Research on the Decomposition Characteristics of Methane Hydrates Exploited by the NH4Cl/NaNO2 System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
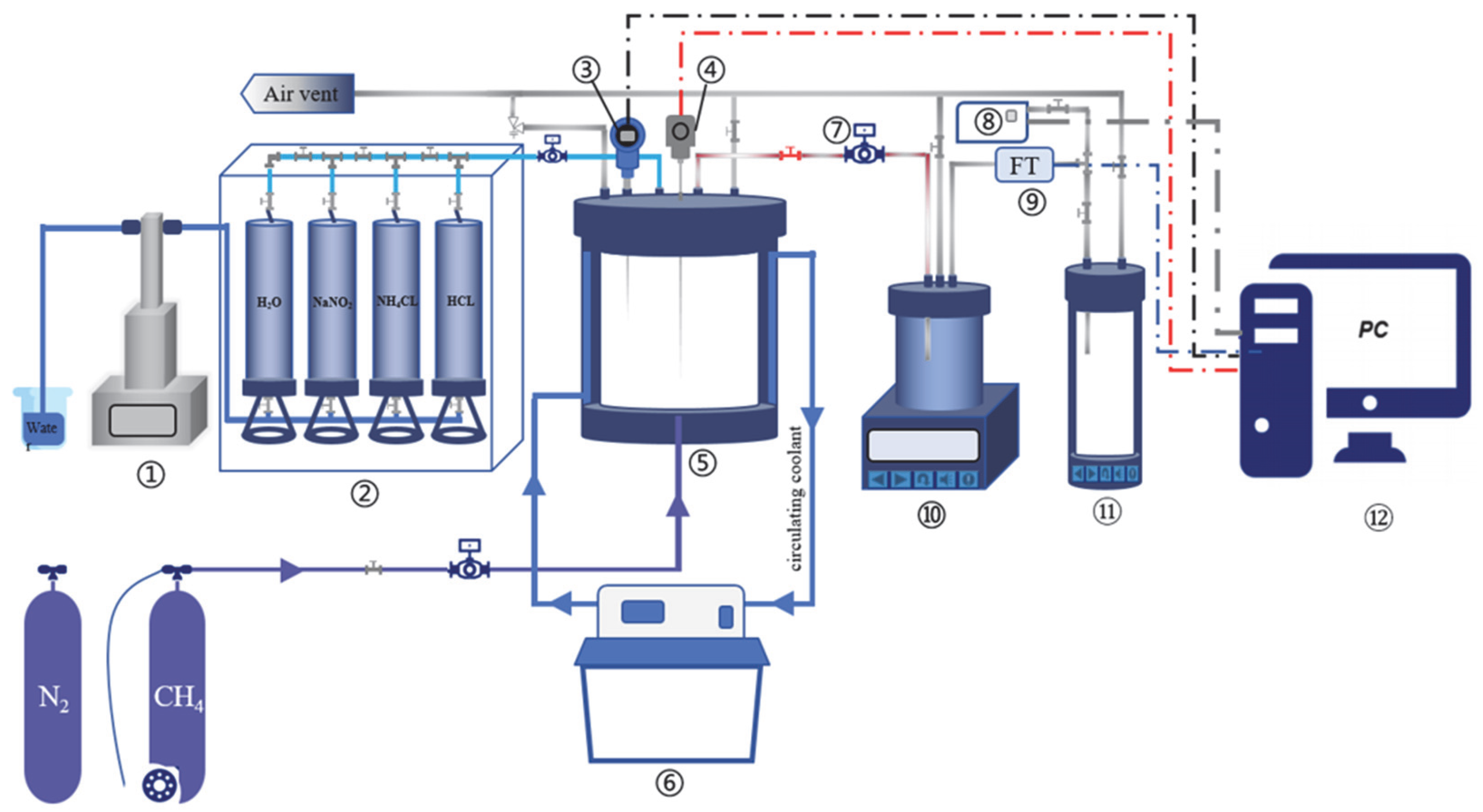
2.2.1. Experimental Apparatus
2.2.2. Experimental Operation Process
- Methane hydrate synthesis process.
- The process of hydrate decomposition by the self-heating system.
- Deionized water was used to prepare a NH4Cl solution (3 mol/L, 200 mL), NaNO2 solution (3 mol/L, 200 mL), and HCl solution (0.0225 mol/L, 10 mL). They were placed in the intermediate containers of the high-pressure pistons and kept at a constant temperature of 3 °C for 12 h of standby.
- The outlet valve was opened, and the PID pressure controller was used to reduce the initial pressure after the completion of hydrate formation to 8 MPa and stabilize it for 1 h.
- The injection pump was opened, and 100 mL of NH4Cl solution and 100 mL of NaNO2 solution were injected into the reactor in sequence at an injection rate of 30 mL/min, and then, 1.17 mL of HCl solution was injected at an injection rate of 0.1 mL/min. The back pressure at the outlet was controlled to 8 MPa, and excess methane gas was discharged until the pressure stabilized. This was recorded as the zero point of the reaction time, and the injection valve was closed.
- The multiphase fluid separation and gas collection system was opened; the volume of the excessive methane gas discharged in steps b) and c) was recorded; and the changes in experimental data, such as pressure, temperature, and gas production volume, during the decomposition process were recorded at an interval of 1 min. The gas chromatograph was used to sample and analyze the gas components and volume fractions in the collector every 30 min (about 1 × 10−5 m3 of gas was collected each time).
- When the gas flowmeter detected that the gas production was 0 or the sampling analysis results of the gas chromatograph did not contain or contained a very small amount of methane gas, the decomposition experiment was completed.
- After the end of the decomposition experiment, the number of moles of methane gas collected by the gas chromatograph and the number of moles of methane gas in the collection device were recorded and calculated, and the total number of moles of methane gas produced was calculated.
2.2.3. Calculation Method
- Instantaneous decomposition efficiency and overall decomposition efficiency.
- Energy efficiency.
3. Results
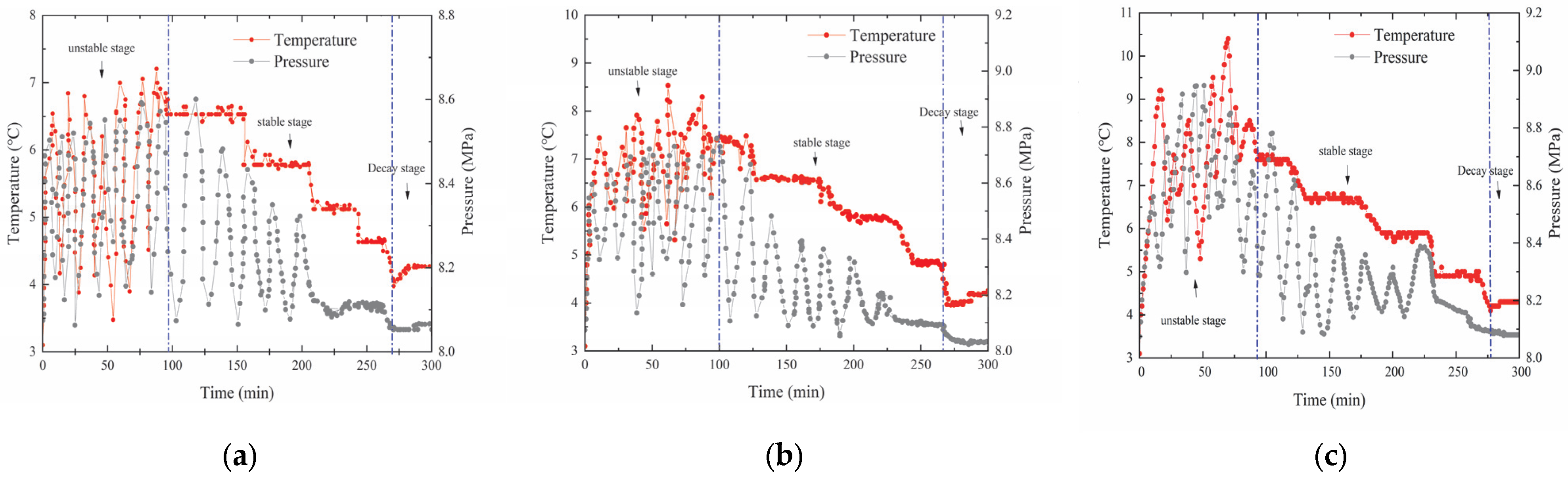
3.1. The Decomposition Characteristics of Hydrate Under Different Decomposition Conditions
- Unstable stage: in the early stage of decomposition, the temperature and pressure in the reactor changed drastically. Analysis of the reasons: The external temperature of the reactor was stable due to the influence of the circulating cooling device, while the experimental temperature in the reactor was unstable, which was quite different from the temperature change in the conventional hot water injection process [18]. The reason is that during the decomposition of hydrates by the self-heating system, a large amount of heat was released when the two substances first came into contact with each other (relevant studies have shown that the heat released during the initial reaction process between the two substances can result in a sharp rise in the ambient temperature [14]). The self-protection effect of the hydrates’ “quasi-liquid film” inhibited the diffusion and mass transfer of methane hydrate. Above the freezing point, the higher the temperature, the thinner the quasi-liquid film thickness, the lower the mass transfer resistance, and the faster the methane diffusion rate, which made the hydrate decomposition rate faster. Compared with the constant-temperature steady-state process of hot water injection, the temperature change in the chemical reaction was a dynamic process and was more drastic. The whole system was in an unsteady state, and the system temperature affected the intensity of the chemical reaction. Therefore, the thickness of the hydrates’ quasi-liquid film was changed accordingly, and it ultimately showed the instability of the decomposition rate, the system temperature, and the pressure.
- Stable stage: This stage was the main decomposition stage of methane hydrate. At this time, the gas production and heat generation of the chemical reaction system made the solid methane hydrate decompose gradually. With the decrease in the chemical reaction rate and the hydrate content, the decomposition pressure and decomposition temperature also gradually decreased. It was observed that the temperature changed in steps over time in the reaction process. This phenomenon proves that as the chemical reaction enters a stable state, the hydrate decomposition process gradually becomes stable, thus forming a dynamic change law involving an exothermic chemical system and endothermic decomposition of the self-heating system. At the later stage of decomposition, the decrease in the chemical reaction rate reduced the heat production, and the temperature in local areas decreased accordingly. The overall temperature in the reactor dropped earlier than the phase equilibrium temperature of methane hydrate, resulting in a reduction in the stable decomposition time of hydrates.
- Decay stage: During this stage, the pressure and the temperature were higher than the back pressure value, and a small ’funnel’ rise in temperature reflected that the chemical reaction system in the self-heating system still maintained a relatively low reaction rate. At this time, the reaction conditions had decreased until they were almost unable to continue to break through the phase equilibrium of methane hydrate, so the stable generation of N2 during this stage made no significant changes in the pressure and temperature in the system, which marked the end of the decomposition experiment.
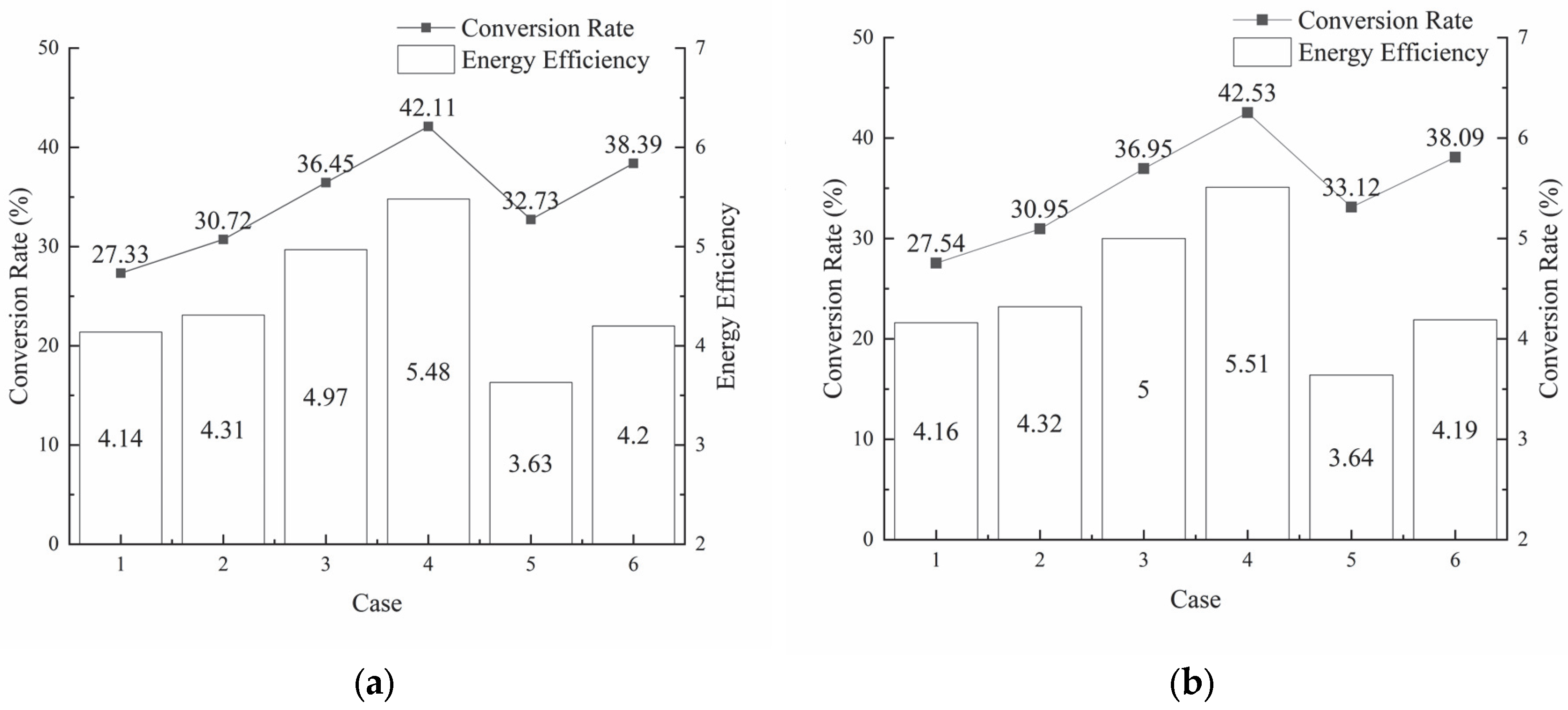
3.2. Effects of Different Decomposition Conditions on Decomposition Efficiency
3.2.1. Effects of Reactant Concentration on Decomposition Efficiency
3.2.2. Effects of H+ Concentration on Decomposition Efficiency
3.3. Analysis of Energy Efficiency Under Different Decomposition Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Symbol Explanation | |
| initial concentration of reactants in the reaction system, mol/L | |
| (H+) | concentration of H+ in the reaction system, mol/L |
| enthalpy of combustion of methane gas, J/mol | |
| decomposition heat of methane gas, J/mol | |
| enthalpy of the NH4Cl and NaNO2 system, J/mol | |
| mass of deionized water consumed during hydrate synthesis, g | |
| molar mass of water, g/mol | |
| - init | number of moles of methane initially contained in the solid hydrate after hydrate synthesis, mol |
| the mole number of excess methane gas after hydrate synthesis at the reaction zero point, mol | |
| the mole number of methane gas in the mixed gas in the collector at time t − 1 and time t, respectively, mol | |
| the number of moles of methane in the mixed gas in the collector at the end of decomposition (, mol | |
| total number of moles of methane gas produced during the decomposition of methane hydrate, mol | |
| number of moles of the NH4Cl/NaNO2 system, mol | |
| hydration index; methane hydrate is taken as 6.12 [21] | |
| reaction zero point, gas mixture pressure at moment t, Pa | |
| heat generated by the combustion of methane gas, J | |
| heat generated by the decomposition of methane gas, J | |
| total heat produced in the reaction of the NH4Cl/NaNO2 system, J | |
| value of heat change during the replacement of hydrate by nitrogen, J | |
| ideal gas constant, taking 8.314 J/(molK) | |
| temperature of the gas in the collector at the zero point of the reaction and at the moment of t, K | |
| decomposition temperature in the reactor at the end of decomposition, K | |
| volume of excess methane gas discharged from the reactor at the end of hydrate synthesis at the zero point of the reaction, m3 | |
| volume occupied by the gas mixture in the collector, m3 | |
| methane gas compression factor (calculated from the Pitzer correlation equation) | |
| Molar fraction of methane in the mixed gas in the collector at time t (measured by gas chromatography), % | |
| instantaneous decomposition efficiency and overall decomposition efficiency of methane hydrate, respectively, % | |
| energy efficiency in the process of decomposition of methane hydrate by an autogenous thermal system | |
| conversion rate of the reaction system, % | |
References
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Pinero, E.; Marquardt, M.; Hensen, C.; Haeckel, M.; Wallmann, K. Estimation of the global inventory of methane hydrates in marine sediments using transfer functions. Biogeosciences 2013, 10, 959–975. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Guo, X.; Wei, R.; Yu, T.; Xu, L.; Sun, L.; Yang, L. Gas production behavior from hydrate-bearing fine natural sediments through optimized step-wise depressurization. Appl. Energy 2020, 260, 114275. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.D.; Liang, Y.P.; Liu, H. The use of electrical heating for the enhancement of gas recovery from methane hydrate in porous media. Appl. Energy 2018, 227, 694–702. [Google Scholar] [CrossRef]
- Tupsakhare, S.S.; Kattekola, S.; Castaldi, M.J. An Application of the Results from the Large Scale Thermal Stimulation Method of Methane Hydrate Dissociation to the Field Tests. Ind. Eng. Chem. Res. 2017, 56, 4588–4599. [Google Scholar] [CrossRef]
- Boswell, R.; Schoderbek, D.; Collett, T.S.; Ohtsuki, S.; White, M.; Anderson, B.J. The Inik Sikumi Field Experiment, Alaska North Slope: Design, Operations, and Implications for CO2-CH4 Exchange in Gas Hydrate Reservoirs. Energy Fuels 2017, 31, 140–153. [Google Scholar] [CrossRef]
- Shi, M.; Woodley, J.M.; von Solms, N. An Experimental Study on Improved Production Performance by Depressurization Combined with CO2-Enriched Air Injection. Energy Fuels 2020, 34, 7329–7339. [Google Scholar] [CrossRef]
- Khlebnikov, V.N.; Antonov, S.V.; Mishin, A.S.; Bakulin, D.A.; Khamidullina, I.V.; Liang, M.; Vinokurov, V.A.; Gushchin, P.A. A new method for the replacement of CH4 with CO2 in natural gas hydrate production. Nat. Gas Ind. B 2016, 3, 445–451. [Google Scholar] [CrossRef]
- Zhang, L.X.; Yang, L.; Wang, J.Q.; Zhao, J.; Dong, H.; Yang, M.; Liu, Y.; Song, Y. Enhanced CH4 recovery and CO2 storage via thermal stimulation in the CH4/CO2 replacement of methane hydrate. Chem. Eng. J. 2017, 308, 40–49. [Google Scholar] [CrossRef]
- Yu, W. Research on In-situ Self-Generated Heat Enhanced CO2 Replacement for Natural Gas Hydrate Exploitation. Master’s Thesis, South China University of Technology, Guangzhou, China, 2022. [Google Scholar] [CrossRef]
- Lee, J. Experimental Study on the Dissociation Behavior and Productivity of Gas Hydrate by Brine Injection Scheme in Porous Rock. Energy Fuels 2010, 24, 456–463. [Google Scholar] [CrossRef]
- Aminnaji, M.; Tohidi, B.; Burgass, R.; Atilhan, M. Effect of injected chemical density on hydrate blockage removal in vertical pipes: Use of MEG/MeOH mixture to remove hydrate blockage. J. Nat. Gas Sci. Eng. 2017, 45, 840–847. [Google Scholar] [CrossRef]
- Zhang, Y. Experimental Study on the Self—Heating Exploitation of Natural Gas Hydrates. Master’s Thesis, Chongqing University, Chongqing, China, 2019. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, C.; Ji, Z.; Yan, F.; Yang, Z.; Ding, M.; Chen, W. Reaction Characteristics of Sodium Nitrite and Ammonium Chloride System. Acta Pet. Sin. 2020, 41, 226–234. [Google Scholar]
- Zhan, Y.; Luo, M.; Fu, C.; Liu, J. Reaction Kinetics of Sodium Nitrite and Ammonium Chloride System Considering Conversion Rate. J. China Univ. Pet. (Ed. Nat. Sci.) 2023, 47, 173–180. [Google Scholar]
- Geng, L.T.; Cai, J.; Lu, C.; Qin, X.; Qi, R.; Meng, F.; Xie, Y.; Sha, Z.; Wang, X.; Sun, C. Phase equilibria of natural gas hydrates in bulk brine and marine sediments from the south china sea. J. Chem. Eng. Data 2021, 66, 4064–4074. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.; Lee, J.; Lee, H.; Seo, Y. CH4 recovery and CO2 sequestration using flue gas in natural gas hydrates as revealed by a micro-differential scanning calorimeter. Appl. Energy 2015, 150, 120–127. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Li, Y.X.; Li, S.D.; He, J.; Li, X.; Xu, T.; Lu, C.; Qin, X. Optimization of the natural gas hydrate hot water injection production method: Insights from numerical and phase equilibrium analysis. Appl. Energy 2024, 361, 122963. [Google Scholar] [CrossRef]
- Wu, A.; Chen, M.; Gu, S.; Wang, W. Research on the Reaction Kinetics of NaNO2 and NH4Cl and Its Application in Oilfields. Oil Drill. Prod. Technol. 1995, 60–64+107. [Google Scholar] [CrossRef]
- Nguyen, D.A.; Iwabim, M.A.; Fogler, H.S. Kinetics and mechanism of the reaction between ammonium and nitriteions: Experimental and theoretical studies. Chem. Eng. Sci. 2003, 58, 4351–4362. [Google Scholar] [CrossRef]
- Ye, C.L.; Meng, Y.G.; Liu, Q.G. Determination of hydration number of methane hydrates using micro-laser raman spectroscopy. Spectrosc. Spectr. Anal. 2010, 30, 963–966. [Google Scholar]


| Case | HCl Concentrations (mol/L) | Reactant Concentrations (mol/L) |
|---|---|---|
| NH4Cl/NaNO2 (1:1) | ||
| 1 | 0.0225 | 3 |
| 2 | 0.0225 | 4 |
| 3 | 0.0225 | 5 |
| 4 | 0.0225 | 6 |
| 5 | 0.0178 | 5 |
| 6 | 0.0356 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wan, Y.; Li, M.; Wang, Y.; Tan, X. Research on the Decomposition Characteristics of Methane Hydrates Exploited by the NH4Cl/NaNO2 System. Energies 2025, 18, 1294. https://doi.org/10.3390/en18051294
Zhang J, Wan Y, Li M, Wang Y, Tan X. Research on the Decomposition Characteristics of Methane Hydrates Exploited by the NH4Cl/NaNO2 System. Energies. 2025; 18(5):1294. https://doi.org/10.3390/en18051294
Chicago/Turabian StyleZhang, Jihong, Yi Wan, Ming Li, Yanan Wang, and Xinjian Tan. 2025. "Research on the Decomposition Characteristics of Methane Hydrates Exploited by the NH4Cl/NaNO2 System" Energies 18, no. 5: 1294. https://doi.org/10.3390/en18051294
APA StyleZhang, J., Wan, Y., Li, M., Wang, Y., & Tan, X. (2025). Research on the Decomposition Characteristics of Methane Hydrates Exploited by the NH4Cl/NaNO2 System. Energies, 18(5), 1294. https://doi.org/10.3390/en18051294






