Mussel-Inspired Dopamine and Carbon Nanotube Leading to a Biocompatible Self-Rolling Conductive Hydrogel Film
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Synthesis of Dopamine-MBA Crosslinker
2.3. Preparation of MWCNT-Dopamine-PEG Hydrogels
2.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.5. Cell Culture
2.6. Cell Growth on Hydrogels
2.7. Cell Viability
2.8. Cell Morphology
2.9. Electrical Conductivity Test
2.10. Quantitative Real-Time PCR Analysis
3. Result and Discussion
3.1. Characterization of Self-Folding Film
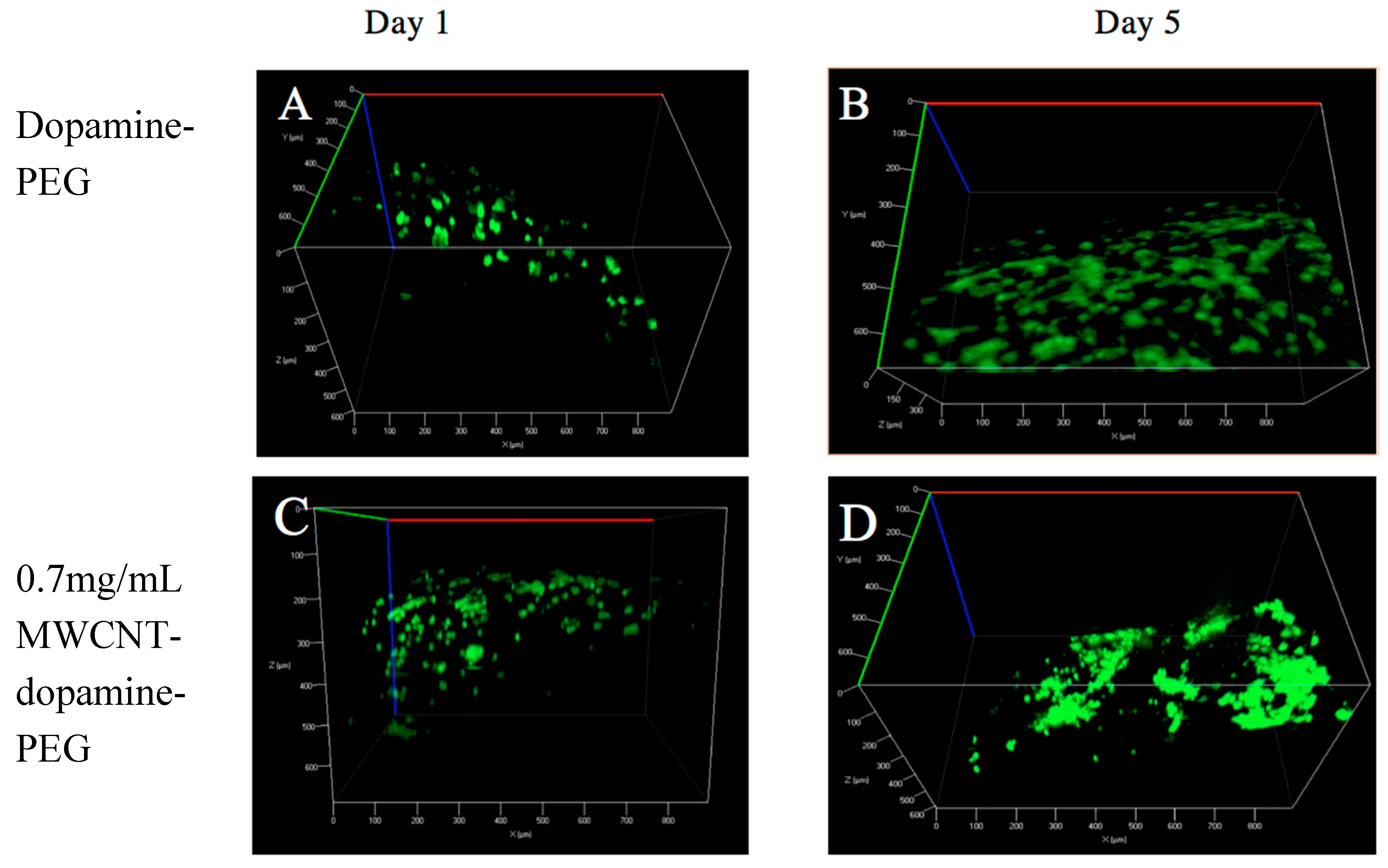
3.2. Live/Dead Assay
3.3. MTT Assay
3.4. Cell Morphology
3.5. Conductivity
3.6. RTPCR
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elcin, A.E.; Elcin, Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Mahadik, B.P.; Harley, B.A.C. Engineering the hematopoietic stem cell niche: Frontiers in biomaterial science. Biotechnol. J. 2015, 10, 1529–1545. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, L.G.; Fisher, J.P. Extracellular Matrix-Based Biohybrid Materials for Engineering Compliant, Matrix-Dense Tissues. Adv. Healthc. Mater. 2015, 4, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Asti, A.; Gioglio, L. Natural and synthetic biodegradable polymers: Different scaffolds for cell expansion and tissue formation. Int. J. Artif. Organs 2014, 37, 187–205. [Google Scholar] [PubMed]
- Garg, T.; Goyal, A.K. Biomaterial-based scaffolds-current status and future directions. Expert Opin. Drug Deliv. 2014, 11, 767–789. [Google Scholar] [CrossRef] [PubMed]
- Maier, G.P.; Butler, A. Siderophores and mussel foot proteins: The role of catechol, cations, and metal coordination in surface adhesion. JBIC J. Biol. Inorg. Chem. 2017, 22, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Huang, K.; Nunalee, F.N.; Shull, K.R.; Messersmith, P.B. Synthesis of 3,4-dihydroxyphenylalanine (DOPA) containing monomers and their co-polymerization with PEG-diacrylate to form hydrogels. J. Biomater. Sci. Polym. Ed. 2004, 15, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, M.; Weng, H.; Gyawali, D.; Tang, L.P.; Yang, J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials 2012, 33, 7972–7983. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Li, B.J.; Yi, N.; Su, B.H.; Yin, Z.H.; Zhang, F.L.; Li, J.; Zhao, C.S. Improving the blood compatibility of material surfaces via biomolecule-immobilized mussel-inspired coatings. J. Biomed. Mater. Res. A 2011, 96A, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.K.; Shi, Z.L.; Lim, T.Y.; Neoh, K.G.; Wang, W. The effect of VEGF functionalization of titanium on endothelial cells in vitro. Biomaterials 2010, 31, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Cai, K.Y.; Zhao, L.; Chen, X.Y.; Hou, Y.H.; Yang, Z.X. Surface Functionalization of TiO2 Nanotubes with Bone Morphogenetic Protein 2 and Its Synergistic Effect on the Differentiation of Mesenchymal Stem Cells. Biomacromolecules 2011, 12, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Ali, F.; Zhao, J.S.; Rhee, K.; Mou, C.C.; Bettinger, C.J. Ultrasound-Mediated Self-Healing Hydrogels Based on Tunable Metal-Organic Bonding. Biomacromolecules 2017, 18, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Liu, K.Z.; Wang, K.F.; Fang, L.M.; Weng, L.T.; Zhang, H.P.; Tang, Y.H.; Ren, F.Z.; Zhao, C.C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, H.; Konst, S.; Sarmiento, R.; Rajachar, R.; Lee, B.P. Injectable Dopamine-Modified Poly(ethylene glycol) Nanocomposite Hydrogel with Enhanced Adhesive Property and Bioactivity. ACS Appl. Mater. Interfaces 2014, 6, 16982–16992. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.Y.; Wang, J.; Yao, X.; Peng, Y.; Tu, X.X.; Du, P.F.; Zheng, Z.; Wang, X.L. Facile Preparation of Mussel-Inspired Polyurethane Hydrogel and Its Rapid Curing Behavior. ACS Appl. Mater. Interfaces 2014, 6, 12495–12504. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Lee, J.S.; Park, C.B. Spatial Control of Cell Adhesion and Patterning through Mussel-Inspired Surface Modification by Polydopamine. Langmuir 2010, 26, 15104–15108. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Monaco, A.M.; Giugliano, M. Carbon-based smart nanomaterials in biomedicine and neuroengineering. Beilstein J. Nanotechnol. 2014, 5, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Haniu, H.; Usui, Y.; Aoki, K.; Hara, K.; Takanashi, S.; Shimizu, M.; Narita, N.; Okamoto, M.; Kobayashi, S.; et al. Safe Clinical Use of Carbon Nanotubes as Innovative Biomaterials. Chem. Rev. 2014, 114, 6040–6079. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Bosi, S.; Alshatwi, A.; Prato, M. Carbon nanotubes for organ regeneration: An electrifying performance. Nano Today 2016, 11, 398–401. [Google Scholar] [CrossRef]
- Marchesan, S.; Melchionna, M.; Prato, M. Carbon Nanostructures for Nanomedicine: Opportunities and Challenges. Fuller. Nanotub. Carbon Nanostruct. 2014, 22, 190–195. [Google Scholar] [CrossRef]
- Caoduro, C.; Hervouet, E.; Girard-Thernier, C.; Gharbi, T.; Boulandour, H.; Delage-Mourroux, R.; Pudlo, M. Carbon nanotubes as gene carriers: Focus on internalization pathways related to functionalization and properties. Acta Biomater. 2017, 49, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Melchionna, M.; Prato, M. Wire Up on Carbon Nanostructures! How To Play a Winning Game. ACS Nano 2015, 9, 9441–9450. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Hampel, S.; Spizzirri, U.G.; Parisi, O.I.; Picci, N.; Iemma, F. Carbon Nanotubes Hybrid Hydrogels in Drug Delivery: A Perspective Review. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.; Bosi, S.; Melchionna, M.; Da Ros, T.; Marchesan, S. The Glitter of Carbon Nanostructures in Hybrid/Composite Hydrogels for Medicinal Use. Curr. Top. Med. Chem. 2016, 16, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ren, K.; Zhang, H.; Ji, J. Nano-structured Multilayer Films Assembled by Poly(dopamine)-Coated Carbon Nanotubes for Controlling Cell Behavior. Chemnanomat 2017. [Google Scholar] [CrossRef]
- Lee, M.; Ku, S.H.; Ryu, J.; Park, C.B. Mussel-inspired functionalization of carbon nanotubes for hydroxyapatite mineralization. J. Mater. Chem. 2010, 20, 8848–8853. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.L.; Zhang, L.Q.; Liu, L.; Dai, Y.J.; Wang, W.C. Preparation and characterization of silver nanoparticles immobilized on multi-walled carbon nanotubes by poly(dopamine) functionalization. J. Nanopart. Res. 2012, 14, 938. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.G. Emerging biological materials through molecular self-assembly. Biotechnol Adv. 2002, 20, 321–339. [Google Scholar] [CrossRef]
- Liu, V.A.; Bhatia, S.N. Three-dimensional photopatterning of hydrogels containing living cells. Biomed. Microdevices 2002, 4, 257–266. [Google Scholar] [CrossRef]
- Yu, T.Y.; Ober, C.K. Methods for the topographical patterning and patterned surface modification of hydrogels based on hydroxyethyl methacrylate. Biomacromolecules 2003, 4, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shoichet, M.S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater. 2004, 3, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Laflin, K.E.; Jamal, M.; Fernandes, R.; Gracias, D.H. Self-folding micropatterned polymeric containers. Biomed. Microdevices 2011, 13, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Tsang, V.L.; Chen, A.A.; Cho, L.M.; Jadin, K.D.; Sah, R.L.; DeLong, S.; West, J.L.; Bhatia, S.N. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007, 21, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.A.; Lo, E.; Ali, S.; Khademhosseini, A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. USA 2008, 105, 9522–9527. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, J.S.; Kanu, L.N.; Singh, G.; Gracias, D.H. Importance of Surface Patterns for Defect Mitigation in Three-Dimensional Self-Assembly. Langmuir 2010, 26, 12534–12539. [Google Scholar] [CrossRef] [PubMed]
- Zakharchenko, S.; Sperling, E.; Ionov, L. Fully Biodegradable Self-Rolled Polymer Tubes: A Candidate for Tissue Engineering Scaffolds. Biomacromolecules 2011, 12, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Bunting, S.C.J.; Davies, H.A.; Loughlin, A.J.; Golding, J.P.; Phillips, J.B. Engineered neural tissue for peripheral nerve repair. Biomaterials 2013, 34, 7335–7343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Filion, T.M.; Kutikov, A.B.; Song, J. Facile Stem Cell Delivery to Bone Grafts Enabled by Smart Shape Recovery and Stiffening of Degradable Synthetic Periosteal Membranes. Adv. Funct. Mater. 2017, 27, 1604784. [Google Scholar] [CrossRef]
- Cheng, S.; Jin, Y.; Wang, N.; Cao, F.; Zhang, W.; Bai, W.; Zheng, W.; Jiang, X. Self-Adjusting, Polymeric Multilayered Roll that can Keep the Shapes of the Blood Vessel Scaffolds during Biodegradation. Adv. Mater. 2017, 29, 1700171. [Google Scholar] [CrossRef] [PubMed]
- Luchnikov, V.; Sydorenko, O.; Stamm, M. Self-rolled polymer and composite polymer/metal micro- and nanotubes with patterned inner walls. Adv. Mater. 2005, 17, 1177–1182. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Crosby, A.J. Adaptive polymer particles. Appl. Phys. Lett. 2008, 93, 103. [Google Scholar] [CrossRef]
- Simpson, B.; Nunnery, G.; Tannenbaum, R.; Kalaitzidou, K. Capture/release ability of thermo-responsive polymer particles. J. Mater. Chem. 2010, 20, 3496–3501. [Google Scholar] [CrossRef]
- Stoychev, G.; Puretskiy, N.; Ionov, L. Self-folding all-polymer thermoresponsive microcapsules. Soft Matter 2011, 7, 3277–3279. [Google Scholar] [CrossRef]
- Emilitri, E.; Ranucci, E.; Ferruti, P. New poly(amidoamine)s containing disulfide linkages in their main chain. J. Polym. Sci. Polym. Chem. 2005, 43, 1404–1416. [Google Scholar] [CrossRef]
- Chun, C.; Lim, H.J.; Hong, K.-Y.; Park, K.-H.; Song, S.-C. The use of injectable, thermosensitive poly(organophosphazene)–RGD conjugates for the enhancement of mesenchymal stem cell osteogenic differentiation. Biomaterials 2009, 30, 6295–6308. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef] [PubMed]









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Huang, Y.; Wang, Y.; Xu, H.; Xing, M.; Zhong, W. Mussel-Inspired Dopamine and Carbon Nanotube Leading to a Biocompatible Self-Rolling Conductive Hydrogel Film. Materials 2017, 10, 964. https://doi.org/10.3390/ma10080964
Jiang J, Huang Y, Wang Y, Xu H, Xing M, Zhong W. Mussel-Inspired Dopamine and Carbon Nanotube Leading to a Biocompatible Self-Rolling Conductive Hydrogel Film. Materials. 2017; 10(8):964. https://doi.org/10.3390/ma10080964
Chicago/Turabian StyleJiang, Junzi, Yong Huang, Yitian Wang, Hui Xu, Malcolm Xing, and Wen Zhong. 2017. "Mussel-Inspired Dopamine and Carbon Nanotube Leading to a Biocompatible Self-Rolling Conductive Hydrogel Film" Materials 10, no. 8: 964. https://doi.org/10.3390/ma10080964
APA StyleJiang, J., Huang, Y., Wang, Y., Xu, H., Xing, M., & Zhong, W. (2017). Mussel-Inspired Dopamine and Carbon Nanotube Leading to a Biocompatible Self-Rolling Conductive Hydrogel Film. Materials, 10(8), 964. https://doi.org/10.3390/ma10080964



