Impact of Cooling Strategies and Cell Housing Materials on Lithium-Ion Battery Thermal Management Performance
Abstract
:1. Introduction
2. Materials and Methods
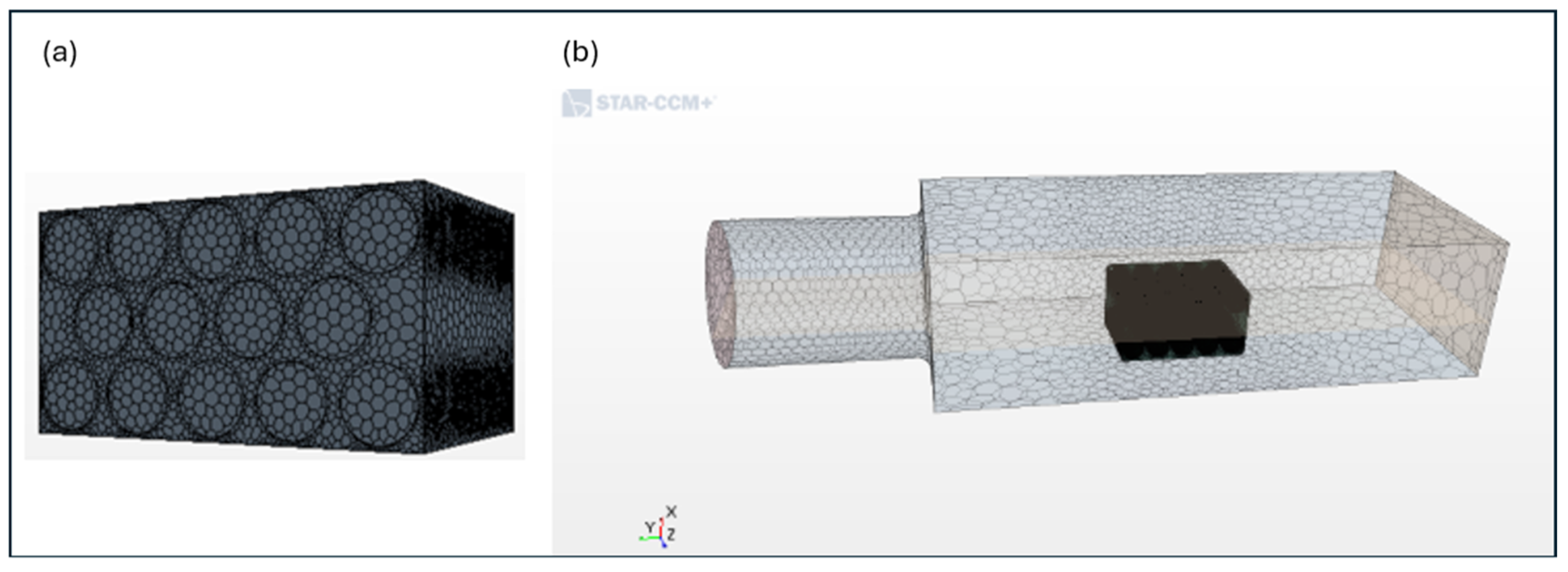
2.1. Battery Pack Description
2.2. Experimental Setup
2.3. Numerical Method
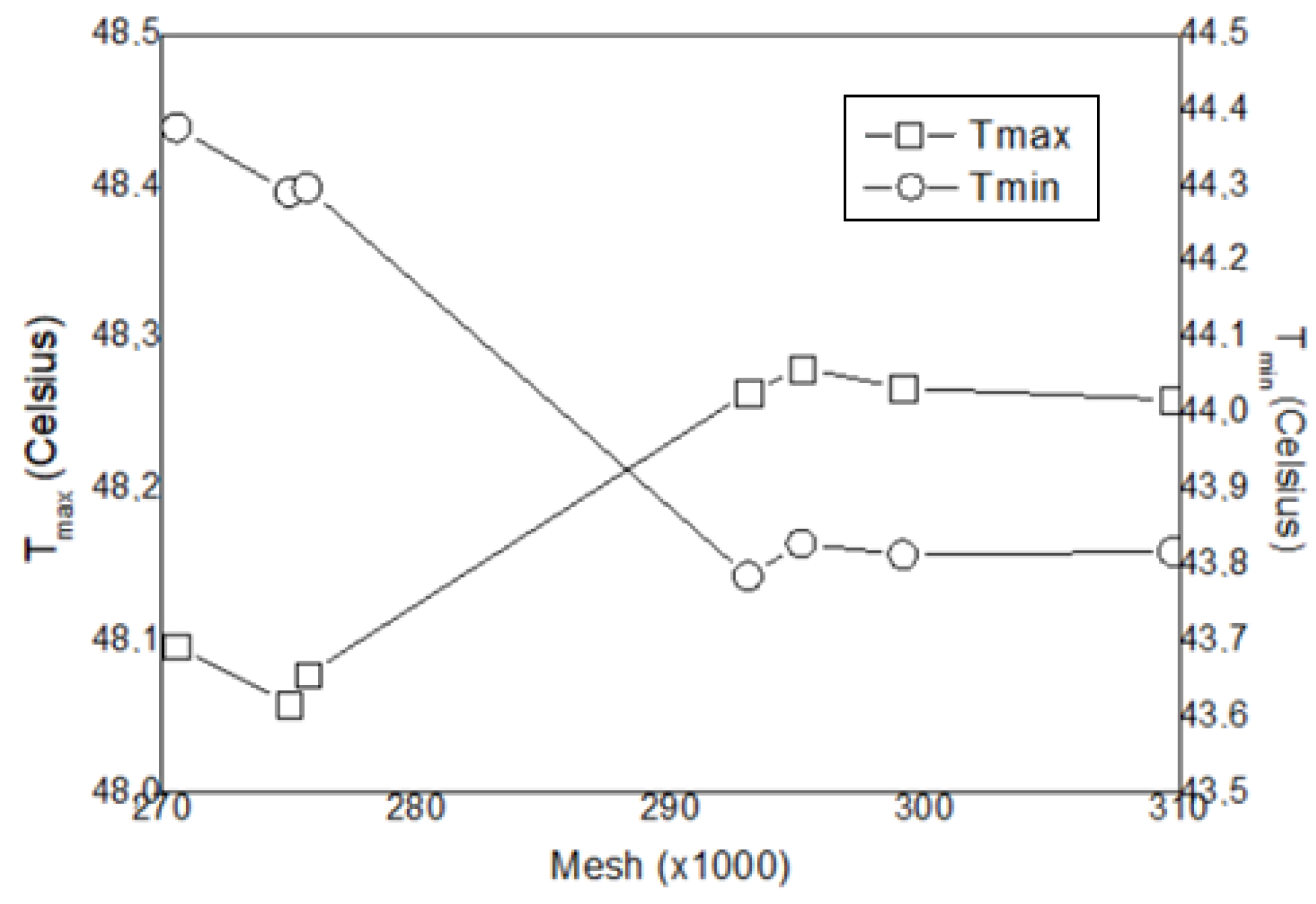
2.4. Mesh Validity Analysis
3. Results
4. Discussion
- 1.
- Effect of Discharge Rate:
- ○
- In tests conducted at room temperature with 1C and 2C discharge rates, the average heat generation powers of the battery were calculated as 0.2116 W and 0.834 W, respectively.
- ○
- The increased current values due to the higher discharge rates increases the heat generation, which leads to higher maximum temperatures in the battery.
- 2.
- Effect of Cooling Methods:
- ○
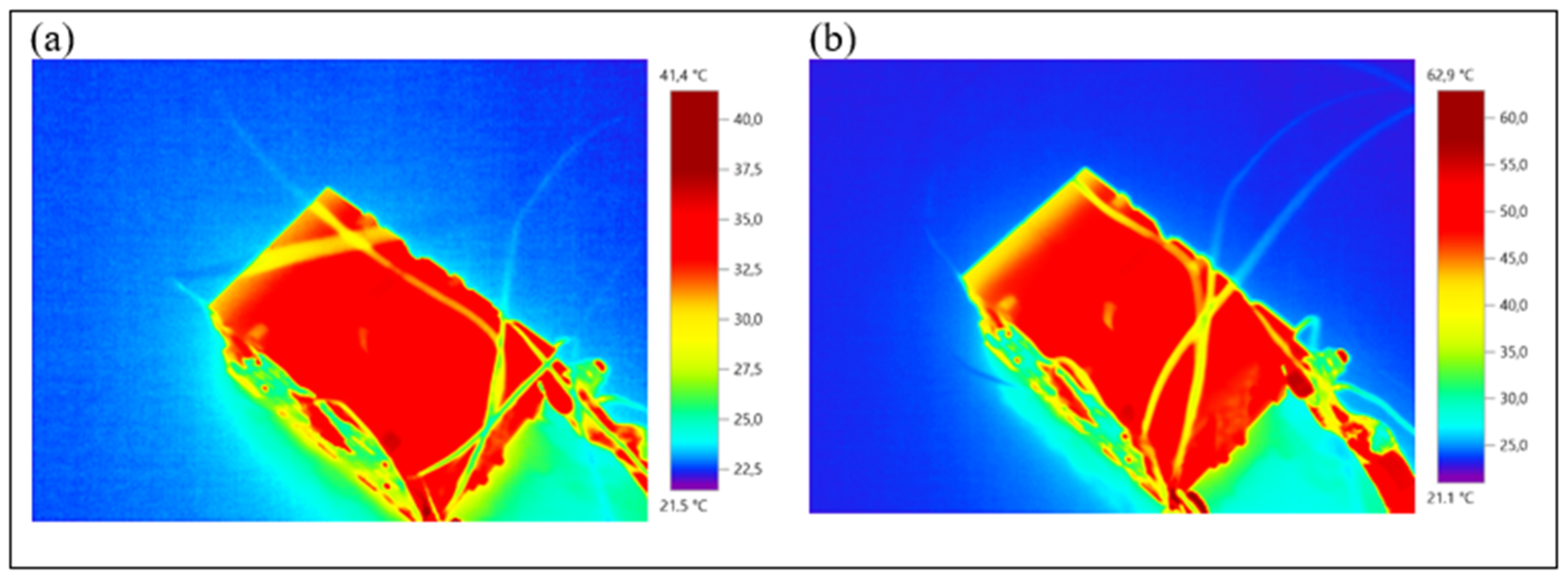
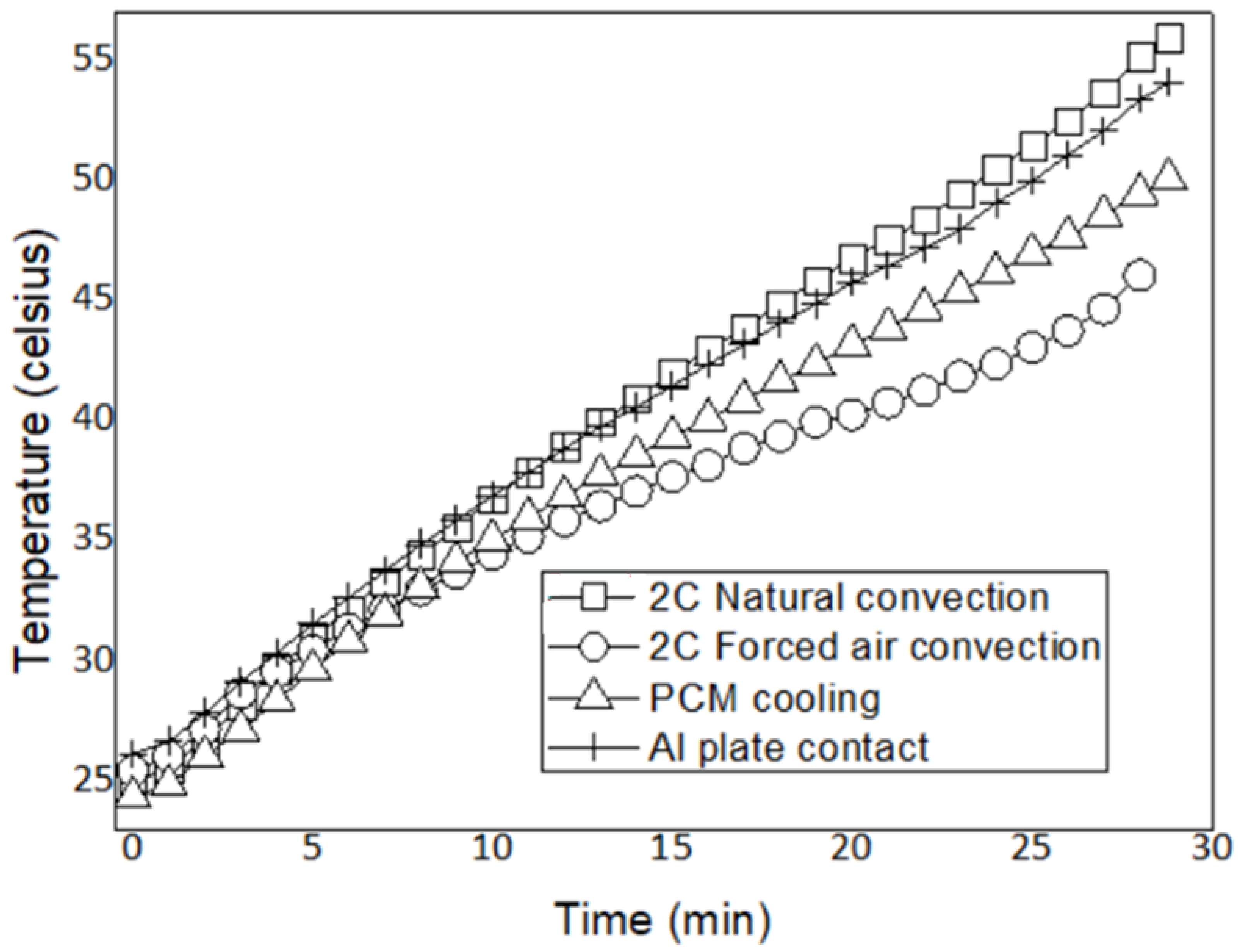
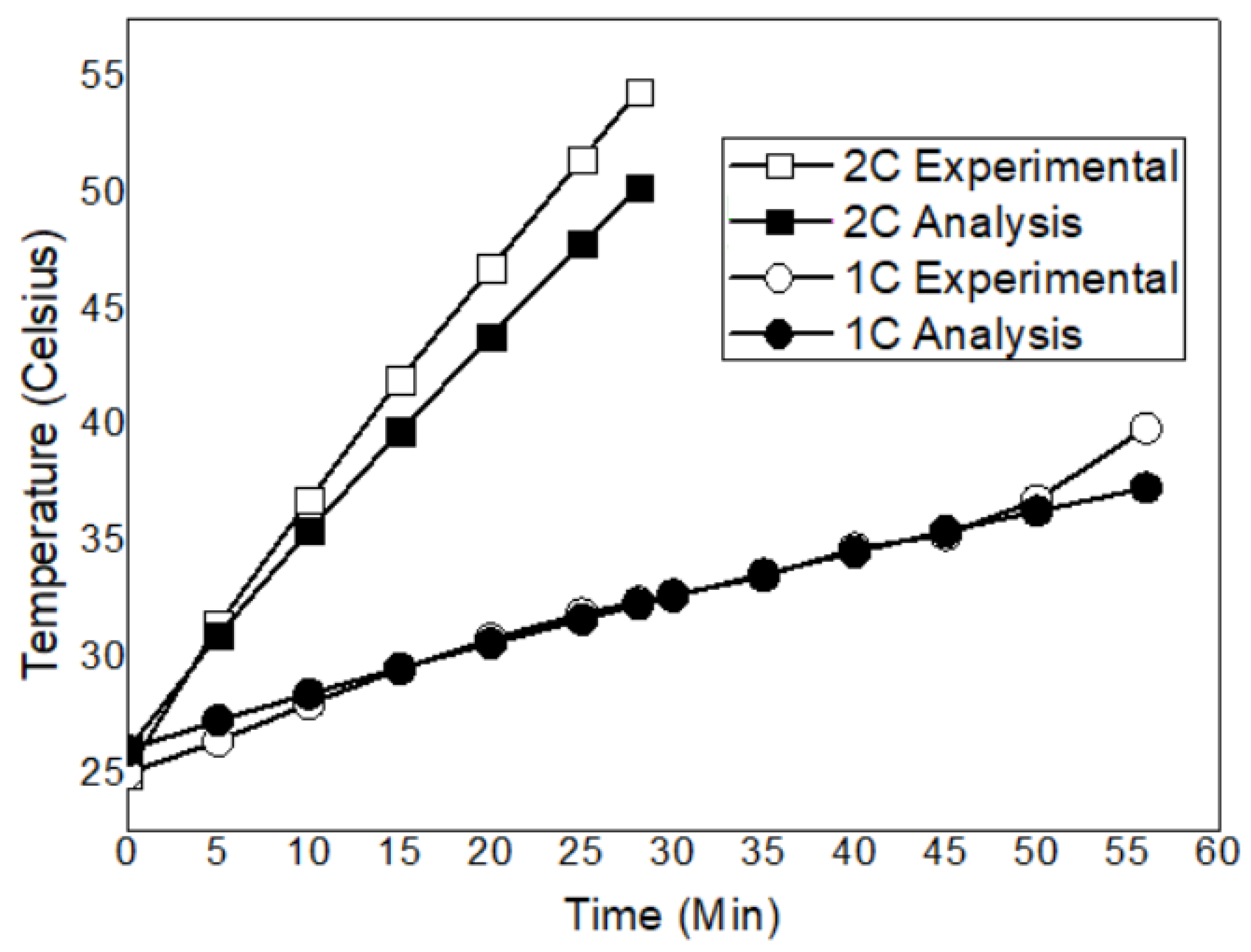
- Forced air convection is the most effective method for maintaining the lowest battery temperature and achieving the highest thermal control efficiency. At a 1C discharge rate, it reduces the battery maximum temperature to 32.9 °C, keeping the battery within the optimal operating range. By contrast, the maximum temperatures with natural air convection and PCM cooling were measured as 39.9 °C and 38.8 °C, respectively.
- ○
- At a 2C discharge rate, the increased heat generation leads to increased battery maximum temperatures. The maximum temperatures were recorded as 55.4 °C for natural air convection, 46.1 °C for forced air convection, and 53.4 °C for PCM cooling. While natural convection ensures the safe operating temperature range at both discharge rates, it exceeds the optimal operating range. PCM cooling slightly reduces the maximum temperature compared to natural convection and could be considered a suitable BTMS alternative, particularly at low discharge rates.
- ○
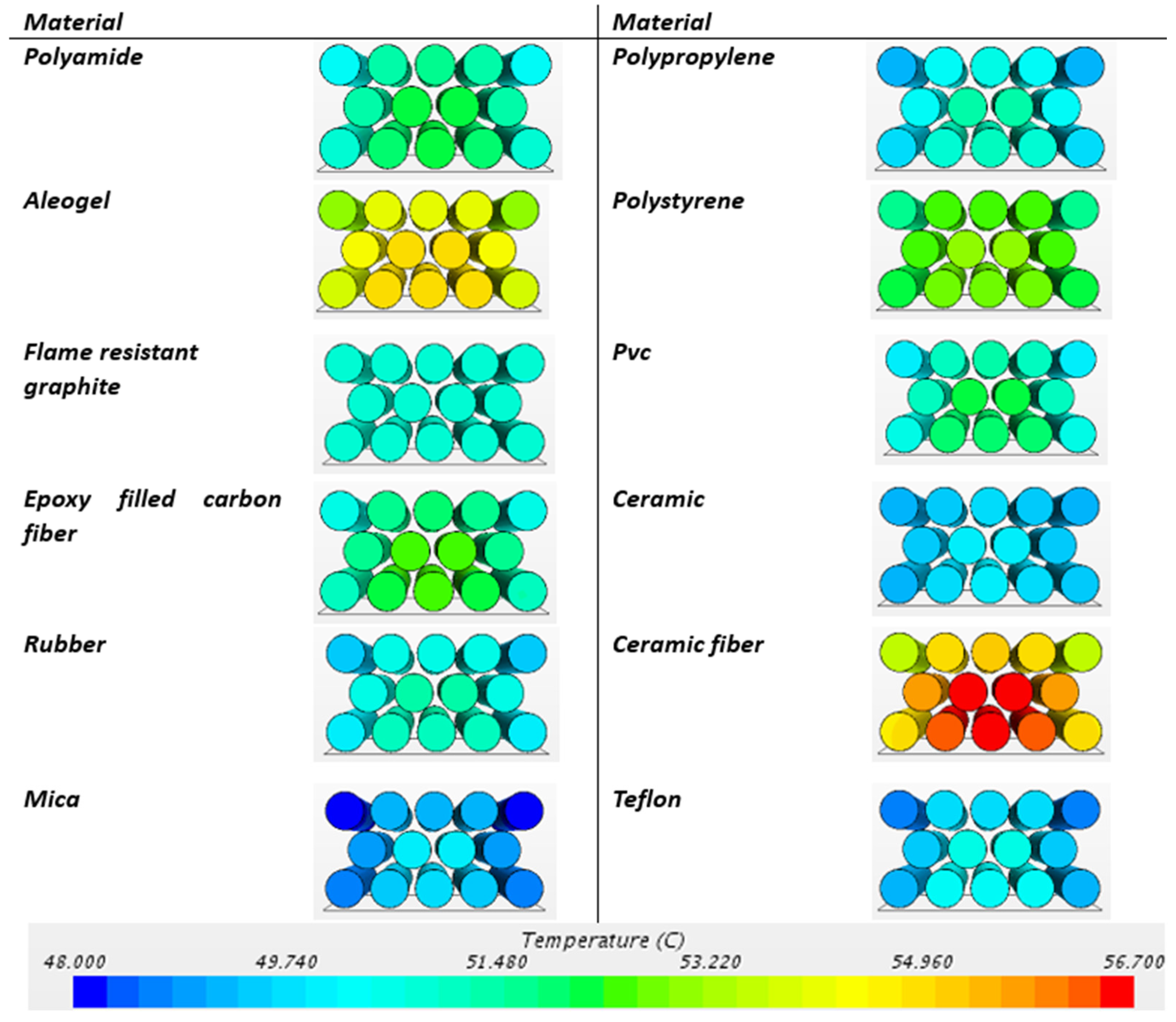
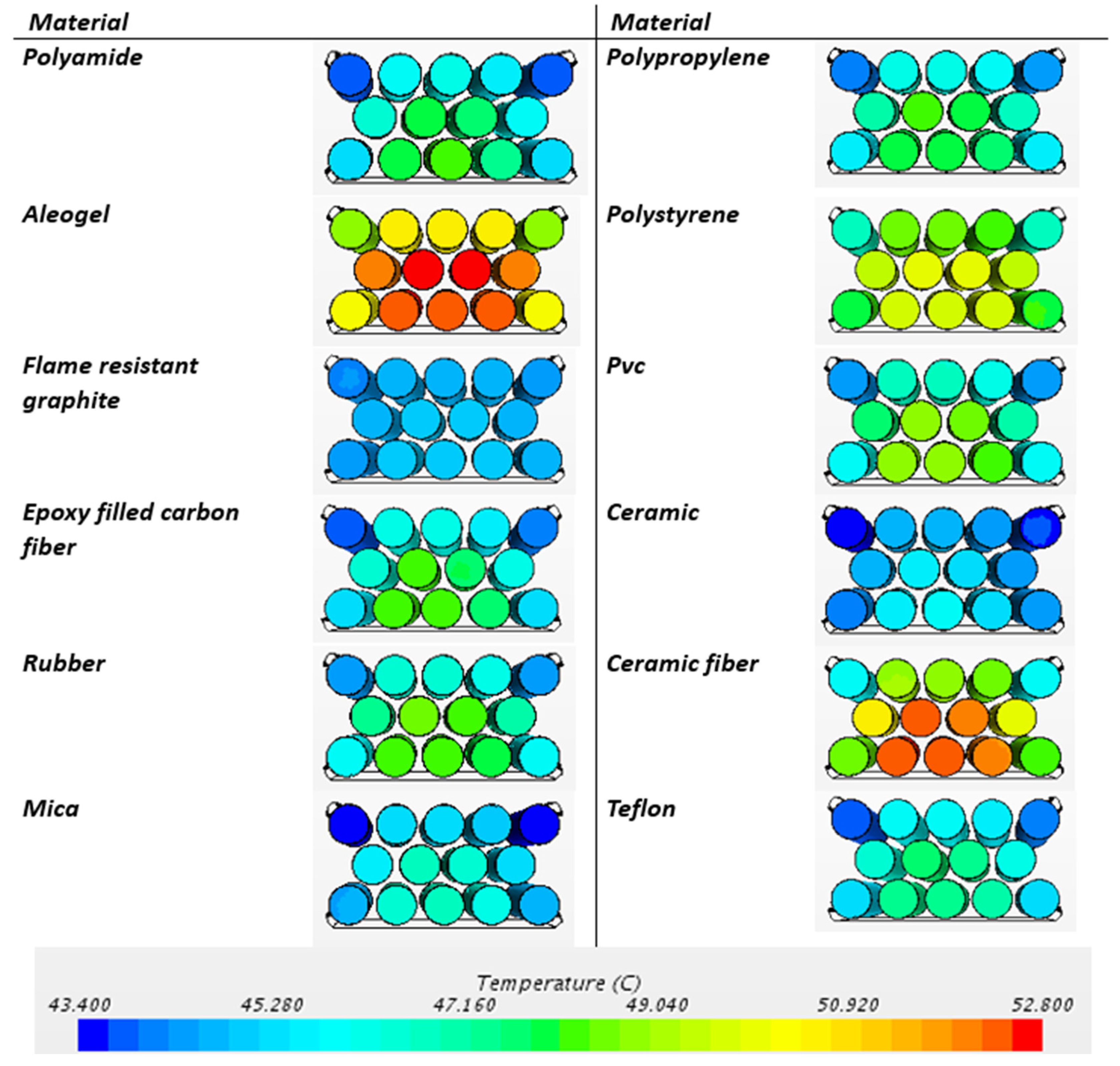
- Passive BTMSs (natural air convection and PCM cooling) ensure more homogeneous temperature distribution within the battery module.
- ○
- Depending on the thermal limits of the air and cell holder material, as the air flow rate increases up to a certain threshold value, the maximum temperature inside the battery and thermal homogeneity decrease. After exceeding the threshold, the maximum temperature and thermal homogeneity inside the battery remain constant.
- ○
- In the battery with a polyamide cell holder, the maximum and minimum temperatures inside the battery were reduced up to flow velocity of 15 m/s. However, at air flow velocity above 15 m/s, the maximum temperature and temperature difference inside the battery remained approximately constant. The maximum temperature inside the battery, which was 51.30 °C without cooling, decreased by 3.24% to 49.5 °C at 1 m/s. At 15 m/s, the maximum temperature decreased by 8.17% to 47.1 °C, and at 45 m/s, it decreased by 8.96% to 46.7 °C. The maximum temperature differences inside the battery at 1, 15, and 45 m/s were 3.6 °C, 4.3 °C, and 4.3 °C, respectively. Considering operational costs and noise, since there was no significant increase in cooling efficiency, an air flow velocity above 15 m/s is not recommended.
- 3.
- Effect of Cell Housing Materials:
- ○
- The thermophysical limits of the cell housing material play an important role in transferring the heat from the battery first to the cell housing and then to the cooling air. The effect of thermal resistance in the housing limits the cooling performance.
- ○
- The use of alternative materials could yield and alternative solutions to overcome these limitations.
- ○
- Among cell housings such as polyamide, aerogel, graphite composite, epoxy-filled carbon fiber, rubber, mica, polypropylene, polystyrene, PVC, ceramic, ceramic fiber, and Teflon, the ceramic and flame-resistant graphite composite materials increased the temperature homogeneity inside the battery and significantly reduced the temperature differences within the battery. When considering concerns about short circuit and thermal runaway safety, the electrically insulating and flame-resistant ceramic material is the most suitable module cell housing material in terms of battery safety and thermal management.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TMS | Thermal management system |
| BTMS | Battery thermal management system |
| PCM | Phase change material |
References
- Ghazal, M.; Akmal, M.; Iyanna, S.; Ghoudi, K. Smart plugs: Perceived usefulness and satisfaction: Evidence from United Arab Emirates. Renew. Sustain. Energy Rev. 2016, 55, 1248–1259. [Google Scholar] [CrossRef]
- Li, K.; Sun, C.; Zhang, M.; Wang, S.; Wei, B.; Cheng, Y.; Ju, X.; Xu, C. A Study of the Thermal Management and Discharge Strategies of Lithium-Ion Batteries in a Wide Temperature Range. Energies 2024, 17, 2319. [Google Scholar] [CrossRef]
- Worighi, I.; Maach, A.; Hafid, A.; Hegazy, O.; Mierlo, J.V. Integrating renewable energy in smart grid system: Architecture, virtualization and analysis. Sustain. Energy Grids Netw. 2019, 18, 100226. [Google Scholar] [CrossRef]
- Baroutaji, A.; Arjunan, A.; Ramadan, M.; Robinson, J.; Alaswad, A.; Abdelkareem, M.A.; Olabi, A.-G. Advancements and prospects of thermal management and waste heat recovery of PEMFC. Int. J. Thermofluids 2021, 9, 100064. [Google Scholar] [CrossRef]
- Allella, F.; Chiodo, E.; Giannuzzi, G.M.; Lauria, D.; Mottola, F. On-Line Estimation Assessment of Power Systems Inertia with High Penetration of Renewable Generatio. IEEE Access 2017, 8, 62689–62697. [Google Scholar] [CrossRef]
- Wang, J.; Lv, D.; Sha, H.; Lai, C.; Zeng, J.; Gao, T.; Yang, H.; Wu, H.; Jiang, Y. Numerical Investigation on the Thermal Performance of a Battery Pack by Adding Ribs in Cooling Channels. Energies 2024, 17, 4451. [Google Scholar] [CrossRef]
- Conte, F.; Giallongo, M.; Kaza, D.; Natrella, G.; Tachibana, R.; Tsuji, S.; Silvestro, F.; Vichi, G. Experimental Validation of Electrothermal and Aging Parameter Identification for Lithium-Ion Batteries. Energies 2024, 17, 2269. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Z.; Gong, M.; Tang, K.; Shen, Y.; Zhang, D. Commercial operation mode of shared energy storage system considering power transaction satisfaction of renewable energy power plants. J. Energy Storage 2025, 105, 114738. [Google Scholar] [CrossRef]
- Khaboshan, H.N.; Jaliliantabar, F.; Abdullah, A.A.; Panchal, S. Improving the cooling performance of cylindrical lithium-ion battery using three passive methods in a battery thermal management system. Appl. Therm. Eng. 2023, 227, 120320. [Google Scholar] [CrossRef]
- Shahid, S.; Munib, M.; Uzair, M.; Alkhedher, M. Numerical Simulation of Lithium-ion battery aging mechanisms and charging-discharging cycle. In Proceedings of the 2021 12th International Renewable Engineering Conference (IREC), Amman, Jordan, 14–15 April 2021. [Google Scholar]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Väyrynen, A.; Salminen, J. Lithium ion battery production. J. Chem. Thermodyn. 2012, 46, 80–85. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Bahiraei, F.; Nazri, G.-A.; Saif, M. Electrochemical–Thermal Model of Pouch-Type Lithium-Ion Batteries. Electrochim. Acta 2017, 247, 569–587. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Xi, L.; Li, Y.; Gao, J. A Review of Thermal Management and Heat Transfer of Lithium-Ion Batteries. Energies 2024, 17, 3873. [Google Scholar] [CrossRef]
- Feng, H.; Song, D. A health indicator extraction based on surface temperature for lithium-ion batteries remaining useful life prediction. J. Energy Storage 2021, 34, 102118. [Google Scholar] [CrossRef]
- Quan, T.; Xia, Q.; Wei, X.; Zhu, Y. Recent Development of Thermal Insulating Materials for Li-Ion Batteries. Energies 2024, 17, 4412. [Google Scholar] [CrossRef]
- Mahmood, A.; Cockerill, T.; Boer, G.; Voss, J.; Thompson, H. Heat Transfer Modeling and Optimal Thermal Management of Electric Vehicle Battery Systems. Energies 2024, 17, 4575. [Google Scholar] [CrossRef]
- Hosseinzadeh, E.; Genieser, R.; Worwood, D.; Barai, A.; Marco, J.; Jennings, P. A systematic approach for electrochemical-thermal modelling of a large format lithium-ion battery for electric vehicle application. J. Power Sources 2018, 382, 77–94. [Google Scholar] [CrossRef]
- Khan, M.; Alkhedher, M.; Ramadan, M.; Ghaza, M. Hybrid PCM-based thermal management for lithium-ion batteries: Trends and challenges. J. Energy Storage 2023, 73, 108775. [Google Scholar] [CrossRef]
- Isfahani, M.S.; Gharehghani, A.; Saeedipour, S.; Rabiei, M. PCM/metal foam and microchannels hybrid thermal management system for cooling of Li-ion battery. J. Energy Storage 2023, 72, 108789. [Google Scholar] [CrossRef]
- Hwang, F.; Confrey, T.; Reidy, C.; Picovici, D.; Callaghan, D.; Culliton, D.; Nolan, C. Review of battery thermal management systems in electric vehicles. Renew. Sustain. Energy Rev 2024, 192, 114171. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Wu, W.; Chen, K.; Hong, S.; Lai, Y. A critical review of battery thermal performance and liquid based battery thermal management. Energy Convers. Manag. 2019, 182, 262–281. [Google Scholar] [CrossRef]
- Sahin, R.C.; Gocmen, S.; Çetkin, E. Thermal management system for air-cooled battery packs with flow-disturbing structures. J. Power Sources 2022, 551, 232214. [Google Scholar] [CrossRef]
- Aydin, S.; Yiğit, K.S.; Savci, İ.H. Survey of air battery thermal management on the autonomous mobile robots. Therm. Sci. 2023, 27, 3561–3577. [Google Scholar] [CrossRef]
- Jeon, H.; Hong, S.; Yun, J.; Han, J. Cooling Strategy Optimization of Cylindrical Lithium-Ion Battery Pack via Multi-Counter Cooling Channels. Energies 2023, 16, 7860. [Google Scholar] [CrossRef]
- Kalkan, O.; Celen, A.; Bakırcı, K. Experimental and numerical investigation of the LiFePO4 battery cooling by natural convection. J. Energy Storage 2021, 40, 102796. [Google Scholar] [CrossRef]
- Yu, X.; Lu, Z.; Wei, L.; Zhang, L.; Cui, X.; Jin, L. Experimental Study on Transient Thermal Characteristics of Stagger-Arranged Lithium-Ion. Int. J. Heat Mass Transf. 2019, 143, 118576. [Google Scholar] [CrossRef]
- Yang, T.; Yang, N.; Zhang, X.; Li, G. Investigation of the thermal performance of axial-flow air cooling for the lithium-ion battery pack. Int. J. Therm. Sci. 2016, 108, 132–144. [Google Scholar] [CrossRef]
- Li, X.; He, F.; Zhang, G.; Huang, Q.; Zhou, D. Experiment and Simulation for Pouch Battery with Silica Cooling Plates and Copper Mesh based air cooling thermal management system. Appl. Therm. Eng. 2019, 146, 866–880. [Google Scholar] [CrossRef]
- Gocmen, S.; Cetkin, E. Emergence of elevated battery positioning in air cooled battery packs for temperature uniformity in ultra-fast dis/charging applications. J. Energy Storage 2022, 45, 103516. [Google Scholar] [CrossRef]
- Akula, R.; Minnikanti, A.; Balaji, C. Pin fin-PCM composite heat sink solution for thermal management of cylindrical Li-ion battery. Appl. Therm. Eng. 2024, 248 Pt A, 123146. [Google Scholar] [CrossRef]
- Yan, J.; Li, K.; Chen, H.; Wang, Q.; Sun, J. Experimental study on the application of phase change material in the dynamic cycling of battery pack system. Energy Convers. Manag. 2016, 128, 12–19. [Google Scholar] [CrossRef]
- Gu, J.; Du, J.; Li, Y.; Li, J.; Chen, L.; Chai, Y.; Li, Y. Preparation and Characterization of n-Octadecane@SiO2/GO and n-Octadecane@SiO2/Ag Nanoencapsulated Phase Change Material for Immersion Cooling of Li-Ion Battery. Energies 2023, 16, 1498. [Google Scholar] [CrossRef]
- Wang, C.L.; Leong, J.C. Analysis of Thermal Management Strategies for 21700 Lithium-Ion Batteries Incorporating Phase Change Materials and Porous Copper Foam with Different Battery Orientations. Energies 2024, 17, 1553. [Google Scholar] [CrossRef]
- Huynh, V.T.; Chang, K.; Lee, S.W. Numerical Investigation of the Thermal Performance of a Hybrid Phase Change Material and Forced Air Cooling System for a Three-Cell Lithium-Ion Battery Module. Energies 2023, 16, 7967. [Google Scholar] [CrossRef]
- El-Haddad, L.A.; İbrahim, L.; El-Sessy, A.; Jaber, A.A.; El-Haddad, S.A.; Hüsrozadeh, R. Thermal heat flux distribution prediction in an electrical vehicle battery cell using finite element analysis and neural network. Green Energy Intell. Transp. 2024, 3, 100155. [Google Scholar] [CrossRef]
- Zhong, Q.; Gao, L.; Li, W.; Garg, A. Transfer learning based topology optimization of battery cooling channels design for improved thermal performance. Appl. Therm. Eng. 2025, 263, 125400. [Google Scholar] [CrossRef]
- ASPİLSAN INR18650A28. Available online: https://www.aspilsan.com/cozumler/uretimini-yaptigimiz-sarj-edilebilir-lityum-iyon-pil/inr18650a28/ (accessed on 1 February 2025).





| Unit | Value | |
|---|---|---|
| Cell dimensional properties; | ||
| Cell diameter | mm | 18 + 0.10/−0.20 |
| Cell length | mm | 65 ± 0.2 |
| Cell Weight | g | 44.5 ± 0.7 |
| Cell chemistry; | ||
| Anode | Graphite | |
| Cathode | NiMnCo (NMC) | |
| Cell thermo-physical specification; | ||
| Density | 2746 | |
| Specific heat | 1065.71 | |
| Thermal conductivity | 33 | |
| Cell performance specification; | ||
| Discharge capacity | mA.h | 2800 (nominal) |
| mA.h | 2700 (minimum) | |
| Nominal Voltage | V | 3.65 |
| Charge end voltage | V | 4.2 |
| Charge cut-off current | mA.h | 140 |
| Discharge end voltage | V | 2.5 |
| Operation temperature | °C | 0/60 (charge) |
| −30/60 (discharge) |
| Discharge Rate | Time (s) | Temperature Rise (°C) | Heat Generation (W) |
|---|---|---|---|
| 1C | 3114 | 14 | 0.2146 |
| 2C | 1809 | 31.6 | 0.834 |
| Parameter | Value |
|---|---|
| Initial temperature | 24.5 °C |
| Ambient temperature | 23 °C |
| Wall heat flux | none |
| Discharge rate | 1C, 2C |
| Turbulence model | k-ω turbulence model |
| Cell and air density | Constant density |
| Battery Maximum Cell Temperature | ||||
|---|---|---|---|---|
| Natural Convection | Forced Air Convection | |||
| 2C | 1C | 2C | 1C | |
| Analysis | 52.3 °C | 38.7 °C | 48.42 °C | 34.44 °C |
| Test | 55.4 °C | 39.9 °C | 46.1 °C | 33 °C |
| Percentage error | 5.6% | 3% | 5% | 4.4% |
| Material | Tmax (°C) | Tmin (°C) | (°C) |
|---|---|---|---|
| Polyamide | 48.28 | 43.829 | 4.451 |
| Aerogel | 52.729 | 48.725 | 4.004 |
| Flame-resistant graphite | 45.047 | 44.268 | 0.779 |
| Epoxy-filled carbon fiber | 48.461 | 43.886 | 4.575 |
| Rubber | 48.547 | 44.293 | 4.254 |
| Mica | 46.951 | 43.401 | 3.55 |
| Polypropylene | 48.369 | 44.178 | 4.191 |
| Polystyrene | 50.103 | 46.776 | 3.327 |
| PVC | 49.051 | 44.342 | 4.709 |
| Ceramic | 45.838 | 43.506 | 2.332 |
| Ceramic fiber | 52.628 | 45.812 | 6.816 |
| Teflon | 47.677 | 43.877 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydın, S.; Samancıoğlu, U.E.; Savcı, İ.H.; Yiğit, K.S.; Çetkin, E. Impact of Cooling Strategies and Cell Housing Materials on Lithium-Ion Battery Thermal Management Performance. Energies 2025, 18, 1379. https://doi.org/10.3390/en18061379
Aydın S, Samancıoğlu UE, Savcı İH, Yiğit KS, Çetkin E. Impact of Cooling Strategies and Cell Housing Materials on Lithium-Ion Battery Thermal Management Performance. Energies. 2025; 18(6):1379. https://doi.org/10.3390/en18061379
Chicago/Turabian StyleAydın, Sevgi, Umut Ege Samancıoğlu, İsmail Hakkı Savcı, Kadri Süleyman Yiğit, and Erdal Çetkin. 2025. "Impact of Cooling Strategies and Cell Housing Materials on Lithium-Ion Battery Thermal Management Performance" Energies 18, no. 6: 1379. https://doi.org/10.3390/en18061379
APA StyleAydın, S., Samancıoğlu, U. E., Savcı, İ. H., Yiğit, K. S., & Çetkin, E. (2025). Impact of Cooling Strategies and Cell Housing Materials on Lithium-Ion Battery Thermal Management Performance. Energies, 18(6), 1379. https://doi.org/10.3390/en18061379






