Safety Analysis of Hydrogen-Powered Train in Different Application Scenarios: A Review
Abstract
:1. Introduction
2. Demonstration Cases of Hydrogen-Powered Train Operation
2.1. Hydrogen-Powered Trains in China
2.2. Hydrogen-Powered Trains in the United States
2.3. Hydrogen-Powered Trains in Europe
2.4. Hydrogen-Powered Trains in Japan and South Korea
3. Hydrogen Leakage Hazard Chain
3.1. Hydrogen Leakage and Diffusion
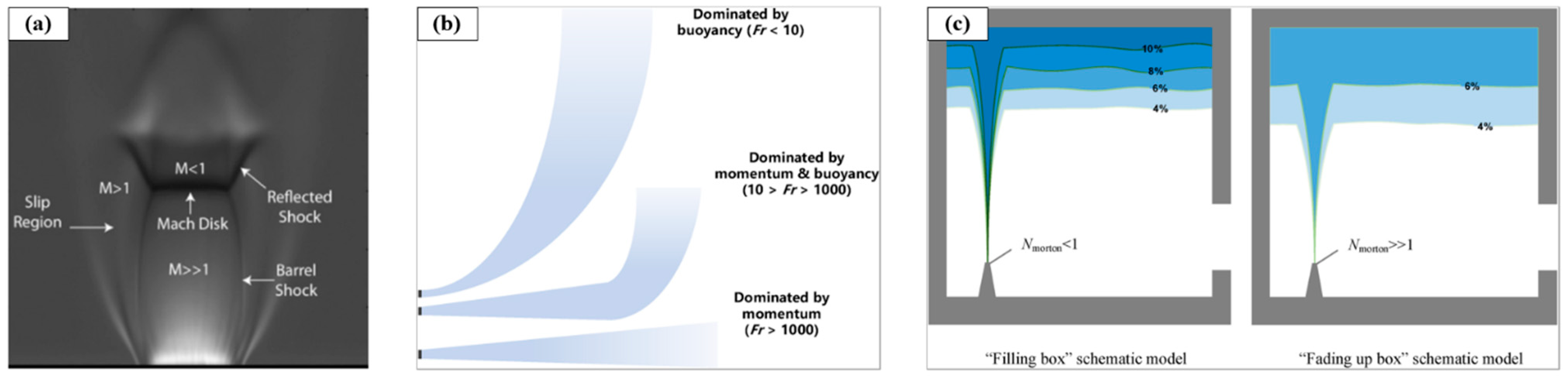
3.2. Self-Ignition of High-Pressure Hydrogen Pipeline Release
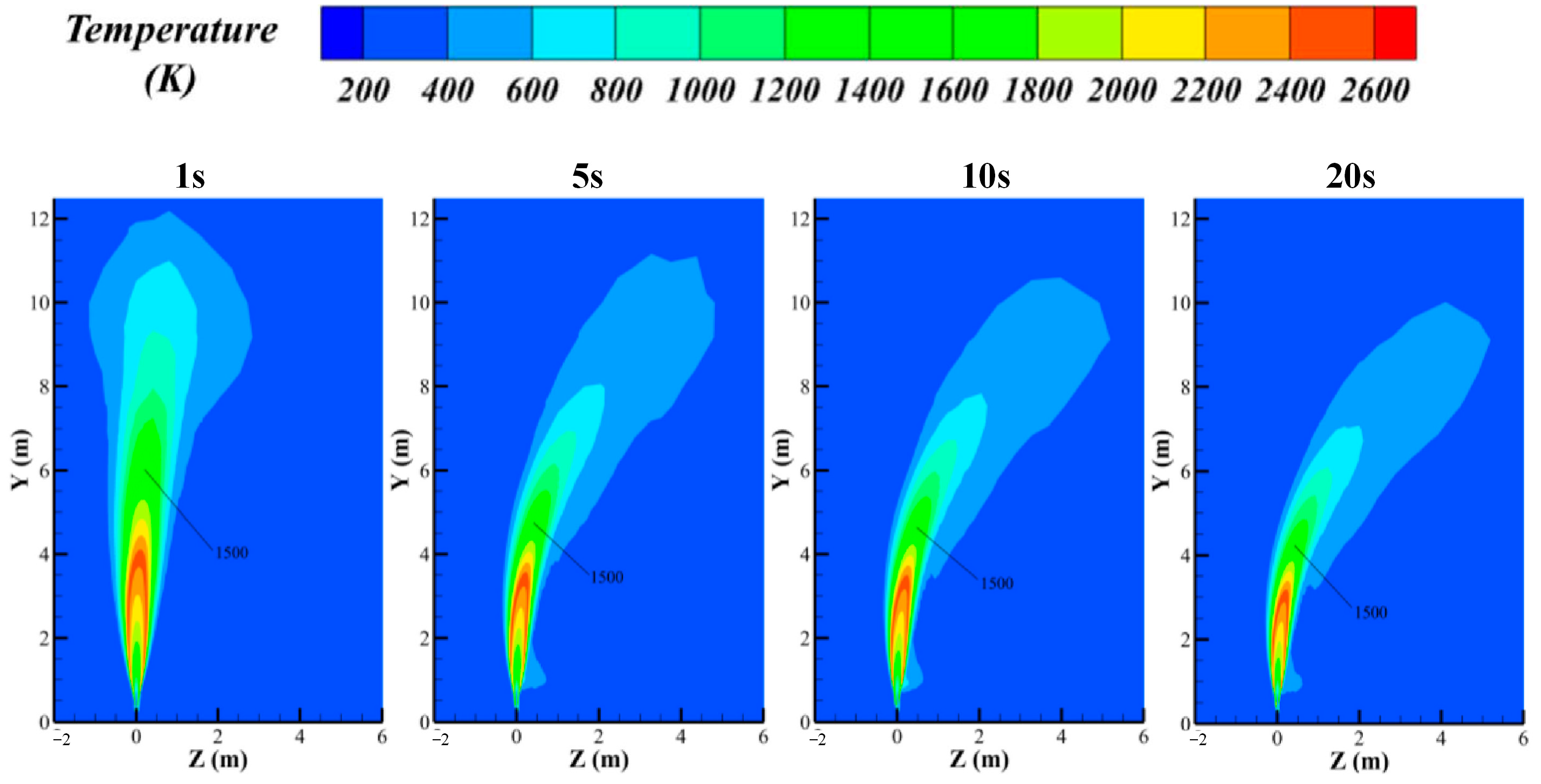
3.3. Hydrogen Jet Flame
3.4. Hydrogen Cloud Explosion
4. Safety Analysis of Hydrogen-Powered Train Scenarios
4.1. The Scenario of Electrified Railways
4.2. The Scenario of Tunnels
4.3. The Scenario of a Train Station and Refueling Station
4.4. The Scenario of Garages
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BOG | Boil-off gas |
| CFD | Computational fluid dynamics |
| DDT | Deflagration to detonation |
| EMUs | Electric multiple units |
| EIS | Electrochemical impedance spectroscopy |
| GDL | Gas diffusion layer |
| HFCVs | Hydrogen fuel cell vehicles |
| LBM | Lattice Boltzmann method |
| LCC | Life cycle costing |
| LOHCs | Liquid organic hydrogen carriers |
| MOFs | Metal–organic frameworks |
| MPL | Microporous layer |
| PHM | Prognosis and health management |
| PEMFC | Proton exchange membrane fuel cell |
| SOH | State of health |
| TPRDs | Thermal pressure relief devices |
References
- Adu, D.; Jianguo, D.; Asomani, S.N.; Abbey, A. Energy generation and carbon dioxide emission-The role of renewable energy for green development. Energy Rep. 2024, 12, 1420–1430. [Google Scholar] [CrossRef]
- Fan, J.Q.; Meng, X.S.; Tian, J.X.; Xing, C.H.; Wang, C.; Wood, J. A review of transportation carbon emissions research using bibliometric analyses. J. Traffic Transp. Eng. 2023, 10, 878–899. [Google Scholar] [CrossRef]
- Anand, C.; Chandraja, B.; Nithiya, P.; Akshaya, M.; Tamizhdurai, P.; Shoba, G.; Subramani, A.; Kumaran, R.; Yadav, K.K.; Gacem, A.; et al. Green hydrogen for a sustainable future: A review of production methods, innovations, and applications. Int. J. Hydrogen Energy 2025, 111, 319–341. [Google Scholar] [CrossRef]
- Sebbahi, S.; Assila, A.; Alaoui Belghiti, A.; Laasri, S.; Kaya, S.; Hlil, E.K.; Rachidi, S.; Hajjaji, A. A comprehensive review of recent advances in alkaline water electrolysis for hydrogen production. Int. J. Hydrogen Energy 2024, 82, 583–599. [Google Scholar] [CrossRef]
- Zhang, W.W.; Li, Y.F.; Li, H.F.; Liu, S.Y.; Zhang, J.Y.; Kong, Y. Systematic review of life cycle assessments on carbon emissions in the transportation system. Environ. Impact Assess. 2024, 109, 107618. [Google Scholar] [CrossRef]
- Ahmad, S.; Ullah, A.; Samreen, A.; Qasim, M.; Nawaz, K.; Ahmad, W.; Alnaser, A.; Kannan, A.M.; Egilmez, M. Hydrogen production, storage, transportation and utilization for energy sector: A current status review. J. Energy Storage 2024, 101, 113733. [Google Scholar]
- Agyekum, E.B.; Odoi-Yorke, F.; Abbey, A.A.; Ayetor, G.K. A review of the trends, evolution, and future research prospects of hydrogen fuel cells—A focus on vehicles. Int. J. Hydrogen Energy 2024, 72, 918–939. [Google Scholar] [CrossRef]
- Shi, Y.; Qiu, Z.H.; Snyder, S.W.; Wang, Y.X. A perspective on broad deployment of hydrogen-fueled vehicles for ground freight transportation with a comparison to electric vehicles. Energy Strategy Rev. 2024, 56, 101565. [Google Scholar]
- Yamchi, H.B.; Kandidayeni, M.; Kelouwani, S.; Boulon, L. Constrained exploration method for optimal energy management in hybrid multi-stack fuel cell vehicles. Energy Convers. Manag. 2024, 316, 118841. [Google Scholar] [CrossRef]
- Pu, J.; Xie, Q.; Li, J.; Zhao, Z.; Lai, J.; Li, K.; Zhou, F. Research on the technical scheme of multi-stack common rail fuel cell engine based on the demand of commercial vehicle. Energy Ai 2024, 16, 100353. [Google Scholar] [CrossRef]
- Tang, X.; Shi, L.; Li, M.; Xu, S.; Sun, C. Health State Estimation and Long-Term Durability Prediction for Vehicular PEM Fuel Cell Stacks Under Dynamic Operational Conditions. IEEE Trans. Power Electron. 2025, 40, 4498–4509. [Google Scholar]
- Wallnöfer-Ogris, E.; Poimer, F.; Köll, R.; Macherhammer, M.-G.; Trattner, A. Main degradation mechanisms of polymer electrolyte membrane fuel cell stacks—Mechanisms, influencing factors, consequences, and mitigation strategies. Int. J. Hydrogen Energy 2024, 50, 1159–1182. [Google Scholar]
- Zhao, L.; Hong, J.; Yuan, H.; Ming, P.; Wei, X.; Dai, H. Dynamic inconsistent analysis and diagnosis of abnormal cells within a high-power fuel cell stack. Electrochim. Acta 2023, 464, 142897. [Google Scholar]
- Zhao, L.; Yuan, H.; Xie, J.; Jiang, S.; Wei, X.; Tang, W.; Ming, P.; Dai, H. Inconsistency evaluation of vehicle-oriented fuel cell stacks based on electrochemical impedance under dynamic operating conditions. Energy 2023, 265, 126162. [Google Scholar]
- Meng, X.; Sun, C.; Mei, J.; Tang, X.; Hasanien, H.M.; Jiang, J.; Fan, F.; Song, K. Fuel cell life prediction considering the recovery phenomenon of reversible voltage loss. J Power Sources 2025, 625, 235634. [Google Scholar] [CrossRef]
- Hua, Z.; Zheng, Z.; Pahon, E.; Péra, M.-C.; Gao, F. Remaining useful life prediction of PEMFC systems under dynamic operating conditions. Energy Convers Manag. 2021, 231, 113825. [Google Scholar]
- Pourrahmani, H.; Van Herle, J. Water management of the proton exchange membrane fuel cells: Optimizing the effect of microstructural properties on the gas diffusion layer liquid removal. Energy 2022, 256, 124712. [Google Scholar]
- Chen, H.; Zhang, R.; Xia, Z.; Weng, Q.; Zhang, T.; Pei, P. Experimental investigation on PEM fuel cell flooding mitigation under heavy loading condition. Appl. Energy 2023, 349, 121632. [Google Scholar] [CrossRef]
- Yan, S.; Yang, M.; Sun, C.; Xu, S. Liquid Water Characteristics in the Compressed Gradient Porosity Gas Diffusion Layer of Proton Exchange Membrane Fuel Cells Using the Lattice Boltzmann Method. Energies 2023, 16, 6010. [Google Scholar] [CrossRef]
- Baz, F.B.; Elzohary, R.M.; Osman, S.; Marzouk, S.A.; Ahmed, M. A review of water management methods in proton exchange membrane fuel cells. Energy Convers Manag. 2024, 302, 118150. [Google Scholar] [CrossRef]
- Gao, Z.; Lang, J.T.; Wei, F.; Iskandarani, B.; Qi, Y.; Secanell, M.; Su, J.; Guo, L.; Zenyuk, I.V.; Morimoto, Y. A design of gas diffusion media for polymer electrolyte membrane fuel cell: Characterization and water management investigation. J. Power Sources 2024, 606, 234507. [Google Scholar]
- Ji, Z.X.; Miao, X.Y. Overall scheme design of a closed solid oxide fuel cell hybrid engine for ships. Energy Convers Manag. 2024, 314, 118682. [Google Scholar]
- Ding, D.N.; Wu, X.Y. Hydrogen fuel cell electric trains: Technologies, current status, and future. Appl. Energy Combust. Sci. 2024, 17, 100255. [Google Scholar] [CrossRef]
- Kapetanović, M.; Núñez, A.; van Oort, N.; Goverde, R.M.P. Analysis of hydrogen-powered propulsion system alternatives for diesel-electric regional trains. J. Rail Transp. Plan. Manag. 2022, 23, 100338. [Google Scholar]
- Yang, F.; Wang, T.; Deng, X.; Dang, J.; Huang, Z.; Hu, S.; Li, Y.; Ouyang, M. Review on hydrogen safety issues: Incident statistics, hydrogen diffusion, and detonation process. Int. J Hydrogen Energy 2021, 46, 31467–31488. [Google Scholar]
- Sun, Y.; Anwar, M.; Hassan, N.M.S.; Spiryagin, M.; Cole, C. A review of hydrogen technologies and engineering solutions for railway vehicle design and operations. Railway Eng. Sci. 2021, 29, 212–232. [Google Scholar]
- Chen, W.; Peng, F.; Liu, Z.; Li, Q.; Dai, C. System integration of China’s first PEMFC locomotive. J. Mod. Transp. 2013, 21, 163–168. [Google Scholar]
- Peng, F.; Chen, W.; Liu, Z.; Li, Q.; Dai, C. System integration of China’s first proton exchange membrane fuel cell locomotive. Int. J. Hydrogen Energy 2014, 39, 13886–13893. [Google Scholar]
- China produces first hydrogen fuel cell tram, with Ballard unit. Fuel Cells Bulletin 2015, 2015, 5.
- Mining Locomotive Runs on Hydrogen. Available online: https://www.sandia.gov/labnews/2002/05/03/key05-03-02-storieslocomotive/ (accessed on 11 March 2025).
- A Forerunner to the Current Interest in Hydrogen Propulsion Provided the First Test of the Concept. Available online: https://www.trains.com/trn/railroads/locomotives/bnsfs-first-hydrogen-locomotive/ (accessed on 11 March 2025).
- Stadler hydrogen fuel cell train for California. Fuel Cells Bulletin 2019, 2019, 1. [CrossRef]
- Stadler Presents FLIRT H2 Hydrogen Train for San Bernardino. Available online: https://railway-news.com/stadler-presents-flirt-h2-hydrogen-train-for-san-bernardino/ (accessed on 11 March 2025).
- Coradia iLint fuel cell train approved for commercial operation on German railways. Fuel Cells Bulletin 2018, 2018, 6–7. [CrossRef]
- HydroFLEX train in first UK mainline trial. Fuel Cells Bulletin 2020, 2020, 5. [CrossRef]
- Wurster, R. Fuels—Hydrogen—Hydrogen Storage|Regulations, Codes, and Standards. In Encyclopedia of Electrochemical Power Sources, 2nd ed.; Garche, J., Ed.; Elsevier: Oxford, UK, 2025; pp. 346–359. [Google Scholar]
- Moretto, P.; Quong, S. Chapter 7—Legal requirements, technical regulations, codes, and standards for hydrogen safety. In Hydrogen Safety for Energy Applications; Kotchourko, A., Jordan, T., Eds.; Butterworth-Heinemann: Oxford, UK, 2022; pp. 345–396. [Google Scholar]
- Ma, X.; Li, B.; Han, B.; Liu, Y.; Song, C. Safety discharge strategies of vehicle-mounted type III high-pressure hydrogen storage tanks under fire scenarios. Int. J. Hydrogen Energy 2024, 93, 1227–1239. [Google Scholar] [CrossRef]
- Offer, G.J.; Howey, D.; Contestabile, M.; Clague, R.; Brandon, N.P. Comparative analysis of battery electric, hydrogen fuel cell and hybrid vehicles in a future sustainable road transport system. Energy Policy 2010, 38, 24–29. [Google Scholar] [CrossRef]
- Horvath, S.; Fasihi, M.; Breyer, C. Techno-economic analysis of a decarbonized shipping sector: Technology suggestions for a fleet in 2030 and 2040. Energy Convers Manag. 2018, 164, 230–241. [Google Scholar] [CrossRef]
- Correa, L.; Razi, F.; Sadiq, R.; Hewage, K. Life cycle costing analysis of a retrofitted hydrogen-powered locomotive: Canadian context. Transp. Res. Part D Transp. Environ. 2024, 133, 104295. [Google Scholar] [CrossRef]
- Li, H.; Cao, X.; Liu, Y.; Shao, Y.; Nan, Z.; Teng, L.; Peng, W.; Bian, J. Safety of hydrogen storage and transportation: An overview on mechanisms, techniques, and challenges. Energy Rep. 2022, 8, 6258–6269. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Sonde, C.U.; Charlie, D.; Usibe, B.E.; Raimi, M.; Obike, A.I.; Magu, T.O. A review on exploring the potential of liquid hydrogen as a fuel for a sustainable future. Sustain. Chem. One World 2024, 3, 100022. [Google Scholar] [CrossRef]
- Berstad, D.O.; Stang, J.H.; Nekså, P. Large-scale hydrogen liquefier utilising mixed-refrigerant pre-cooling. Int. J. Hydrogen Energy 2010, 35, 4512–4523. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Khan, T.; Adegboye, O.R.; Odoi-Yorke, F.; Okonkwo, P.C. Analyzing the research trends in the direction of hydrogen storage—A look into the past, present and future for the various technologies. Int. J. Hydrogen Energy 2024, 74, 259–275. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Zhu, K.; Wang, Y.; Wang, L.; Tan, H. Investigation on no-vent filling process of liquid hydrogen tank under microgravity condition. Int. J. Hydrogen Energy 2017, 42, 8264–8277. [Google Scholar] [CrossRef]
- Wang, J.; Alkhaledi, A.N.; Hughes, T.J.; Webley, P.A. Technoeconomic investigation of optimal storage pressure and boil-off gas utilisation in large liquid hydrogen carriers. Appl. Energy 2025, 384, 125356. [Google Scholar] [CrossRef]
- Choi, M.; Jung, W.; Ji, S.; Lee, J. Optimization and analysis of reliquefaction system utilizing hydrogen as refrigerant for liquid hydrogen carriers. Appl. Energy 2025, 384, 125490. [Google Scholar] [CrossRef]
- Morales-Ospino, R.; Celzard, A.; Fierro, V. Strategies to recover and minimize boil-off losses during liquid hydrogen storage. Renew. Sustain. Energy Rev. 2023, 182, 113360. [Google Scholar] [CrossRef]
- Fernández, A.; Arzac, G.M.; Vogt, U.F.; Hosoglu, F.; Borgschulte, A.; Jiménez de Haro, M.C.; Montes, O.; Züttel, A. Investigation of a Pt containing washcoat on SiC foam for hydrogen combustion applications. Appl. Catal. B Environ. 2016, 180, 336–343. [Google Scholar] [CrossRef]
- Arzac, G.M.; Montes, O.; Fernández, A. Pt-impregnated catalysts on powdery SiC and other commercial supports for the combustion of hydrogen under oxidant conditions. Appl. Catal. B Environ. 2017, 201, 391–399. [Google Scholar] [CrossRef]
- Ikeuba, A.I. TOF-SIMS and AFM analysis of pH effect on the interfacial films on η-phase in aqueous salt solutions. Appl. Surf. Sci. Adv. 2023, 15, 100410. [Google Scholar]
- Munyentwali, A.; Tan, K.C.; He, T. Advancements in the development of liquid organic hydrogen carrier systems and their applications in the hydrogen economy. Prog. Nat. Sci. Mater. Int. 2024, 34, 825–839. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Zhang, B.; Wang, J.; Han, E.H.; Ke, W.; Okafor, P.C. SVET and SIET study of galvanic corrosion of Al/MgZn2 in aqueous solutions at different pH. J. Electrochem. Soc. 2018, 165, C180–C194. [Google Scholar] [CrossRef]
- Deng, J.; Fan, Y.; Wang, C.; Yang, N. Advances in hydrogen leakage jets for hydrogen storage systems. Int. J. Hydrogen Energy 2024, 93, 585–606. [Google Scholar] [CrossRef]
- Han, S.H.; Chang, D.; Kim, J.S. Experimental investigation of highly pressurized hydrogen release through a small hole. Int. J. Hydrogen Energy 2014, 39, 9552–9561. [Google Scholar]
- Stewart, J.R. CFD modelling of underexpanded hydrogen jets exiting rectangular shaped openings. Process Saf. Environ. 2020, 139, 283–296. [Google Scholar]
- Schefer, R.W.; Houf, W.G.; Williams, T.C. Investigation of small-scale unintended releases of hydrogen: Momentum-dominated regime. Int. J. Hydrogen Energy 2008, 33, 6373–6384. [Google Scholar]
- Ruggles, A.J.; Ekoto, I.W. Ignitability and mixing of underexpanded hydrogen jets. Int. J. Hydrogen Energy 2012, 37, 17549–17560. [Google Scholar]
- Xu, B.P.; Wen, J.X.; Dembele, S.; Tam, V.H.Y.; Hawksworth, S.J. The effect of pressure boundary rupture rate on spontaneous ignition of pressurized hydrogen release. J. Loss Prev. Proc. 2009, 22, 279–287. [Google Scholar]
- Astbury, G.R.; Hawksworth, S.J. Spontaneous ignition of hydrogen leaks: A review of postulated mechanisms. Int. J. Hydrogen Energy 2007, 32, 2178–2185. [Google Scholar]
- Mogi, T.; Wada, Y.; Ogata, Y.; Hayashi, A.K. Self-ignition and flame propagation of high-pressure hydrogen jet during sudden discharge from a pipe. Int. J. Hydrogen Energy 2009, 34, 5810–5816. [Google Scholar] [CrossRef]
- Li, H.; Cao, X.W.; Xu, Z.Y.; Cao, H.G.; Teng, L.; Bian, J. Impact of mixing low-reactivity gases on the mechanism of hydrogen spontaneous combustion: A ReaxFF MD study. Int. J. Hydrogen Energy 2024, 81, 497–511. [Google Scholar]
- Kim, Y.R.; Lee, H.J.; Kim, S.; Jeung, I.S. A flow visualization study on self-ignition of high pressure hydrogen gas released into a tube. Proc. Combust. Inst. 2013, 34, 2057–2064. [Google Scholar] [CrossRef]
- Kitabayashi, N.; Wada, Y.; Mogi, T.; Saburi, T.; Hayashi, A.K. Experimental study on high pressure hydrogen jets coming out of tubes of 0.1–4.2 m in length. Int. J. Hydrogen Energy 2013, 38, 8100–8107. [Google Scholar]
- Wang, Z.; Pan, X.; Wang, Q.; Jiang, Y.; Xu, X.; Yan, W.; Jiang, J. Experimental study on spontaneous ignition and flame propagation of high-pressure hydrogen release through tubes. Int. J. Hydrogen Energy 2019, 44, 22584–22597. [Google Scholar]
- Jiang, Y.; Pan, X.; Yan, W.; Wang, Z.; Wang, Q.; Hua, M.; Jiang, J. Pressure dynamics, self-ignition, and flame propagation of hydrogen jet discharged under high pressure. Int. J. Hydrogen Energy 2019, 44, 22661–22670. [Google Scholar]
- Zhou, S.Y.; Luo, Z.M.; Wang, T.; He, M.Y.; Li, R.K.; Su, B. Research progress on the self-ignition of high-pressure hydrogen discharge: A review. Int. J. Hydrogen Energy 2022, 47, 9460–9476. [Google Scholar]
- UN GTR No.13; Global Technical Regulation Concerning the Hydrogen and FuelCell Vehicles. UN/WP29:2013. UNECE: Geneva, Switzerland, 2013.
- GB/T 35544-2017; Fully-Wrapped Carbon Fiber Reinforced Cylinders with an Aluminum Liner for the On-Board Storage of Compressed Hydrogen as a Fuel for Land Vehicles. Standardization Administration of the People’s Republic of China: Beijing, China, 2017.
- JARI S 001; Technical Standards for Containers for Compressed Hydrogen Vehicle Fuel Device. Japan Automobile Standards Internationalization Center: Shinjuku City, Japan, 2004.
- Wang, X.Y.; Li, B.; Han, B.; Jin, X.; Zhang, D.W.; Bi, M.S. Explosion of high pressure hydrogen tank in fire: Mechanism, criterion, and consequence assessment. J. Energy Storage 2023, 72, 108455. [Google Scholar]
- Zou, L.; Li, B.; Han, B.; Liu, Y.; Wang, X.; Bi, M.; Shu, C.-M. Release of high-pressure hydrogen from type III tank in a fire scenario: Analysis and prediction of jet flame length and thermal response characteristics. Fuel 2024, 372, 132153. [Google Scholar]
- Molkov, V.; Dadashzadeh, M.; Kashkarov, S.; Makarov, D. Performance of hydrogen storage tank with TPRD in an engulfing fire. Int. J. Hydrogen Energy 2021, 46, 36581–36597. [Google Scholar]
- Delichatsios, M.A. Transition from Momentum to Buoyancy-Controlled Turbulent Jet Diffusion Flames and Flame Height Relationships. Combust. Flame 1993, 92, 349–364. [Google Scholar]
- Hu, Q.C.; Zhang, X.H.; Hao, H. A review of hydrogen-air cloud explosions: The fundamentals, overpressure prediction methods, and influencing factors. Int. J. Hydrogen Energy 2023, 48, 13705–13730. [Google Scholar]
- Yang, P.; Wang, T.; Sheng, Y.; Yu, Y.; Li, R.; Su, B.; Cheng, F.; Qu, J.; Deng, J.; Luo, Z. Recent advances in hydrogen process safety: Deflagration behaviors and explosion mitigation strategies. Process Saf. Environ. 2024, 188, 303–316. [Google Scholar]
- Groethe, M.; Merilo, E.; Colton, J.; Chiba, S.; Sato, Y.; Iwabuchi, H. Large-scale hydrogen deflagrations and detonations. Int. J. Hydrogen Energy 2007, 32, 2125–2133. [Google Scholar]
- Boeck, L.R.; Berger, F.M.; Hasslberger, J.; Sattelmayer, T. Detonation propagation in hydrogen-air mixtures with transverse concentration gradients. Shock Waves 2016, 26, 181–192. [Google Scholar]
- Klebanoff, L.E.; Pratt, J.W.; LaFleur, C.B. Comparison of the safety-related physical and combustion properties of liquid hydrogen and liquid natural gas in the context of the SF-BREEZE high-speed fuel-cell ferry. Int. J. Hydrogen Energy 2017, 42, 757–774. [Google Scholar]
- Chen, Y.; Li, Z.; Ji, C.; Liu, X. Effects of hydrogen concentration, non-homogenous mixtures and obstacles on vented deflagrations of hydrogen-air mixtures in a 27 m3 chamber. Int. J. Hydrogen Energy 2020, 45, 7199–7209. [Google Scholar] [CrossRef]
- Yuan, J.X.; Peng, L.W.; Zhou, H.; Gan, D.L.; Qu, K. Recent research progress and application of energy storage system in electrified railway. Electr. Power Syst. Res. 2024, 226, 109893. [Google Scholar] [CrossRef]
- Song, Y.; Antunes, P.; Pombo, J.; Liu, Z.G. A methodology to study high-speed pantograph-catenary interaction with realistic contact wire irregularities. Mech. Mach. Theory 2020, 152, 103940. [Google Scholar]
- Song, Y.; Rønnquist, A.; Jiang, T.J.; Nåvik, P. Identification of short-wavelength contact wire irregularities in electrified railway pantograph-catenary system. Mech. Mach. Theory 2021, 162, 104338. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, P.; Cheng, C.; Zhang, M.; Ma, R. Influence of arc discharge on the temperature and wear behaviors of the contact strip in pantograph-rigid catenary systems under AC conditions. Wear 2024, 546–547, 205368. [Google Scholar] [CrossRef]
- Wu, J. Chapter 6—Electric Contact Properties of Pantograph and Contact Line. In Pantograph and Contact Line System; Wu, J., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 193–238. [Google Scholar]
- Sezavar, H.R.; Fahimi, N.; Hasanzadeh, S.; Akmal, A.A.S. Risk assessment of contaminated composite insulators in pre-flashover conditions. Electr. Power Syst. Res. 2024, 230, 110256. [Google Scholar] [CrossRef]
- Kim, D.-M.; Lee, H.J. Estimation of flammable region through experimental observation of liquid hydrogen cloud leaked under various atmospheric conditions. Int. J. Hydrogen Energy 2024, 61, 1199–1211. [Google Scholar] [CrossRef]
- Shen, Y.; Lv, H.; Zheng, T.; Liu, Y.; Zhou, W.; Zhang, C. Temporal and spatial evolution of hydrogen leakage and diffusion from tube fittings on fuel cell vehicles under the effect of ambient wind. Renew. Sustain. Energy Rev. 2023, 185, 113596. [Google Scholar] [CrossRef]
- Tamura, Y.; Takeuchi, M.; Sato, K. Effectiveness of a blower in reducing the hazard of hydrogen leaking from a hydrogen-fueled vehicle. Int. J. Hydrogen Energy 2014, 39, 20339–20349. [Google Scholar] [CrossRef]
- GB/T 29729-2022; Essential Requirements for the Safety of Hydrogen Systems. Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- Li, X.; Hao, Y.; Wu, F.; Xing, Z.; Zhuang, S.; Wang, X. Numerical simulation of leakage jet flame hazard of high-pressure hydrogen storage bottle in open space. Int. J. Hydrogen Energy 2024, 62, 706–721. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, H.; Sun, Y.; Yue, S.; Zhu, X.; Jiang, X. Flashover characteristics and altitude correction of railway insulators at high altitude and polluted areas. Electr. Power Syst. Res. 2023, 224, 109724. [Google Scholar] [CrossRef]
- Bhavani, J.; Ch, S.P.K. Finite Element Modeling of Voltage and Electric Field Distribution along the Insulators. In Proceedings of the 2019 4th International Conference on Recent Trends on Electronics, Information, Communication & Technology (RTEICT), Bangalore, India, 17–18 May 2019; pp. 225–229. [Google Scholar]
- Xie, Y.L.; Lv, N.; Wang, X.J.; Wu, D.J.; Wang, S.M. Thermal and fire characteristics of hydrogen jet flames in the tunnel at longitudinal ventilation strategies. Fuel 2021, 306, 121659. [Google Scholar] [CrossRef]
- Gong, L.; Wang, H.; Yang, W.; Zhang, C.; Yang, Z.; Yang, X. Effect of the leakage mass flow rate and ignition time on the explosion characteristics of hydrogen leaked from hydrogen-powered train in tunnel. Saf. Sci. 2025, 184, 106762. [Google Scholar] [CrossRef]
- Yan, M.H.; Wang, W.; Tian, S.X.; Yang, J.T.; Liu, J.; Jiang, Z.B. Hydrogen leakage distribution and migration analysis under multiple factors in underground cross-river tunnel. Tunn. Undergr. Space Technol. 2024, 152, 105947. [Google Scholar] [CrossRef]
- Houf, W.G.; Evans, G.H.; Merilo, E.; Groethe, M.; James, S.C. Releases from hydrogen fuel-cell vehicles in tunnels. Int. J. Hydrogen Energy 2012, 37, 715–719. [Google Scholar] [CrossRef]
- Xie, Y.; Lv, N.; Huang, Y.; Wu, D.; Gong, L.; Yang, X.; Zeng, Y. Comparative analysis on temperature characteristics of hydrogen-powered and traditional fossil-fueled vehicle fires in the tunnel under longitudinal ventilations. Int. J. Hydrogen Energy 2022, 47, 24107–24118. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, J.; Pan, Y.; Ni, Y.; Ma, C.; Zhou, W.; Wang, Y. Hazard analysis on tunnel hydrogen jet fire based on CFD simulation of temperature field and concentration field. Saf. Sci. 2020, 122, 104532. [Google Scholar] [CrossRef]
- Sato, Y.; Iwabuchi, H.; Groethe, M.; Merilo, E.; Chiba, S. Experiments on hydrogen deflagration. J. Power Sources 2006, 159, 144–148. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, J.; Zhang, H.; Breitung, W.; Travis, J.; Kuznetsov, M.; Jordan, T. Numerical analysis of hydrogen release, dispersion and combustion in a tunnel with fuel cell vehicles using all-speed CFD code GASFLOW-MPI. Int. J. Hydrogen Energy 2021, 46, 12474–12486. [Google Scholar]
- Kudriakov, S.; Studer, E.; Bernard-Michel, G.; Bouix, D.; Domergue, L.; Forero, D.; Gueguen, H.; Ledier, C.; Manicardi, P.; Martin, M.; et al. Full-scale tunnel experiments: Blast wave and fireball evolution following hydrogen tank rupture. Int. J. Hydrogen Energy 2022, 47, 18911–18933. [Google Scholar]
- Tian, T.; Lu, C.; Ha, W.; Zhao, Y.; Hua, Z.; Peng, W.; Gu, C. Cfd Study on High-Pressure Hydrogen Leakage and Explosion of Hydrogen Energy Tram in Tunnel. In Proceedings of the ASME 2023 Pressure Vessels & Piping Conference, Atlanta, GA, USA, 16–21 July 2023; Volume 3. PVP2023-106868. [Google Scholar]
- Wang, F.; Xiao, J.; Kuznetsov, M.; Breitung, W.; He, B.; Rui, S.; Zhou, S.; Jordan, T.; Song, K.; Zhang, L. Deterministic risk assessment of hydrogen leak from a fuel cell truck in a real-scale hydrogen refueling station. Int. J. Hydrogen Energy 2024, 50, 1103–1118. [Google Scholar]
- Liu, K.; He, C.X.; Yu, Y.Z.; Guo, C.Y.; Lin, S.M.; Jiang, J.Y. A study of hydrogen leak and explosion in different regions of a hydrogen refueling station. Int. J. Hydrogen Energy 2023, 48, 14112–14126. [Google Scholar] [CrossRef]
- Cui, W.Y.; Yuan, Y.P.; Tong, L.; Shen, B.Y. Numerical simulation of hydrogen leakage diffusion in seaport hydrogen refueling station. Int. J. Hydrogen Energy 2023, 48, 24521–24535. [Google Scholar] [CrossRef]
- Choi, G.H.; Jerng, D.W.; Kim, T.W. Analyses of hydrogen risk in containment filtered venting system using MELCOR. Nucl. Eng. Technol. 2022, 54, 177–185. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, M.B.; Ba, Q.X.; Christopher, D.M.; Li, X.F. Modeling of hydrogen dispersion from hydrogen fuel cell vehicles in an underground parking garage. Int. J. Hydrogen Energy 2022, 47, 686–696. [Google Scholar] [CrossRef]
- Choi, J.; Hur, N.; Kang, S.; Lee, E.D.; Lee, K.B. A CFD simulation of hydrogen dispersion for the hydrogen leakage from a fuel cell vehicle in an underground parking garage. Int. J. Hydrogen Energy 2013, 38, 8084–8091. [Google Scholar]
- Li, Y.H.; Jiang, J.C.; Yu, Y.; Zhang, Q.W. Numerical simulation of dispersion and distribution behaviors of hydrogen leakage in the garage with a crossbeam. Simulation 2019, 95, 1229–1238. [Google Scholar] [CrossRef]
- Venetsanos, A.G.; Papanikolaou, E.; Delichatsios, M.; Garcia, J.; Hansen, O.R.; Heitsch, M.; Huser, A.; Jahn, W.; Jordan, T.; Lacome, J.M.; et al. An inter-comparison exercise on the capabilities of CFD models to predict the short and long term distribution and mixing of hydrogen in a garage. Int. J. Hydrogen Energy 2009, 34, 5912–5923. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, T.; Liu, C.; Chen, M.; Ji, S.; Christopher, D.M.; Li, X. Leak localization using distributed sensors and machine learning for hydrogen releases from a fuel cell vehicle in a parking garage. Int. J. Hydrogen Energy 2021, 46, 1420–1433. [Google Scholar] [CrossRef]












| Category of Hydrogen-Powered Vehicles | ||||
|---|---|---|---|---|
Hydrogen Car | Hydrogen Train | Hydrogen Ship | Hydrogen Aircraft | |
| Operating route | Land (free route) | Railway track (fixed route) | Water surface (fixed route) | Air (more fixed route) |
| Passenger capacity | Scale of less than a dozen people | Hundred-person scale | Scale of 100 to 1000 | Hundred-person scale |
| Operating speed | Up to 200 km/h | Up to 250 km/h | Up to 50 km/h | Up to 1000 km/h |
| System power | 100 kW scale | 100 kW scale | 1000 kW scale | 1000 kW scale |
| Hydrogen storage characteristics | Small hydrogen storage capacity; mainly stored as gas; located under the seats or toward the rear of the car | Larger hydrogen storage capacity; mainly stored as gas; located at the top of the cabin | Large hydrogen storage capacity; stored in gaseous or liquid form; located on the deck of the ship | Larger hydrogen storage capacity; stored in gaseous or liquid form; typically located inside the fuselage |
| Scenarios | Hydrogen Leakage Boundary | Research Methods | Hazard Type | Main Conclusion | Ref |
|---|---|---|---|---|---|
| Electrified railways (refer to open-air scene) | 70 MPa, 48 L hydrogen cylinder; release valve diameter: 2 mm | Experiment | Jet flame | The maximum length and width of the jet flame are 4.93 m and 1.65 m, respectively. | [73] |
| Leakage hole diameter: 12.7 mm; flow rate: 1.28 LPM | Experiment | Leakage | Flammable areas are most affected by wind speed. The increase in ambient temperature helps to reduce the flammable areas. | [88] | |
| 35 MPa, 251 L hydrogen cylinder; leakage apertures: 0.5 mm, 1 mm, 2 mm, 3 mm | CFD | Jet flame | The size of the leakage aperture is positively correlated with the size of the jet flame. | [92] | |
| Tunnels | Leakage mass flow rates: 0.5 kg/m2s−1, 1 kg/m2s−1, and 2 kg/m2s−1; leakage hole areas: 1 cm2, 12.5 cm2, 25 cm2 | CFD | Leakage | The higher the longitudinal ventilation speed inside the tunnel, the faster the diffusion rate of hydrogen. | [97] |
| 13.79 MPa hydrogen cylinder | CFD and experiment | Deflagration | The overpressure distribution of delayed ignition is characterized. Results of the simulations were found to be in good agreement with the experimental data. | [98] | |
| Leakage hole diameter: 31.05 mm; leakage mass flow rates: 0.13 kg/s | CFD | Jet flame | The high-temperature zone of the pool fire only exists above the ceiling of the vehicle. | [99] | |
| Leakage area: 0.125 m2, 0.25 m2, 0.5 m2; leakage rate: 0.169 kg/m2s−1, 0.845 kg/m2s−1, 1.69 kg/m2s−1 | CFD | Jet flame | As the hydrogen release rate increases, the rate of temperature rise and the hydrogen diffusion rate within the tunnel also increase. If the hydrogen release rate is too high, the hydrogen will fail to diffuse into the downstream tunnel. | [100] | |
| 70 MPa hydrogen cylinder; leakage hole diameter: 0.1 m | CFD | Jet flame and detonation | Hydrogen accumulates below the ceiling, forming a thin layer with a strong concentration gradient. For the case of delayed ignition, the pressure wave is at an overpressure of 8 bar. | [102] | |
| 50 L, 185 bar hydrogen cylinder; 78 L, 650 bar hydrogen cylinder | Experiment | Detonation | The mechanical energy of the compressed gas and, to a small extent, the chemical energy contribute to the explosion wave strength. | [103] | |
| Hydrogen refueling stations | Leakage hole diameter: 3 cm, 39 MPa hydrogen cylinder | CFD | Leakage and explosion | The effect of wind speed on hydrogen fire probability in different regions of a hydrogen refueling station is different. An increase in the delayed ignition time may result in an increase in the intensity of the explosion. | [105] |
| Leakage hole diameter: 1 cm | CFD | Leakage | Compared to flat roofs, sloped roofs are more effective in reducing the volume of combustible hydrogen clouds. | [106] | |
| Garages | Leakage hole diameter: 16.9 mm; leakage rate: 0.003 kg/s | CFD | Leakage | The hydrogen concentration distributions are not uniform in the gas-mixture layer along the ceiling. | [108] |
| Leakage area: 25 cm2; leakage rate: 131 L/min | CFD | Leakage | The volume of the combustible region grows nonlinearly in time with a delay period. | [109] | |
| Leakage hole diameter: 20 mm; leakage rate: 1 g/s | Experiment and CFD | Leakage | The difference between CFD simulation results and experimental data is mainly due to the turbulence model and numerical accuracy. | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Li, Y.; Zhang, W.; Ma, T.; Jing, X. Safety Analysis of Hydrogen-Powered Train in Different Application Scenarios: A Review. Energies 2025, 18, 1743. https://doi.org/10.3390/en18071743
Xu L, Li Y, Zhang W, Ma T, Jing X. Safety Analysis of Hydrogen-Powered Train in Different Application Scenarios: A Review. Energies. 2025; 18(7):1743. https://doi.org/10.3390/en18071743
Chicago/Turabian StyleXu, Lei, Yankun Li, Wenchao Zhang, Tiancai Ma, and Xiuhui Jing. 2025. "Safety Analysis of Hydrogen-Powered Train in Different Application Scenarios: A Review" Energies 18, no. 7: 1743. https://doi.org/10.3390/en18071743
APA StyleXu, L., Li, Y., Zhang, W., Ma, T., & Jing, X. (2025). Safety Analysis of Hydrogen-Powered Train in Different Application Scenarios: A Review. Energies, 18(7), 1743. https://doi.org/10.3390/en18071743






