Preparation of Highly Efficient and Stable All-Inorganic Perovskite Solar Cells in Atmosphere Environment
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Fabrication of Carbon-Based CsPbI1.8Br1.2 PSCs
2.3. Characterization of Perovskite Solar Cells
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, J.; Wang, C.; Wang, Y.; Xu, Z.; Lu, Z.; Ma, Y.; Zhu, H.; Hu, Y.; Xiao, C.; Yi, X.; et al. All-inorganic perovskite solar cells. J. Am. Chem. Soc. 2016, 138, 15829–15832. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Xia, W.; Zhou, S.; Sun, L.; Cheng, J.; Xu, C.; Lu, Y. Solvent engineering for ambient-air-processed, phase-stable CsPbI3 in perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 3603–3608. [Google Scholar] [CrossRef]
- Yang, W.; Long, H.; Sha, X.; Sun, J.; Zhao, Y.; Guo, C.; Peng, X.; Shou, C.; Yang, X.; Sheng, J.; et al. Unlocking voltage potentials of mixed-halide perovskite solar cells via phase segregation suppression. Adv. Funct. Mater. 2022, 32, 2110698. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.; Lam, Y.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Wu, Z.; Cai, H.; Wu, T.; Xu, J.; Wang, Z.; Du, H.; Zhao, J.; Huang, F.; Cheng, Y.; Zhong, J. The tricyclic alkaloid catalyzed crystallization of α-FAPbI 3 for high performance antisolvent-free perovskite solar cells. Energy Environ. Sci. 2024, 17, 4670–4680. [Google Scholar] [CrossRef]
- Lin, J.; Lai, M.; Dou, L.; Kley, C.S.; Chen, H.; Peng, F.; Sun, J.; Lu, D.; Hawks, S.A.; Xie, C.; et al. Thermochromic halide perovskite solar cells. Nat. Mater. 2018, 17, 261–267. Available online: https://www.nature.com/articles/s41563-017-0006-0#citeas (accessed on 20 April 2025). [CrossRef]
- Beal, R.E.; Slotcavage, D.J.; Leijtens, T.; Bowring, A.R.; Belisle, A.R.; Nguyen, W.H.; Burkhard, G.F.; Hoke, E.T.; McGehee, M.D. Cesium lead halide perovskites with improved stability for tandem solar cells. J. Phys. Chem. Lett. 2016, 7, 746–751. [Google Scholar] [CrossRef]
- Straus, D.B.; Guo, S.; Abeykoon, A.M.M.; Cava, R.J. Understanding the Instability of the Halide Perovskite CsPbI3 through Temperature-Dependent Structural Analysis. Adv. Mater. 2020, 32, 2001069. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, P.; Wang, C.; Wang, Y.; Hu, Y.; Zhu, G.; Ma, L.; Liu, J.; Jin, Z. CsPb0.9Sn0.1IBr2 based all-inorganic perovskite solar cells with exceptional efficiency and stability. J. Am. Chem. Soc. 2017, 139, 14009–14012. [Google Scholar] [CrossRef]
- Zhang, J.; Hodes, G.; Jin, Z.; Liu, S. All-inorganic CsPbX3 perovskite solar cells: Progress and prospects. Angew. Chem. Int. Ed. 2019, 58, 15596–15618. [Google Scholar] [CrossRef]
- Lau, C.F.J.; Wang, Z.; Sakai, N.; Zheng, J.; Liao, C.H.; Green, M.; Huang, S.; Snaith, H.; Baillie, A.H. Fabrication of efficient and stable CsPbI3 perovskite solar cells through cation exchange process. Adv. Energy Mater. 2019, 9, 1901685. [Google Scholar] [CrossRef]
- Lassoued, M.S.; Ahmad, F.; Zheng, Y. Film thickness effect on 2D lead-free hybrid double perovskite properties: Band gap, photocurrent and stability. Chin. Chem. Lett. 2025, 36, 110477. [Google Scholar] [CrossRef]
- Yang, X.; Han, J.; Ruan, W.; Hu, Y.; He, Z.; Jia, X.; Zhang, S.; Wang, D. Low temperature fabrication for high-performance semitransparent CsPbI2Br perovskite solar cells. Chin. Chem. Lett. 2022, 33, 1425–1429. [Google Scholar] [CrossRef]
- Guo, Z.; Jena, A.K.; Takei, I.; Kim, G.M.; Kamarudin, M.A.; Sanehira, Y.; Ishii, A.; Numata, Y.; Hayase, S.; Miyasaka, T. VOC over 1.4 V for amorphous tin-oxide-based dopant-free CsPbI2Br perovskite solar cells. J. Am. Chem. Soc. 2020, 142, 9725–9734. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, Y.B.; Ge, Z. Understanding of perovskite crystal growth and film formation in scalable deposition processes. Chem. Soc. Rev. 2020, 49, 1653–1687. [Google Scholar] [CrossRef]
- Qin, M.; Chan, P.F.; Lu, X. A systematic review of metal halide perovskite crystallization and film formation mechanism unveiled by in situ GIWAXS. Adv. Mater. 2021, 33, 2105290. [Google Scholar] [CrossRef]
- Liu, X.; Lian, H.; Zhou, Z.; Zou, C.; Xie, J.; Zhang, F.; Yuan, H.; Yang, S.; Hou, Y.; Yang, H. Stoichiometric dissolution of defective CsPbI2Br surfaces for inorganic solar cells with 17.5% efficiency. Adv. Energy Mater. 2022, 12, 2103933. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, C.; Zhang, X.; Rujisamphan, N.; Liu, Y.; Li, Y.; Yuan, J.; Ma, W. Dual interfacial engineering enables efficient and reproducible CsPbI2Br all-inorganic perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 31659–31666. [Google Scholar] [CrossRef]
- Zhang, B.; Bi, W.; Wu, Y.; Chen, C.; Li, H.; Song, Z.; Dai, Q.; Xu, L.; Song, H. High-performance CsPbIBr2 perovskite solar cells: Effectively promoted crystal growth by antisolvent and organic ion strategies. ACS Appl. Mater. Interfaces 2019, 11, 33868–33878. [Google Scholar] [CrossRef]
- Dong, C.; Han, X.; Li, W.; Qiu, Q.; Wang, J. Anti-solvent assisted multi-step deposition for efficient and stable carbon-based CsPbI2Br all-inorganic perovskite solar cell. Nano Energy 2019, 59, 553–559. [Google Scholar] [CrossRef]
- Konstantakou, M.; Perganti, D.; Falaras, P.; Stergiopoulos, T. Anti-solvent crystallization strategies for highly efficient perovskite solar cells. Crystals 2017, 7, 291. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, F.; Chen, H.; Ji, L.; Wang, Y.; Chun, L.; Raschke, M.; Li, S. Mediator–antisolvent strategy to stabilize all-inorganic CsPbI3 for perovskite solar cells with efficiency exceeding 16%. ACS Energy Lett. 2020, 5, 1619–1627. [Google Scholar] [CrossRef]
- Gou, Y.; Tang, S.; Yun, C.; Zhao, P.; Chen, J.; Yu, H. Research progress of green antisolvent for perovskite solar cells. Mater. Horiz. 2024, 11, 3465–3481. [Google Scholar] [CrossRef] [PubMed]
- Asuo, I.M.; Varposhti, A.M.; Gomez, E.D.; Doumon, N.Y. Anti-solvent engineering enables efficient ambient-processed halide perovskite solar cells. J. Mater. Chem. C 2024, 12, 7562–7571. [Google Scholar] [CrossRef]
- Bian, J.; Wu, Y.; Bi, W.; Liu, L.; Su, X.; Zhang, B. Efficient CsPbIBr2 perovskite solar cells: Precise control of film growth through the application of organic iodized salt and anti-solvent. Energy Fuels 2020, 34, 11472–11478. [Google Scholar] [CrossRef]
- Vaynzof, Y. The future of perovskite photovoltaics—Thermal evaporation or solution processing? Adv. Energy Mater. 2020, 10, 2003073. [Google Scholar] [CrossRef]
- Yang, S.; Wu, M.; Lei, X.; Wang, J.; Han, Y.; He, X.; Liu, S.; Liu, Z. Ultra-High 1.27 VV OC of Pure CsPbI3 Perovskite Solar Cells with an Efficiency of 21.8%. ACS Energy Lett. 2024, 9, 4817–4826. [Google Scholar] [CrossRef]
- Frolova, L.A.; Davlethanov, A.I.; Dremova, N.N.; Zhidkov, I.; Akbulatov, A.F.; Kurmaev, E.Z.; Aldoshin, S.M.; Stevenson, K.J.; Troshin, P.A. Efficient and stable MAPbI3-based perovskite solar cells using polyvinylcarbazole passivation. J. Phys. Chem. Lett. 2020, 16, 6772–6778. [Google Scholar] [CrossRef]
- Zhao, P.; Kim, B.J.; Ren, X.; Lee, D.G.; Bang, G.J.; Jeon, J.B.; Kim, W.B.; Jung, H.S. Antisolvent with an ultrawide processing window for the one-step fabrication of efficient and large-area perovskite solar cells. Adv. Mater. 2018, 30, 1802763. [Google Scholar] [CrossRef]
- Taylor, A.D.; Sun, Q.; Goetz, K.P.; An, Q.; Schramm, T.; Hofstetter, Y.; Litterst, M.; Paulus, F.; Vaynzof, Y. A general approach to high-efficiency perovskite solar cells by any antisolvent. Nat. Commun. 2021, 12, 1878. Available online: https://www.nature.com/articles/s41467-021-22049-8#citeas (accessed on 20 April 2025). [CrossRef]
- Long, J.; Sheng, W.; Dai, R.; Huang, Z.; Yang, J.; Zhang, J.; Li, X.; Tan, L.; Chen, Y. Understanding the mechanism between antisolvent dripping and additive doping strategies on the passivation effects in perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 56151–56160. [Google Scholar] [CrossRef] [PubMed]
- Manion, J.G.; Proppe, A.H.; Hicks, G.E.J.; Sargent, E.H.; Seferos, D.S. High-throughput screening of antisolvents for the deposition of high-quality perovskite thin films. ACS Appl. Mater. Interfaces 2020, 12, 26026–26032. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.C.; Cai, C.; Liu, B.; Yang, H.; Lee, R. Cellulose Nanocrystal-Incorporated MAPbI3 for Inverted Perovskite Solar Cells with Enhanced Efficiency and Stability. ACS Appl. Energy Mater. 2024, 7, 12092–12102. [Google Scholar] [CrossRef]
- Kodalle, T.; Byranvand, M.M.; Goudreau, M.; Das, C.; Roy, R.; Kot, M.; Briesenick, S.; Zohdi, M.; Rai, M.; Tamura, N.; et al. An integrated deposition and passivation strategy for controlled crystallization of 2D/3D halide perovskite films. Adv. Mater. 2024, 36, 2309154. [Google Scholar] [CrossRef]
- Wu, J.; Dong, J.J.; Chen, S.X.; Hao, H.Y.; Xing, J.; Liu, H. Fabrication of efficient organic-inorganic perovskite solar cells in ambient air. Nanoscale Res. Lett. 2018, 13, 293. Available online: https://link.springer.com/article/10.1186/s11671-018-2714-z#citeas (accessed on 20 April 2025). [CrossRef]
- Lee, S.; Tang, M.C.; Munir, R.; Barrit, D.; Kim, Y.J.; Kang, R.; Yun, J.M.; Smilgies, D.M.; Amassian, A.; Kim, D.y. In situ study of the film formation mechanism of organic–inorganic hybrid perovskite solar cells: Controlling the solvate phase using an additive system. J. Mater. Chem. A 2020, 8, 7695–7703. [Google Scholar] [CrossRef]



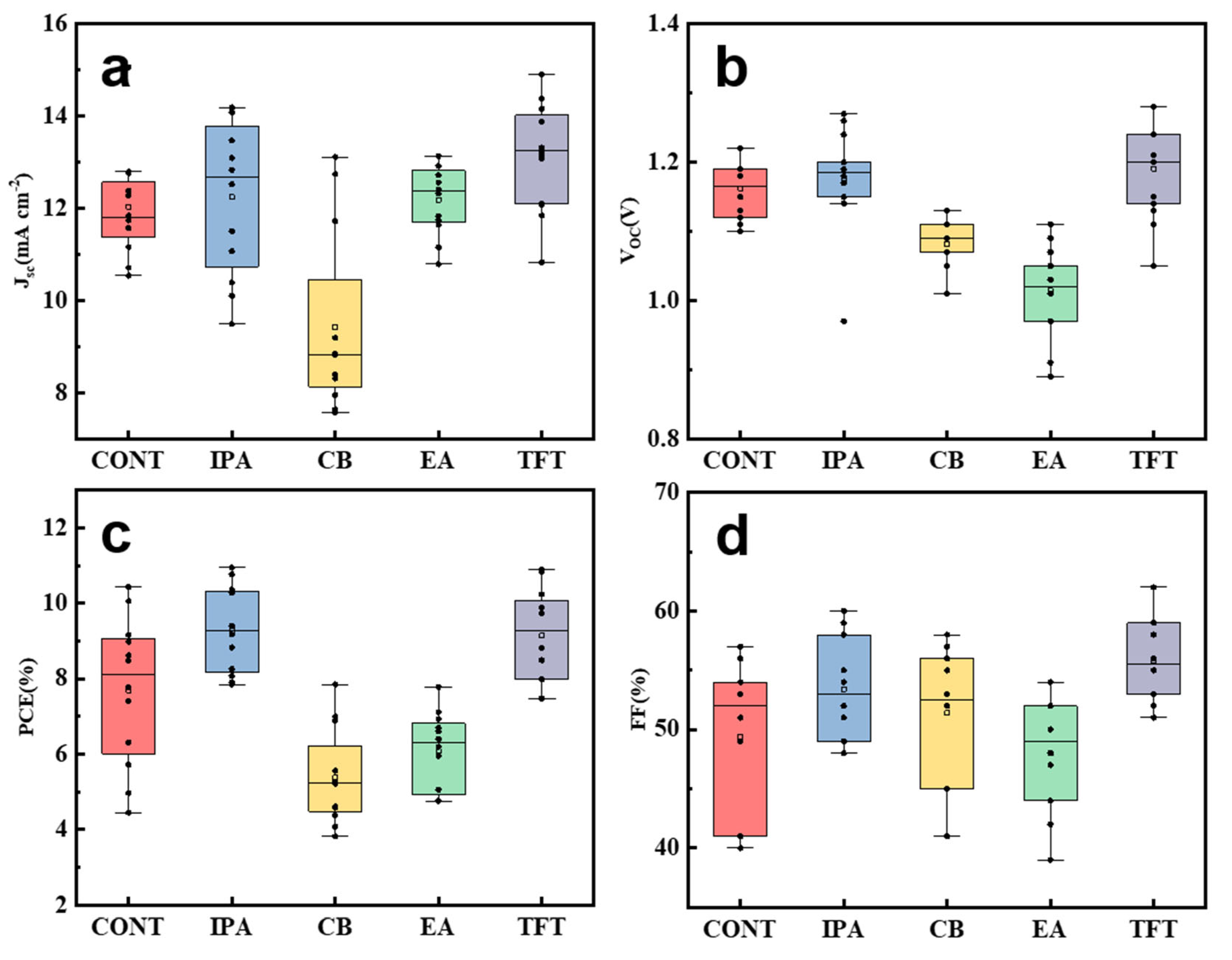
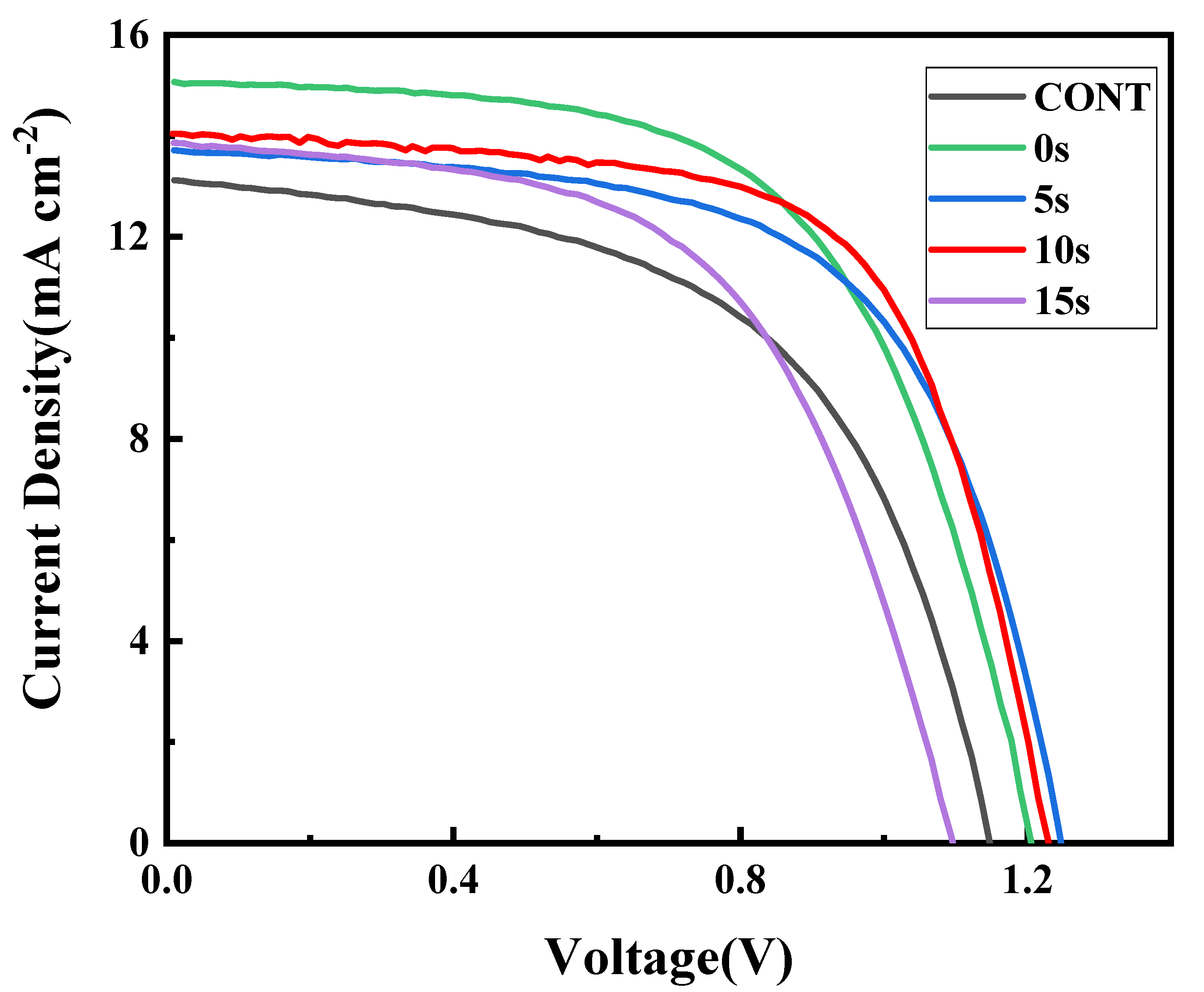

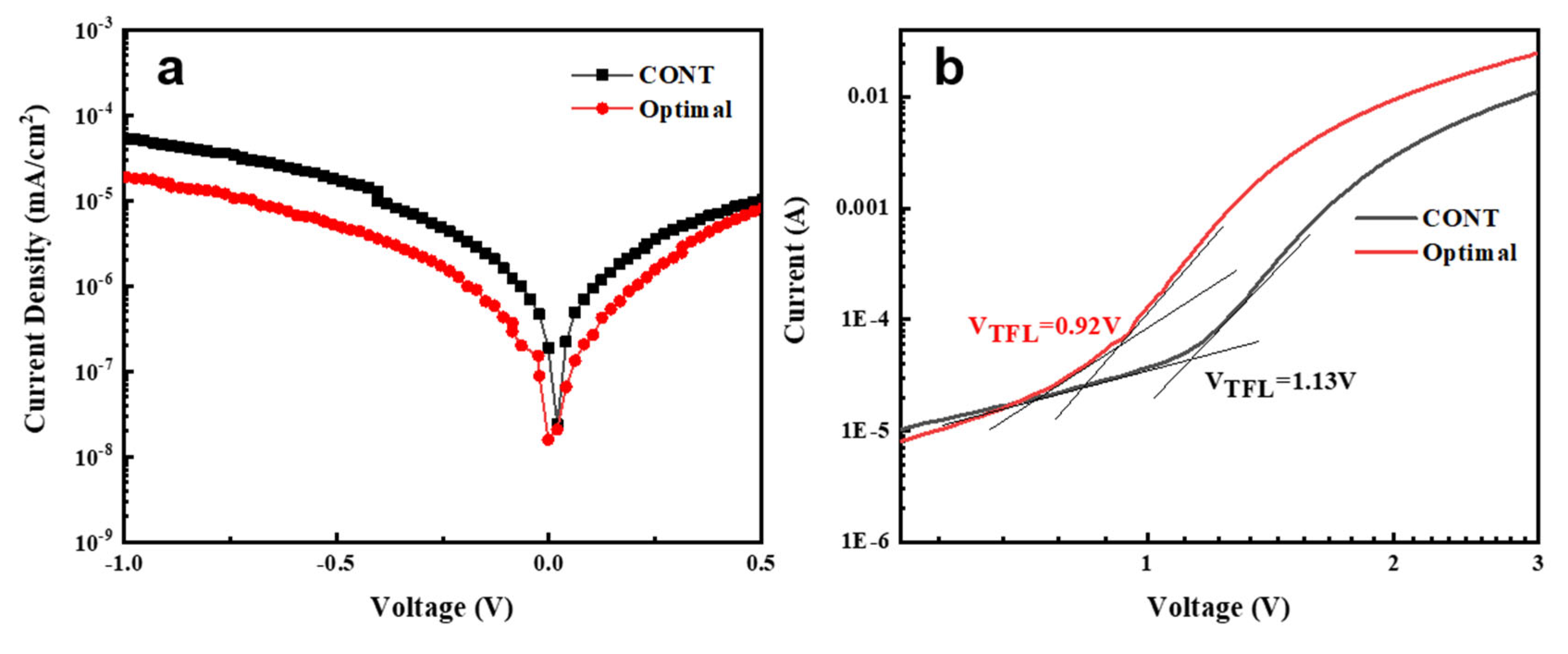
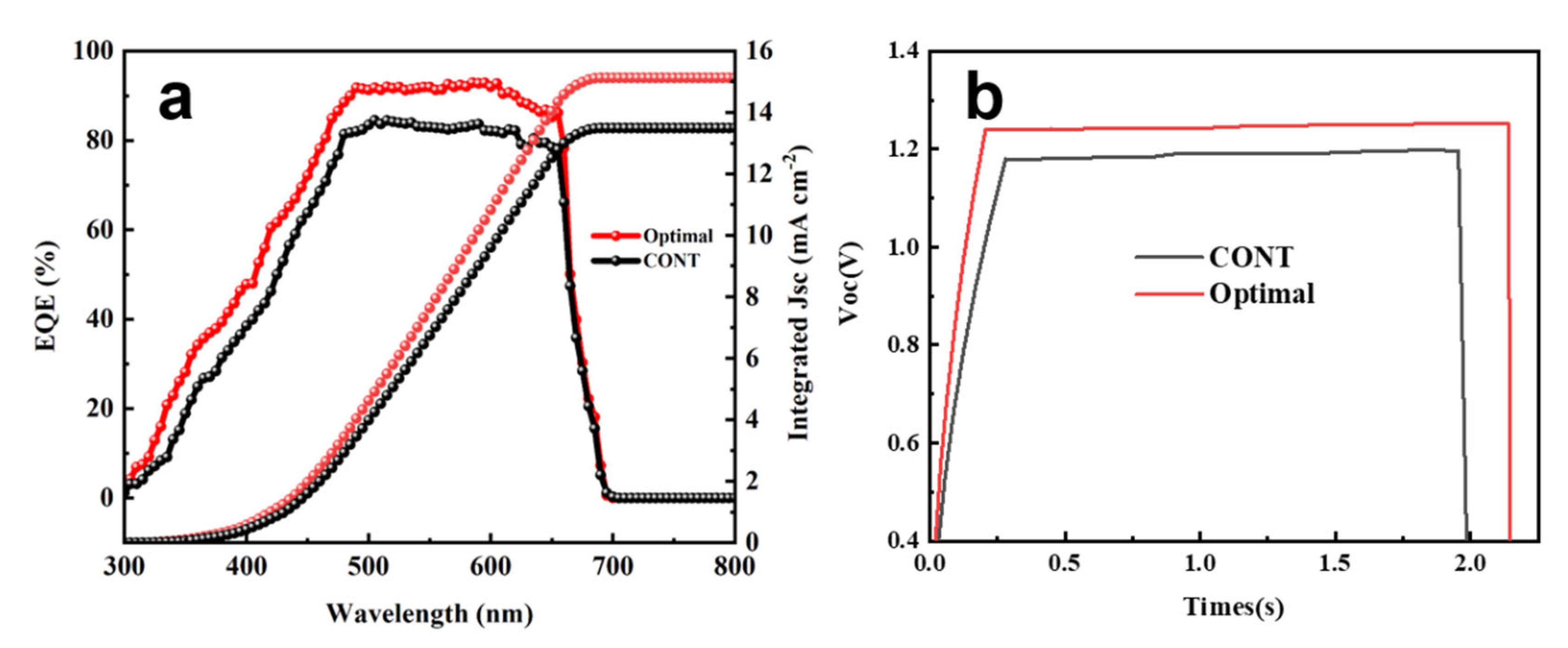

| Anti-Solvent Type | JSC (mA cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| CONT | 12.52 | 1.19 | 57.9 | 8.62 |
| IPA | 13.08 | 1.3 | 60.9 | 10.36 |
| CB | 13.11 | 1.07 | 56.0 | 7.85 |
| EA | 12.92 | 1.11 | 54.2 | 7.78 |
| TFT | 15.06 | 1.2 | 60.3 | 10.89 |
| Application Timing | JSC (mA cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| CONT | 13.12 | 1.14 | 58.9 | 8.81 |
| 0 s | 15.06 | 1.2 | 60.3 | 10.89 |
| 5 s | 13.71 | 1.24 | 63.5 | 10.79 |
| 10 s | 13.97 | 1.21 | 66.3 | 11.21 |
| 15 s | 13.84 | 1.09 | 60.5 | 9.13 |
| Additive Quantity | JSC (mA cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 0% | 13.63 | 1.23 | 64.9 | 10.88 |
| 0.5% | 14.42 | 1.23 | 65.3 | 11.58 |
| 1% | 14.77 | 1.24 | 65.7 | 12.04 |
| 2% | 15.53 | 1.26 | 64.8 | 12.68 |
| 4% | 14.76 | 1.31 | 62.5 | 12.09 |
| 8% | 14.34 | 1.3 | 60.6 | 11.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Deng, D.; Dong, J. Preparation of Highly Efficient and Stable All-Inorganic Perovskite Solar Cells in Atmosphere Environment. Energies 2025, 18, 2162. https://doi.org/10.3390/en18092162
Jiang Y, Deng D, Dong J. Preparation of Highly Efficient and Stable All-Inorganic Perovskite Solar Cells in Atmosphere Environment. Energies. 2025; 18(9):2162. https://doi.org/10.3390/en18092162
Chicago/Turabian StyleJiang, Yufan, Dongdong Deng, and Jingjing Dong. 2025. "Preparation of Highly Efficient and Stable All-Inorganic Perovskite Solar Cells in Atmosphere Environment" Energies 18, no. 9: 2162. https://doi.org/10.3390/en18092162
APA StyleJiang, Y., Deng, D., & Dong, J. (2025). Preparation of Highly Efficient and Stable All-Inorganic Perovskite Solar Cells in Atmosphere Environment. Energies, 18(9), 2162. https://doi.org/10.3390/en18092162







