Rheological Properties of Diesel-Based Fuels with Tyre Pyrolysis Oil as Admixture
Abstract
:1. Introduction
1.1. Used Car Tires as an Environmental Problem
1.2. Properties of Pyrolytic Oil from Car Tires
1.3. Importance of Assessing Rheological Properties of Fuels
2. Materials and Methods
2.1. Basic Indices Describing the Fluidity of Fuels
2.2. Fuels Used in the Experiment
2.3. Methods and Apparatus Used in the Experiment
3. Results and Discussion
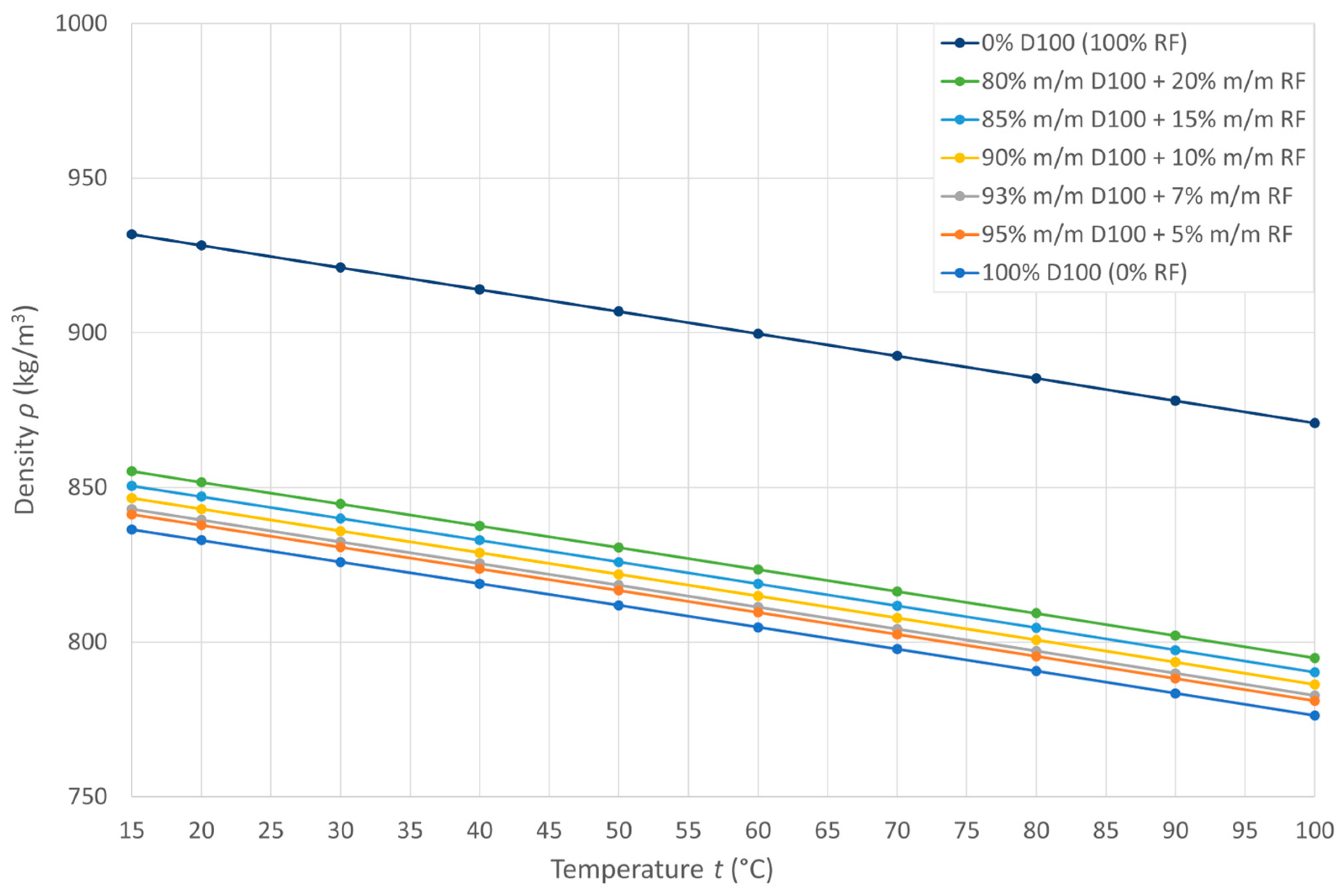
3.1. Density
3.2. Kinematic Viscosity
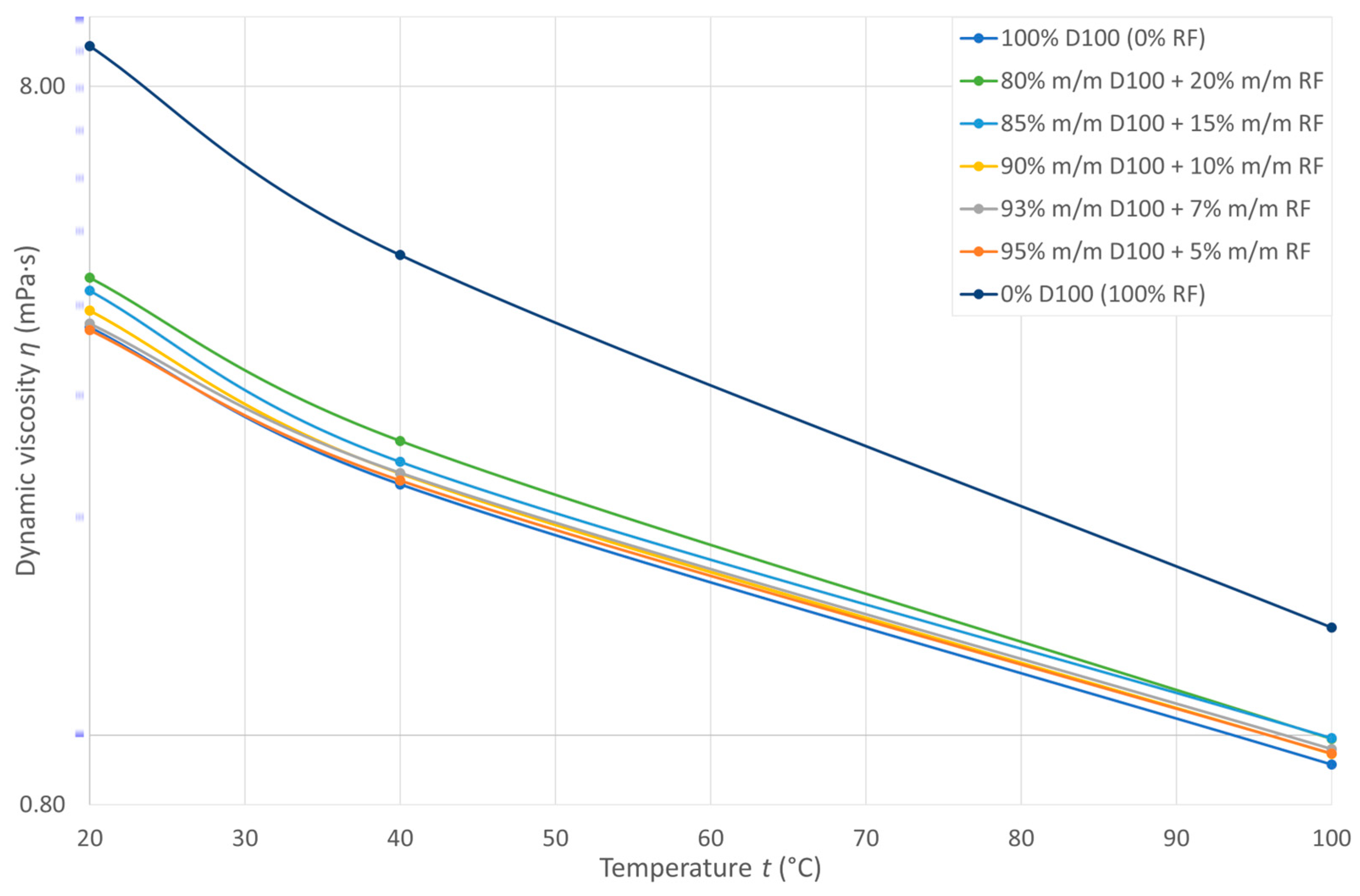
3.3. Dynamic Viscosity
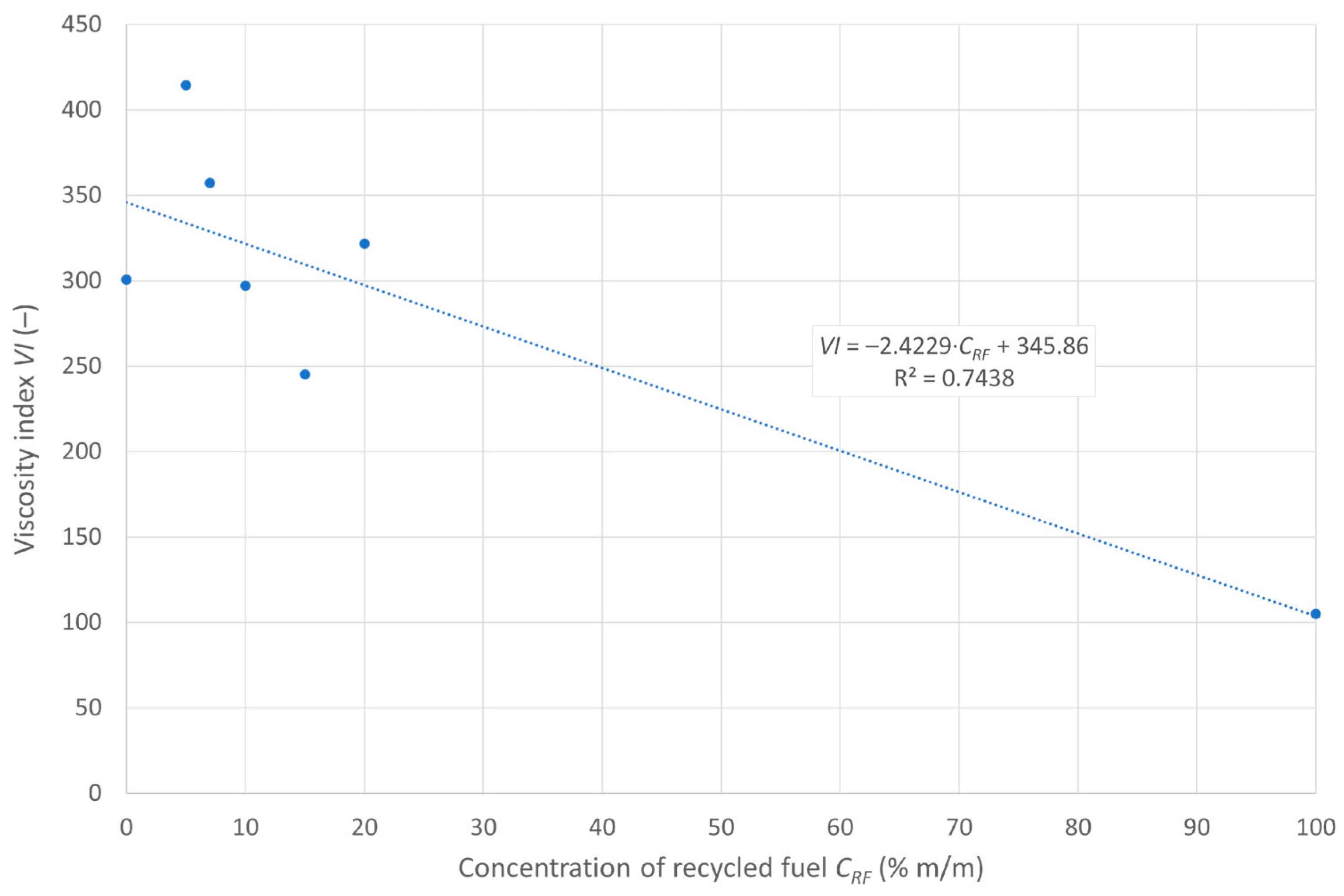
3.4. Variation in Viscosity as a Function of Temperature
3.5. Characteristics of Fuels at Low Temperatures
3.6. Evaluation of the Uncertainty of the Obtained Results
3.7. Assessment of Compliance with Normative Requirements by the Tested Fuels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAS | Chemical Abstracts Service Registry Number |
| CS | sulfur content in the fuels under study |
| CRF | mass percentage of recycled oil in the diesel blend |
| Cw | water content in the fuels under study |
| D100 | pure diesel oil without FAME additives |
| DFA, DFB, DFZ, DMA, DMB, DMZ, DMX | categories of distillation fuels indicated in the standard ISO 8217:2024 marked as ISO-F-… |
| EU | European Union |
| FAME | fatty acid methyl esters |
| HC | hydrocarbons with general designation |
| HFRR | high-frequency reciprocating rig |
| ISO | International Organization for Standardization |
| n/a | not applicable |
| NOx | generic determination of NO and NO2 nitrogen oxides |
| RF | index for the parameters measured or calculated for recycled fuel TPO (short for recycled fuel) |
| RMG | Regulation of the Ministry of Economy of Poland on the properties of fuels |
| RT | retention time |
| SI | Similarity Index |
| tCFPP | cold filter plugging point temperature |
| tFP | flash point temperature |
| TPO | tire pyrolysis oil |
| u | uncertainty |
| W | lower heat value |
| WS1.4 | average diameter of wear scar during HFRR lubricity test corrected to a reference pressure of 1.4 kPa |
| XA | ash residue |
| XCR | coking residue with 10% distillation residue |
| XS | total sediment by hot filtration |
| ν40 | kinematic viscosity at 40 °C |
| ν100 | kinematic viscosity at 100 °C |
| ρt | density |
Appendix A. Pyrolysis Oil Data
| Retention Time RT (min) | Name of the Component | Chemical Abstracts Service Registry Number (CAS) | Similarity Index SI (–) |
|---|---|---|---|
| 4.1 | Cyclobutane, (1-methylethylidene)- | 1528-22-9 | 92 |
| 4.3 | Toluene | 108-88-3 | 97 |
| 5.7 | Ethylbenzene | 100-41-4 | 96 |
| 5.8 | o-Xylene | 95-47-6 | 96 |
| 6.1 | Benzene, 1,3-dimethyl- | 108-38-3 | 84 |
| 6.5 | Benzene, (1-methylethyl)- | 98-82-8 | 88 |
| 7.0 | Benzene, 1-ethyl-4-methyl- | 622-96-8 | 87 |
| 7.3 | Tricyclo[3.1.0.0(2,4)]hex-3-ene-3-carbonitrile | 103495-51-8 | 70 |
| 7.4 | Benzene, 1,2,4-trimethyl- | 95-63-6 | 85 |
| 7.8 | D-Limonene | 5989-27-5 | 93 |
| 8.5 | Benzene, (2-methyl-1-propenyl)- | 768-49-0 | 84 |
| 9.2 | Benzene, 1-methyl-2-(2-propenyl)- | 1587-04-8 | 88 |
| 9.6 | 1H-Indene, 2,3-dihydro-4,7-dimethyl- | 6682-71-9 | 76 |
| 10.8 | Naphthalene, 1-methyl- | 90-12-0 | 87 |
| 11.2 | 2,4,4,6,6,8,8-Heptamethyl-2-nonene | 39761-73-4 | 75 |
| 11.5 | 1H-Indene, 1,1,3-trimethyl- | 2177-45-9 | 83 |
| 11.6 | Tetradecane | 629-59-4 | 66 |
| 12.0 | Naphthalene, 1,6-dimethyl- | 575-43-9 | 92 |
| 12.9 | Naphthalene, 1,6,7-trimethyl- | 2245-38-7 | 96 |
| 14.0 | Heneicosane | 629-94-7 | 91 |
| 15.5 | Hexadecanenitrile | 629-79-8 | 93 |
| 16.9 | Octadecanenitrile | 638-65-3 | 93 |
References
- The Invisible Impact: Exposing the Environmental Cost of Tires. Available online: https://www.fluidtruck.com/blog/the-invisible-impact-exposing-the-environmental-cost-of-tires (accessed on 17 February 2025).
- Han, W.; Han, D.; Chen, H. Pyrolysis of Waste Tires: A Review. Polymers 2023, 15, 1604. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.R.; Rodrigue, D. Application of Waste Tire in Construction: A Road towards Sustainability and Circular Economy. Sustainability 2024, 16, 3852. [Google Scholar] [CrossRef]
- Patrício, J.; Andersson-Sköld, Y.; Gustafsson, M. End-of-Life Tyres Applications: Technologies and Environmental Impacts; Statens Väg-och Transportforskningsinstitut: Linköping, Sweden, 2021. [Google Scholar]
- Abdullah, Z.T. Remanufactured Waste Tire By-Product Valorization: Quantitative–Qualitative Sustainability-Based Assessment. Results Eng. 2024, 22, 102229. [Google Scholar] [CrossRef]
- European Commission. Directive 2000/53/EC of the European Parliament and of the Council of 18 September 2000 on End-of Life Vehicles—Commission Statements; European Commission: Brussels, Belgium, 2000.
- European Commission. Council Directive 1999/31/EC of 26 April 1999 on the Landfill of Waste; European Commission: Brussels, Belgium, 1999.
- Campuzano, F.; Abdul Jameel, A.G.; Zhang, W.; Emwas, A.-H.; Agudelo, A.F.; Martínez, J.D.; Sarathy, S.M. On the Distillation of Waste Tire Pyrolysis Oil: A Structural Characterization of the Derived Fractions. Fuel 2021, 290, 120041. [Google Scholar] [CrossRef]
- Rahman, M.M.; Yu, Y.; Wu, H. Valorisation of Waste Tyre via Pyrolysis: Advances and Perspectives. Energy Fuels 2022, 36, 12429–12474. [Google Scholar] [CrossRef]
- Antoniou, N.; Zabaniotou, A. Features of an Efficient and Environmentally Attractive Used Tyres Pyrolysis with Energy and Material Recovery. Renew. Sustain. Energy Rev. 2013, 20, 539–558. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, T.H.; Nguyen, H.P. Scrap Tire Pyrolysis as a Potential Strategy for Waste Management Pathway: A Review. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 6305–6322. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Hossain, F.M.; Rasul, M.G.; Chowdhury, A.A. A Review on the Thermochemical Recycling of Waste Tyres to Oil for Automobile Engine Application. Energies 2021, 14, 3837. [Google Scholar] [CrossRef]
- Laresgoiti, M.F.; Caballero, B.M.; de Marco, I.; Torres, A.; Cabrero, M.A.; Chomón, M.J. Characterization of the Liquid Products Obtained in Tyre Pyrolysis. J. Anal. Appl. Pyrolysis 2004, 71, 917–934. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Jamil, M.A.; Gulzar, M. Potential of Tire Pyrolysis Oil as an Alternate Fuel for Diesel Engines: A Review. J. Energy Inst. 2021, 96, 205–221. [Google Scholar] [CrossRef]
- Ali, M.H.; Moral, M.N.A. Pyrolytic Fuel Extraction from Tire and Tube: Analysis of Parameters on Product Yield. Case Stud. Chem. Environ. Eng. 2022, 6, 100273. [Google Scholar] [CrossRef]
- Uğuz, G.; Ayanoğlu, A. Characterization of Waste Tire Pyrolysis Products by GC, ICP-MS, TGA and DSC. Bitlis Eren Üniversitesi Fen Bilim. Derg. 2021, 10, 930–942. [Google Scholar] [CrossRef]
- de Oliveira Neto, G.C.; Chaves, L.E.C.; Pinto, L.F.R.; Santana, J.C.C.; Amorim, M.P.C.; Rodrigues, M.J.F. Economic, Environmental and Social Benefits of Adoption of Pyrolysis Process of Tires: A Feasible and Ecofriendly Mode to Reduce the Impacts of Scrap Tires in Brazil. Sustainability 2019, 11, 2076. [Google Scholar] [CrossRef]
- Alsaleh, A.; Sattler, M.L. Waste Tire Pyrolysis: Influential Parameters and Product Properties. Curr. Sustain. Renew. Energy Rep. 2014, 1, 129–135. [Google Scholar] [CrossRef]
- Yaqoob, H.; Ali, H.M. Sustainability Analysis of Neat Waste Tire Oil Powered Diesel Engine: A Thermodynamics Approach. Process Saf. Environ. Prot. 2024, 182, 1121–1129. [Google Scholar] [CrossRef]
- Arya, S.; Sharma, A.; Rawat, M.; Agrawal, A. Tyre Pyrolysis Oil as an Alternative Fuel: A Review. Mater. Today Proc. 2020, 28, 2481–2484. [Google Scholar] [CrossRef]
- Chybowski, L.; Szczepanek, M.; Pusty, T.; Brożek, P.; Pełech, R.; Borowski, P. Evaluation of the Ignition Properties of Fuels Based on Oil Diesel Fuel with the Addition of Pyrolytic Oil from Tires. Energy 2025, 18, 860. [Google Scholar] [CrossRef]
- Karagöz, M.; Ağbulut, Ü.; Sarıdemir, S. Waste to Energy: Production of Waste Tire Pyrolysis Oil and Comprehensive Analysis of Its Usability in Diesel Engines. Fuel 2020, 275, 117844. [Google Scholar] [CrossRef]
- Mikulski, M.; Hunicz, J.; Duda, K.; Kazimierski, P.; Suchocki, T.; Rybak, A. Tyre Pyrolytic Oil Fuel Blends in a Modern Compression Ignition Engine: A Comprehensive Combustion and Emissions Analysis. Fuel 2022, 320, 123869. [Google Scholar] [CrossRef]
- Krause, P.; Labuda, R. The Influence of Liquid Viscosity on Atomized Fuel Mean Droplet Size Determined by the Laser Diffraction Method. New Trends Prod. Eng. 2018, 1, 435–441. [Google Scholar] [CrossRef]
- Alazemi, A.A.; Alajmi, A.F.; Al-Salem, S.M. Investigation of Chemical, Physical, and Tribological Properties of Pyrolysis Oil Derived from End-of-Life Tires (ELTs) against Conventional Engine Oil. Lubricants 2024, 12, 188. [Google Scholar] [CrossRef]
- Alazemi, A.A.; Alajmi, A.F.; Al-Salem, S.M. Viable Use of Tire Pyrolysis Oil as an Additive to Conventional Motor Oil: A Tribological and Physical Study. Lubricants 2025, 13, 64. [Google Scholar] [CrossRef]
- ISO 8217:2024; Petroleum Products—Fuels (Class F)—Specifications of Marine Fuels. ISO: Geneva, Switzerland, 2024.
- ISO 12185:2024; Crude Petroleum, Petroleum Products and Related Products—Determination of Density—Laboratory Density Meter with an Oscillating U-Tube Sensor. ISO: Geneva, Switzerland, 2024.
- Piotrowski, I.; Witkowski, K. Okrętowe Silniki Spalinowe, 3rd ed.; Trademar: Gdynia, Poland, 2013; ISBN 83-900731-1-9. [Google Scholar]
- ISO 3104:2023; Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. ISO: Geneva, Switzerland, 2023.
- Arrhenius, S. Über Die Innere Reibung Verdünnter Wässeriger Lösungen. Z. Für Phys. Chem. 1887, 1, 285–298. [Google Scholar] [CrossRef]
- Murugan, S.; Ramaswamy, M.C.; Nagarajan, G. Performance, Emission and Combustion Studies of a DI Diesel Engine Using Distilled Tyre Pyrolysis Oil-Diesel Blends. Fuel Process. Technol. 2008, 89, 152–159. [Google Scholar] [CrossRef]
- ASTM D2270; Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 °C and 100 °C. ASTM: Pennsylvania, PA, USA, 2016.
- ISO 2909:2002; Petroleum Products—Calculation of Viscosity Index from Kinematic Viscosity. ISO: Geneva, Switzerland, 2002.
- Chybowski, L.; Szczepanek, M.; Ćwirko, K.; Marosek, K. Analytical Method for Determining the Viscosity Index of Engine Lubricating Oils. Energy 2024, 17, 4908. [Google Scholar] [CrossRef]
- RP, M.G. Rozporządzenie Ministra Gospodarki z Dnia 9 Października 2015 r. w Sprawie Wymagań Jakościowych Dla Paliw Ciekłych; Ministerstwo Gospodarki RP: Warszawa, Poland, 2015.
- Polski Komitet Normalizacyjny. Przetwory Naftowe: Oznaczanie Ciepła Spalania Paliw Ciekłych w Bombie Kalorymetrycznej i Obliczanie Wartości Opałowej z Zastosowaniem Wzorów Empirycznych; Polski Komitet Normalizacyjny: Warszawa, Poland, 2018; ISBN 9788327594679. [Google Scholar]
- ISO 2719:2016-08/A1:2021-06; Determination of Flash Point—Pens. ISO: Geneva, Switzerland, 2021.
- ISO 3015:2019-06; Petroleum Products and Similar Products of Syn. ISO: Geneva, Switzerland, 2019.
- ISO 3016:2019; Petroleum and Related Products from Natural or Synthetic Sources—Determination of Pour Point. ISO: Geneva, Switzerland, 2019.
- ISO 12937:2005; Petroleum Products—Determination of Water—Coulometric Karl Fischer Titration Method. ISO: Geneva, Switzerland, 2005.
- ISO 8754:2003; Petroleum Products—Determination of Sulfur Content—Energy-Dispersive X-Ray Fluorescence Spectrometry. ISO: Geneva, Switzerland, 2003.
- ISO 12156-1:2023; Diesel Fuel—Assessment of Lubricity Using the High-Frequency Reciprocating Rig (HFRR)—Part 1: Test Method. ISO: Geneva, Switzerland, 2023.
- ISO 10370:2014; Petroleum Products—Determination of Carbon Residue—Micro Method. ISO: Geneva, Switzerland, 2014.
- ISO 6245:2008; Petroleum Products—Determination of Ash. ISO: Geneva, Switzerland, 2008.
- ISO 10307-1:2009; Petroleum Products—Total Sediment in Residual Fuel OilsPart 1: Determination by Hot Filtration. ISO: Geneva, Switzerland, 2009.
- ASTM 6595-17; Standard Test Method for Determination of Wear Metals and Contaminants in Used Lubricating Oils or Used Hydraulic Fluids by Rotating Disc Electrode Atomic Emission Spectrometry. ASTM: Pennsylvania, PA, USA, 2022.
- JCGM 100:2008; Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement. JCGM: Sèvres, France, 2008.
- EN 116:2015; Diesel and Domestic Heating Fuels—Determination of Cold Filter Plugging Point—Stepwise Cooling Bath Method. European Committee for Standardization: Brussels, Belgium, 2015.
- ZN-ORLEN-5; Przetwory Naftowe. Olej Napędowy EFECTA DIESEL. PKN Orlen S.A.: Płock, Poland, 2019.



| Parameter | Unit of Measurement | Measurement Standard | Diesel Oil (D100) | Tyre Pyrolytic Oil (TPO) | |
|---|---|---|---|---|---|
| Density @ 15 °C ρ15 | kg/m3 | ISO 12185:2002 [28] | 836.4 | 931.9 | |
| Kinematic viscosity @ 40 °C ν40 | mm2/s | ISO 3104:2023 [30] | 2.728 | 5.096 | |
| Kinematic viscosity @ 100 °C ν100 | mm2/s | ISO 3104:2023 [30] | 1.173 | 1.620 | |
| Lower heating value W | MJ/kg | PN-C-04062:2018-05 [37] | 45.46 | 42.16 | |
| Flashpoint tFP | °C | ISO 2719:2016-08+A1:2021-06 [38] | 64 | 38 | |
| Cloud point tCP | °C | ISO 3015:2019-06 [39] | 8. | n/a * | |
| Pour point tPP | °C | ISO 3016:2019-06 [40] | –32 | n/a | |
| Water content Cw | % m/m | ISO 12937:2005+Ap1:2021-11P [41] | 0.002 | 0.020 | |
| Sulfur content CS | % m/m | ISO 8754:2003+Ap1:2014-02P [42] | 0.000 | 0.822 | |
| Lubricity (corrected average wear trace from HFRR test at 60 °C) WS1.4 | μm | ISO 12156-1:2023 [43] | 327 | 185 | |
| Coking residue (from 10% distillation residue) XCR | % m/m | ISO 10370:2014-12 [44] | 0.015 | 0.604 | |
| Incineration residue XA | % m/m | ISO 6245:2008 [45] | 0.004 | 0.052 | |
| Total sediment in hot filtration XS | % m/m | ISO 10307-2:2010 [46] | 0.00 | 0.008 | |
| Elemental composition | Fe | ppm | ASTM D6595-17 [47] | 0.0 | 0.0 |
| Cr | 1.1 | 0.1 | |||
| Pb | 7.3 | 10.2 | |||
| Cu | 0.0 | 1.2 | |||
| Sn | 7.4 | 10.0 | |||
| Al | 2.5 | 0.6 | |||
| Ni | 7.6 | 13.0 | |||
| Ag | 0.5 | 0.1 | |||
| Si | 27.0 | 1.4 | |||
| B | 1.0 | 1.5 | |||
| Mg | 0.0 | 0.1 | |||
| Ba | 0.0 | 0.0 | |||
| P | 0.0 | 0.0 | |||
| Zn | 9.5 | 1.2 | |||
| Mo | 1.3 | 1.4 | |||
| Ti | 1.7 | 0.9 | |||
| V | 0.0 | 0.0 | |||
| Step Number | Stage Characteristics | Realization Time |
|---|---|---|
| I | A clean glass vessel was placed on the balance, after which the scale was tared | n/a |
| II | Using a laboratory pipette, a precisely measured mass of pyrolytic oil mRF was added to the glass vessel on the scale, after which the scale was tared | n/a |
| III | Using a laboratory pipette, a precisely measured mass of diesel oil mDO was added to the glass vessel on the scale, after which the scale was tared | n/a |
| IV | The resulting mixture of diesel and pyrolysis oil was mixed using a magnetic stirrer | 15 min |
| Parameter | Unit | Blend of Pyrolysis Oil with Diesel Oil | ||||
|---|---|---|---|---|---|---|
| Mass share of pyrolytic oil in CRF blend | % m/m | 5 | 7 | 10 | 15 | 20 |
| Masa diesel oil mDO | g | 190 | 186 | 180 | 170 | 160 |
| Masa recycled oil mRF | g | 10 | 14 | 20 | 30 | 40 |
| Type B standard uncertainty of the determined mass um | g | 0.0023 | 0.0023 | 0.0023 | 0.0023 | 0.0023 |
| Type B standard uncertainty of the mass proportion of pyrolysis oil in the blend with diesel oil | % m/m | 0.0019 | 0.0010 | 0.0005 | 0.0002 | 0.0001 |
| Parameter | Designation | Unit of Measurement | Method Used | Apparatus Used |
|---|---|---|---|---|
| Density | ρ | kg/m3 | ISO 12185:2024 [28] | DMA 4500 density analyzer (Anton Paar GmbH, Graz, Austria) |
| Kinematic viscosity | ν | mm2/s | ISO 3104:2023 [30] | Cannon-Fenske Opaque glass capillary viscometer (Paradise Scientific Company Ltd., Dhaka, Bangladesh) and a TV 2000 viscometric bath (Labovisco bv, Zoetermeer, The Netherlands) |
| Dynamic viscosity | η | mPa s | ISO 12185:2024 [28] and ISO 3104:2023 [30] | Value calculated from the measured density and kinematic viscosity at the same temperature |
| Viscosity index | VI | – | ASTM D2270-10(2016) [33], ISO 3104:2021-03 [30] | Value calculated from the kinematic viscosity at reference temperatures |
| Cloud point | tCP | °C | ISO 3015:2019-06 [39] | Automatic apparatus for the determination of pour point and cloud point with the integrated cooling system CPP 5Gs (ISL, Verson, France) |
| Pour point | tPP | °C | ISO 3016:20159-06 [40] | |
| Cold filter plugging point | tCFPP | °C | EN 116:2015-09 [49] | Automatic cold-filter plugging-point-determination apparatus with integrated cooling system FPP 5Gs (ISL, Verson, France) |
| Pyrolytic Oil Content in Fuel CRF (% m/m) | ρ15 (kg/m3) | ε (kg/(m3 °C)) | R2 (–) |
|---|---|---|---|
| 0 | 836.4 | 0.7082 | 0.9999 |
| 5 | 841.3 | 0.7094 | 0.9999 |
| 7 | 843.0 | 0.7094 | 0.9999 |
| 10 | 846.5 | 0.7082 | 0.9999 |
| 15 | 850.5 | 0.7094 | 0.9999 |
| 20 | 855.3 | 0.7106 | 0.9999 |
| 100 | 931.9 | 0.7188 | 0.9999 |
| Pyrolytic Oil Content in Fuel CRF (% m/m) | a1 (mm2/s) | a2 (1/°C) | R2 (–) |
|---|---|---|---|
| 0 | 5.6231 | –0.016 | 0.9761 |
| 5 | 5.6515 | –0.016 | 0.9717 |
| 7 | 5.7141 | –0.016 | 0.9769 |
| 10 | 5.9275 | –0.016 | 0.9689 |
| 15 | 6.2457 | –0.016 | 0.9625 |
| 20 | 6.6347 | –0.017 | 0.9741 |
| 100 | 13.7780 | –0.022 | 0.9713 |
| Pyrolytic Oil Content in Fuel CRF (% m/m) | b1 (mPa s) | b2 (1/°C) | R2 (–) |
|---|---|---|---|
| 0 | 4.8294 | –0.017 | 0.9752 |
| 5 | 4.7594 | –0.016 | 0.9771 |
| 7 | 4.8833 | –0.017 | 0.9788 |
| 10 | 5.0861 | –0.017 | 0.9714 |
| 15 | 5.3661 | –0.017 | 0.9644 |
| 20 | 5.7704 | –0.018 | 0.9770 |
| 100 | 12.9990 | –0.023 | 0.9729 |
| Parameter | Symbol | Unit | Concentration of Recycled Fuel CRF (% m/m) (max. ±0.002% m/m) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 7 | 10 | 15 | 20 | 100 | |||
| Density at 15 °C | u(ρ15) | kg/m3 | 0.363 | 0.364 | 0.365 | 0.366 | 0.367 | 0.369 | 0.392 |
| Kinematic viscosity at 40 °C | u(ν40) | mm2/s | 0.009 | 0.009 | 0.008 | 0.009 | 0.008 | 0.006 | 0.010 |
| Kinematic viscosity at 100 °C | u(ν100) | mm2/s | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.012 |
| Dynamic viscosity at 40 °C | u(η40) | mPa s | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.005 | 0.010 |
| Dynamic viscosity at 100 °C | u(η100) | mPa s | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.011 |
| Viscosity index | u(VI) | – | 0.723 | 2.544 | 2.985 | 1.539 | 3.919 | 2.729 | 3.129 |
| Cold filter plugging point | U(tCF) | °C | 2.875 | 2.733 | 2.733 | 2.804 | 2.804 | 2.804 | n/a |
| Cloud point | u(tCP) | °C | 2.030 | 2.030 | 2.030 | 2.030 | 2.119 | 2.119 | N/A |
| Pour point | u(tPP) | °C | 2.940 | 2.940 | 2.940 | 2.986 | 2.940 | 2.940 | N/A |
| Specification | TPO Content in the Tested Blend CRF (% m/m) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Document | Parameter | Limit | 0 | 5 | 7 | 10 | 15 | 20 | 100 | |
| ISO 8217:2024 | Density Ρ15 at 15 °C | Max. | 890.0 | + | + | + | + | + | + | − |
| Viscosity ν40 at 40 °C (ISO-F-DMX category fuel) | Max. Min. | 5.500 1.400 | + | + | + | + | + | + | + | |
| Cold filter plugging point | – | n/a | Value to be reported | |||||||
| Pour point | – | n/a | Value to be reported | |||||||
| Cloud point | Max. | –6 | + | + | + | + | + | + | + | |
| Ministry of Economy of Poland | Density Ρ15 at 15 °C (standard-grade diesel oils) | Max. Min. | 845.0 820.0 | + | + | + | − | − | − | − |
| Density Ρ15 at 15 °C (premium-grade diesel oils) | Max. Min. | 840.0 800.0 | + | − | − | − | − | − | − | |
| Viscosity ν40 at 40 °C (standard-grade fuel) | Max. Min. | 4.500 2.000 | + | + | + | + | + | + | − | |
| Viscosity ν40 at 40 °C (premium-grade fuel) | Max. Min. | 4.000 1.500 | + | + | + | + | + | + | − | |
| Cold filter plugging point tCF (standard-grade fuel) | Max. | 0–20 | + | + | + | + | + | + | + | |
| Cold filter plugging point tCF (standard-grade fuel) | Max. | –32 | − | − | − | − | − | − | − | |
| Cloud point tCP (standard-grade fuel) | – | n/a | Not specified | |||||||
| Cloud point tCP (premium-grade fuel) | Max. | –22 | − | − | − | − | − | − | − | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chybowski, L.; Szczepanek, M.; Pusty, T.; Brożek, P.; Pełech, R. Rheological Properties of Diesel-Based Fuels with Tyre Pyrolysis Oil as Admixture. Energies 2025, 18, 1993. https://doi.org/10.3390/en18081993
Chybowski L, Szczepanek M, Pusty T, Brożek P, Pełech R. Rheological Properties of Diesel-Based Fuels with Tyre Pyrolysis Oil as Admixture. Energies. 2025; 18(8):1993. https://doi.org/10.3390/en18081993
Chicago/Turabian StyleChybowski, Leszek, Marcin Szczepanek, Tomasz Pusty, Piotr Brożek, and Robert Pełech. 2025. "Rheological Properties of Diesel-Based Fuels with Tyre Pyrolysis Oil as Admixture" Energies 18, no. 8: 1993. https://doi.org/10.3390/en18081993
APA StyleChybowski, L., Szczepanek, M., Pusty, T., Brożek, P., & Pełech, R. (2025). Rheological Properties of Diesel-Based Fuels with Tyre Pyrolysis Oil as Admixture. Energies, 18(8), 1993. https://doi.org/10.3390/en18081993








