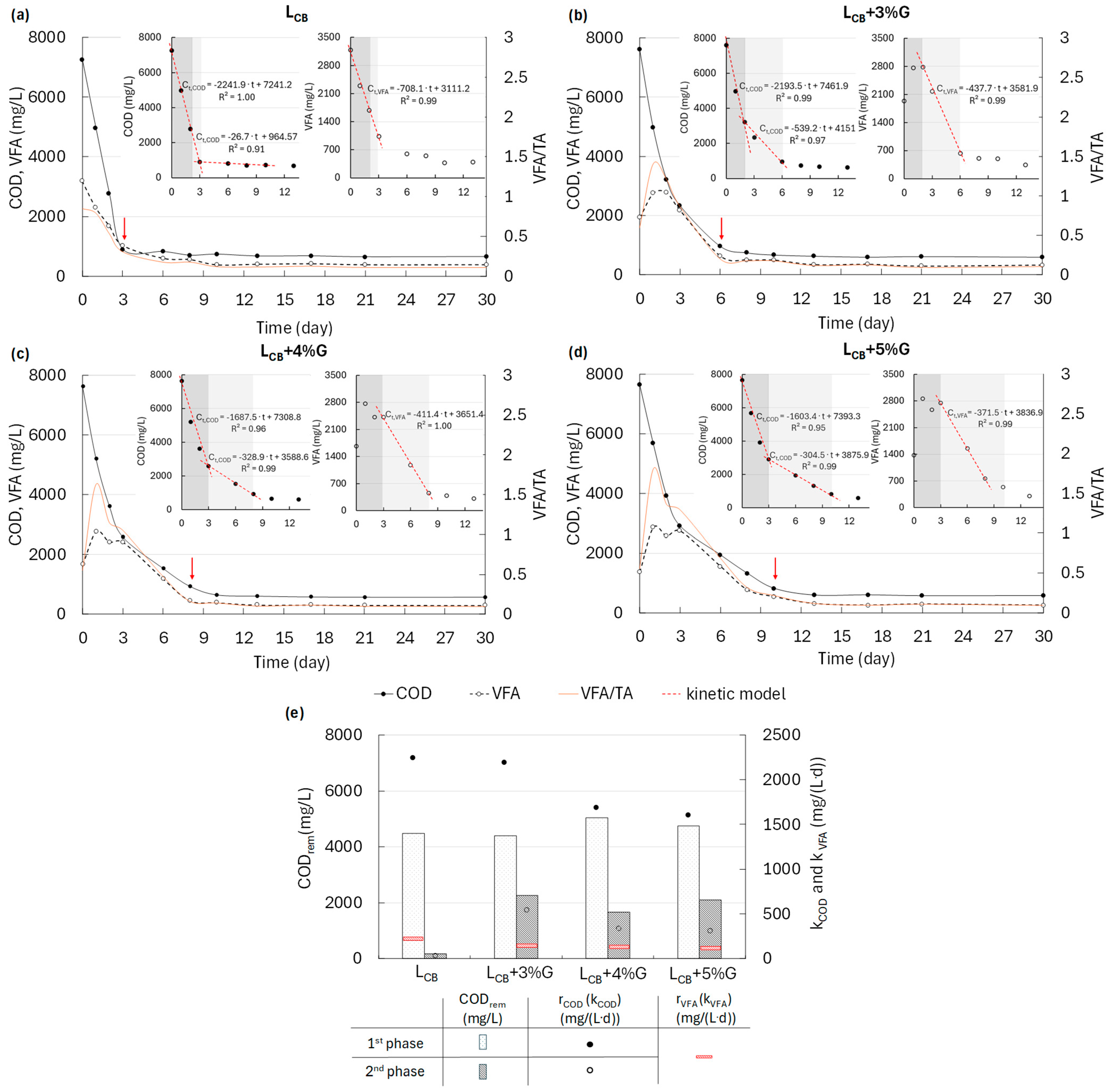
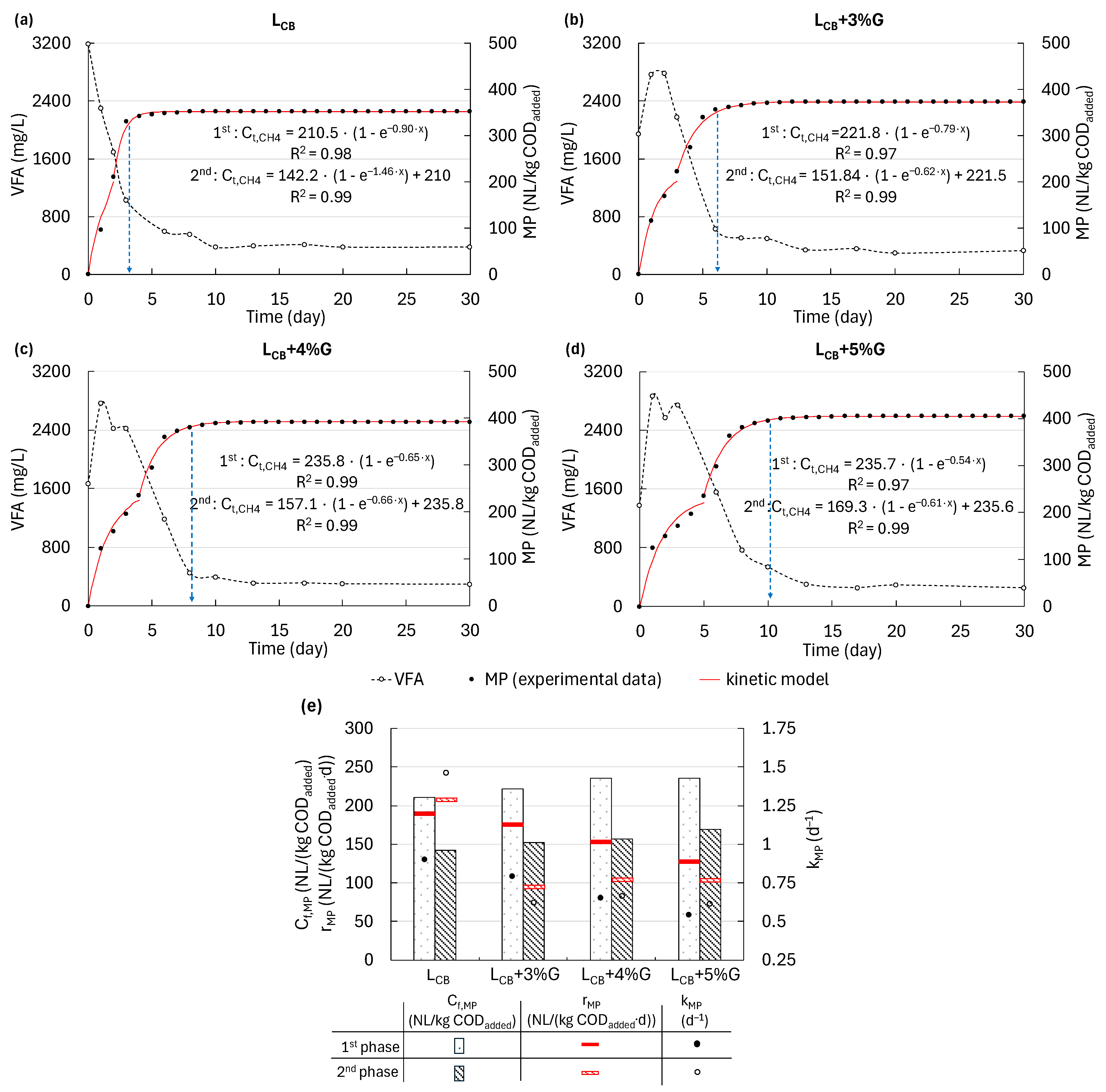
3.2. Changes in the Concentrations of Total VFA and COD During MP
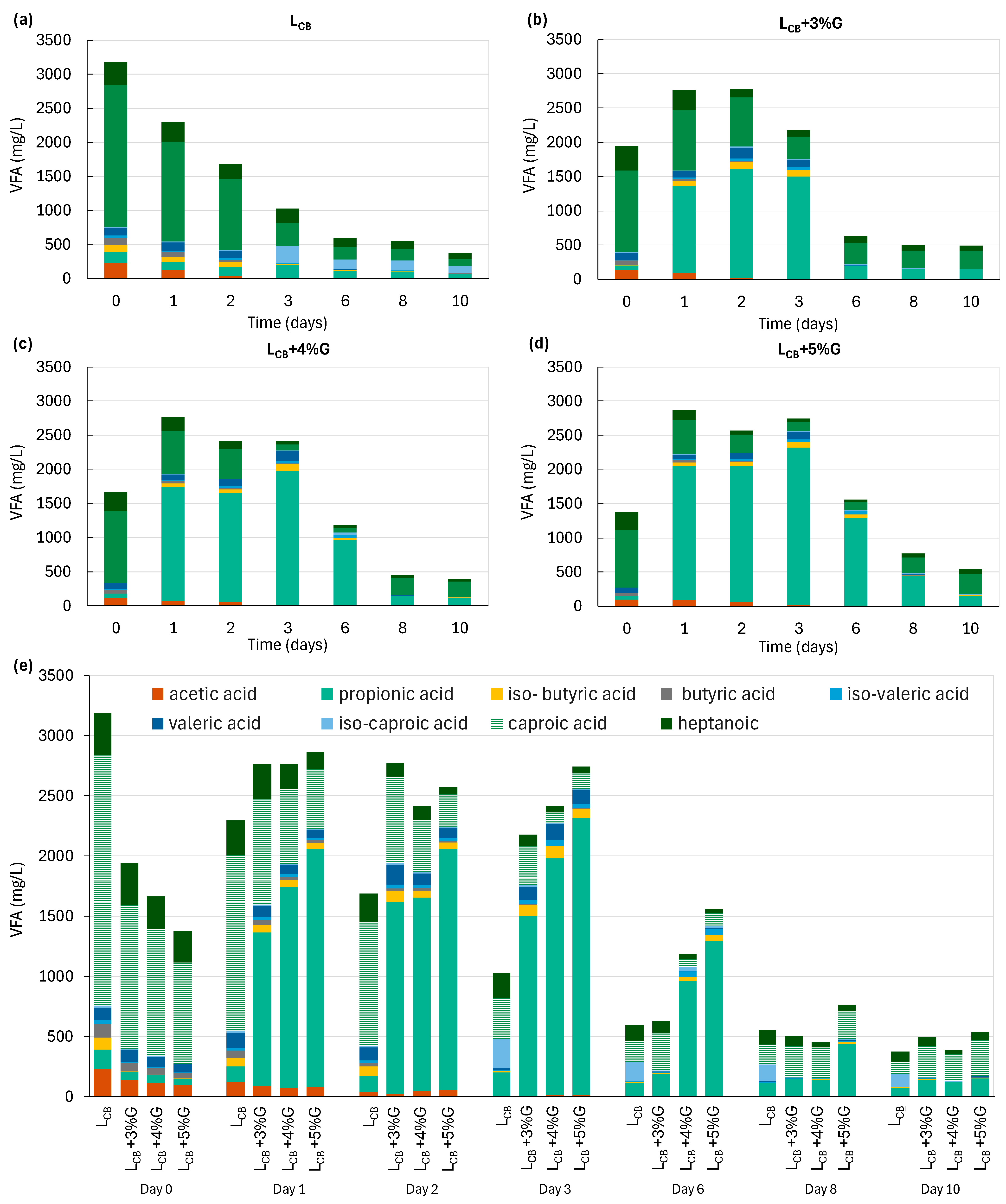
3.3. Changes in the Individual VFA Concentration and the Effect of VFA Accumulation on MP
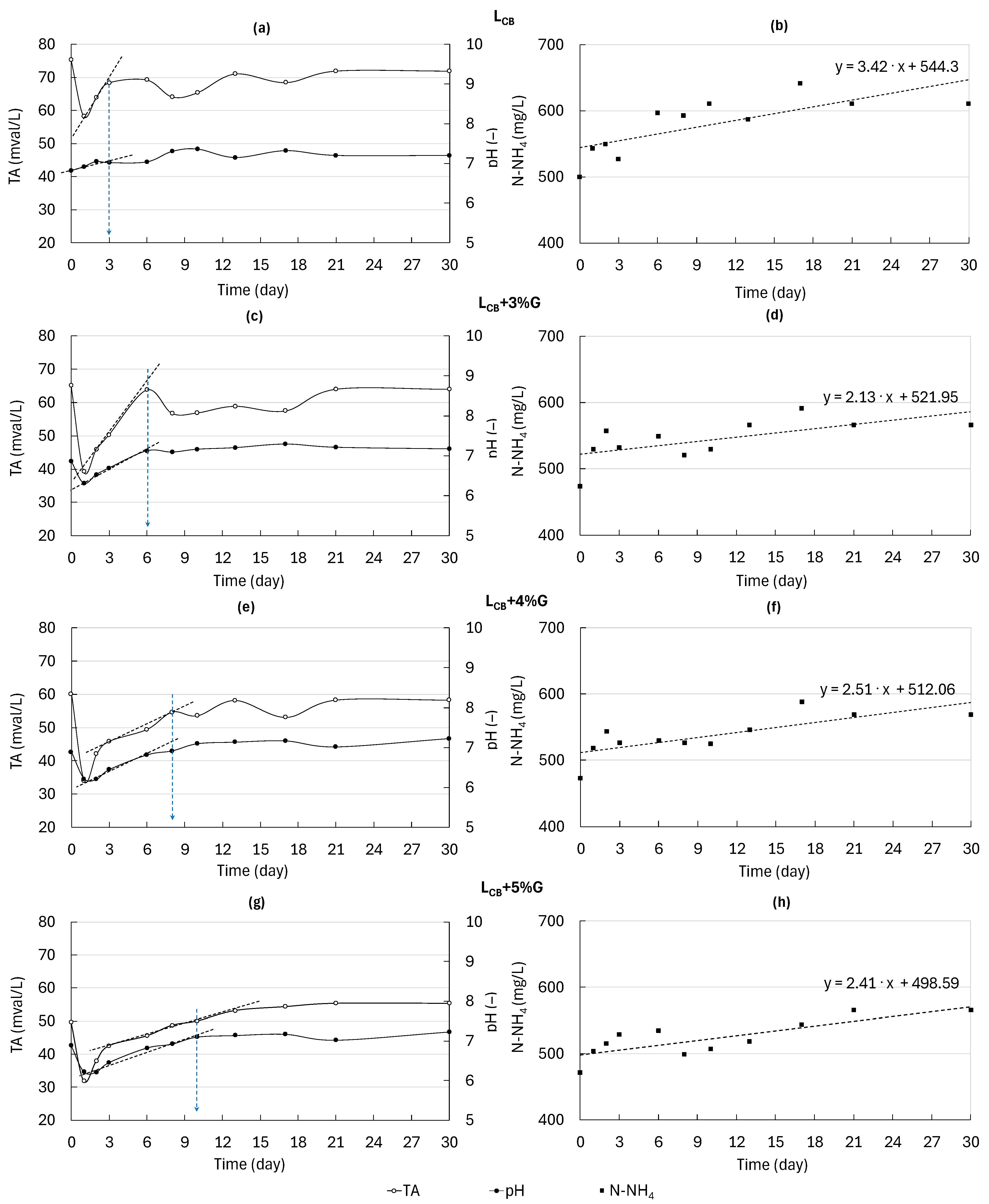
3.4. Environmental Conditions of AD of LCB and Co-Digestion of LCB and G
3.5. MP During AD of LCB and Co-Digestion of LCB and G
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Union. Directive (EU) 2018/850 of the European Parliament and of the Council of 30 May 2018. Amending Directive 1999/31/EC on the Landfill of Waste (Text with EEA Relevance). Off. J. Eur. Union. 2018, 150, 100–108. [Google Scholar]
- Eurostat. Municipal Waste Statistics. 2022. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Municipal_waste_statistics (accessed on 16 January 2025).
- Bayard, R.; Benbelkacem, H.; Gourdon, R.; Buffiere, P. Characterization of selected municipal solid waste components to estimate their biodegradability. J. Environ. Manag. 2018, 216, 4–12. [Google Scholar] [CrossRef]
- Bernat, K.; Wojnowska-Baryła, I.; Kamińska, A.; Zaborowska, M. Towards a circular economy for stabilized residual from organic municipal solid waste processed at an MBT installation—The potential of SR recycling and recovery. Desalin. Water Treat. 2021, 244, 63–76. [Google Scholar] [CrossRef]
- Bernat, K.; Zaborowska, M.; Wojnowska-Baryła, I.; Samul, I. Insight into the composition of the stabilized residual from a full-scale mechanical-biological treatment (MBT) plant in terms of the potential recycling and recovery of its contaminants. Sustainability 2021, 13, 5432. [Google Scholar] [CrossRef]
- Jędrczak, A.; den Boer, E.; Kamińska-Borak, J.; Kozłowska, B.; Szpadt, R.; Mierzwinski, A.; Krzysków, A.; Kundegórski, M. Analysis of Waste Management Costs—Assessment of Investment Needs in the Country in the Field of Waste Prevention and Waste Management in Connection with the New Eu Financial Perspective 2021–2027; IOS’-PIB, NFOS’iGW: Warsaw, Poland, 2020. (In Polish) [Google Scholar]
- Połomka, J.; Jędrczak, A. Efficiency of waste processing in the MBT system. Waste. Manag. 2019, 96, 9–14. [Google Scholar] [CrossRef]
- Zero Waste Europe. 2021. Available online: https://zerowasteeurope.eu/wp-content/uploads/2022/06/ZWE-Annual-Report-2021.pdf (accessed on 16 January 2025).
- European Commission (EC). Directive 2008/98/EC on Waste (Waste Framework Directive)—Environment. 2008. Available online: http://data.europa.eu/eli/dir/2008/98/oj (accessed on 16 January 2025).
- European Commission (EC). The European Green DealCOM 640 Final, Brussels, 11 December 2019b. 2019. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:b828d165-1c22-11ea-8c1f-01aa75ed71a1.0002.02/DOC_1&format=PDF (accessed on 16 January 2025).
- ECN (Ed.) ECN Data Report 2022-Compost and Digestate for a Circular Economy; European Compost Network (ECN) e.V.: Bochum, Germany, 2022. [Google Scholar]
- Defra. Mechanical Biological Treatment of Municipal Solid Waste, PB13890. Department for Environment, Food and Rural Affairs, London. 2013. Available online: https://assets.publishing.service.gov.uk/media/5a7c918640f0b626628acfb3/pb13890-treatment-solid-waste.pdf (accessed on 16 January 2025).
- EPA. Approaches to Composting. Available online: https://www.epa.gov/sustainable-management-food/approaches-composting (accessed on 16 January 2025).
- Čeh, B.; Luskar, L.; Hladnik, A.; Trošt, Ž.; Polanšek, J.; Naglič, B. The Quantity and Composition of Leachate from Hop Plant Biomass during Composting Process. Appl. Sci. 2022, 12, 2375. [Google Scholar] [CrossRef]
- Nayono, S.E.; Winter, J.; Gallert, C. Anaerobic digestion of pressed off leachate from the organic fraction of municipal solid waste. Waste. Manag. 2010, 30, 1828–1833. [Google Scholar] [CrossRef]
- Limonti, C.; Curcio, G.M.; Siciliano, A.; Le Pera, A.; Demirer, G.N. Kinetic study of anaerobic digestion of compost leachate from organic fraction of municipal solid waste. Fermentation 2023, 9, 297. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossaini, H.; Amini, J. Operational parameters influenced on biogas production in zeolite/anaerobic baffled reactor for compost leachate treatment. J. Environ. Health Sci. Eng. 2021, 30, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- He, X.S.; Xi, B.D.; Zhang, Z.Y.; Gao, R.T.; Tan, W.B.; Cui, D.Y.; Yuan, Y. Composition, removal, redox, and metal complexation properties of dissolved organic nitrogen in composting leachates. J. Hazard. Mater. 2015, 283, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sanadi, N.F.A.; Fan, Y.V.; Lee, C.T.; Ibrahim, N.; Li, C.; Gao, Y.; Ong, P.Y.; Klemes, J.J. Nutrient in leachate of biowaste compost and its availability for plants. Chem. Eng. Trans. 2019, 76, 1369–1374. [Google Scholar] [CrossRef]
- National Waste Management Plan 2028 (Polish Monitor of 12 June 2023, Official Journal, Item 702), Resolution No. 96 of the Council of Ministers of the 12th of July 2023 Regarding to the National Waste Plan 2028. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WMP20230000702/O/M20230702.pdf (accessed on 16 January 2025).
- EEA. Waste Recycling in Europe. 2021. Available online: https://www.eea.europa.eu/ims/waste-recycling-in-europe (accessed on 16 January 2025).
- Moujanni, A.E.; Qarraey, I.; Ouatmane, A. Anaerobic codigestion of urban solid waste fresh leachate and domestic wastewaters: Biogas production potential and kinetic. Environ. Eng. Res. 2019, 24, 38. [Google Scholar] [CrossRef]
- Bernat, K.; Zaborowska, M.; Zielińska, M.; Wojnowska-Baryła, I.; Ignalewski, W. Biological treatment of leachate from stabilization of biodegradable municipal solid waste in a sequencing batch biofilm reactor. Int. J. Environ. Sci. Technol. 2021, 18, 1047–1060. [Google Scholar] [CrossRef]
- Stegmann, R.; Heyer, K.U.; Cossu, R. Leachate treatment. In Proceedings of the Sardinia 2005, Tenth International Waste Management and Landfill Symposium, S. Margherita di Pula, Cagliari, Italy, 3–7 October 2005. [Google Scholar]
- Zayen, A.; Schories, G.; Sayadi, S. Incorporation of an anaerobic digestion step in a multistage treatment system for sanitar landfill leachate. Waste Manag. 2016, 53, 32–39. [Google Scholar] [CrossRef]
- Freire, R.C.M.; Aguiar, A.C.M.; Nascimento, M.A.; Cruz, F.S.O.; Mounteer, A.H.; Silva, A.A.; Lopes, R.P. Anaerobically treated leachate from a composting plant: Characterization and evaluation as a biofertilizer. J. Braz. Chem. Soc. 2024, 35, 1–9. [Google Scholar] [CrossRef]
- Kontodimos, I.; Ketikidis, C.; Grammelis, P. Valorization of food waste leachates through anaerobic digestion. Eng. Proc. 2023, 31, 25. [Google Scholar] [CrossRef]
- de Castro, T.M.; Torres, D.G.B.; Arantes, E.J.; de Carvalho, K.Q.; Passig, F.H.; Christ, D.; Gomes, S.D. Anaerobic co-digestion of industrial landfill leachate and glycerin: Methanogenic potential, organic matter removal and process optimization. Environ. Technol. 2019, 41, 2583–2593. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2016, 22, 261–265. [Google Scholar] [CrossRef]
- Attarbachi, T.; Kingsley, M.D.; Spallina, V. New trends on crude glycerol purification: A review. Appl. Eng. Agric. 2016, 22, 261–265. [Google Scholar] [CrossRef]
- Vásquez, J.; Nakasaki, K. Effects of shock loading versus stepwise acclimation on microbial consortia during the anaerobic digestion of glycerol. Biomass Bioenergy 2016, 86, 129–135. [Google Scholar] [CrossRef]
- López, J.A.S.; Martín Santos, M.d.l.A.; Chica Pérez, A.F.; Martín Martín, A. Anaerobic digestion of glycerol derived from biodiesel manufacturing. Bioresour. Technol. 2009, 100, 5609–5615. [Google Scholar] [CrossRef]
- Viana, M.B.; Freitas, A.V.; Leitão, R.C.; Pinto, G.A.S.; Santaella, S.T. Anaerobic digestion of crude glycerol: A review. Environ. Technol. Rev. 2012, 1, 81–92. [Google Scholar] [CrossRef]
- de Castro, T.M.; Arantes, E.J.; de Mendonça Costa, M.S.S.; Gotardo, J.T.; Passig, F.H.; Carvalho, K.Q.; Gomes, S.D. Anaerobic co-digestion of industrial waste landfill leachate and glycerin in a continuous anaerobic bioreactor with a fixed-structured bed (ABFSB): Effects of volumetric organic loading rate and alkaline supplementation. Renew. Energy 2021, 164, 1436–1446. [Google Scholar] [CrossRef]
- DIN EN 1899-1/EN 1899-2 EPA Method, OxiTop WTW Wissenschaftlich-Technische Werkstätten GmbH, Weilheim, Germany. Available online: https://envcoglobal.com/wp-content/uploads/2014/10/oxitop-spec-sheet-2008.pdf (accessed on 16 January 2025).
- Greenberg, A.E.; Clesceri, L.S.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association (APHA): Washington, DC, USA, 1992. [Google Scholar]
- Roy, D.; Benkaraache, S.; Azaïs, A.; Drogui, P.; Rajeshwar, D.T. Leachate treatment: Assessment of the systemic changes in the composition and biodegradability of leachates originating in an open co-composting facility in Canada. J. Environ. Chem. Eng. 2019, 7, 1103056. [Google Scholar] [CrossRef]
- Simonič, M. Compost leachate pretreatment by coagulation/flocculation followed by Filter press. J. Chem. Chem. Eng. Croat. 2023, 72, 545–549. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, W.; Zhang, X.; Yu, L.; Zhou, J.; Xu, Y.; Qian, G. Phosphate enhancing fermentative hydrogen production from substrate with municipal solid waste composting leachate as a nutrient. Biores. Technol. 2015, 190, 431–437. [Google Scholar] [CrossRef]
- Ibrahim, I.C.; Prudent, P.; Théraulaz, F.; Farnet Da Silva, A.M.; Asia, L.; Gori, D.; Vassalo, L.; Durand, A.; Demelas, C.; Höhener, P.; et al. Treatment of sewage sludge compost leachates on a green waste biopile: A case study for an on-site application. Processes 2022, 10, 1196. [Google Scholar] [CrossRef]
- Bakhshoodeh, R.; Alavi, N.; Majlesi, M.; Paydary, P. Compost leachate treatment by a pilot-scale subsurface horizontal flow constructed wetland. Ecol. Eng. 2017, 105, 7–14. [Google Scholar] [CrossRef]
- Henze, M.; Mladenovski, C. Hydrolysis of particulate substrate by activated sludge under aerobic, anoxic and anaerobic conditions. Water Res. 1994, 25, 61–64. [Google Scholar] [CrossRef]
- Menzel, T.; Neubauer, P.; Junne, S. Role of microbial hydrolysis in anaerobic digestion. Energies 2020, 13, 5555. [Google Scholar] [CrossRef]
- Park, J.G.; Lee, B.; Jo, S.Y.; Lee, J.S.; Jun, H.B. Control of accumulated volatile fatty acids by recycling nitrified effluent. J. Environ. Health Sci. Eng. 2018, 6, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.J.P.; Mishra, R.K.; Chinnam, S.; Binnal, P.; Dwivedi, N. A comprehensive study on anaerobic digestion of organic solid waste: A review on configurations, operating parameters, techno-economic analysis and current trends. Biotechnol. Notes. 2024, 5, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Biebl, H.; Menzel, K.; Zeng, A.P.; Deckwer, W.D. Microbial production of 1,3-propanediol. Appl. Microbiol. Biotechnol. 1999, 52, 289–297. [Google Scholar] [CrossRef]
- Thierry, A.; Maillard, M.B.; Yvon, M. Conversion of L-leucine to isovaleric acid by Propionibacterium freudenreichii TL 34 and ITGP23. Appl. Environ. Microbiol. 2002, 68, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Pogozheva, I.D.; Armstrong, G.A.; Kong, L.; Hartnagel, T.J.; Carpino, C.A.; Gee, S.E.; Picarello, D.M.; Rubin, A.S.; Lee, J.; Park, S.; et al. Comparative molecular dynamics simulation studies of realistic eukaryotic, prokaryotic, and archaeal membranes. J. Chem. Inf. Model. 2022, 62, 1036–1051. [Google Scholar] [CrossRef]
- Wang, Q.; Kuninobu, M.; Ogawa, H.I.; Kato, Y. Degradation of volatile fatty acids in highly efficient anaerobic digestion. Biomass Bioenergy 1999, 16, 6407–6416. [Google Scholar] [CrossRef]
- Chakraborty, D.; Karthikeyan, O.P.; Selvam, A.; Wong, J.W. Co-digestion of food waste and chemically enhanced primary treated sludge in a continuous stirred tank reactor. Biomass Bioenergy 2018, 111, 232–240. [Google Scholar] [CrossRef]
- Cavalcante, W.A.; Leitão, R.C.; Gehring, T.A.; Angenent, L.T.; Santaella, S.T. Anaerobic fermentation for n-caproic acid production: A review. Process Biochem. 2017, 54, 106–119. [Google Scholar] [CrossRef]
- Lee, D.H.; Behera, S.K.; Kim, J.W.; Park, H.S. Methane production potential of leachate generated from Korean food waste recycling facilities: A lab-scale study. Waste Manag. 2009, 29, 876–882. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, S.; Zhong, D.; Zhu, J.; Liao, L. Anaerobic co-digestion of food waste and landfill leachate in single-phase batch reactors. Waste Manag. 2014, 34, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Takeda, P.Y.; Gotardo, J.T.; Gomes, S.D. Anaerobic co-digestion of leachate and glycerol for renewable energy generation. Environ. Technol. 2020, 43, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Astals, S.; Ariso, M.; Galí, A.; Mata-Alvarez, J. Co-digestion of pig manure and glycerine: Experimental and modelling study. J. Environ. Manag. 2011, 92, 1091–1096. [Google Scholar] [CrossRef]
- Simm, S.; Orrico, A.C.A.; Junior, M.A.P.O.; Sunada, N.S.; Schwingel, A.W.; Costa, M.S.S.M. Crude glycerin in anaerobic co-digestion of dairy cattle manure increases methane production. Sci. Agric. 2016, 74, 175–179. [Google Scholar] [CrossRef]
- Pazuch, F.A.; Siqueira, J.; Friedrich, L.; Lenz, A.M.; Nogueira, C.E.C.; Souza, S.N.M. Co-digestion of crude glycerin associated with cattle manure in biogas production in the State of Paraná, Brazil. Acta Sci. Technol. 2017, 39, 149–159. [Google Scholar] [CrossRef]
- Amon, T.H.; Amon, B.; Kryvoruchko, V.; Bodiroza, V.; Pötsch, E.; Zollitsch, W. Optimising methane yield from anaerobic digestion of manure: Effects of dairy systems and of glycerine supplementation. Int. Congr. Ser. 2006, 1293, 217–220. [Google Scholar] [CrossRef]
- Robra, S.; da Cruz, R.S.; De Oliveira, A.M.; Neto, J.A.; Santos, J.V. Generation of biogas using crude glycerin from biodiesel production as a supplement to cattle slurry. Biomass Bioenergy 2010, 34, 1330–1335. [Google Scholar] [CrossRef]
- Chou, Y.C.; Su, J.J. Biogas production by anaerobic co-digestion of dairy wastewater with the crude glycerol from slaughterhouse sludge cake transesterification. Animals 2019, 9, 618. [Google Scholar] [CrossRef]
| Characteristic | Unit | Glycerine | Inoculum |
|---|---|---|---|
| DM | % | – | 1.42 |
| OM | %DM | – | 66.5 |
| pH | – | 6.86 | 7.03 |
| TA | mval/L | 4.80 | 52.50 |
| COD | mg/L | 100,000.00 | 1076.00 |
| BOD7 | mg/L | 70,730.00 | – |
| VFA | mg/L | 1008.00 | 591.00 |
| TKN | mg/L | 160.00 | – |
| N-NH4 | mg/L | 14.00 | 466.20 |
| COD/TKN | – | ~625.00 | – |
| Density in 20 °C | kg/L | 1.26 | – |
| Glycerol content | % | 94.10 | – |
| Characteristic | Unit | LCB | LCB+3%G | LCB+4%G | LCB+5%G |
|---|---|---|---|---|---|
| pH | – | 5.64 | 6.87 | 6.89 | 6.90 |
| TA | mval/L | 76.85 | 65.20 | 60.20 | 49.60 |
| COD | mg/L | 41,260.00 | 58,882.00 | 64,756.00 | 70,630.00 |
| BOD7 | mg/L | 29,866.00 | 42,125.2 | 46,211.65 | 50,297.98 |
| BOD7/COD | – | 0.72 | 0.72 | 0.71 | 0.71 |
| VFA | mg/L | 17,464.29 | 12,528.03 | 10,941.97 | 8733.20 |
| TKN | mg/L | 1438.00 | 1066.60 | 942.80 | 819.37 |
| COD/TKN | – | 28.70 | 55.20 | 68.70 | 86.20 |
| Doses of L/G | mL | 54.53 | 26.75/11.46 | 20.85/13.90 | 15.93/15.93 |
| Inoculum/substrate ratio | – | 5.50 | 7.85 | 8.63 | 9.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.C.T.; Bernat, K.; Pokój, T.; Kulikowska, D. Closing the Loop of Biowaste Composting by Anaerobically Co-Digesting Leachate, a By-Product from Composting, with Glycerine. Energies 2025, 18, 537. https://doi.org/10.3390/en18030537
Le TCT, Bernat K, Pokój T, Kulikowska D. Closing the Loop of Biowaste Composting by Anaerobically Co-Digesting Leachate, a By-Product from Composting, with Glycerine. Energies. 2025; 18(3):537. https://doi.org/10.3390/en18030537
Chicago/Turabian StyleLe, Thi Cam Tu, Katarzyna Bernat, Tomasz Pokój, and Dorota Kulikowska. 2025. "Closing the Loop of Biowaste Composting by Anaerobically Co-Digesting Leachate, a By-Product from Composting, with Glycerine" Energies 18, no. 3: 537. https://doi.org/10.3390/en18030537
APA StyleLe, T. C. T., Bernat, K., Pokój, T., & Kulikowska, D. (2025). Closing the Loop of Biowaste Composting by Anaerobically Co-Digesting Leachate, a By-Product from Composting, with Glycerine. Energies, 18(3), 537. https://doi.org/10.3390/en18030537








