Hydrogen Purity: Influence of Production Methods, Purification Techniques, and Analytical Approaches
Abstract
1. Introduction
2. Hydrogen Production Based on Feedstock
3. Reactions and Purity in Hydrogen Production Processes
| Steam Reforming | CnHm + nH2O ↔ nCO + (n + 0.5m)H2 | (1) |
| Partial Oxidation | CnHm + 0.5nO2 ↔ nCO + 0.5mH2 | (2) |
| Auto-Reforming | CnHm + 0.5nH2O + 0.25nO2 ↔ nCO + 0.5(n + m)H2 | (3) |
| Thermochemical | Biomass + Air → H2 + CO + N2 + CH4 +H2O + Tar+ Charcoal Biomass + Steam → H2 + CO + N2 + CH4 + Tar+ Charcoal | (4) |
| Biological (Photodecomposition) | H2O + Light → 2H2 + O2 | (5) |
| Biological (Fermentation) | C6H12O6 + 2H2O → 2CH3COOH + 4H2 + 2CO2 | (6) |
| Electrolysis | PEM | (7) |
| Anode 2H2O → O2 + 4H+ + 4e−/Cathode 4H+ + 4e− → 2H2 | ||
| Alkaline | ||
| Anode 4OH− →O2 +2H2O + 4e−/Cathode 2H2O + 2e− → 2OH− + H2 | ||
| SOEC | ||
| Anode 2O2− → O2 +4e−/Cathode H2O + 2e− → H2 + O2− | ||
| Pyrolysis | H2O + Heat → H2 + 0.5O2 | (8) |
| Photovoltaic | Anode 2p+ + H2O → 0.5O2 + 2H+/Cathode 2H+ + 2e− → H2 | (9) |
4. Hydrogen Purity Measurement Equipment
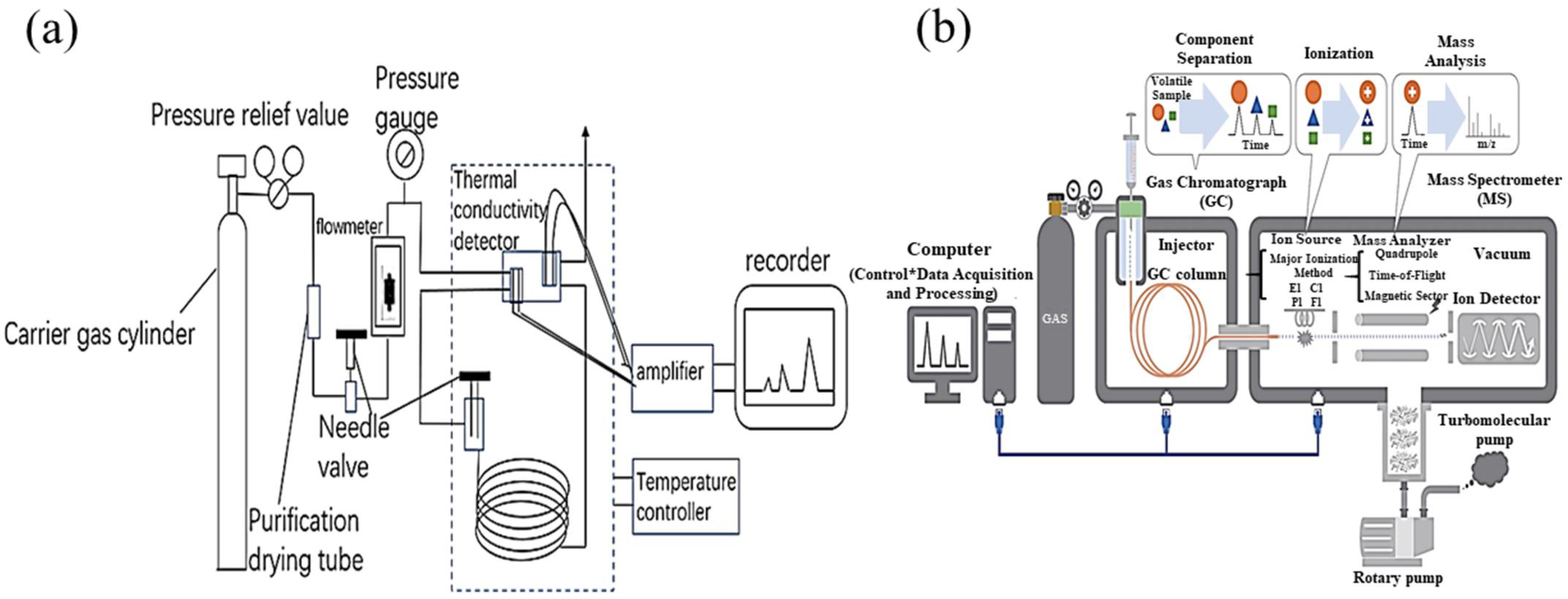
4.1. Gas Chromatography (GC)
4.2. Gas Chromatography–Mass Spectrometry (GC-MS)
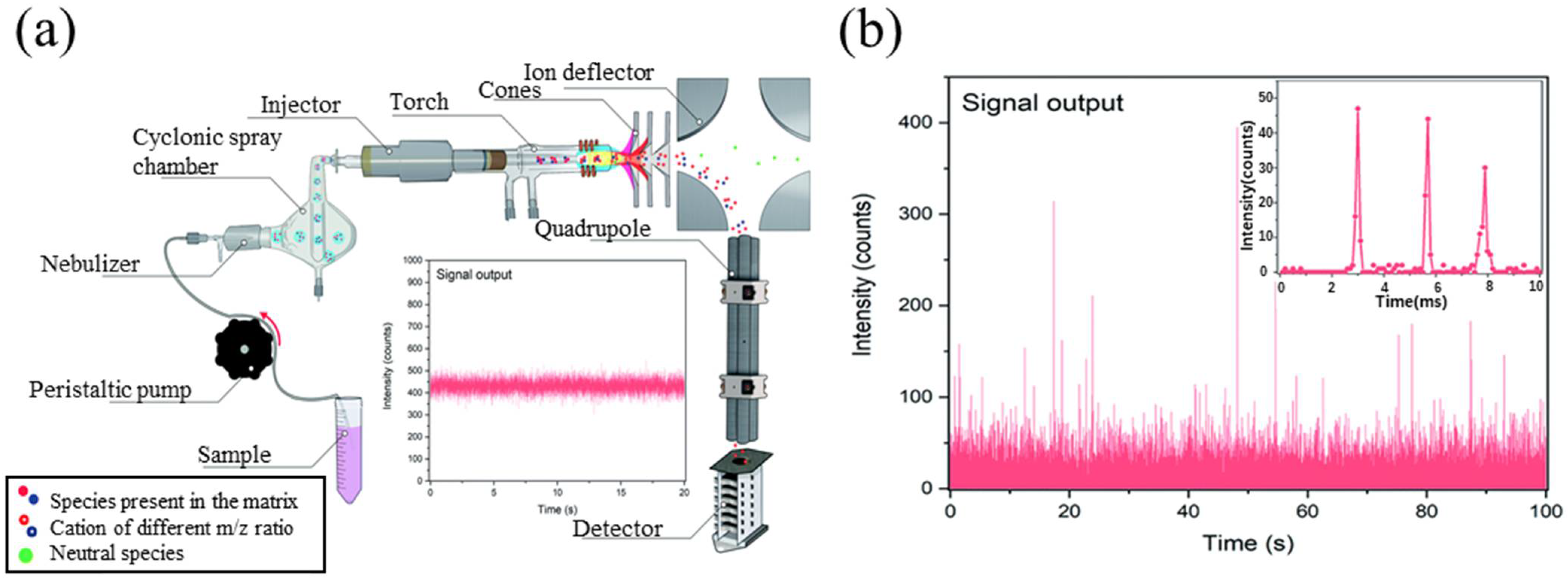
4.3. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
4.4. Fourier Transform Infrared Spectroscopy (FT-IR)
4.5. Cavity Ring-Down Spectroscopy (CRDS)
4.6. Ion Chromatography (IC)
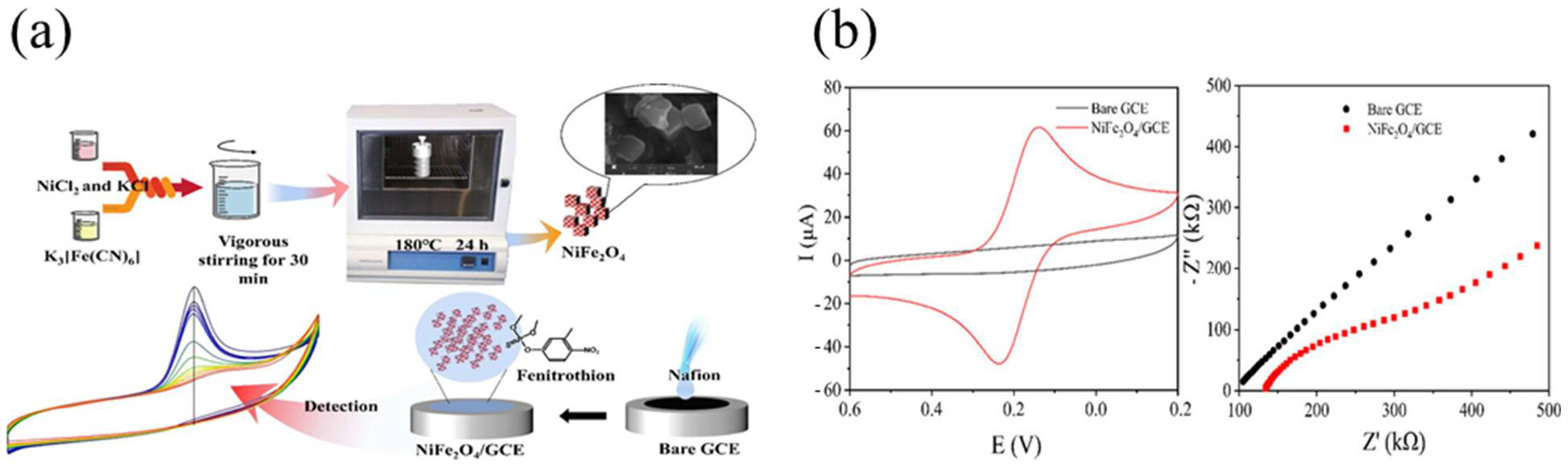
4.7. Electrochemical Sensors
4.8. Gravimetric Analysis (GA)
5. Purification Technologies
5.1. Adsorption Method
5.1.1. Principle of PSA (Pressure Swing Adsorption)
5.1.2. Temperature Swing Adsorption (TSA)
5.1.3. Vacuum Swing Adsorption (VSA)
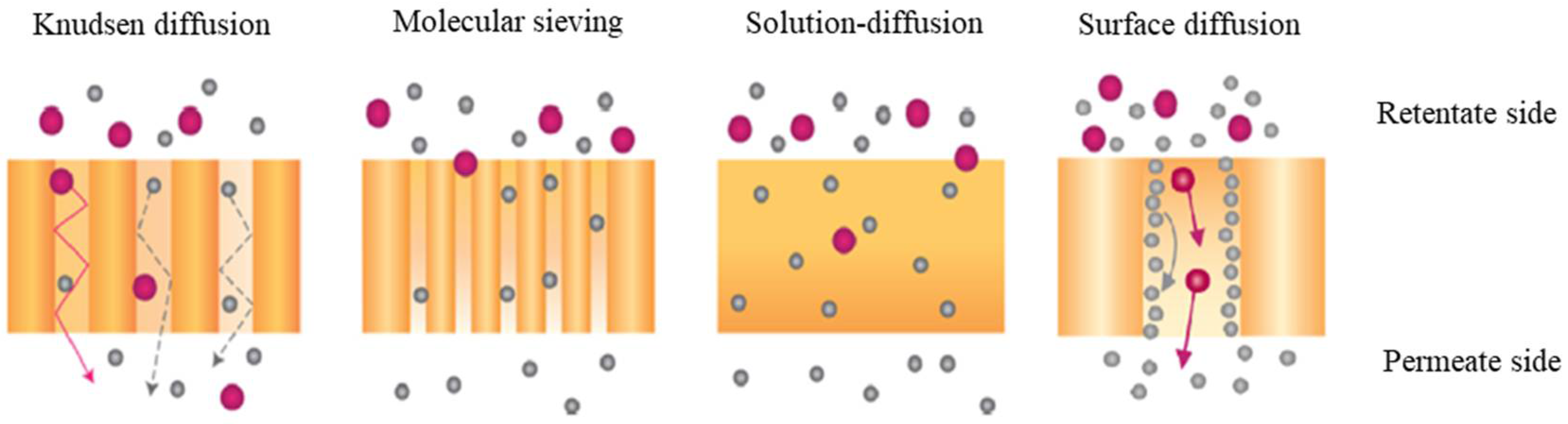
5.2. Membrane Separation Method
5.2.1. Polymer Membranes
5.2.2. Metal Membranes
5.2.3. Ceramic Membranes
5.2.4. Composite Membranes
5.3. Cryogenic Distillation Method
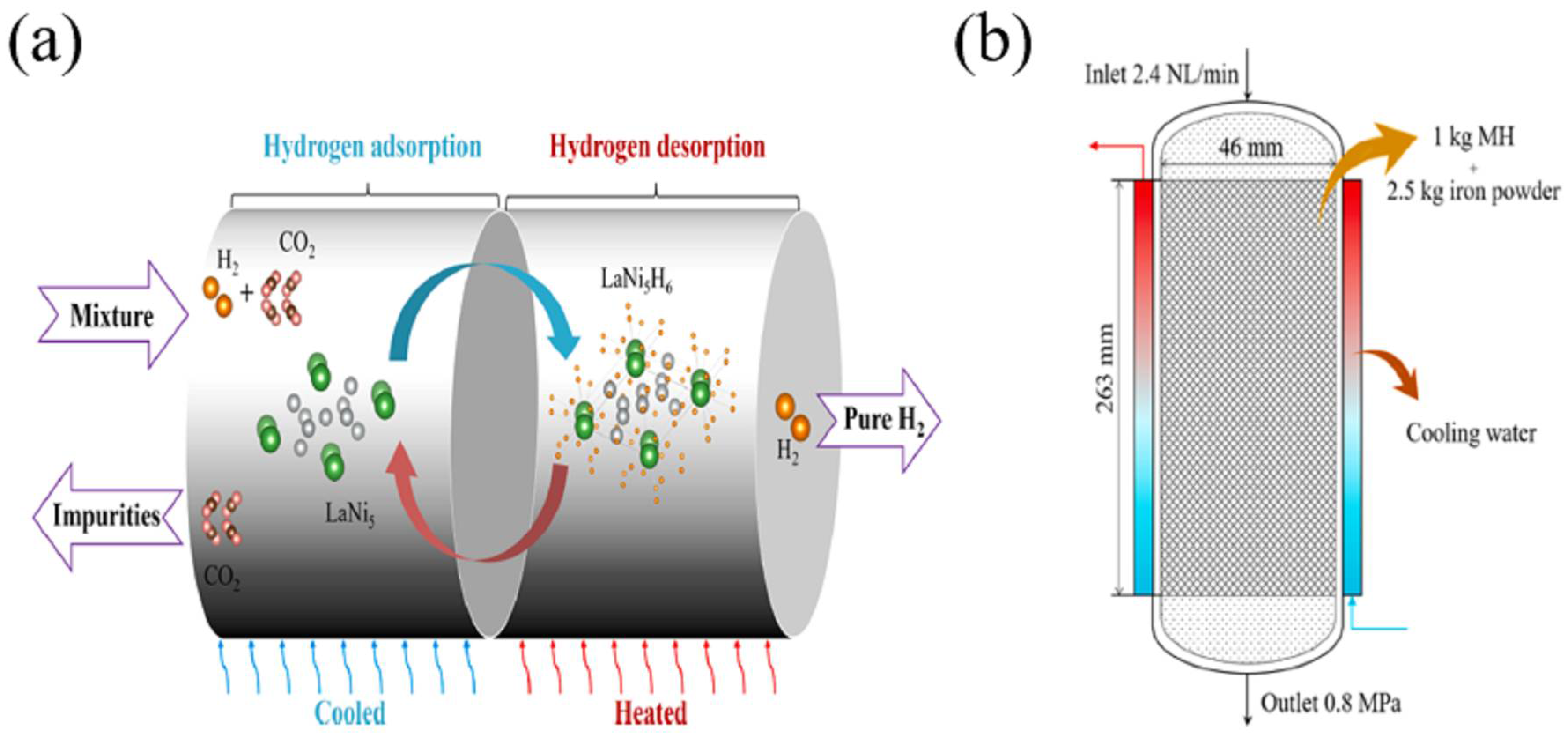
5.3.1. Metal Hydride Separation
5.3.2. Catalytic Purification
6. Purification Based on Production Method
6.1. Steam Methane Reforming (SMR)
6.2. Electrolysis
6.3. Coal Gasification
6.4. Biomass Gasification
6.5. Water–Gas Shift Reaction (WGS)
7. Hydrogen Purification Based on Purity Levels
7.1. Low Purity Feed Gas (40–70% Hydrogen)
7.2. Intermediate Purity Feed Gas (70–90% Hydrogen)
7.3. High-Purity Feed Gas (90–99% Hydrogen)
7.4. Ultra-High-Purity Feed Gas (99% + Hydrogen)
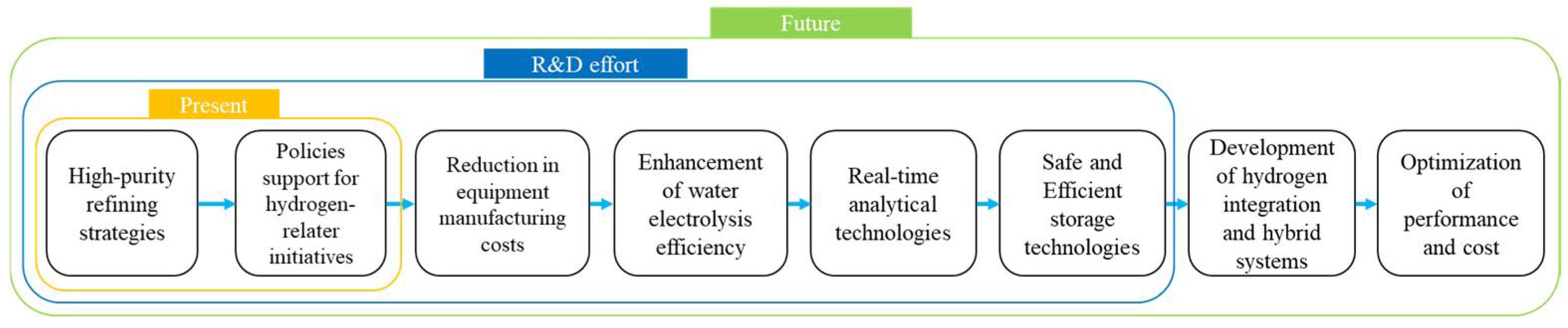
8. Conclusions
- -
- Development of High-Efficiency, Low-Cost Technologies: Innovations in membrane materials, catalysts, and hybrid processes aim to enhance efficiency and reduce operational costs.
- -
- Integration with Renewable Energy: Clean hydrogen production technologies, such as biomass gasification and advanced water electrolysis, are expected to leverage renewable energy sources like solar, wind, and hydro.
- -
- Advances in Ultra-High-Purity Hydrogen Production: Hybrid processes combining PSA, membrane technologies, and metal hydride separation will enable production tailored to specific applications.
- -
- Scalable Production Technologies: Large-scale hydrogen production technologies will address growing global demand and expand industrial applications.
- -
- Digitalization and Real-Time Monitoring: Advanced digital tools and monitoring systems will improve the precision and safety of hydrogen purification processes.
- -
- International Collaboration and Standardization: Global cooperation and standardization will ensure the seamless integration of hydrogen technologies across markets and regions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hosseini, S.E. Hydrogen fuel, a game changer for the world’s energy scenario. Int. J. Green Energy 2024, 21, 1366–1382. [Google Scholar] [CrossRef]
- Hosseini, S.E. An outlook on the global development of renewable and sustainable energy at the time of COVID-19. Energy Res. Soc. Sci. 2020, 68, 101633. [Google Scholar] [CrossRef]
- Hosseini, S.E. Transition away from fossil fuels toward renewables: Lessons from Russia-Ukraine crisis. Future Energy 2020, 1, 2–5. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Green hydrogen: A pathway to a sustainable energy future. Int. J. Hydrogen Energy 2024, 50, 310–333. [Google Scholar] [CrossRef]
- Bernardo, G.; Araujo, T.; da Silva Lopes, T.; Sousa, J.; Mendes, A. Recent advances in membrane technologies for hydrogen purification. Int. J. Hydrogen Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Soltani, S.M.; Lahiri, A.; Bahzad, H.; Clough, P.; Gorbounov, M.; Yan, Y. Sorption-enhanced steam methane reforming for combined CO2 capture and hydrogen production: A state-of-the-art review. Carbon Capture Sci. Technol. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C. Fuel and hydrogen related problems for conventional steam reforming of natural gas. In Current Trends and Future Developments on (Bio-) Membranes. Membranes in Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–89. [Google Scholar] [CrossRef]
- Im, O.-G. Technical trends of hydrogen production. Clean Technol. 2017, 23, 121–132. [Google Scholar] [CrossRef]
- Suchocki, T. Energy utilization of rapeseed biomass in Europe: A review of current and innovative applications. Energies 2024, 17, 6177. [Google Scholar] [CrossRef]
- Rodrigues, J.A.P.; Pinto, N.A.B.T.; Leite, L.A.D.S.B.; Pereira, A.O., Jr. Production of bio-oil via pyrolysis of banana peel and tire waste for energy utilization. Energies 2024, 17, 6149. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Wang, J.; Wang, S. High-performance membranes comprising polyaniline nanoparticles incorporated into polyvinylamine matrix for CO2/N2 separation. J. Membr. Sci. 2015, 25, 203–215. [Google Scholar] [CrossRef]
- Im, K.S.; Son, T.Y.; Jeong, H.N.; Kwon, D.J.; Nam, S.Y. A research trend on diaphragm membranes alkaline water electrolysis system. Membr. J. 2021, 31, 133–144. [Google Scholar] [CrossRef]
- Ganguli, A.; Bhatt, V. Hydrogen production using advanced reactors by steam methane reforming: A review. Front. Therm. Eng. 2023, 3, 1143987. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Mishra, K.; Siwal, S.S.; Saini, A.K.; Thakur, V.K. Recent update on gasification and pyrolysis processes of lignocellulosic and algal biomass for hydrogen production. Fuel 2023, 332, 126169. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Ni, M. Morphology control and electronic tailoring of CoxAy (A = P, S, Se) electrocatalysts for water splitting. Chem. Eng. J. 2023, 460, 141674. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Ni, M. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Terlouw, T.; Bauer, C.; McKenna, R.; Mazzotti, M. Large-scale hydrogen production via water electrolysis: A techno-economic and environmental assessment. Energy Environ. Sci. 2022, 15, 3583–3602. [Google Scholar] [CrossRef]
- Simoes, S.G.; Catarino, J.; Picado, A.; Lopes, T.F.; Di Berardino, S.; Amorim, F.; de Leao, T.P. Water availability and water usage solutions for electrolysis in hydrogen production. J. Clean. Prod. 2021, 315, 128124. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.; Byun, M.; Lim, H. Comparative techno-economic analysis for steam methane reforming in a sorption-enhanced membrane reactor: Simultaneous H2 production and CO2 capture. Chem. Eng. Res. Des. 2021, 171, 383–394. [Google Scholar] [CrossRef]
- Navas-Anguita, Z.; Garcia-Gusano, D.; Dufour, J.; Iribarren, D. Revisiting the role of steam methane reforming with CO2 capture and storage for long-term hydrogen production. Sci. Total Environ. 2021, 771, 145432. [Google Scholar] [CrossRef]
- Leu, J.H.; Su, A.; Kuo, S.C.; Chen, K.C. The observation of performance trends by numerical simulation with different parameters in a nonlinear reversible solid oxide cell system. Energies 2024, 17, 6083. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, H.; Yang, J.; He, Z.; Yan, K.; Liu, T.; Wen, C. Experimental study and optimization analysis of operating conditions on photo-thermochemical cycle of water splitting for hydrogen production based on CeO2 catalyst. Energies 2024, 17, 6314. [Google Scholar] [CrossRef]
- Du, Z.; Liu, C.; Zhai, J.; Guo, X.; Xiong, Y.; Su, W.; He, G. A review of hydrogen purification technologies for fuel cell vehicles. Catalysts 2021, 11, 393. [Google Scholar] [CrossRef]
- Krol, A.; Gajec, M.; Holewa-Rataj, J.; Kukulska-Zajc, E.; Rataj, M. Hydrogen purification technologies in the context of its utilization. Energies 2024, 17, 3794. [Google Scholar] [CrossRef]
- Kim, T.; Song, Y.; Kang, J.; Kim, S.K.; Kim, S. A review of recent advances in hydrogen purification for selective removal of oxygen: Deoxo catalysts and reactor systems. Int. J. Hydrogen Energy 2022, 47, 24817–24834. [Google Scholar] [CrossRef]
- Kheimi, M.; Salamah, S.K. Simulation of temperature swing adsorption process to purify hydrogen for fuel cell uses by SAPO34 as adsorbent. Chemosphere 2023, 338, 139454. [Google Scholar] [CrossRef]
- Raganati, F.; Chirone, R.; Ammendola, P. CO2 capture by temperature swing adsorption: Working capacity as affected by temperature and CO2 partial pressure. Ind. Eng. Chem. Res. 2020, 59, 3593–3605. [Google Scholar] [CrossRef]
- Xiao, J.; Mei, A.; Tao, W.; Ma, S.; Benard, P.; Chahine, R. Hydrogen purification performance optimization of vacuum pressure swing adsorption on different activated carbons. Energies 2021, 14, 2450. [Google Scholar] [CrossRef]
- Zhang, N.; Benard, P.; Chahine, R.; Yang, T.; Xiao, J. Optimization of pressure swing adsorption for hydrogen purification based on Box-Behnken design method. Int. J. Hydrogen Energy 2021, 46, 5403–5417. [Google Scholar] [CrossRef]
- Valdes-Lopez, V.F.; Mason, T.; Shearing, P.R.; Brett, D.J. Carbon monoxide poisoning and mitigation strategies for polymer electrolyte membrane fuel cells—A review. Prog. Energy Combust. Sci. 2020, 79, 100842. [Google Scholar] [CrossRef]
- Dushina, A.; Schmies, H.; Schonvogel, D.; Dyck, A.; Wagner, P. The influence of hydrogen sulphide contamination on platinum catalyst used in polymer electrolyte membrane fuel cells during potential cycling at 0.05–1.05 V vs RHE: An RRDE study. Int. J. Hydrogen Energy 2020, 45, 35073–35084. [Google Scholar] [CrossRef]
- Kakoulaki, G.; Kougias, I.; Taylor, N.; Dolci, F.; Moya, J.; Jager-Waldau, A. Green hydrogen in Europe—A regional assessment: Substituting existing production with electrolysis powered by renewables. Energy Convers. Manag. 2021, 228, 113649. [Google Scholar] [CrossRef]
- Matthes, C.; Aruffo, V.; Retby-Pradeau, L. The Risks and Opportunities of Green Hydrogen Production and Export from the MENA Region to Europe: Study on Behalf. Friedrich-Ebert-Stiftung 2020. Available online: https://library.fes.de/pdf-files/bueros/amman/16998-20210322.pdf (accessed on 14 September 2020).
- Bianco, E.; Blanco, H. Green Hydrogen: A Guide to Policy Making. IRENA 2020. Available online: https://www.irena.org/publications/2020/Nov/Green-hydrogen (accessed on 25 November 2020).
- Blanco, D.; Rivera, Y.; Berna-Escriche, C.; Munoz-Cobo, J.L. Economy decarbonization using green hydrogen and electricity, forecasts and sensitivity analysis for the Canarian Islands in 2040. J. Energy Storage 2024, 80, 110232. [Google Scholar] [CrossRef]
- Curtis, A.J.; McLellan, B.C. Potential Domestic Energy System Vulnerabilities from Major Exports of Green Hydrogen: A Case Study of Australia. Energies 2023, 16, 5881. [Google Scholar] [CrossRef]
- Gongora Orellana, C.A.; Bannura Jorquera, C.A. Compania de Innovacion Tecnologica H2 Chile SPA: Desarrolladores de Proyectos de Hidrogeno Verde 2021. Available online: https://repositorio.uchile.cl/handle/2250/182748 (accessed on 17 November 2021).
- IRENA, R. Green Hydrogen Supply: A Guide to Policy Making; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2021; Available online: https://www.irena.org/publications/2021/May/Green-Hydrogen-Supply-A-Guide-To-Policy-Making (accessed on 27 May 2021).
- Gielen, D.; Taibi, E.; Miranda, R. Hydrogen: A Renewable Energy Perspective; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019; Available online: https://www.irena.org/publications/2019/Sep/Hydrogen-A-renewable-energy-perspective (accessed on 30 September 2019).
- Kim, J.H.; Kim, K.; Nam, S.Y. Research trends of polybenzimidazole-based membranes for hydrogen purification applications. Appl. Chem. Eng. 2020, 31, 453–466. [Google Scholar] [CrossRef]
- Scholes, C.A. Challenges for CO2 capture by membranes. In Advances in Carbon Capture; Woodhead Publishing: Sawston, UK, 2020; pp. 357–377. [Google Scholar] [CrossRef]
- Sazali, N. A review of the application of carbon-based membranes to hydrogen separation. J. Mater. Sci. 2020, 55, 11052–11070. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, T.W.; Byun, S.G.; Seo, D.W.; Hwang, H.J.; Kim, H.; Ryi, S.K. Hydrogen purification from methanol reforming gas for submarine fuel cells using a Pd-composite membrane. Clean Technol. 2022, 28, 54–62. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Mizrahi Rodriguez, K.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-based membranes for gas separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef] [PubMed]
- Vermaak, L.; Neomagus, H.W.; Bessarabov, D.G. Recent advances in membrane-based electrochemical hydrogen separation: A review. Membranes 2021, 11, 127. [Google Scholar] [CrossRef]
- Li, A.; Boyd, T.; Lim, J.C.; Grace, J.R. Development of palladium-alloy membranes for hydrogen separation and purification. J. Membr. Sci. Res. 2020, 6, 99–106. [Google Scholar] [CrossRef]
- Aasadnia, M.; Mehrpooya, M.; Ghorbani, B. A novel integrated structure for hydrogen purification using the cryogenic method. J. Clean. Prod. 2021, 278, 123872. [Google Scholar] [CrossRef]
- IEA. The Future of Hydrogen. 2019. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 14 July 2019).
- Gallardo, F.I.; Ferrario, A.M.; Lamagna, M.; Bocci, E.; Garcia, D.A.; Baeza-Jeria, T.E. A Techno-Economic Analysis of solar hydrogen production by electrolysis in the north of Chile and the case of exportation from Atacama Desert to Japan. Int. J. Hydrogen Energy 2021, 46, 13709–13728. [Google Scholar] [CrossRef]
- IEA, Global Hydrogen Review 2021. Available online: https://www.iea.org/reports/global-hydrogen-review-2021/executive-summary (accessed on 4 October 2021).
- Loisel, R.; Simon, C.; Woznicki, M.; Guerineau, M.; Baranger, L.; Lemiale, L.; Le Solliec, G. Green Hydrogen Multi-Market Optimisation: Real Complementarities or Temporary Transaction Costs? IEEE: Piscataway, NJ, USA, 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Yates, J.; Daiyan, R.; Patterson, R.; Egan, R.; Amal, R.; Ho-Baille, A.; Chang, N.L. Techno-economic analysis of hydrogen electrolysis from off-grid stand-alone photovoltaics incorporating uncertainty analysis. Cell Rep. Phys. Sci. 2020, 1, 100209. [Google Scholar] [CrossRef]
- Ghatak, S.R.; Sannigrahi, S.; Acharjee, P. Design framework for optimal deployment of photovoltaic hydrogen system with battery storage in unbalanced distribution system. In Proceedings of the 2020 IEEE International Conference on Computing, Greater Noida, India, 2–4 October 2020; pp. 473–479. [Google Scholar] [CrossRef]
- Naquash, A.; Qyyum, M.A.; Chaniago, Y.D.; Riaz, A.; Yehia, F.; Lim, H.; Lee, M. Separation and purification of syngas-derived hydrogen: A comparative evaluation of membrane-and cryogenic-assisted approaches. Chemosphere 2023, 313, 137420. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Lau, K.K.; Shariff, A.M.; Murshid, G. Process simulation and optimal design of membrane separation system for CO2 capture from natural gas. Comput. Chem. Eng. 2012, 36, 119–128. [Google Scholar] [CrossRef]
- Shukla, U. Fourier transform infrared spectroscopy: A power full method for creating fingerprint of molecules of nanomaterials. J. Mol. Struct. 2024, 1322, 140454. [Google Scholar] [CrossRef]
- Luberti, M.; Ahn, H. Review of Polybed pressure swing adsorption for hydrogen purification. Int. J. Hydrogen Energy 2022, 47, 10911–10933. [Google Scholar] [CrossRef]
- Urm, J.J.; Park, D.; Choi, J.H.; Lee, J.U.; Chang, M.H.; Lee, J.M. Dynamic Optimization Study for Cryogenic Distillation in Hydrogen Isotope Separation System. IFAC-Pap. 2022, 55, 168–173. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Z.; Wang, H.; Yan, H.; Yang, F.; Cheng, G.; Zhang, Z. Efficient hydrogen recovery and purification from industrial waste hydrogen to high-purity hydrogen based on metal hydride powder. Chem. Eng. J. 2023, 455, 140689. [Google Scholar] [CrossRef]
- Ma, P.; Li, J.; Chen, Y.; Zhou Montano, B.A.; Luo, H.; Zhang, D.; Chen, Z. Non-invasive exhaled breath diagnostic and monitoring technologies. Microw. Opt. Technol. Lett. 2023, 65, 1475–1488. [Google Scholar] [CrossRef]
- Resano, M.; Aramendia, M.; Garcia-Ruiz, E.; Bazo, A.; Bolea-Fernandez, E.; Vanhaecke, F. Living in a transient world: ICP-MS reinvented via time-resolved analysis for monitoring single events. Chem. Sci. 2022, 13, 4436–4473. [Google Scholar] [CrossRef]
- Bhargava, R.; Wang, S.Q.; Koenig, J.L. FTIR microspectroscopy of polymeric systems. In Liquid Chromatography/FTIR Microspectroscopy/Microwave Assisted Synthesis; Springer: Berlin/Heidelberg, Germany, 2003; pp. 137–191. [Google Scholar] [CrossRef]
- Browne, D.E.; Peverall, R.; Ritchie, G.A.; Viles, H.A. Determining water transport kinetics in limestone by dual-wavelength cavity ring-down spectroscopy. Anal. Chem. 2022, 94, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Xu, Z.; Liu, Y.; Guo, X.; Ou, M.; Xu, X. Highly efficient removal of uranium (VI) from wastewater by polyacrylic acid hydrogels. RSC Adv. 2017, 7, 6278–6287. [Google Scholar] [CrossRef]
- Morganti, A.; Becagli, S.; Castellano, E.; Severi, M.; Traversi, R.; Udisti, R. An improved flow analysis; ion chromatography method for determination of cationic and anionic species at trace levels in Antarctic ice cores. Anal. Chim. Acta 2007, 603, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Liemberger, W.; Groß, M.; Miltner, M.; Harasek, M. Experimental analysis of membrane and pressure swing adsorption (PSA) for the hydrogen separation from natural gas. J. Clean. Prod. 2017, 167, 896–907. [Google Scholar] [CrossRef]
- Speight, J.G. Heavy Oil Recovery and Upgrading; Gulf Professional Publishing: Houston, TX, USA, 2019. [Google Scholar]
- Karousos, D.S.; Qadir, D.; Sapalidis, A.A.; Ahmad, F.; Favvas, E.P. Polymeric, metallic and carbon membranes for hydrogen separation: A review. Gas Sci. Eng. 2023, 120, 205167. [Google Scholar] [CrossRef]
- Pal, N.; Agarwal, M. Advances in materials process and separation mechanism of the membrane towards hydrogen separation. Int. J. Hydrogen Energy 2021, 46, 27062–27087. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Cardoso, S.P.; Azenha, I.S.; Lin, Z.; Portugal, I.; Rodrigues, A.E.; Silva, C.M. Inorganic membranes for hydrogen separation. Sep. Purif. Rev. 2018, 47, 229–266. [Google Scholar] [CrossRef]
- Liguori, S.; Kian, K.; Buggy, N.; Anzelmo, B.H.; Wilcox, J. Opportunities and challenges of low-carbon hydrogen via metallic membranes. Prog. Energy Combust. Sci. 2020, 80, 100851. [Google Scholar] [CrossRef]
- Kamble, A.R.; Patel, C.M.; Murthy, Z.V.P. A review on the recent advances in mixed matrix membranes for gas separation processes. Renew. Sustain. Energy Rev. 2021, 145, 111062. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Lee, J.; Bae, T.H. Graphene-based membranes for H2 separation: Recent progress and future perspective. Membranes 2020, 10, 336. [Google Scholar] [CrossRef]
- Xiang, L.; Li, H.; Chen, Z.; Tang, J. A spinel metal oxide (Ni-Fe-O) decorated glassy carbon electrode as a sensitive sensor for the electrochemical detection of fenitrothion. J. Electrochem. Soc. 2023, 170, 117518. [Google Scholar] [CrossRef]
- Kruszewski, J.; White, G.T.; O’Hara, M.; Smith, L.C. Hydrogen membrane separation processes in large-scale applications. Environ. Prog. Sustain. Energy 2023, 42, 1–10. [Google Scholar] [CrossRef]
- Hu, D.; Xie, Y.; Wang, S.; Liu, X. Review of membrane-based technologies for hydrogen recovery and purification. Membranes 2020, 9, 391. [Google Scholar]
- Wibowo, S.T.; Kurniawan, T.A. Membrane separation technology for hydrogen purification and its applications in fuel cells. Renew. Sustain. Energy Rev. 2020, 24, 307–316. [Google Scholar]
- Sheshadri, A.; Boiarski, J.J.; Pfeiffer, S.; Wang, S. Optimization of pressure swing adsorption for hydrogen purification. Chem. Eng. Res. Des. 2020, 164, 344–357. [Google Scholar] [CrossRef]
- Patel, A.K.; Acharya, N.K. Thermally rearranged (TR) HAB-6FDA nanocomposite membranes for hydrogen separation. Int. J. Hydrogen Energy 2020, 45, 18685–18692. [Google Scholar] [CrossRef]
- Zito, P.F.; Brunetti, A.; Barbieri, G. Hydrogen concentration and purification by membrane process: A multistage analysis. Renew. Energy 2023, 218, 119243. [Google Scholar] [CrossRef]
- An, N.; Gu, B.; Kim, J.; Ga, S. Advancing green hydrogen purification: Multiscale evaluation of membrane processes using novel software, pySembrane. Renew. Sustain. Energy Rev. 2025, 208, 114998. [Google Scholar] [CrossRef]
- Franco, A.; Giovannini, C. Recent and future advances in water electrolysis for green hydrogen generation: Critical Analysis and Perspectives. Sustainability 2023, 15, 16917. [Google Scholar] [CrossRef]
- Munoz Diaz, M.T.; Chavez Orostica, H.; Guajardo, J. Economic analysis: Green hydrogen production systems. Processes 2023, 11, 1390. [Google Scholar] [CrossRef]
- Yan, X.; Zheng, W.; Wei, Y.; Yan, Z. Current Status and Economic Analysis of Green Hydrogen Energy Industry Chain. Processes 2024, 12, 315. [Google Scholar] [CrossRef]
- Risco-Bravo, A.; Varela, C.; Bartels, J.; Zondervan, E. From green hydrogen to electricity: A review on recent advances, challenges, and opportunities on power-to-hydrogen-to-power systems. Renew. Sustain. Energy Rev. 2024, 189, 113930. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, G.; Yu, M.; Liu, Y.; Wang, Z.; Song, H. Development of a vacuum swing adsorption process for high-purity hydrogen. Ind. Eng. Chem. Res. 2020, 59, 4267–4277. [Google Scholar]
- Ozdemir, G.; Ku, S.W.; Park, Y. Techniques for CO2 removal and purification in hydrogen production: A review. Chem. Eng. J. 2020, 402, 126108. [Google Scholar] [CrossRef]
- Lo, W.C.; Liao, S.S.; Cheng, C.C.; Hsieh, L.F. Hydrogen production and purification by methane reforming using palladium membranes. Energy Fuels 2020, 33, 847–855. [Google Scholar]
- Verkerk, M.; Ma, T.; Kang, Y.; Lee, J.K.; Donkers, B.; Keller, B. Recent advances in the hydrogen purification process using palladium-alloy membranes. Materials 2020, 13, 370. [Google Scholar] [CrossRef]
- Boury, F.; Moal, C.; Bouaifi, A.; Alario, J.P. Comparison of hydrogen separation technologies. J. Membr. Sci. 2020, 499, 165–178. [Google Scholar] [CrossRef]
- Giffin, B.; Demkowicz, M.; Lee, J.; Li, X. Palladium-based membranes for hydrogen purification and production: A review. J. Appl. Membr. Sci. 2020, 7, 28–39. [Google Scholar] [CrossRef]
- Rojas, F.; Diaz, J.A.; Garcia, S.; Rodriguez, A.J. Hydrogen purification by adsorption on activated carbon: A review. Energy 2020, 187, 116455. [Google Scholar]
- Prat, D.; Pardos, M.; Cosovanu, A.; Venturini, D. Advanced hydrogen purification technologies in cryogenic distillation. Cryogenics 2020, 98, 56–69. [Google Scholar] [CrossRef]
- Jaffar, M.; Sarli, S.; Fatah, M.; Chen, C.S. Recent developments in the hydrogen purification and separation technology. J. Appl. Chem. 2022, 7, 22–35. [Google Scholar]
- Taylor, J.; Evans, R.G.; Singh, N.S. Design of palladium membranes for hydrogen purification. Mater. Sci. Eng. A 2020, 545, 119–128. [Google Scholar] [CrossRef]
- Linga, P.; Astarita, T.; Ruan, Y.; Lee, K.; Choi, W. Hydrogen purification by absorption processes: Recent advances and future directions. Energy Technol. 2020, 10, 1217–1235. [Google Scholar]
- Naquash, A.; Riaz, A.; Yehia, F.; Chaniago, Y.D.; Lim, H.; Lee, M. Hydrogen Purification Through a Membrane–Cryogenic Integrated Process: A 3 E’s (Energy, Exergy, and Economic) Assessment. Gases 2023, 3, 92–105. [Google Scholar] [CrossRef]
- Kalman, V.; Voigt, J.; Jordan, C.; Harasek, M. Hydrogen purification by pressure swing adsorption: High-pressure PSA performance in recovery from seasonal storage. Sustainability 2022, 14, 14037. [Google Scholar] [CrossRef]
- Erdöl, H.B.; Şahin, F.I.; Acaralı, N. Novel Study on Cryogenic Distillation Process and Application by Using CHEMCAD Simulation. ACS Omega 2024, 9, 15165–15174. [Google Scholar] [CrossRef]
- Dehdari, L.; Yang, J.; Xiao, P.; Li, G.K.; Webley, P.A.; Singh, R. Separation of hydrogen from 10% hydrogen+ methane mixtures using double-stage Vacuum Swing Adsorption (VSA). Chem. Eng. J. 2024, 489, 151032. [Google Scholar] [CrossRef]
- Blinov, D.V.; Borzenko, V.I.; Bezdudny, A.V.; Kazakov, A.N. Metal hydride hydrogen storage and purification technologies. J. Phys. Conf. Ser. 2021, 2039, 012005. [Google Scholar] [CrossRef]
- Plaza, M.G.; Rubiera, F.; Pevida, C. Evaluating the feasibility of a TSA process based on steam stripping in combination with structured carbon adsorbents to capture CO2 from a coal power plant. Energy Fuels 2017, 31, 9760–9775. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]





| Feedstock | Energy Source | Chemical Reaction |
|---|---|---|
| Fuels (Natural Gas, LPG, Naphtha, Coal, etc.) | Reforming Reaction | Gas Fuel (LNG, LPG) Byproduct Gas Synthesis Fuel (Methanol, DME) |
| Gasification Reaction | Coal/Petcoke Gasification | |
| Biological Conversion Reaction | Biological CO Conversion | |
| Biomass, Waste Resources | Combustible Waste Gasification | |
| Biomass Gasification | ||
| Biological Fermentation | ||
| Water | Electrolysis (Electrolysis) | Alkaline Electrolysis (AEC) Proton-Exchange Membrane Electrolysis (PEMEC) Solid Oxide Electrolysis (SOEC) |
| Photo-chemical Decomposition | Photoelectrochemical (PEC) Photocatalytic Photobiological | |
| Thermal Decomposition | Thermochemical Cycles Redox Cycles | |
| Nuclear | High-Temperature Gas Reactors | |
| Test Item | GC/GC-MS (ppb~ppm) | FT-IR (ppm~%) | ICP-MS (ppt~ppb) | IC (ppb~ppm) |
|---|---|---|---|---|
| Purity | O | X | X | X |
| Moisture (H2O) | X | O | X | O |
| Total Hydrocarbons (THC) | O(FID) | X | X | X |
| Oxygen (O2) | O(TCD) | X | X | X |
| Helium (He) | O(TCD) | X | X | X |
| Nitrogen/Argon (N2/Ar) | O(TCD) | X | X | X |
| Carbon Dioxide (CO2) | O(TCD) | O | X | O |
| Carbon Monoxide (CO) | O(TCD) | O | X | X |
| Total Sulfur (Total S) | X | X | O | O |
| Organic Acids | O | O | X | O |
| Formaldehyde (CH2O) | O | O | X | X |
| Ammonia (NH3) | O | O | X | O |
| Halogen Compounds (HX) | O | O | X | O |
| Particle Concentration | X | X | O | X |
| Refining Technologies | Advantages | Disadvantages | Purity (%) |
|---|---|---|---|
| PSA | High hydrogen recovery rates Widely used commercially | High energy consumption Expensive for low-purity | 99.999 |
| Membrane Separation | High hydrogen recovery rates Widely used commercially | High operating costs Membrane lifespan issues | 95.8–99.9 |
| Cryogenic Distillation | High hydrogen recovery rates Widely used commercially | High energy consumption Complex cooling systems Expensive to operate | 99.999 |
| TSA | High hydrogen recovery rates Widely used commercially | Limited application fields Restricted to the removal of certain lightweight impurities | 95–98 |
| VSA | High efficiency at low pressures Low energy consumption Suitable for small-scale applications | Unsuitable for large-scale applications | 95–98 |
| Metal Hydride Separation | High selectivity Operable at low temperatures Capable of storage and purification | Unsuitable for large-scale applications | 99.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Yang, H. Hydrogen Purity: Influence of Production Methods, Purification Techniques, and Analytical Approaches. Energies 2025, 18, 741. https://doi.org/10.3390/en18030741
Kim Y, Yang H. Hydrogen Purity: Influence of Production Methods, Purification Techniques, and Analytical Approaches. Energies. 2025; 18(3):741. https://doi.org/10.3390/en18030741
Chicago/Turabian StyleKim, Yunji, and Heena Yang. 2025. "Hydrogen Purity: Influence of Production Methods, Purification Techniques, and Analytical Approaches" Energies 18, no. 3: 741. https://doi.org/10.3390/en18030741
APA StyleKim, Y., & Yang, H. (2025). Hydrogen Purity: Influence of Production Methods, Purification Techniques, and Analytical Approaches. Energies, 18(3), 741. https://doi.org/10.3390/en18030741








