Enhancing the Performance of Natural Ester Insulating Liquids in Power Transformers: A Comprehensive Review on Antioxidant Additives for Improved Oxidation Stability
Abstract
:1. Introduction
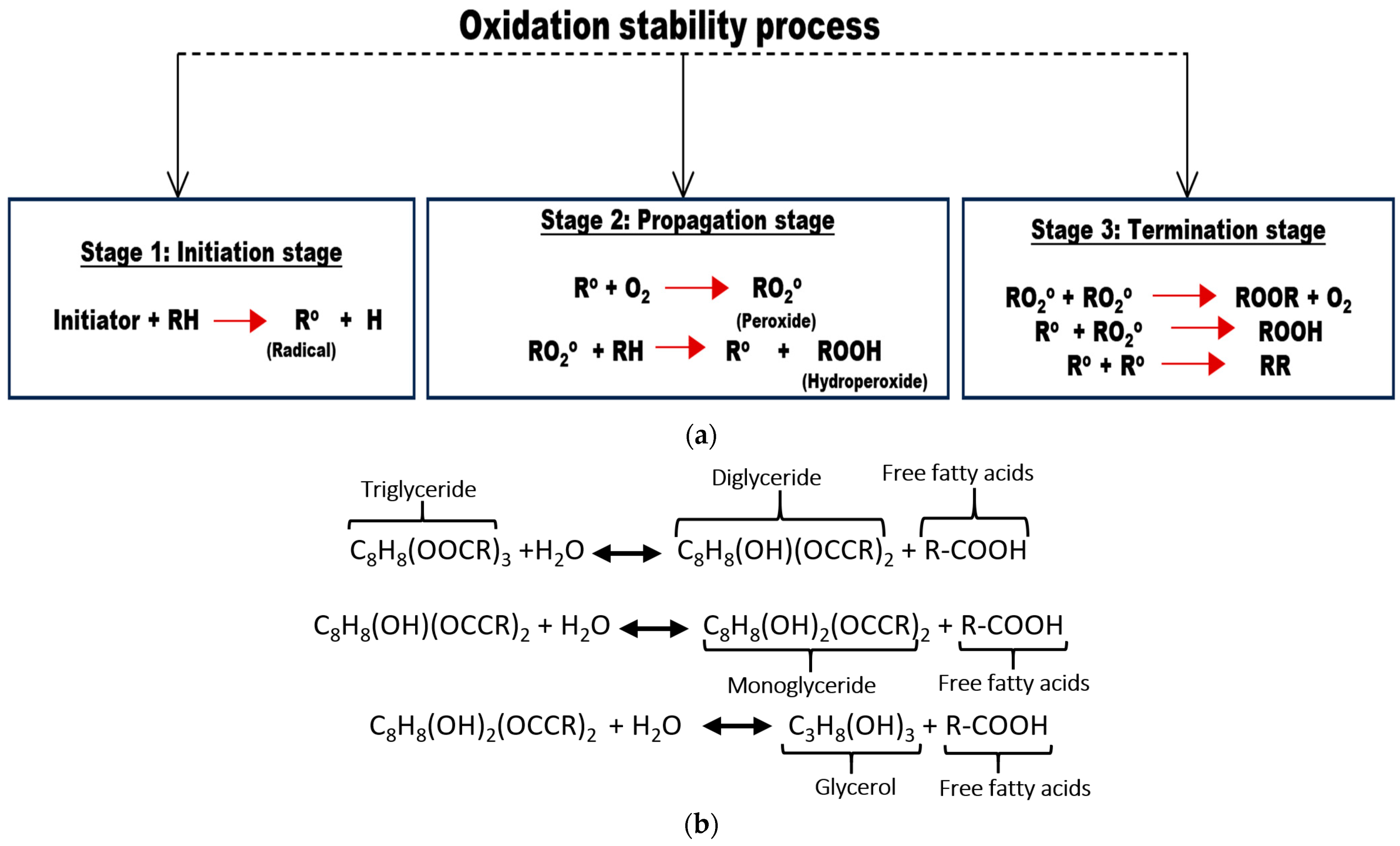
2. Oxidation Mechanism in Natural Esters
2.1. Effect of Oxidation on Transformer Useful Life and Sustainability
2.1.1. Impact of Oxidation Reaction on Oil Properties
2.1.2. Impact of Liquid Oxidation on Insulating Paper
3. Antioxidants Classification
3.1. Natural Antioxidant
3.1.1. Phenolics
3.1.2. Flavonoids
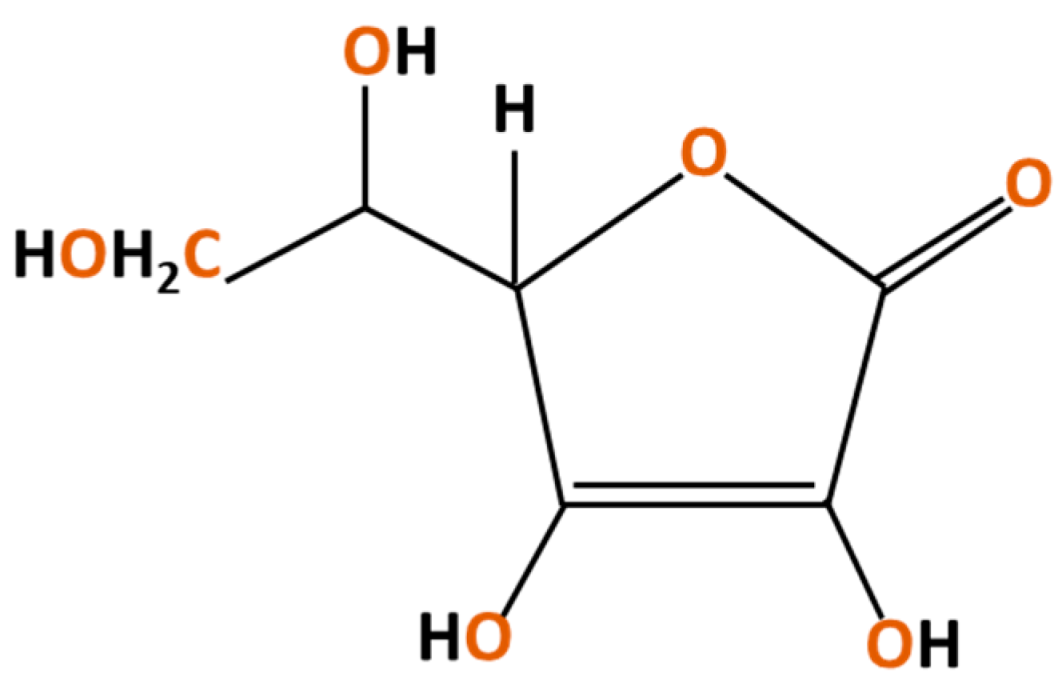
3.1.3. Ascorbic Acid
3.2. Synthetic Antioxidants
3.3. Antioxidant Activity Assessment
3.3.1. The Hydrogen Atom Transfer (HAT) Reaction Method
3.3.2. The Single Electron Transfer (SET) Test
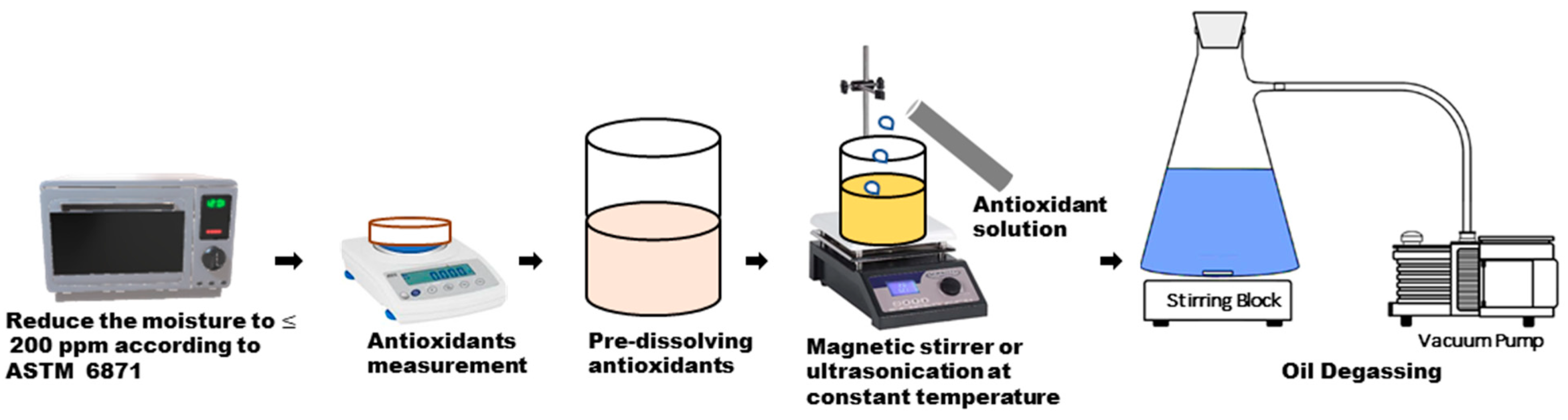
3.4. Natural Esters–Antioxidant Preparation
3.4.1. Moisture Content
3.4.2. Stirring Time and Temperature
3.5. Antioxidant Mechanism in Insulating Liquids
3.6. Natural Esters’ Oxidation Stability Assessment
- i.
- ii.
- RBOT: Oxidation assessment of natural esters can be determined using the rotating bomb oxidation test (RBOT). The natural ester sample is aged in a pressurized vessel at 120 °C in the presence of oxygen following the ASTM 2272 [114]. The completion of the test is indicated by the reduction in pressure within the vessel and the measurement of the corresponding time interval in minutes. The oxidation assessment value for mineral insulating liquids is 300 min, whereas for natural esters, it is less than 40 min [6,115]. A table comparing the oxidation induction time of different types of esters is presented in [116]. Table 5 shows the oxidation induction time of natural esters and other insulating liquids. Although the RBOT is an in-depth test for determining the oxidation stability of the insulating liquids, comparing the thermo–oxidative stability of different liquids when considering this method might be a challenge [115].
- iii.
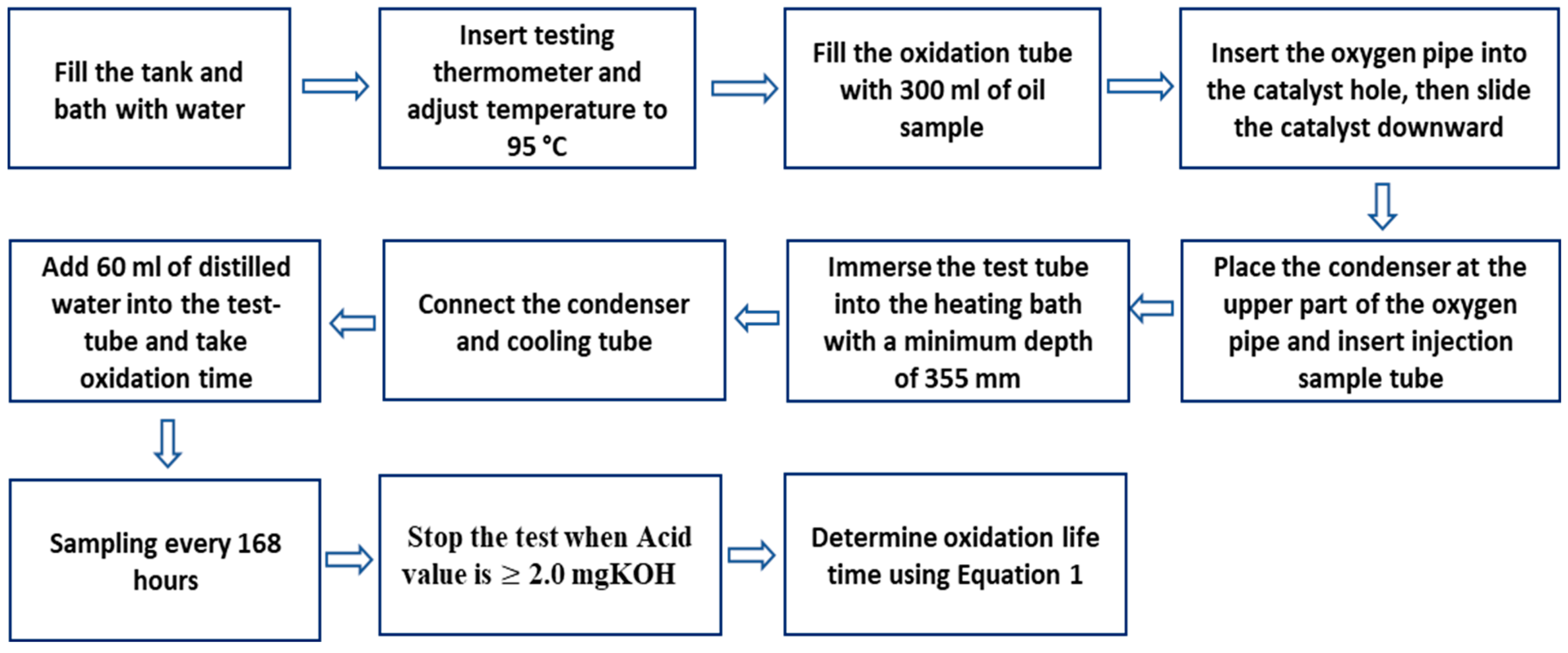
- The turbine oil oxidation stability test (TOST): This approach efficiently assesses the oxidative stability of oils and has proven effective in evaluating the oxidation stability of natural ester insulating oils for transformer applications [30]. A schematic diagram illustrating the sequential stages of the turbine oil oxidation stability test (TOST) following ASTM D-943-04a [117] is depicted in Figure 7. As depicted in Figure 7, samples are taken every 168 h, and testing ceases upon reaching an acid value of 2.0 mgKOH/g. The oxidation lifetime of the liquid samples is calculated using the expression given in Equation (1).where A represents the duration in test hours when the acid number was last recorded below 2.0; B represents the duration in test hours when the acid number was recorded above 2.0; C represents the acid number at A hours; and D represents the acid number at B hours [30].
- iv.
- Differential Scanning Calorimetry (DSC): DSC is a widely used technique for evaluating the oxidation stability of natural ester insulating liquids. During oxidation, oxygen reacts with the fatty acid chains in vegetable oils, leading to the generation of heat due to the energy released from the breaking and formation of chemical bonds. At the propagation stage of the oxidation process, the formation of lipid hydroperoxides is typically accompanied by heat release, which can be detected as an exothermic signal. Based on this principle, the oxidation induction time (OIT), defined according to ASTM E1858, is determined from the onset of the exothermic oxidation reaction and serves as a relative indicator of the oil’s oxidative stability [73]. An extended OIT implies improved oxidation resistance, especially when antioxidants are introduced, as their radical scavenging effect delays the onset of oxidation and increases the measured induction time.
4. Antioxidants and Effects on Some Imperative Properties of Natural Ester-Based Insulating Liquids
4.1. AC Breakdown Voltage of Natural Esters Containing Antioxidants
4.2. Ignition Properties of Natural Esters Containing Antioxidants
4.3. Effect of Antioxidants on Cooling Properties of Natural Esters
4.4. Effect of Antioxidants on the Dielectric Dissipation Factor of Natural Esters
4.5. Antioxidant Monitoring in Online Transformers
- i.
- High-Performance Liquid Chromatography (HPLC)
- ii.
- Gas Chromatography-Mass Spectrometry (GC-MS)
- iii.
- Fourier Transform Infrared Spectroscopy (FTIR)
- iv.
- Nuclear Magnetic Resonance (NMR) Spectroscopy
Machine Learning and Artificial Intelligence in Oxidation and Antioxidant Monitoring
- i.
- Predictive Models for Oxidation Stability
- ii.
- Integration with Molecular Dynamics
- iii.
- Real-Time Monitoring of Antioxidants
- iv.
- Fault Detection and Maintenance Optimization
5. Antioxidants and Other Additives in Insulating Liquids
6. Environmental Impact of Antioxidants Used in Natural Ester Oxidation Stability Enhancement
6.1. Antioxidant-Related Contamination Mitigation Strategy
- i.
- It is of utmost importance to understand the environmental effect of specific antioxidants that are being considered in natural ester enhancement by conducting thorough environmental impact assessments before use. This could involve assessing the toxicity of both the antioxidants and their secondary products in the ecosystem.
- ii.
- Effective handling and storage of natural esters containing antioxidants is important regardless of the toxicity of the antioxidants used. This helps prevent spills or leaks of the liquids by ensuring that the containers are securely sealed, labeled, and stored appropriately.
- iii.
- In the event of accidental oil spills or leaks, it is important to have effective means of oil detection and excellent cleaning measures in place to prevent the release of antioxidant-containing natural esters. Installing sensors or oil detection alarms in transformer units enables swift response to oil leakages. Furthermore, regular transformer maintenance and monitoring are vital for detecting wear, corrosion, and damage, allowing a prompt intervention to prevent oil leakage or spills.
- iv.
- When insulating oil reaches the end of its life, it is essential to ensure proper disposal in accordance with recommended regulations and guidelines for used oil disposal. Additionally, if the used oils are being considered for recycling, such as conversion into lubricants and biodiesel, it is important to take proper precautions, especially for workers, by providing reliable personal protective equipment.
6.2. Oxidation Stability Enhancement of Natural Esters for Sustainable Energy
- i.
- Inhibition of Oxidation Reactions: Antioxidants play a critical role in interrupting the oxidation chain reaction within natural esters. By neutralizing free radicals, which are highly reactive species responsible for initiating and propagating oxidation, antioxidants help prevent the degradation of the insulating liquid.
- ii.
- Thermal Stability and Extended Transformer Lifespan: Antioxidants significantly enhance the thermal stability of transformer oils, allowing natural esters to function effectively at high temperatures without experiencing pronounced degradation or thermal aging of insulating components. This improvement in thermo–oxidative stability enables transformers to handle higher and fluctuating loads without compromising efficiency, which is vital for ensuring reliable and sustainable energy.
- iii.
- Increased Compatibility with Existing Transformer Designs: By enhancing the oxidation stability of natural esters, antioxidants also improve their compatibility with existing transformer designs. This increased compatibility supports the broader adoption of natural esters as a replacement for mineral oils, promoting a shift towards more sustainable and environmentally friendly insulating liquids. To achieve sustainable, reliable, and efficient electricity generation and distribution, it is essential to focus on enhancing the oxidation stability of natural esters. The introduction of antioxidants extends the life and performance of transformers and also aligns with the global sustainability goals, making it a key strategy for the future of energy.
6.3. Economic Importance of Antioxidants in Natural Ester Enhancement
7. Challenges and Outlook
8. Conclusions
9. Recommendation
Author Contributions
Funding
Conflicts of Interest
References
- Ghani, S.A.; Noorden, Z.A.; Muhamad, N.A.; Zainuddin, H.; Chua Abdullah, M.I.H.; Chairul, I.S. Dielectric Strength Improvement of Natural Ester Insulation Oil via Mixed Antioxidants: Taguchi Approach. Int. J. Electr. Comput. Eng. 2017, 7, 650–658. [Google Scholar] [CrossRef]
- Fofana, I. 50 years in the development of insulating liquids. IEEE Electr. Insul. Mag. 2013, 29, 13–25. [Google Scholar] [CrossRef]
- Subburaj, S.K.; Rengaraj, M.; Mariappan, R. Evaluating critical characteristics of vegetable oil as a biodegradable insulating oil for transformer. Int. J. Emerg. Electr. Power Syst. 2020, 21, 20200128. [Google Scholar]
- Oparanti, S.O.; Khaleed, A.A.; Abdelmalik, A.A. AC breakdown analysis of synthesized nanofluids for oil-filled transformer insulation. Int. J. Adv. Manuf. Technol. 2021, 117, 1395–1403. [Google Scholar] [CrossRef]
- Bandara, K.; Ekanayake, C.; Saha, T.; Ma, H. Performance of natural ester as a transformer oil in moisture-rich environments. Energies 2016, 9, 258. [Google Scholar] [CrossRef]
- Mehta, D.M.; Kundu, P.; Chowdhury, A.; Lakhiani, V.; Jhala, A. A review on critical evaluation of natural ester vis-a-vis mineral oil insulating liquid for use in transformers: Part 1. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 873–880. [Google Scholar]
- Cilliyuz, Y.; Bicen, Y.; Aras, F.; Aydugan, G. Measurements and performance evaluations of natural ester and mineral oil-immersed identical transformers. Int. J. Electr. Power Energy Syst. 2021, 125, 106517. [Google Scholar]
- Oparanti, S.; Khaleed, A.; Abdelmalik, A. Nanofluid from palm kernel oil for high voltage insulation. Mater. Chem. Phys. 2021, 259, 123961. [Google Scholar]
- Adekunle, A.A.; Oparanti, S.O.; Fofana, I. Performance Assessment of Cellulose Paper Impregnated in Nanofluid for Power Transformer Insulation Application: A Review. Energies 2023, 16, 2002. [Google Scholar] [CrossRef]
- Jimoh, A.; Uba, S.; Ajibola, V.O.; Agbaji, E.B. Nanofluids DC Breakdown Analysis for Transformer Application. Chem. Afr. 2023, 6, 2101–2118. [Google Scholar] [CrossRef]
- Das, A.K. Comparative analysis of AC breakdown properties of Jatropha-based ester and other insulating oils: Commercial natural ester, synthetic ester, and mineral oil. Biomass Convers. Biorefinery 2023, 14, 30329–30341. [Google Scholar] [CrossRef]
- Koutras, K.N.; Peppas, G.D.; Tegopoulos, S.N.; Kyritsis, A.; Yiotis, A.G.; Tsovilis, T.E.; Gonos, I.F.; Pyrgioti, E.C. Ageing Impact on Relative Permittivity, Thermal Properties and Lightning Impulse Voltage Performance of Natural Ester Oil Filled with Semi-conducting Nanoparticles. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 1598–1607. [Google Scholar] [CrossRef]
- Evangelista, J.M., Jr.; Coelho, F.E.B.; Carvalho, J.A.; Araújo, E.M.; Miranda, T.L.; Salum, A. Development of a new bio-based insulating fluid from Jatropha curcas oil for power transformers. Adv. Chem. Eng. Sci. 2017, 7, 235. [Google Scholar] [CrossRef]
- Rafiq, M.; Shafique, M.; Ateeq, M.; Zink, M.; Targitay, D. Natural esters as sustainable alternating dielectric liquids for transformer insulation system: Analyzing the state of the art. Clean Technol. Environ. Policy 2023, 26, 623–659. [Google Scholar] [CrossRef]
- Przybylski, R.; Mag, T.; Eskin, N.; McDonald, B. Canola oil. Bailey’s Ind. Oil Fat Prod. 2005, 2, 61–122. [Google Scholar]
- Oparanti, S.O.; Fofana, I.; Jafari, R.; Zarrougui, R.; Abdelmalik, A. Canola oil: A renewable and sustainable green dielectric liquid for transformer insulation. Ind. Crops Prod. 2024, 215, 118674. [Google Scholar]
- Oparanti, S.O.; Yapi, K.M.L.; Fofana, I.; Rao, U.M. Preliminary studies on Improving the Properties of Canola Oil by Addition of Methyl Ester from a Saturated Vegetable Oil. In Proceedings of the 2023 IEEE Electrical Insulation Conference (EIC), Quebec City, QC, Canada, 18–22 June 2023; pp. 1–4. [Google Scholar]
- Wong, T.; Timoshkin, I.; Given, M.; Wilson, M.; MacGregor, S. Conduction characteristics of MIDEL eN 1204 insulating liquid under DC non-uniform conditions. In Proceedings of the 11th Universities High Voltage Network Colloquium, Athens, Greece, 10–13 September 2018. [Google Scholar]
- Salama, M.M.; Mansour, D.-E.A.; Daghrah, M.; Abdelkasoud, S.M.; Abbas, A.A. Thermal performance of transformers filled with environmentally friendly oils under various loading conditions. Int. J. Electr. Power Energy Syst. 2020, 118, 105743. [Google Scholar] [CrossRef]
- Chahal, J.; Reddy, C. Investigations on mustard oil for its suitability as insulating fluid in transformers. In Proceedings of the 2017 IEEE 19th International Conference on Dielectric Liquids (ICDL), Manchester, UK, 25–29 June 2017; pp. 1–3. [Google Scholar]
- Mahanta, D.K.; Laskar, S. Electrical insulating liquid: A review. J. Adv. Dielectr. 2017, 7, 1730001. [Google Scholar] [CrossRef]
- Hussin, N.; Yeaw, L.K.; Khalil, A.N.M.; Jamil, M.K.M.; Nayan, N.; Arshad, S.N.M.; Azizie, N.A. The effect of semi-conductive and non-conductive nano particles in sunflower oil based insulation. J. Phys. Conf. Ser. 2020, 1432, 012016. [Google Scholar]
- Ab Ghani, S.; Muhamad, N.A.; Noorden, Z.A.; Zainuddin, H.; Bakar, N.A.; Talib, M.A. Methods for improving the workability of natural ester insulating oils in power transformer applications: A review. Electr. Power Syst. Res. 2018, 163, 655–667. [Google Scholar] [CrossRef]
- Romadhona, G.; Wibowo, K.; Royan, R. Study of extra virgin olive oil as liquid insulation in transformer. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Jakarta, Indonesia, 23–25 March 2021; p. 012030. [Google Scholar]
- Banumathi, S.; Chandrasekar, S. Analysis of breakdown strength and physical characteristics of vegetable oils for high voltage insulation applications. J. Adv. Chem. 2016, 12, 4902–4912. [Google Scholar]
- Rao, U.M.; Fofana, I.; Rozga, P.; Picher, P.; Sarkar, D.K.; Karthikeyan, R. Influence of gelling in natural esters under open beaker accelerated thermal aging. IEEE Trans. Dielectr. Electr. Insul. 2022, 30, 413–420. [Google Scholar] [CrossRef]
- Oparanti, S.O.; Rao, U.M.; Fofana, I. Natural esters for green transformers: Challenges and keys for improved serviceability. Energies 2022, 16, 61. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, S.; Liu, Q.; Wang, Z. Oxidation stability assessment of a vegetable transformer oil under thermal aging. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 683–692. [Google Scholar] [CrossRef]
- Szcześniak, D.; Przybylek, P. Oxidation stability of natural ester modified by means of fullerene nanoparticles. Energies 2021, 14, 490. [Google Scholar] [CrossRef]
- Raof, N.A.; Yunus, R.; Rashid, U.; Azis, N.; Yaakub, Z. Effect of molecular structure on oxidative degradation of ester based transformer oil. Tribol. Int. 2019, 140, 105852. [Google Scholar] [CrossRef]
- Rafiq, M.; Lv, Y.; Zhou, Y.; Ma, K.; Wang, W.; Li, C.; Wang, Q. Use of vegetable oils as transformer oils—A review. Renew. Sustain. Energy Rev. 2015, 52, 308–324. [Google Scholar] [CrossRef]
- Bandara, K.; Ekanayake, C.; Saha, T.K.; Annamalai, P.K. Understanding the ageing aspects of natural ester based insulation liquid in power transformer. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 246–257. [Google Scholar] [CrossRef]
- Yan, Z.W.; Matharage, S.; Liu, Q.; Wang, Z. Extraction of low molecular weight acids from transformer liquids using water extraction technique. In Proceedings of the 2018 12th International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Xi’an, China, 20–24 May 2018; pp. 198–201. [Google Scholar]
- Kouassi, K.D.; Fofana, I.; Cissé, L.; Hadjadj, Y.; Yapi, K.M.L.; Diby, K.A. Impact of low molecular weight acids on oil impregnated paper insulation degradation. Energies 2018, 11, 1465. [Google Scholar] [CrossRef]
- Yan, Z.; Kihampa, T.; Matharage, S.; Liu, Q.; Wang, Z. Measuring Low Molecular Weight Acids in Mineral and Ester Transformer Liquids. In Proceedings of the 2020 8th International Conference on Condition Monitoring and Diagnosis (CMD), Phuket, Thailand, 25–28 October 2020; pp. 354–357. [Google Scholar]
- Walker, J.; Valot, A.; Wang, Z.; Yi, X.; Liu, Q. M/DBT, new alternative dielectric liquids for transformers. In Proceedings of the CIGRE D1-107 Colloquium; CIGRE: Paris, France, 2012; pp. 1–10. [Google Scholar]
- Fofana, I.; Sabau, J.; Betie, A. Measurement of the relative Free radical content of Insulating Oils of Petroleum Origin. Energies 2015, 8, 7690–7702. [Google Scholar] [CrossRef]
- Bhat, B.A.; Nisar, S.; Sheikh, B.A.; Mir, W.R.; Mir, M.A. Antioxidants (natural and synthetic) screening assays: An overview. Bentham Briefs Biomed. Pharmacother. Oxidative Stress Nat. Antioxid. 2021, 1, 105–126. [Google Scholar]
- Xia, G.; Wu, G.; Gao, B.; Yin, H.; Yang, F. A new method for evaluating moisture content and aging degree of transformer oil-paper insulation based on frequency domain spectroscopy. Energies 2017, 10, 1195. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Huang, B.; Hao, J.; Chen, G. Review of research progress on the electrical properties and modification of mineral insulating oils used in power transformers. Energies 2018, 11, 487. [Google Scholar] [CrossRef]
- Sbravati, A.; Ignacio, R.; Rapp, K.; Wirtz, K. Long-term performance of natural ester liquids. In Proceedings of the 2022 IEEE 21st International Conference on Dielectric Liquids (ICDL), Sevilla, Spain, 29 May–2 June 2022; pp. 1–4. [Google Scholar]
- Oparanti, S.O.; Adekunle, A.A.; Oteikwu, V.E.; Galadima, A.I.; Abdelmalik, A.A. An experimental investigation on composite methyl ester as a solution to environmental threat caused by mineral oil in transformer insulation. Biomass Convers. Biorefinery 2022, 14, 12933–12943. [Google Scholar] [CrossRef]
- Oparanti, S.; Abdelmalik, A.; Khaleed, A.; Abifarin, J.; Suleiman, M.; Oteikwu, V. Synthesis and characterization of cooling biodegradable nanofluids from non-edible oil for high voltage application. Mater. Chem. Phys. 2022, 277, 125485. [Google Scholar] [CrossRef]
- Oparanti, S.O.; Salaudeen, I.K.; Adekunle, A.A.; Oteikwu, V.E.; Galadima, A.I.; Abdelmalik, A.A. Physicochemical and Dielectric Study on Nigerian Thevetia Peruviana as a Potential Green Alternative Fluid for Transformer Cooling/Insulation. Waste Biomass Valorization 2022, 14, 1693–1703. [Google Scholar] [CrossRef]
- Wilhelm, H.; Stocco, G.; Batista, S. Reclaiming of in-service natural ester-based insulating fluids. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 128–134. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, J.; Zhang, C.; Zheng, P.; Nakanishi, Y.; Wu, T. State-of-art review on chemical indicators for monitoring the aging status of oil-immersed transformer paper insulation. Energies 2023, 16, 1396. [Google Scholar] [CrossRef]
- Wan, F.; Du, L.; Chen, W.; Wang, P.; Wang, J.; Shi, H. A novel method to directly analyze dissolved acetic acid in transformer oil without extraction using Raman spectroscopy. Energies 2017, 10, 967. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Natural antioxidants of plant origin. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 90, pp. 1–81. [Google Scholar]
- Girardi, J.C.; Bariccatti, R.A.; Savada, F.Y.; Borsato, D.; de Souza, S.N.M.; Amaral, C.Z.; Prior, M. Response surface methodology for the optimization of oxidative stability through the use of natural additives. Renew. Energy 2020, 159, 346–355. [Google Scholar] [CrossRef]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.; Serna Saldívar, S.O. Environmentally friendly methods for flavonoid extraction from plant material: Impact of their operating conditions on yield and antioxidant properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar]
- Rani, A.; Kumar, V.; Verma, S.K.; Shakya, A.K.; Chauhan, G. Tocopherol content and profile of soybean: Genotypic variability and correlation studies. J. Am. Oil Chem. Soc. 2007, 84, 377–383. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Crozier, A.; Clifford, M.N.; Ashihara, H. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Blackwell Publishers: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Kaur, P.; Mehta, R.G.; Thind, T.S.; Arora, S. Bentham Briefs in Biomedicine and Pharmacotherapy Oxidative Stress and Natural Antioxidants; Bentham Science Publishers: Sharjah, United Arab Emirates, 2021. [Google Scholar]
- Rial, R.C.; Merlo, T.C.; Santos, P.H.M.; Melo, L.F.D.; Barbosa, R.A.; de Freitas, O.N.; Nazário, C.E.D.; Viana, L.H. Evaluation of oxidative stability of soybean methyl biodiesel using extract of cagaite leaves (Eugenia dysenterica DC.) as additive. Renew. Energy 2020, 152, 1079–1085. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Knothe, G.; Nie, X.; Jiang, J. Synthesis of epoxidized cardanol and its antioxidative properties for vegetable oils and biodiesel. ACS Sustain. Chem. Eng. 2016, 4, 901–906. [Google Scholar] [CrossRef]
- Buosi, G.M.; da Silva, E.T.; Spacino, K.; Silva, L.R.C.; Ferreira, B.A.D.; Borsato, D. Oxidative stability of biodiesel from soybean oil: Comparison between synthetic and natural antioxidants. Fuel 2016, 181, 759–764. [Google Scholar] [CrossRef]
- Valenga, M.G.P.; Boschen, N.L.; Rodrigues, P.R.P.; Maia, G.A.R. Agro-industrial waste and Moringa oleifera leaves as antioxidants for biodiesel. Ind. Crops Prod. 2019, 128, 331–337. [Google Scholar] [CrossRef]
- Kumar, S.S.; Iruthayarajan, M.W.; Bakrutheen, M.; Kannan, S.G. Effect of antioxidants on critical properties of natural esters for liquid insulations. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2068–2078. [Google Scholar]
- Singh, S.K.; Kaldate, R.; Bisht, A. Citric acid, antioxidant effects in health. In Antioxidants Effects in Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–322. [Google Scholar]
- Ryan, E.M.; Duryee, M.J.; Hollins, A.; Dover, S.K.; Pirruccello, S.; Sayles, H.; Real, K.D.; Hunter, C.D.; Thiele, G.M.; Mikuls, T.R. Antioxidant properties of citric acid interfere with the uricase-based measurement of circulating uric acid. J. Pharm. Biomed. Anal. 2019, 164, 460–466. [Google Scholar] [CrossRef]
- Fuchs, J. Potentials and limitations of the natural antioxidants RRR-alpha-tocopherol, L-ascorbic acid and β-carotene in cutaneous photoprotection. Free Radic. Biol. Med. 1998, 25, 848–873. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Ascorbic acid as antioxidant. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2023; Volume 121, pp. 247–270. [Google Scholar]
- Mohamed, R.; Fernandez, J.; Pineda, M.; Aguilar, M. Roselle (Hibiscus sabdariffa) seed oil is a rich source of γ-tocopherol. J. Food Sci. 2007, 72, S207–S211. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.L. Carotenoids—Antioxidant properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gu, X.; Shi, Y. A review on lignin antioxidants: Their sources, isolations, antioxidant activities and various applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhong, W.; Li, P.; Ren, J.; Jiang, K.; Wu, W. Recent advances in lignin antioxidant: Antioxidant mechanism, evaluation methods, influence factors and various applications. Int. J. Biol. Macromol. 2023, 125992. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 1–16. [Google Scholar]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical stability of ascorbic acid integrated into commercial products: A review on bioactivity and delivery technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Raymon, A.; Pakianathan, P.S.; Rajamani, M.; Karthik, R. Enhancing the critical characteristics of natural esters with antioxidants for power transformer applications. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 899–912. [Google Scholar]
- Ab Ghani, S.; Muhamad, N.A.; Noorden, Z.A.; Zainuddin, H.; Ahmad, A.A. Multi-response optimization of the properties of natural ester oil with mixed antioxidants using taguchi-based methodology. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1674–1684. [Google Scholar] [CrossRef]
- Ab Ghani, S.; Muhamad, N.A.; Zainuddin, H.; Noorden, Z.A.; Mohamad, N. Application of response surface methodology for optimizing the oxidative stability of natural ester oil using mixed antioxidants. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 974–983. [Google Scholar] [CrossRef]
- de Oliveira, R.R.; de Lima, K.M.G.; Tauler, R.; de Juan, A. Application of correlation constrained multivariate curve resolution alternating least-squares methods for determination of compounds of interest in biodiesel blends using NIR and UV–visible spectroscopic data. Talanta 2014, 125, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, I.d.M.; Rios, M.A.d.S.; Cavalcante, C.L., Jr.; Luna, F.M.T. Effects of amine and phenolic based antioxidants on the stability of babassu biodiesel using rancimat and differential scanning calorimetry techniques. Ind. Eng. Chem. Res. 2019, 59, 18–24. [Google Scholar] [CrossRef]
- Khezerlou, A.; pouya Akhlaghi, A.; Alizadeh, A.M.; Dehghan, P.; Maleki, P. Alarming impact of the excessive use of tert-butylhydroquinone in food products: A narrative review. Toxicol. Rep. 2022, 9, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bi, Y.; Yang, H.; Wang, D. Antioxidative properties and interconversion of tert-butylhydroquinone and tert-butylquinone in soybean oils. J. Agric. Food Chem. 2017, 65, 10598–10603. [Google Scholar] [CrossRef]
- Gálico, D.; Nova, C.; Guerra, R.; Bannach, G. Thermal and spectroscopic studies of the antioxidant food additive propyl gallate. Food Chem. 2015, 182, 89–94. [Google Scholar] [CrossRef]
- Wang, Q.; de Oliveira, E.F.; Alborzi, S.; Bastarrachea, L.J.; Tikekar, R.V. On mechanism behind UV-A light enhanced antibacterial activity of gallic acid and propyl gallate against Escherichia coli O157: H7. Sci. Rep. 2017, 7, 8325. [Google Scholar] [CrossRef]
- Fazal, M.; Jakeria, M.; Haseeb, A.; Rubaiee, S. Effect of antioxidants on the stability and corrosiveness of palm biodiesel upon exposure of different metals. Energy 2017, 135, 220–226. [Google Scholar] [CrossRef]
- Thangaraj, H.; David, P.W.; Balachandran, G.B.; Sivasekar, G.K. Performance evaluation of natural Olea europaea (olive oil)-based blended esters with butylated hydroxyanisole and butylated hydroxytoluene: Optimization using response surface methodology. Environ. Sci. Pollut. Res. 2024, 31, 4985–5000. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of antioxidants: Mechanisms and pharmaceutical applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef]
- Yu, S. A new antioxidant with higher activity at elevated temperature based on multiple intramolecular synergisms. ChemistrySelect 2023, 8, e202300747. [Google Scholar] [CrossRef]
- Johnson, D. LubricationTribology, Lubricants and Additives; BoD–Books on Demand: Hamburg, Germany, 2018. [Google Scholar]
- Rudnick, L.R. Lubricant Additives: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Tang, J.; You, G.; Ruan, L.; Lu, Y.; Wen, B.; Wu, S. Antioxidant behavior affected by polarity in the olive oil: Experimental and molecular simulation investigations. ACS Omega 2021, 6, 7119–7126. [Google Scholar]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative stability of flaxseed oil: Effect of hydrophilic, hydrophobic and intermediate polarity antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [PubMed]
- Farooq, S.; Abdullah; Zhang, H.; Weiss, J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil–water interface of food emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4277. [Google Scholar] [PubMed]
- Barchan, A.; Bakkali, M.; Arakrak, A.; Pagán, R.; Laglaoui, A. The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 399–412. [Google Scholar]
- Bakhouche, K.; Dhaouadi, Z.; Jaidane, N.; Hammoutène, D. Comparative antioxidant potency and solvent polarity effects on HAT mechanisms of tocopherols. Comput. Theor. Chem. 2015, 1060, 58–65. [Google Scholar]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Moharram, H.; Youssef, M. Methods for determining the antioxidant activity: A review. Alex. J. Food Sci. Technol. 2014, 11, 31–42. [Google Scholar]
- Varghese, S.; Akshaya, C.; Jisha, M. Unravelling the bioprospects of mycoendophytes residing in withania somnifera for productive pharmaceutical applications. Biocatal. Agric. Biotechnol. 2021, 37, 102172. [Google Scholar] [CrossRef]
- Demirci Çekiç, S.; Cetinkaya, A.; Avan, A.N.; Apak, R. Correlation of total antioxidant capacity with reactive oxygen species (ROS) consumption measured by oxidative conversion. J. Agric. Food Chem. 2013, 61, 5260–5270. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Ab Ghani, S.; Muhamad, N.A.; Noorden, Z.A.; Zainuddin, H.; Talib, M.A. Oxidation stability enhancement of natural ester insulation oil: Optimizing the antioxidants mixtures by two-level factorial design. ARPN J. Eng. Appl. Sci. 2017, 12, 1694–1700. [Google Scholar]
- ASTM D6871-17; Standard Specification for Natural (Vegetable Oil) Ester Fluids Used in Electrical Apparatus. ASTM: West Conshohocken, PA, USA, 2003.
- Demirci-Cekic, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of oxidative stress and antioxidant defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- Jadim, R.; Kans, M.; Rehman, S.; Alhems, L. A relevant condition monitoring of corrosive sulphur deposition on the windings of oil-filled electrical transformers. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1736–1742. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Lu, Y.; Xi, Z.; Jin, B.; Li, M.; Ren, C. Reaction mechanism and thermodynamics of the elimination of peroxy radicals by an antioxidant enzyme inhibitor complex. Fuel 2020, 272, 117719. [Google Scholar] [CrossRef]
- Penta, S. Advances in Structure and Activity Relationship of Coumarin Derivatives; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Rao, U.M.; Fofana, I.; N’cho, J.S. On some imperative IEEE standards for usage of natural ester liquids in transformers. IEEE Access 2020, 8, 145446–145456. [Google Scholar] [CrossRef]
- Sabahannur, S.; Alimuddin, S. Identification of Fatty Acids in Virgin Coconut Oil (VCO), Cocoa Beans, Crude Palm Oil (CPO), and Palm Kernel Beans Using Gas Chromatography. IOP Conf. Ser. Earth Environ. Sci. 2022, 1083, 012036. [Google Scholar]
- Souza, L.; Silva, F.; Maria, A.; Belem, A.; Cecchin, D.; Barros, M. Response surface for biodiesel production from soybean oil by ethylic route. Agron. Res. 2020, 18, 1498–1515. [Google Scholar] [CrossRef]
- Chowdhury, K.; Banu, L.A.; Khan, S.; Latif, A. Studies on the fatty acid composition of edible oil. Bangladesh J. Sci. Ind. Res. 2007, 42, 311–316. [Google Scholar]
- IEC 61125; Insulating Liquids—Test Methods for Oxidation Stability—Test Method for Evaluating the Oxidation Stability of Insulating Liquids in the Delivered State. International Electrotechnical Commission: Geneva, Switzerland, 2018.
- IEC 62770; Fluids for Electrotechnical Applications—Unused Natural Esters for Transformers and Similar Electrical Equipment. International Electrotechnical Commission: Geneva, Switzerland, 2013.
- Mustangin, M.; Purwantana, B.; Hidayat, C. Development of high free fatty acid crude palm oil as a biodegradable electrical liquid insulator as an alternative to mineral oil-based insulators. Clean. Eng. Technol. 2024, 18, 100712. [Google Scholar] [CrossRef]
- ASTM D2112-21; Standard Test Method for Oxidation Stability of Inhibited Mineral Insulating Oil by Pressure Vessel. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D2440-19; Standard Test Method for Oxidation Stability of Mineral Insulating Oil. ASTM International: West Conshohocken, PA, USA, 2019.
- Oparanti, S.O.; Fofana, I.; Zarrougui, R.; Jafari, R.; Yapi, K.M.L. Improving some physicochemical characteristics of environmentally friendly insulating liquids for enhanced sustainability in subpolar transformer applications. Sustain. Mater. Technol. 2024, 41, e00996. [Google Scholar] [CrossRef]
- Joseph, P.; Sharma, D. Improvement of thermooxidative stability of non-edible vegetable oils of Indian origin for biodegradable lubricant application. Lubr. Sci. 2010, 22, 149–161. [Google Scholar] [CrossRef]
- Martin, R.; Athanassatou, H.; Duart, J.C.; Perrier, C.; Sitar, I.; Walker, J.; Claiborne, C.; Boche, T.; Cherry, D.; Darwin, A. Experiences in Service with New Insulating Liquids; Cigré Technical Brochure 436; CIGRE: Paris, France, 2010. [Google Scholar]
- Das, A.K.; Chavan, A.S.; Shill, D.C.; Chatterjee, S. Jatropha Curcas oil for distribution transformer–A comparative review. Sustain. Energy Technol. Assess. 2021, 46, 101259. [Google Scholar] [CrossRef]
- ASTM D943-04a; Standard Test Method for Oxidation Characteristics of Inhibited Mineral Oils. ASTM International: West Conshohocken, PA, USA, 2004.
- Raof, N.A.; Yunus, R.; Rashid, U.; Azis, N.; Yaakub, Z. Effects of molecular structure on the physical, chemical, and electrical properties of ester-based transformer insulating liquids. J. Am. Oil Chem. Soc. 2019, 96, 607–616. [Google Scholar] [CrossRef]
- Yaakob, Z.; Narayanan, B.N.; Padikkaparambil, S. A review on the oxidation stability of biodiesel. Renew. Sustain. Energy Rev. 2014, 35, 136–153. [Google Scholar] [CrossRef]
- Amalanathan, A.J.; Sarathi, R.; Zdanowski, M.; Vinu, R.; Nadolny, Z. Review on gassing tendency of different insulating fluids towards transformer applications. Energies 2023, 16, 488. [Google Scholar] [CrossRef]
- Samikannu, R.; Raj, R.A.; Karuppiah, D.; Dasari, N.R.; Subburaj, S.K.; Murugesan, S.; Akbar, S.S. Reclamation of natural esters using nanocarriers as the biodegradable choice for the transformer insulation. Environ. Technol. Innov. 2021, 23, 101634. [Google Scholar] [CrossRef]
- Srinivasa, D.M.; Surendra, U. Investigation of electrical properties of developed indigenous natural ester liquid used as alternate to transformer insulation. Indones. J. Electr. Eng. Comput. Sci. 2023, 29, 609–617. [Google Scholar] [CrossRef]
- Rao, U.M.; Fofana, I.; Jaya, T.; Rodriguez-Celis, E.M.; Jalbert, J.; Picher, P. Alternative dielectric fluids for transformer insulation system: Progress, challenges, and future prospects. IEEE Access 2019, 7, 184552–184571. [Google Scholar] [CrossRef]
- Tiwari, R.; Agrawal, P.S.; Belkhode, P.N.; Ruatpuia, J.V.; Rokhum, S.L. Hazardous effects of waste transformer oil and its prevention: A review. Next Sustain. 2024, 3, 100026. [Google Scholar] [CrossRef]
- Oparanti, S.O.; Fofana, I.; Jafari, R.; Zarrougui, R. A state-of-the-art review on green nanofluids for transformer insulation. J. Mol. Liq. 2024, 124023. [Google Scholar] [CrossRef]
- Abdelmalik, A.A. The Feasibility of Using a Vegetable Oil-Based Fluid as Electrical Insulating Oil. Ph.D. Thesis, University of Leicester, Leicester, UK, 2012. [Google Scholar]
- Raghu, P.; Murugesan, M. Molecular interactions of the antioxidant in the coolant oil for energy transformers: Dielectric and simulation studies. J. Mol. Liq. 2024, 125795. [Google Scholar] [CrossRef]
- Madavan, R.; Balaraman, S. Comparison of antioxidant influence on mineral oil and natural ester properties under accelerated aging conditions. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2800–2808. [Google Scholar] [CrossRef]
- Totzauer, P.; Trnka, P.; Hornak, J.; Kadlec, P.; Pihera, J. Antioxidant variations in the nature ester oil. In Proceedings of the 2017 18th International Scientific Conference on Electric Power Engineering (EPE), Kouty nad Desnou, Czech Republic, 17–19 May 2017; pp. 1–6. [Google Scholar]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Baishya, T.; Das, P.; Ashraf, G.J.; Dua, T.K.; Paul, P.; Nandi, G.; Jajo, H.; Dutta, A.; Kumar, A.; Bhattacharya, M. Antioxidant activity, cytotoxicity assay, and evaluation of bioactive compounds using GC-MS and HPTLC-based bioassay in the extracts of Osbeckia stellata var. crinita (Benth. ex Naudin) grown in Manipur, India. Kuwait J. Sci. 2024, 51, 100229. [Google Scholar] [CrossRef]
- Ali, A.H. High-performance liquid chromatography (HPLC): A review. Ann. Adv. Chem. 2022, 6, 010–020. [Google Scholar] [CrossRef]
- Gupta, M.K.; Ghuge, A.; Parab, M.; Al-Refaei, Y.; Khandare, A.; Dand, N.; Waghmare, N. A comparative review on high-performance liquid chromatography (HPLC), ultra performance liquid chromatography (UPLC) & high-performance thin layer chromatography (HPTLC) with current updates. Curr. Issues Pharm. Med. Sci. 2022, 35, 224–228. [Google Scholar] [CrossRef]
- Tatarczak-Michalewska, M.; Flieger, J. Application of high-performance liquid chromatography with diode array detection to simultaneous analysis of reference antioxidants and 1, 1-diphenyl-2-picrylhydrazyl (DPPH) in free radical scavenging test. Int. J. Environ. Res. Public Health 2022, 19, 8288. [Google Scholar] [CrossRef]
- Adebo, O.A.; Oyeyinka, S.A.; Adebiyi, J.A.; Feng, X.; Wilkin, J.D.; Kewuyemi, Y.O.; Abrahams, A.M.; Tugizimana, F. Application of gas chromatography-mass spectrometry (GC-MS)-based metabolomics for the study of fermented cereal and legume foods: A review. Int. J. Food Sci. Technol. 2021, 56, 1514–1534. [Google Scholar] [CrossRef]
- Rontani, J.-F. Use of gas chromatography-mass spectrometry techniques (GC-MS, GC-MS/MS and GC-QTOF) for the characterization of photooxidation and autoxidation products of lipids of autotrophic organisms in environmental samples. Molecules 2022, 27, 1629. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, H.; Feitosa, L.; Silva, L.; Cabrino, A.; Ramos, L. Evaluation of in-service oxidative stability and antioxidant additive consumption in corn oil based natural ester insulating fluid. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 864–868. [Google Scholar]
- Qian, Y.; Zhao, Y.; Wang, Q. Application of infrared spectroscopy in monitoring the aging process of transformer oil. In Proceedings of the 2022 7th Asia Conference on Power and Electrical Engineering (ACPEE), Virtual, 16–17 April 2022; pp. 844–848. [Google Scholar]
- Rao, U.M.; Fofana, I.; Sarathi, R. Alternative Liquid Dielectrics for High Voltage Transformer Insulation Systems: Performance Analysis and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Gautam, L.; Sarathi, R.; Soundarya, P.; Sekar, G.; Rao, U.M.; Fofana, I. Quantitative Estimation of the Gelling in Thermally Aged Natural Ester Dielectric Liquid. IEEE Trans. Dielectr. Electr. Insul. 2023, 31, 897–903. [Google Scholar] [CrossRef]
- Günther, H. NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- De Graaf, R.A. In Vivo NMR Spectroscopy: Principles and Techniques; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Adekunle, A.A.; Fofana, I.; Picher, P.; Rodriguez-Celis, E.M.; Arroyo-Fernandez, O.H. Analyzing Transformer Insulation Paper Prognostics and Health Management: A Modeling Framework Perspective. IEEE Access 2024, 12, 58349–58377. [Google Scholar] [CrossRef]
- Wong, S.Y.; Ye, X.; Guo, F.; Goh, H.H. Computational intelligence for preventive maintenance of power transformers. Appl. Soft Comput. 2022, 114, 108129. [Google Scholar] [CrossRef]
- Das, S.; Paramane, A.; Rao, U.M.; Chatterjee, S.; Kumar, K.S. Corrosive dibenzyl disulfide concentration prediction in transformer oil using deep neural network. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 1608–1615. [Google Scholar] [CrossRef]
- Firouzimagham, D.; Aminaie, P.; Shayan, Z.; Sabouri, M.; Asemani, M.H. Online transformer oil analysis based on spectroscopy technique and machine learning classifier: Experimental setup. In Proceedings of the 2020 15th International Conference on Protection and Automation of Power Systems (IPAPS), Shiraz, Iran, 30–31 December 2020; pp. 30–36. [Google Scholar]
- Luo, N.; Xu, D.; Xing, B.; Yang, X.; Sun, C. Principles and applications of convolutional neural network for spectral analysis in food quality evaluation: A review. J. Food Compos. Anal. 2024, 128, 105996. [Google Scholar] [CrossRef]
- Tian, W.; Li, Y.; Guzman, C.; Ibba, M.I.; Tilley, M.; Wang, D.; He, Z. Quantification of food bioactives by NIR spectroscopy: Current insights, long-lasting challenges, and future trends. J. Food Compos. Anal. 2023, 105708. [Google Scholar] [CrossRef]
- Wiklund, P.; Levin, M.; Pahlavanpour, B. Copper dissolution and metal passivators in insulating oil. IEEE Electr. Insul. Mag. 2007, 23, 6–14. [Google Scholar]
- Rabelo Neto, R.C.; Lima, D.O.; Pinheiro, T.D.; Almeida, R.m.F.; Castro Dantas, T.N.; Dantas, M.S.; Araujo, M.A.S.; Cavalcante, C.L.; Azevedo, D.C. Thermo-oxidative stability of mineral naphthenic insulating oils: Combined effect of antioxidants and metal passivator. Ind. Eng. Chem. Res. 2004, 43, 7428–7434. [Google Scholar]
- Yang, X.; Wang, H.; Liao, J.; Liu, Z.; Zhu, Z.; Zen, C. Performance Study of Anti-Deterioration Additives in Alkylbenzene-Insulated High-Voltage Cable Oils. In Proceedings of the 2024 International Conference on Energy and Electrical Engineering (EEE), Lanzhou, China, 18–20 October 2024; pp. 1–4. [Google Scholar]
- Dukhi, V.; Bissessur, A.; Martincigh, B. Unique antioxidant and sulfur corrosion retardant properties of transformer oil blended with turmerone extract. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2798–2808. [Google Scholar]
- Jaber, A.; Mehanna, N.; Oweimreen, G.; Abulkibash, A. The effect of DBDS, DBPC, BTA and DBP combinations on the corrosion of copper immersed in mineral transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1–7. [Google Scholar]
- Zhu, C.; Pan, Z.; Du, B.; Liang, B.; He, Y.; Chen, H.; Liu, L.; Zeng, L. Massive emissions of a broad range of emerging hindered phenol antioxidants and sulfur antioxidants from E-waste recycling in urban mining: New insights into an environmental source. Environ. Sci. Technol. Lett. 2021, 9, 42–49. [Google Scholar] [CrossRef]
- Cui, S.; Yu, Y.; Zhan, T.; Gao, Y.; Zhang, J.; Zhang, L.; Ge, Z.; Liu, W.; Zhang, C.; Zhuang, S. Carcinogenic risk of 2, 6-di-tert-butylphenol and its quinone metabolite 2, 6-DTBQ through their interruption of rarβ: In vivo, in vitro, and in silico investigations. Environ. Sci. Technol. 2021, 56, 480–490. [Google Scholar] [CrossRef]
- Naguib, M.; Yassin, M.A. Polymeric antioxidant via ROMP of bioderived tricyclic oxanorbornene based on vanillin and furfurylamine. ACS Appl. Polym. Mater. 2022, 4, 2181–2188. [Google Scholar] [CrossRef]
- Song, X.-C.; Dreolin, N.; Canellas, E.; Goshawk, J.; Nerin, C. Prediction of collision cross-section values for extractables and leachables from plastic products. Environ. Sci. Technol. 2022, 56, 9463–9473. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Rat metabolism study suggests 3-(3, 5-Di-tert-butyl-4-hydroxyphenyl) propionic acid as a potential urinary biomarker of human exposure to representative 3-(3, 5-Di-tert-butyl-4-hydroxyphenyl) propionate antioxidants. Environ. Sci. Technol. 2021, 55, 14051–14058. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic phenolic antioxidants: A review of environmental occurrence, fate, human exposure, and toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar] [CrossRef]
- Hartwig, A.; Arand, M.; Epe, B.; Guth, S.; Jahnke, G.; Lampen, A.; Martus, H.-J.; Monien, B.; Rietjens, I.M.; Schmitz-Spanke, S. Mode of action-based risk assessment of genotoxic carcinogens. Arch. Toxicol. 2020, 94, 1787–1877. [Google Scholar] [CrossRef]
- Kobets, T.; Smith, B.P.; Williams, G.M. Food-borne chemical carcinogens and the evidence for human cancer risk. Foods 2022, 11, 2828. [Google Scholar] [CrossRef]
- Sarmah, R.; Kanta Bhagabati, S.; Dutta, R.; Nath, D.; Pokhrel, H.; Mudoi, L.P.; Sarmah, N.; Sarma, J.; Ahmed, A.M.; Jyoti Nath, R. Toxicity of a synthetic phenolic antioxidant, butyl hydroxytoluene (BHT), in vertebrate model zebrafish embryo (Danio rerio). Aquac. Res. 2020, 51, 3839–3846. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, Y.; Liu, W.; Li, Z.; Souders II, C.L.; Martyniuk, C.J. Butylated hydroxytoluene induces hyperactivity and alters dopamine-related gene expression in larval zebrafish (Danio rerio). Environ. Pollut. 2020, 257, 113624. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.-G.; Hur, S.-W.; Katya, K.; Kim, K.-W.; Lee, B.-J. Assessment of Safety, Effects, and Muscle-Specific Accumulation of Dietary Butylated Hydroxytoluene (BHT) in Paralichthys olivaceus. Aquac. Nutr. 2023, 2023, 1381923. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.; Shafique, M.; Azam, A.; Ateeq, M.; Khan, I.A.; Hussain, A. Sustainable, renewable and environmental-friendly insulation systems for high voltages applications. Molecules 2020, 25, 3901. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, F.; Wang, Z.; Li, J. A critical review of plant-based insulating fluids for transformer: 30-year development. Renew. Sustain. Energy Rev. 2021, 141, 110783. [Google Scholar] [CrossRef]
- Pagger, E.P.; Pattanadech, N.; Uhlig, F.; Muhr, M. Application of New Insulating Liquid in High Voltage Equipment. In Biological Insulating Liquids: New Insulating Liquids for High Voltage Engineering; Springer: Berlin/Heidelberg, Germany, 2023; pp. 141–230. [Google Scholar]
- Brochure, C. 526-Oxidation Stability of Insulating Fluids; WG D1; CIGRE: Paris, France, 2013; Volume 30. [Google Scholar]


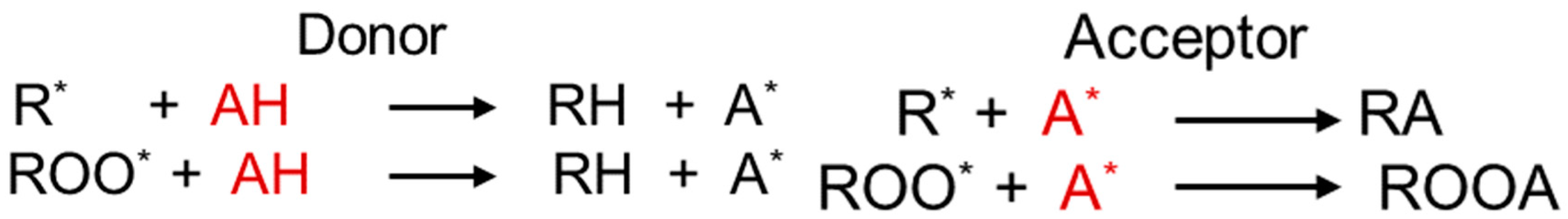
| Sn | Antioxidants | Chemical Formula | Source | Reference |
|---|---|---|---|---|
| 1 | Quercetin | C15H10O7 | Apples, onions and berries | [55] |
| 2 | Cardanol, epoxy cardanol | C15H27OH | Cashew nut shell liquid | [56] |
| 3 | Rosemary | C18H16O8 | Rosmarinus species | [57] |
| 4 | Gallic acid, caffeic acid and ferulic acid | C7H6O5, C9H8O4, C10H10O4 | Macerated barley waste and Moringa Oleifera leaves | [58,59] |
| 5 | Camphor | C10H16O | Camphor plant | [49] |
| 6 | Limonene | C10H16 | Lemon and orange | [49] |
| 7 | Citric acid (anhydrous) | C6H8O7 | Citrus fruits | [60,61] |
| 8 | L-Ascorbic acid | C6H8O6 | Citrus fruits | [62,63] |
| 9 | α-tocopherol | C31H52O3 | Vegetable oils, fruits | [64] |
| 10 | Carotenoids | C40H56 | Fruits, vegetables | [65] |
| 11 | Lignin | - | Forestry residue | [66,67] |
| Sn | Antioxidants | Chemical Formula | Source | Reference |
|---|---|---|---|---|
| 1 | N,N′-disec-butyl-p-phenylenediamine | C22H38N2 | Reaction of p-phenylenediamine with sec-butyl alcohol | [74,75] |
| 2 | Tert-butylhydroquinone | C10H14O2 | Petroleum | [76,77] |
| 3 | Propyl gallate (PG) | C10H12O5 | Esterification of gallic acid | [78,79] |
| 4 | Butylated hydroxy toluene | C15H24O | Petroleum | [80] |
| 5 | 1,2,3-trihydroxybenzene (Pyrogallol) | C6H6O3 | Hydrolysis of gallotannins or oxidation of gallic acid | [80] |
| 6 | Butylated hydroxyl anisole | C11H16O2 | Reaction of p-methoxyphenol and isobutylene | [81] |
| Antioxidants | ||
|---|---|---|
| Donor | Acceptor | Acceptor/Donor |
| Vitamin C (Ascorbic Acid) | Superoxide Dismutase (SOD) | Glutathione |
| Vitamin E (Tocopherol) | Catalase | Trolox |
| Butylated Hydroxytoluene (BHT) | ||
| Butylated Hydroxyanisole (BHA) | ||
| THBQ | ||
| N,N′-disec-butyl-p-phenylenediamine | ||
| Fatty Acid | Chemical Structure | Palm Kernel Oil | Coconut Oil | Canola Oil | Soybean Oil | Cotton Seed Oil | Neem Oil | Sunflower Oil | Rapeseed Oil | Safflower Oil | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidation stability decreases downward | Caprylic acid C8:0 | s | 3.30 | 9.16 | - | - | - | - | - | - | |
| Capric acid C10:0 | s | 3.40 | 6.44 | - | - | - | - | - | - | ||
| Lauric acid C12:0 | s | 48.20 | 45.57 | - | - | - | - | - | - | ||
| Myristic acid C14:0 | s | 16.20 | 16.65 | 0.07 | 0.08 | 1.00 | - | - | - | ||
| Palmitic acid C16:0 | s | 8.40 | 8.21 | 1.29 | 14.04 | 23.70 | 18.10 | 7.00 | 4.00 | 7.00 | |
| Stearic acid C18:0 | s | 2.50 | 3.42 | 2.40 | 4.07 | 3.40 | 18.10 | 5.00 | 2.00 | 2.00 | |
| Palmitoleic acid C16:1 | mus | - | - | 0.29 | 0.09 | 0.60 | - | - | - | ||
| Oleic acid C18:1 | mus | 15.40 | 6.27 | 64.40 | 23.27 | 19.40 | 44.50 | 19.00 | 56.00 | 13.00 | |
| Erucic acid C22:1 | mus | - | - | 0.50 | - | - | - | - | - | ||
| Linoleic acid C18:2 | pus | 2.30 | 1.40 | 17.40 | 52.18 | 53.20 | 18.30 | 68.00 | 22.00 | 78.00 | |
| Linolenic acid C18:3 | pus | - | - | 9.60 | 5.63 | 0.50 | 0.20 | 1.00 | 10.00 | - |
| Types of Insulating Liquids | RBOT Test (Minutes) |
|---|---|
| Silicon fluids | >450 |
| Natural esters | <40 |
| High oleic natural esters | 197 |
| Synthetic esters | 421 |
| Mineral oils | 300 |
| Antioxidants | Ratio of Antioxidants | Soybean Oil (% Increment) | Pongamia Pinnata Oil | Sunflower Oil (% Increment) | Rice Bran Oil (% Increment) | Corn Oil (% Increment) | Rapeseed Oil (% Increment) |
|---|---|---|---|---|---|---|---|
| α-Tocopherol + CA | 1.0:1.0 | +40.0 | - | +44.0 | +23.0 | +50.0 | - |
| 0.5:0.5 | +37.0 | - | +59.0 | 0 | +47.0 | - | |
| α-Tocopherol + BHT | 2.5:2.5 | - | +60 | - | - | - | +63.0 |
| 1.25:1.25 | - | +41 | - | - | - | +53.0 | |
| BHA + BHT | 2.5:2.5 | - | +88 | - | - | - | +53.0 |
| 1.25:1.25 | - | +50 | - | - | - | +59.0 | |
| BHT+ (citric acid) CA | 1.0:1.0 | +48.0 | +32 | −3.0 | +53.0 | ||
| 0.5:0.5 | +44.0 | +12 | +15.0 | +34.0 | |||
| PG (1 mg) + CA (1 mg) + α-Tocopherol (1 mg) | 500 mL of the composite antioxidants in 500 mL of oil (90 °C) | - | - | +20 | - | - | - |
| PG (1 mg) + CA (1 mg) + α-Tocopherol (1 mg) | 1000 mL of the composite antioxidants in 500 mL of oil (90 °C) | +19.2 | - | - | - | - | - |
| PG (1 mg) + CA (1 mg) + α-Tocopherol (1 mg) | 500 mL of the composite antioxidants in 500 mL of oil (90 °C) | - | - | - | −12.0 | - | - |
| Base oil | Antioxidants | Ratio of Antioxidants | Percentage Increment |
|---|---|---|---|
| 25% Olive oil and 75% of Rice bran oil | BHT | 0.25% | +46.6 |
| 75% Olive oil and 25% of Soya bean oil | BHT | 1.00% | +52.54 |
| 50% Olive oil and 50% of Sunflower | BHT | 1.00% | +48.50 |
| 25% Olive oil and 75% of Corn oil | BHT | 1.00% | +48.50 |
| 75% Olive oil and 25% of Rice bran oil | BHA | 1.00% | +62.50 |
| 50% Olive oil and 50% of Soya bean oil | BHA | 1.00% | 57.14 |
| 25% Olive oil and 75% of Sunflower | BHA | 1.00% | +40.63 |
| 50% Olive oil and 50% of Corn oil | BHA | 1.00% | +51.61 |
| Vegetable oil | DBPC | 9 g in 1108 mL of oil | +27.58 |
| Vegetable oil | BHA | 9 g in 1108 mL of oil | +48.27 |
| Vegetable oil | TBHQ | 9 g in 1108 mL of oil | +51.72 |
| Honge oil | GA | 0.50% | +12.14 |
| Neem oil | GA | 0.50% | +11.63 |
| Mustard oil | GA | 0.25% | +25.00 |
| Punna oil | GA | 0.50% | +20.56 |
| Castor oil | GA | 0.25% | +51.61 |
| Sunflower oil | AA | 1.00 g | +24.00 |
| Soya bean oil | AA | 1.00 g, 5.00 g | +56.00, +96.00 |
| Corn oil | PG | 1.00 g, 5.00 g | +72.00, +72.00 |
| Base Oil | Antioxidants | Ratio of Antioxidants | Flash Point Percentage Increment | Fire Point Percentage Increment |
|---|---|---|---|---|
| Sunflower | PG (1 mg) + CA (1 mg) + α-Tocopherol (1 mg) | 500 ppm in 500 mL | +8.70 | +8.00 |
| Soybean | BHT (1 mg) + CA (1 mg) + α-Tocopherol (1 mg) | 500 ppm in 500 mL | −3.70 | −9.67 |
| Rice bran | BHT (1 mg) + CA (1 mg) + α-Tocopherol (1 mg) | 500 ppm in 500 mL | +8.00 | +7.14 |
| 25% Olive oil and 75% of Rice bran oil | BHT | 0.25% | +8.57 | +3.66 |
| 50% Olive oil and 50% of Sunflower | BHT | 0.50% | +5.26 | +3.33 |
| 75% Olive oil and 25% of Corn oil | BHA | 1.00% | +1.66 | +1.94 |
| 75% Olive oil and 25% of Sunflower oil | BHA | 1.00% | +2.75 | +4.33 |
| Honge oil | BHT | 0.75% | +3.36 | +5.81 |
| Neem oil | BHT | 0.75% | +6.94 | +13.80 |
| Mustard oil | BHT | 0.5% | +7.27 | +10 |
| Punna oil | BHT | 0.75% | +12.14 | +8.20 |
| Castor oil | BHT | 0.75% | +6.43 | +9.66 |
| Neem oil | GA | 0.5% | +8.33 | +9.43 |
| Mustard oil | GA | 0.25% | +7.96 | +11.00 |
| Punna oil | GA | 0.50% | +6.43 | +4.26 |
| Castor oil | GA | 0.50% | +13.57 | +14.48 |
| Corn oil | PG | 5.00g | +7.00 | +6.00 |
| Sunflower | AA | 5.00g | +8.00 | +19.00 |
| Sunflower | α-Tocopherol + CA | 1:1 | +12.00 | +11.00 |
| Sunflower | BHT + CA | 0.5:0.5 | 0 | +11.00 |
| Base Liquids | Antioxidants | Ratio of Antioxidants | Percentage Increment/Decrement at Room Temperature |
|---|---|---|---|
| Sunflower oil | PG | 1.00 g | −15.00 |
| AA | 1.00 g | −8.00 | |
| BHT + CA | 0.5:0.5 | −17.00 | |
| α-Tocopherol + CA | 0.5:0.5 | −17.00 | |
| Corn oil | PG | 1.00 g | −19.00 |
| α-Tocopherol + CA | 0.5:0.5 | −22.00 | |
| BHT + CA | 0.5:0.5 | −7.00 | |
| AA | 5.00 g | −7.00 | |
| Castor oil | BHT | 0.25% | +35.12 |
| BHA | 0.25% | +40.13 | |
| GA | 0.25% | +35.12 | |
| Punna oil | BHT | 0.25% | −20.12 |
| BHA | 1.00% | −32.22 | |
| GA | 0.50% | −14.48 | |
| Mustard oil | BHT | 1.00% | +21.81 |
| BHA | 0.25% | −10.93 | |
| GA | 1.00% | +9.82 | |
| Neem oil | BHT | 0.50% | +35.95 |
| BHA | 0.25% | −21.61 | |
| GA | 0.25% | +0.79 | |
| Honge oil | BHT | 1.00% | −14.55 |
| BHA | 1.00% | +8.95 | |
| GA | 1.00% | +6.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obebe, E.O.; Hadjadj, Y.; Oparanti, S.O.; Fofana, I. Enhancing the Performance of Natural Ester Insulating Liquids in Power Transformers: A Comprehensive Review on Antioxidant Additives for Improved Oxidation Stability. Energies 2025, 18, 1690. https://doi.org/10.3390/en18071690
Obebe EO, Hadjadj Y, Oparanti SO, Fofana I. Enhancing the Performance of Natural Ester Insulating Liquids in Power Transformers: A Comprehensive Review on Antioxidant Additives for Improved Oxidation Stability. Energies. 2025; 18(7):1690. https://doi.org/10.3390/en18071690
Chicago/Turabian StyleObebe, Esther Ogwa, Yazid Hadjadj, Samson Okikiola Oparanti, and Issouf Fofana. 2025. "Enhancing the Performance of Natural Ester Insulating Liquids in Power Transformers: A Comprehensive Review on Antioxidant Additives for Improved Oxidation Stability" Energies 18, no. 7: 1690. https://doi.org/10.3390/en18071690
APA StyleObebe, E. O., Hadjadj, Y., Oparanti, S. O., & Fofana, I. (2025). Enhancing the Performance of Natural Ester Insulating Liquids in Power Transformers: A Comprehensive Review on Antioxidant Additives for Improved Oxidation Stability. Energies, 18(7), 1690. https://doi.org/10.3390/en18071690







