Investigation the Corrosion Inhibition Effect of Itraconazole on Copper in H2SO4 at Different Temperatures: Combining Experimental and Theoretical Studies
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Preparation
2.2. Surface Investigation by Scanning Electron Microscope, Atomic Force Microscope
2.3. Electrochemical Experiments
2.4. Theoretical Calculation
3. Results and Discussion
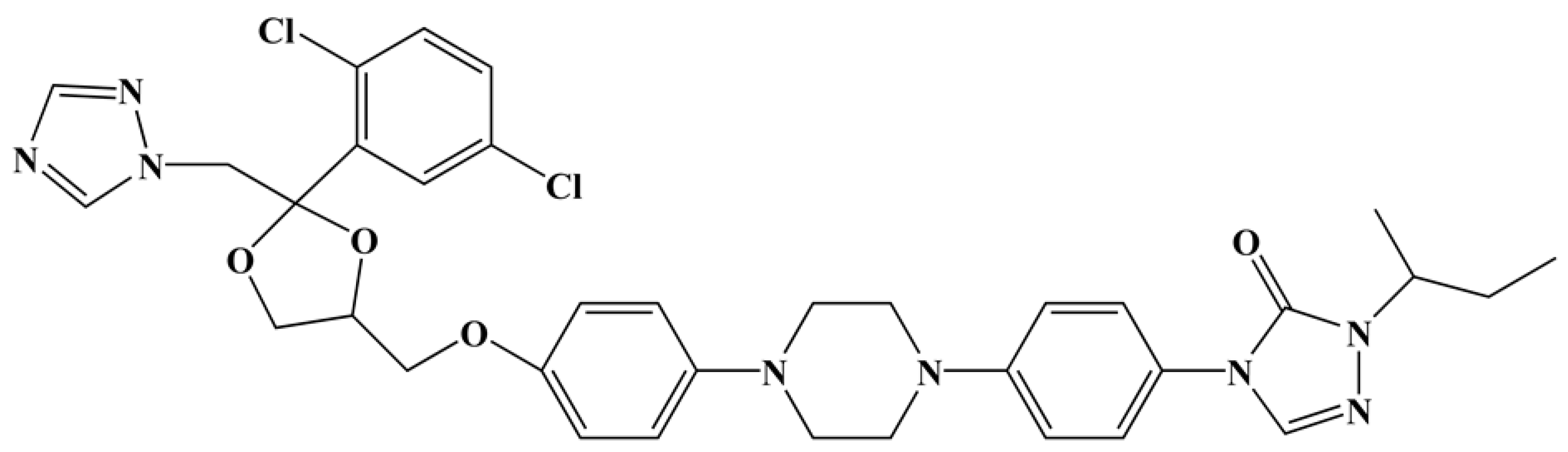
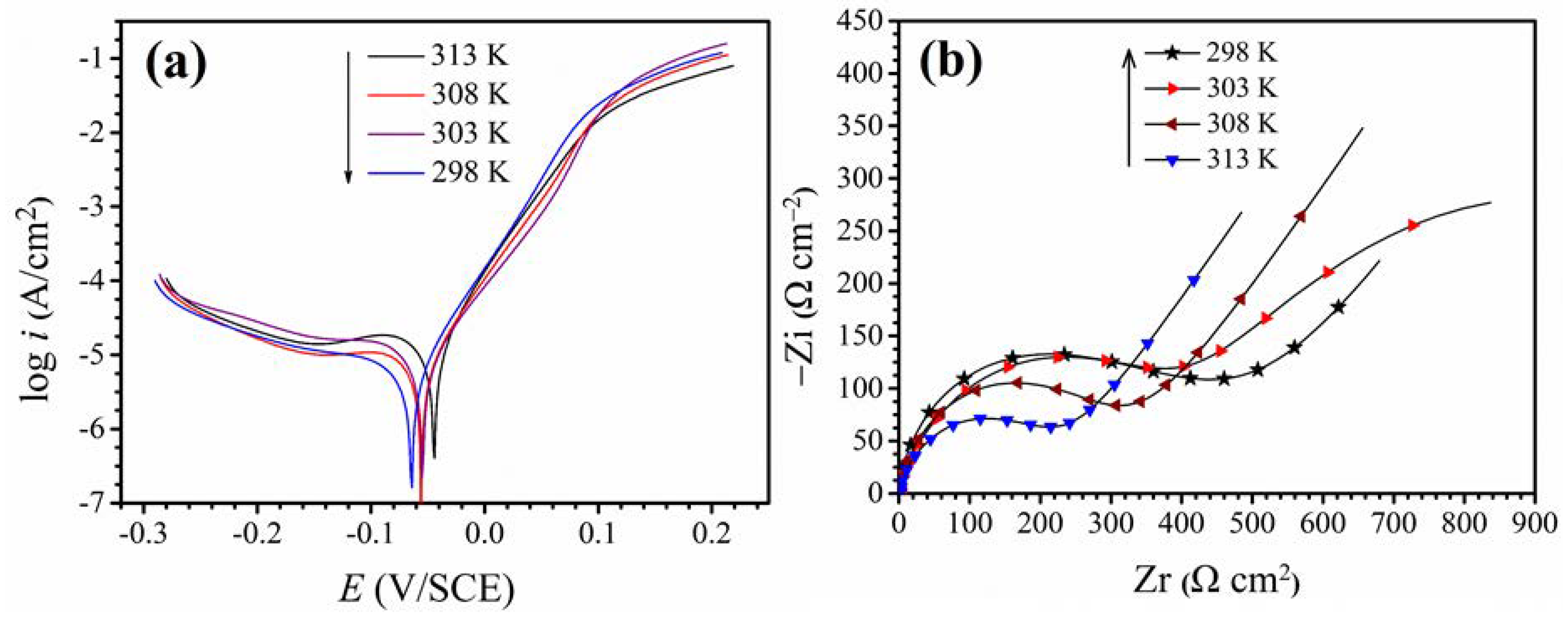
3.1. Blank Solution Electrochemical Analysis at Different Temperatures
3.2. Polarization Curves
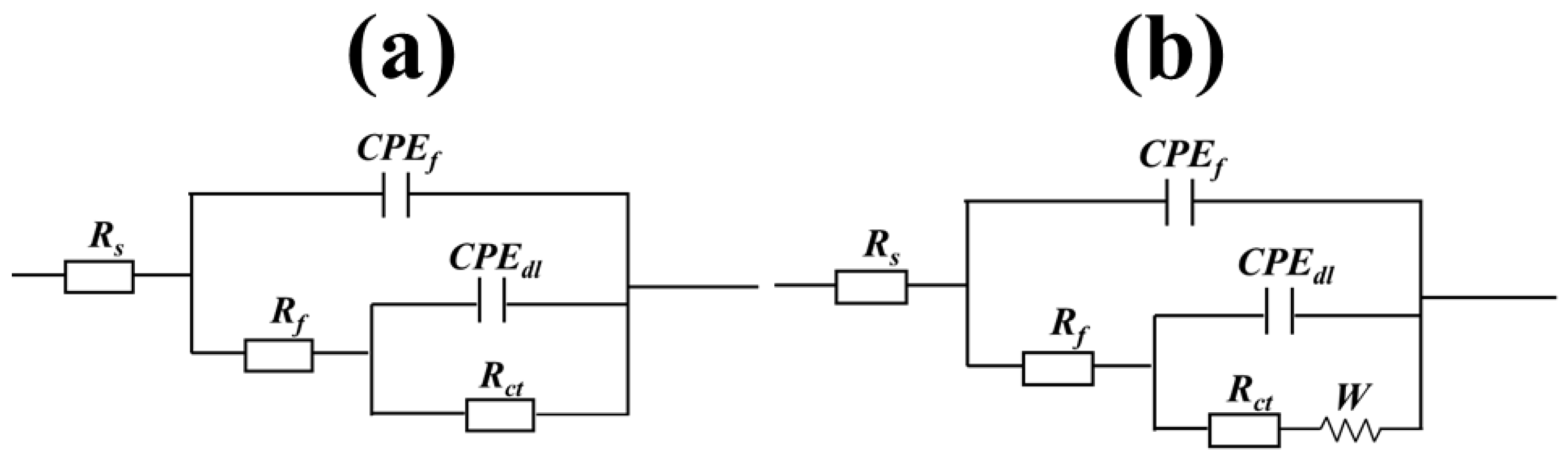
3.3. Electrochemical Impedance Spectroscopy Test
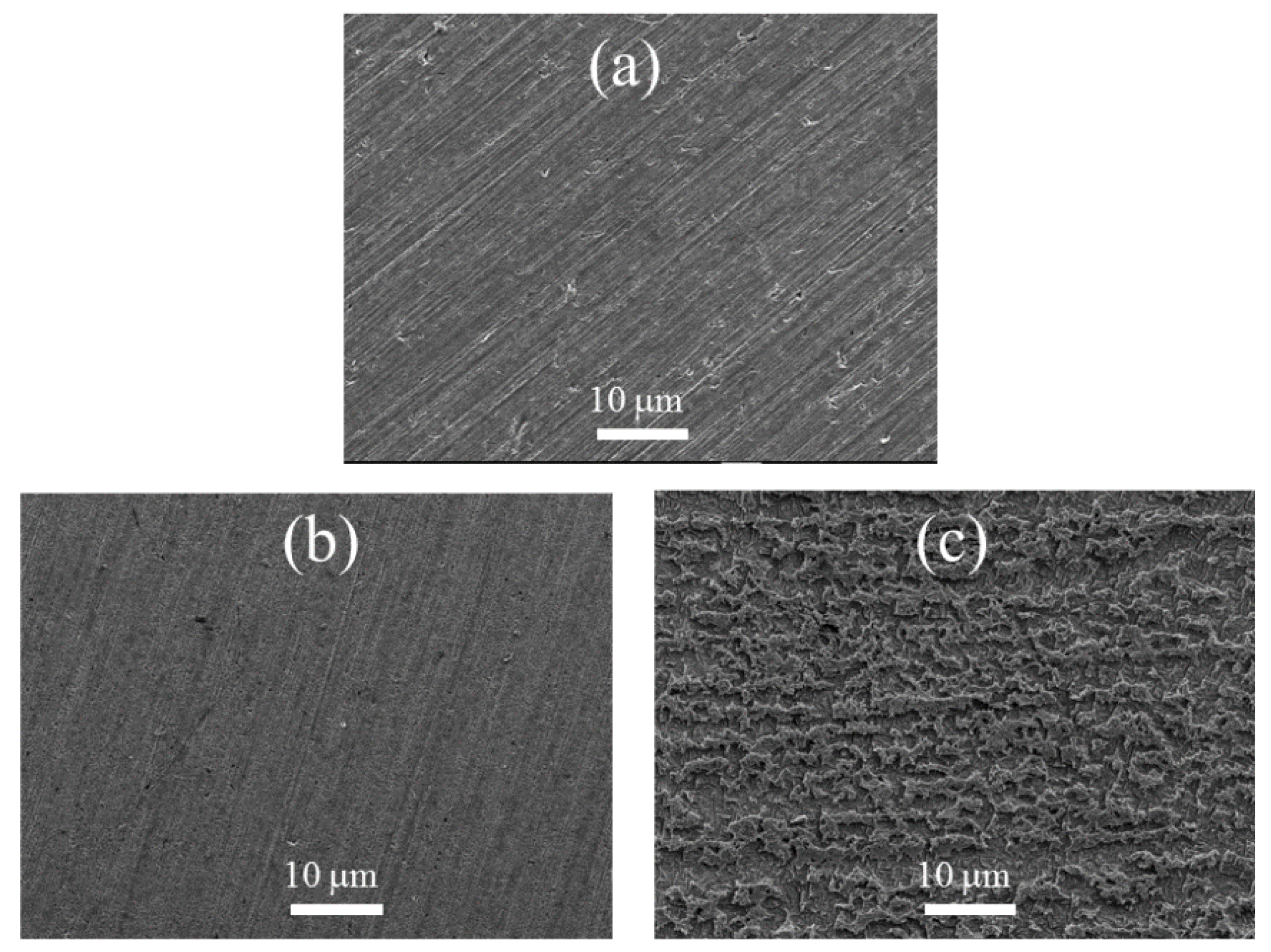
3.4. SEM Surface Investigate
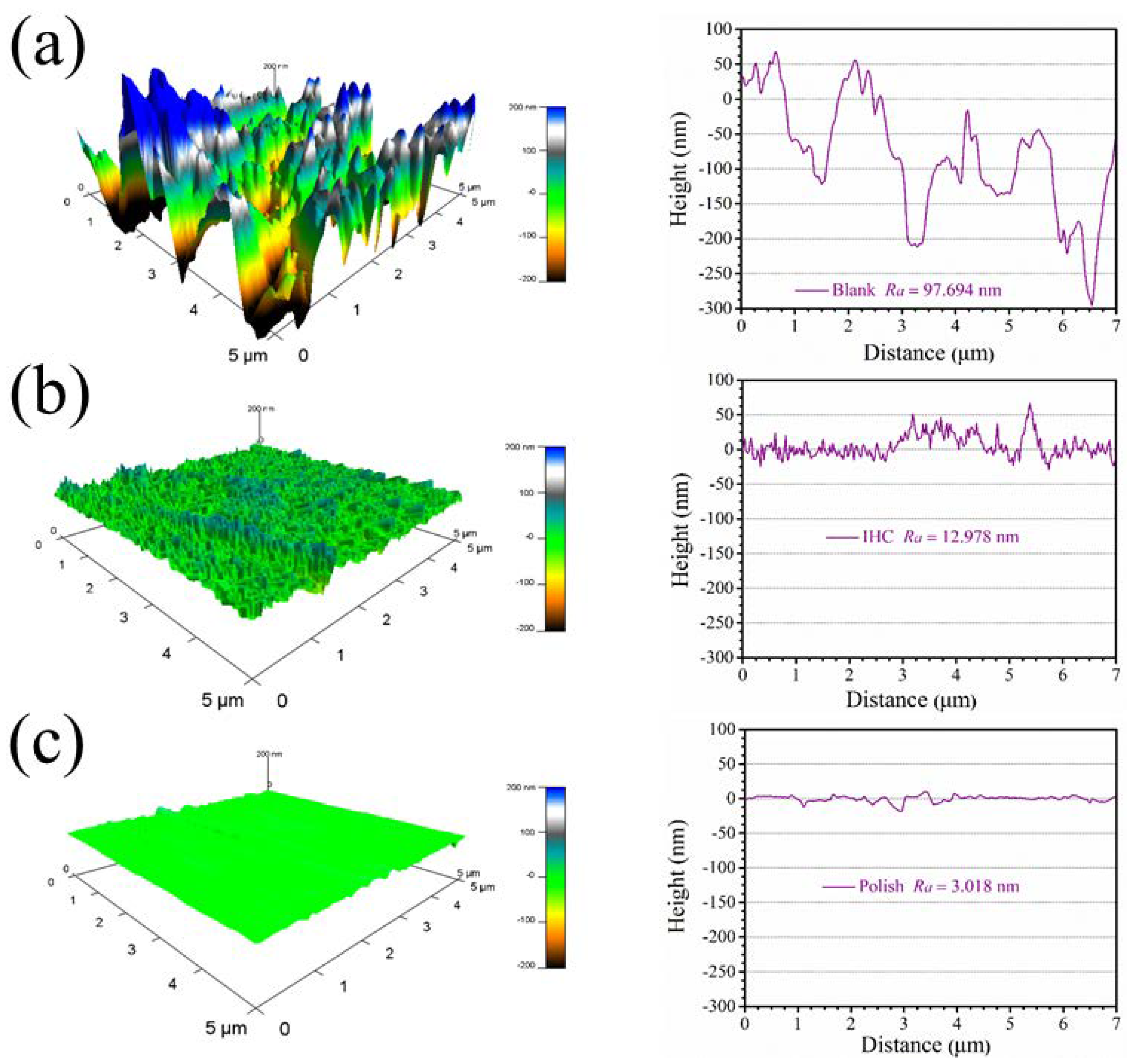
3.5. AFM Surface Analysis
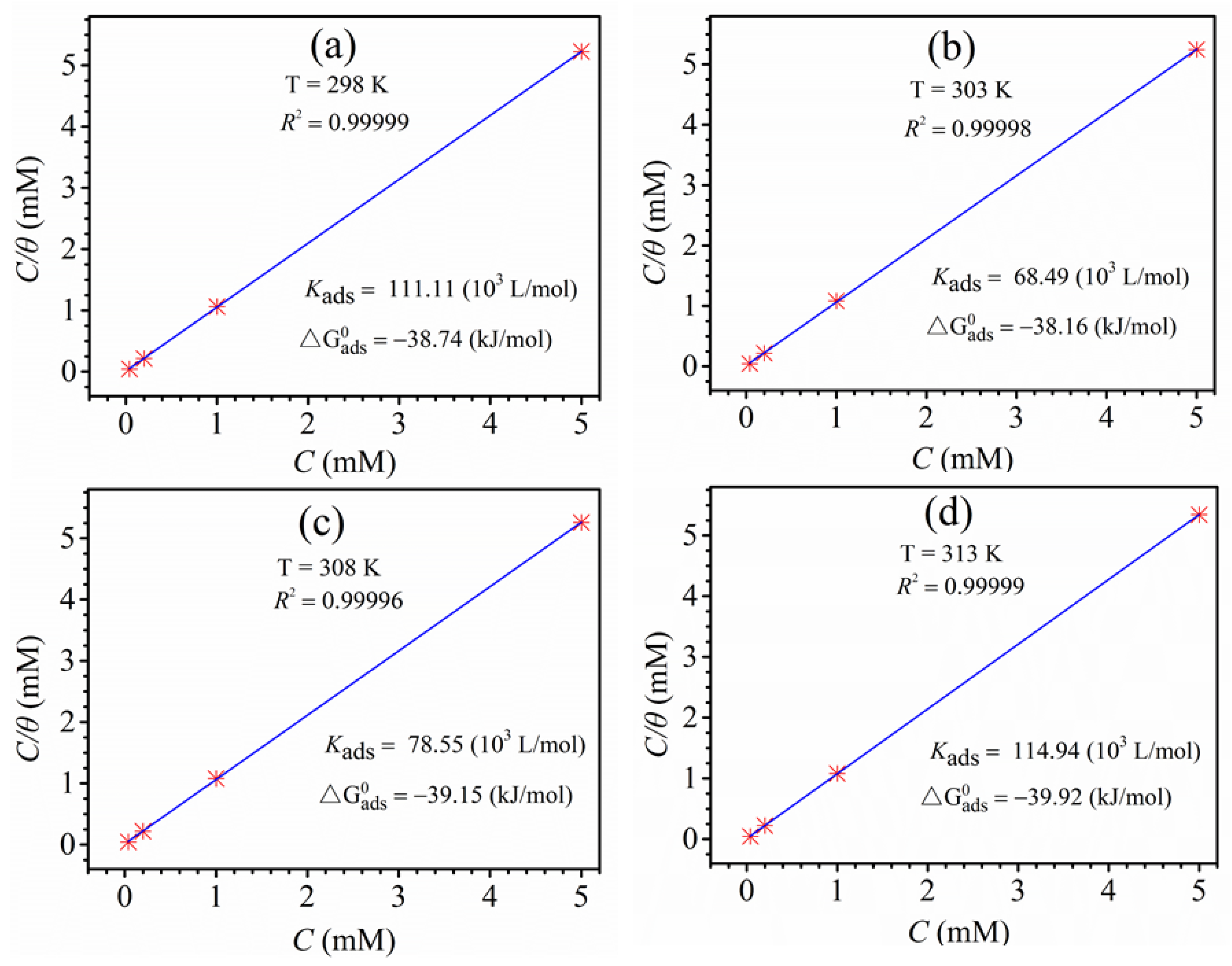
3.6. Adsorption Isotherm Research
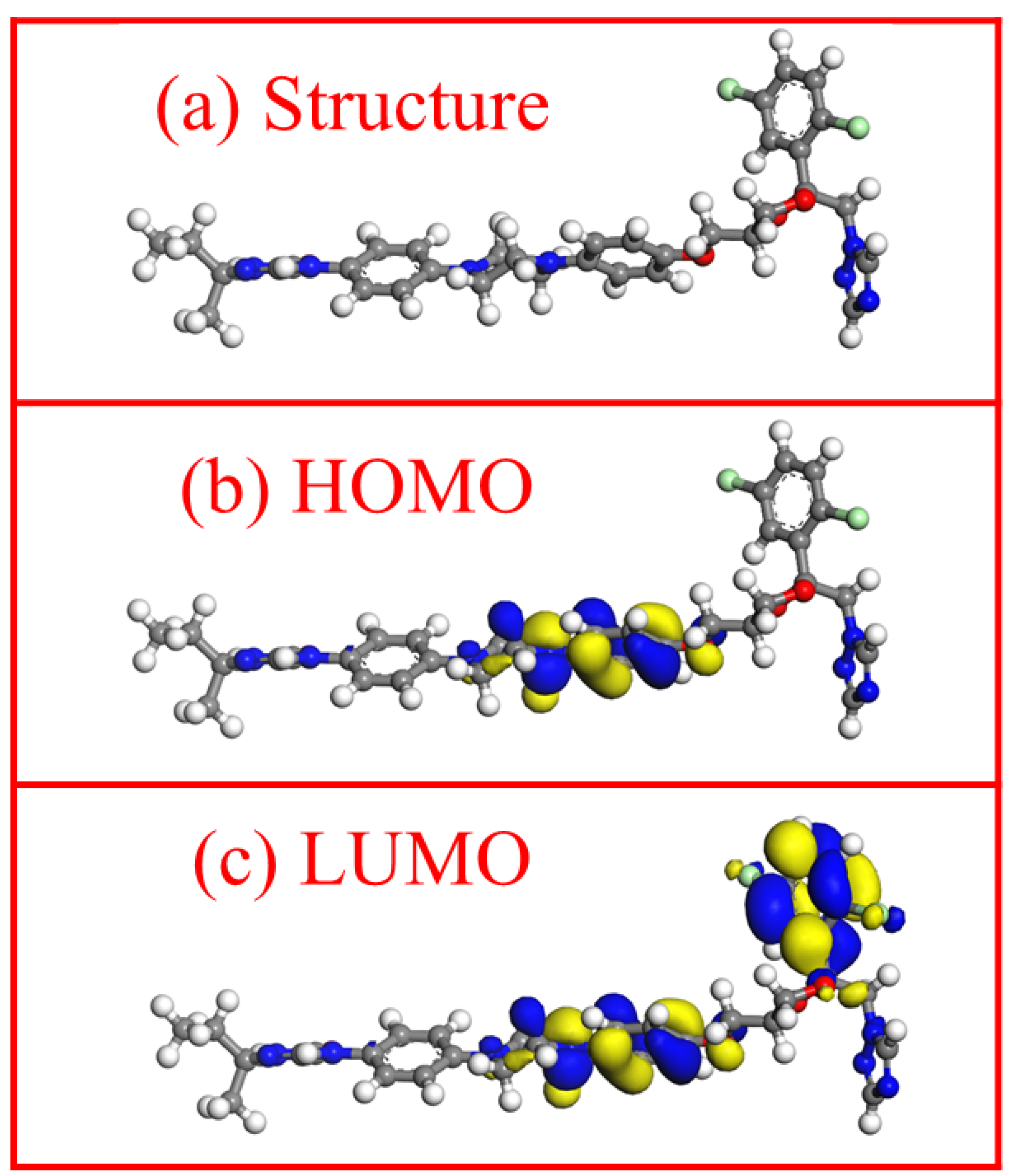
3.7. Quantum Chemical Calculation Analysis
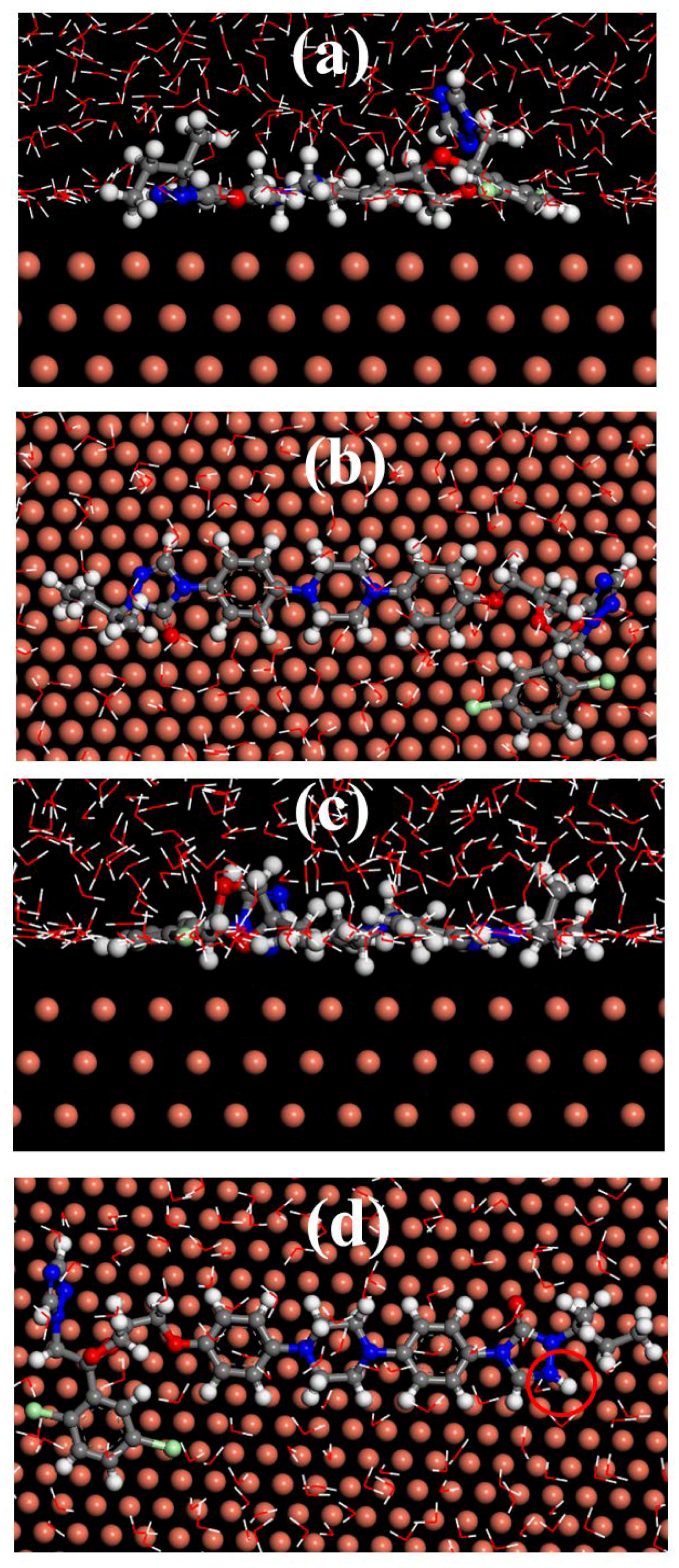
3.8. Molecular Dynamics Simulation
4. Conclusions
- (1)
- Electrochemical experiments show that itraconazole is a mixed corrosion inhibitor for Cu in H2SO4. The corrosion inhibition efficiency can still be maintained at about 90% within a certain temperature range.
- (2)
- The SEM and AFM images demonstrate that the itraconazole molecules form a compact film on the copper surface to effectively inhibit copper corrosion.
- (3)
- Itraconazole molecules adsorb on the Cu surface conforms to the Langmuir adsorption isotherm model and is dominated by chemisorption.
- (4)
- Theoretical calculations show that itraconazole adsorbs on the surface of Cu via a parallel manner.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A. Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: A review. J. Mol. Liq. 2018, 251, 100–118. [Google Scholar] [CrossRef]
- Chira, A.; Bucur, B.; Radu, G.L. Electrodeposited Organic Layers Formed from Aryl Diazonium Salts for Inhibition of Copper Corrosion. Materials 2017, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, S.; Qiang, Y.; Guo, L.; Feng, L.; Liao, C.; Xu, Y.; Chen, S. A combined experimental and theoretical study of the inhibition effect of three disulfide-based flavouring agents for copper corrosion in 0.5 M sulfuric acid. J. Colloid Interface Sci. 2018, 526, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, W.; Liu, A.; Gao, X.; Han, P.; Ding, R.; Yang, C.; Wang, D. Controlled delivery of multi-substituted triazole by metal-organic framework for efficient inhibition of mild steel corrosion in neutral chloride solution. Corros. Sci. 2018, 131, 1–16. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Fitoz, A.; Nazır, H.; Özgür, M.; Emregül, E.; Emregül, K.C. An experimental and theoretical approach towards understanding the inhibitive behavior of a nitrile substituted coumarin compound as an effective acidic media inhibitor. Corros. Sci. 2018, 133, 451–464. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Gong, Y.; Gao, F.; Luo, Z.; Zhang, S.; Li, H. Water soluble corrosion inhibitors for copper in 3.5 wt% sodium chloride solution. Corros. Sci. 2017, 123, 339–350. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Qiang, Y.; Xu, Y.; Guo, L.; Madkour, L.H.; Chen, S. Experimental and Theoretical Investigation of Thiazolyl Blue as a Corrosion Inhibitor for Copper in Neutral Sodium Chloride Solution. Materials 2018, 11, 1042. [Google Scholar] [CrossRef] [PubMed]
- Petrović Mihajlović, M.B.; Radovanović, M.B.; Tasić, Ž.Z.; Antonijević, M.M. Imidazole based compounds as copper corrosion inhibitors in seawater. J. Mol. Liq. 2017, 225, 127–136. [Google Scholar] [CrossRef]
- Shi, S.C.; Su, C.C. Corrosion Inhibition of High Speed Steel by Biopolymer HPMC Derivatives. Materials 2016, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.S.H.; Hien, P.V.; Man, T.V.; Hanh Thu, V.T.; Tri, M.D.; Nam, N.D. A Study on Corrosion Inhibitor for Mild Steel in Ethanol Fuel Blend. Materials 2017, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Zhang, S.; Qiang, Y.; Feng, L.; Liao, C.; Xu, Y.; Chen, S. Investigation of the inhibition effect of Montelukast Sodium on the copper corrosion in 0.5 mol/L H2SO4. J. Mol. Liq. 2017, 248, 902–910. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Q.; Xu, K.; Yin, Y.; Bao, H.; Li, X.; Niu, L.; Chen, S. Enhanced Corrosion Resistance of Carbon Steel in Hydrochloric Acid Solution by Eriobotrya Japonica Thunb. Leaf Extract: Electrochemical Study. Materials 2017, 10, 956. [Google Scholar] [CrossRef] [PubMed]
- Sherif, E.M.; Abdo, H.S.; Abedin, S.Z.E. Corrosion Inhibition of Cast Iron in Arabian Gulf Seawater by Two Different Ionic Liquids. Materials 2015, 8, 3883–3895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannan, P.; Rao, T.S.; Rajendran, N. Improvement in the corrosion resistance of carbon steel in acidic condition using naphthalen-2-ylnaphthalene-2-carboxammide inhibitor. J. Colloid Interface Sci. 2018, 512, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.M.; Umoren, S.A. In-situ preparation, characterization and anticorrosion property of polypropylene glycol/silver nanoparticles composite for mild steel corrosion in acid solution. J. Colloid Interface Sci. 2016, 462, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Obot, I.B.; Zheng, X.; Shen, X.; Qiang, Y.; Kaya, S.; Kaya, C. Theoretical insight into an empirical rule about organic corrosion inhibitors containing nitrogen, oxygen, and sulfur atoms. Appl. Surf. Sci. 2017, 406, 301–306. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Guo, L.; Zheng, X.; Xiang, B.; Chen, S. Experimental and theoretical studies of four allyl imidazolium-based ionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros. Sci. 2017, 119, 68–78. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Tao, G. A novel and high-efficiency inhibitor of 5-(4-methoxyphenyl)-3h-1,2-dithiole-3-thione for copper corrosion inhibition in sulfuric acid at different temperatures. J. Mol. Liq. 2018, 272, 369–379. [Google Scholar] [CrossRef]
- Ramya, K.; Joseph, A. Synergistic effects and hydrogen bonded interaction of alkyl benzimadazoles and thiourea pair on mild steel in hydrochloric acid. J. Taiwan Inst. Chem. Eng. 2015, 52, 127–139. [Google Scholar]
- Liao, L.L.; Mo, S.; Luo, H.Q.; Li, N.B. Corrosion protection for mild steel by extract from the waste of lychee fruit in HCl solution: Experimental and theoretical studies. J. Colloid Interface Sci. 2018, 520, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bedair, M.A.; El-Sabbah, M.M.B.; Fouda, A.S.; Elaryian, H.M. Synthesis, electrochemical and quantum chemical studies of some prepared surfactants based on azodye and Schiff base as corrosion inhibitors for steel in acid medium. Corros. Sci. 2017, 128, 54–72. [Google Scholar] [CrossRef]
- Ehsani, A.; Mahjani, M.G.; Hosseini, M.; Safari, R.; Moshrefi, R.; Mohammad Shiri, H. Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory. J. Colloid Interface Sci. 2017, 490, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Mashuga, M.; Olasunkanmi, L.; Adekunle, A.; Yesudass, S.; Kabanda, M.; Ebenso, E. Adsorption. Thermodynamic and Quantum Chemical Studies of 1-hexyl-3-methylimidazolium Based Ionic Liquids as Corrosion Inhibitors for Mild Steel in HCl. Materials 2015, 8, 3607–3632. [Google Scholar] [CrossRef]
- Mendonça, G.L.F.; Costa, S.N.; Freire, V.N.; Casciano, P.N.S.; Correia, A.N.; Lima-Neto, P.D. Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid medium by amino acids using electrochemical techniques allied to molecular modelling methods. Corros. Sci. 2017, 115, 41–55. [Google Scholar] [CrossRef]
- Khaled, K.F. Adsorption and inhibitive properties of a new synthesized guanidine derivative on corrosion of copper in 0.5 M H2SO4. Appl. Surf. Sci. 2008, 255, 1811–1818. [Google Scholar] [CrossRef]
- Quartarone, G.; Battilana, M.; Bonaldo, L.; Tortato, T. Investigation of the inhibition effect of indole-3-carboxylic acid on the copper corrosion in 0.5 M H2SO4. Corros. Sci. 2008, 50, 3467–3474. [Google Scholar] [CrossRef]
- Quartarone, G.; Bellomi, T.; Zingales, A. Inhibition of copper corrosion by isatin in aerated 0.5 M H2SO4. Corros. Sci. 2003, 45, 715–733. [Google Scholar] [CrossRef]
- Solmaz, R.; Altunbaş Şahin, E.; Döner, A.; Kardaş, G. The investigation of synergistic inhibition effect of rhodanine and iodide ion on the corrosion of copper in sulphuric acid solution. Corros. Sci. 2011, 53, 3231–3240. [Google Scholar] [CrossRef]
- Asegbeloyin, J.; Ejikeme, P.; Olasunkanmi, L.; Adekunle, A.; Ebenso, E. A Novel Schiff Base of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran-2-one and 2,2’-(ethylenedioxy)diethylamine as Potential Corrosion Inhibitor for Mild Steel in Acidic Medium. Materials 2015, 8, 2918–2934. [Google Scholar] [CrossRef]
- Jing, C.; Wang, Z.; Gong, Y.; Huang, H.; Ma, Y.; Xie, H.; Li, H.; Zhang, S.; Gao, F. Photo and thermally stable branched corrosion inhibitors containing two benzotriazole groups for copper in 3.5 wt.% sodium chloride solution. Corros. Sci. 2018, 138, 353–371. [Google Scholar] [CrossRef]
- Eyu, G.D.; Will, G.; Dekkers, W.; MacLeod, J. The Synergistic Effect of Iodide and Sodium Nitrite on the Corrosion Inhibition of Mild Steel in Bicarbonate-Chloride Solution. Materials 2016, 9, 868. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, J.; Krakowiak, S.; Ryl, J. Evaluation of citric acid corrosion inhibition efficiency and passivation kinetics for aluminium alloys in alkaline media by means of dynamic impedance monitoring. Electrochim. Acta 2017, 258, 1463–1475. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Haque, J.; Dohare, P.; Lgaz, H.; Salghi, R.; Quraishi, M.A. Effect of electron donating functional groups on corrosion inhibition of mild steel in hydrochloric acid: Experimental and quantum chemical study. J. Taiwan Inst. Chem. Eng. 2018, 82, 233–251. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Xu, S.; Li, W. Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution. J. Colloid Interface Sci. 2016, 472, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Usman, B.J.; Umoren, S.A.; Gasem, Z.M. Inhibition of API 5L X60 steel corrosion in CO2 -saturated 3.5% NaCl solution by tannic acid and synergistic effect of KI additive. J. Mol. Liq. 2017, 237, 146–156. [Google Scholar] [CrossRef]
- Nwankwo, H.U.; Ateba, C.N.; Olasunkanmi, L.O.; Adekunle, A.S.; Isabirye, D.A.; Onwudiwe, D.C.; Ebenso, E.E. Synthesis, Characterization, Antimicrobial Studies and Corrosion Inhibition Potential of 1,8-dimethyl-1,3,6,8,10,13-hexaazacyclotetradecane: Experimental and Quantum Chemical Studies. Materials 2016, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M.; Motamedi, M. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: Experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 2018, 255, 185–198. [Google Scholar] [CrossRef]
- Javadian, S.; Darbasizadeh, B.; Yousefi, A.; Ektefa, F.; Dalir, N.; Kakemam, J. Dye-surfactant aggregates as corrosion inhibitor for mild steel in NaCl medium: Experimental and theoretical studies. J. Taiwan Inst. Chem. Eng. 2017, 71, 344–354. [Google Scholar] [CrossRef]
- Lgaz, H.; Salghi, R.; Jodeh, S.; Hammouti, B. Effect of clozapine on inhibition of mild steel corrosion in 1.0 M HCl medium. J. Mol. Liq. 2017, 225, 271–280. [Google Scholar] [CrossRef]
- Gerengi, H.; Mielniczek, M.; Gece, G.; Solomon, M.M. Experimental and Quantum Chemical Evaluation of 8-Hydroxyquinoline as a Corrosion Inhibitor for Copper in 0.1 M HCl. Ind. Eng. Chem. Res. 2016, 55, 9614–9624. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, S.; Gong, M.; Li, W. Experimental and Theoretical Study on the Corrosion Inhibition of Mild Steel by 1-Octyl-3-methylimidazolium l-Prolinate in Sulfuric Acid Solution. Ind. Eng. Chem. Res. 2014, 53, 16349–16358. [Google Scholar] [CrossRef]
- Yousefi, A.; Javadian, S.; Neshati, J. A New Approach to Study the Synergistic Inhibition Effect of Cationic and Anionic Surfactants on the Corrosion of Mild Steel in HCl Solution. Ind. Eng. Chem. Res. 2014, 53, 5475–5489. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, W.; Xu, B.; Chen, Y.; Yin, X.; Liu, Y.; Zuo, H. Inhibitory effect of konjac glucomanan on pitting corrosion of AA5052 aluminium alloy in NaCl solution. J. Colloid Interface Sci. 2018, 517, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Mourya, P.; Mondal, D.; Pal, S.; Udayabhanu, G. Grafting effect of gum acacia on mild steel corrosion in acidic medium: Gravimetric and electrochemical study. J. Mol. Liq. 2018, 251, 470–479. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Yan, S.; Zou, X.; Chen, S. Three indazole derivatives as corrosion inhibitors of copper in a neutral chloride solution. Corros. Sci. 2017, 126, 295–304. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Sanaei, Z.; Bahlakeh, G.; Ramezanzadeh, B. Highly effective inhibition of mild steel corrosion in 3.5% NaCl solution by green Nettle leaves extract and synergistic effect of eco-friendly cerium nitrate additive: Experimental, MD simulation and QM investigations. J. Mol. Liq. 2018, 256, 67–83. [Google Scholar] [CrossRef]
- Fu, J.; Zang, H.; Wang, Y.; Li, S.; Chen, T.; Liu, X. Experimental and Theoretical Study on the Inhibition Performances of Quinoxaline and Its Derivatives for the Corrosion of Mild Steel in Hydrochloric Acid. Ind. Eng. Chem. Res. 2012, 51, 6377–6386. [Google Scholar] [CrossRef]
- Mishra, A.; Verma, C.; Lgaz, H.; Srivastava, V.; Quraishi, M.A.; Ebenso, E.E. Synthesis, characterization and corrosion inhibition studies of N-phenyl-benzamides on the acidic corrosion of mild steel: Experimental and computational studies. J. Mol. Liq. 2018, 251, 317–332. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, Y.; Jing, C.; Huang, H.; Li, H.; Zhang, S.; Gao, F. Synthesis of dibenzotriazole derivatives bearing alkylene linkers as corrosion inhibitors for copper in sodium chloride solution: A new thought for the design of organic inhibitors. Corros. Sci. 2016, 113, 64–77. [Google Scholar] [CrossRef]
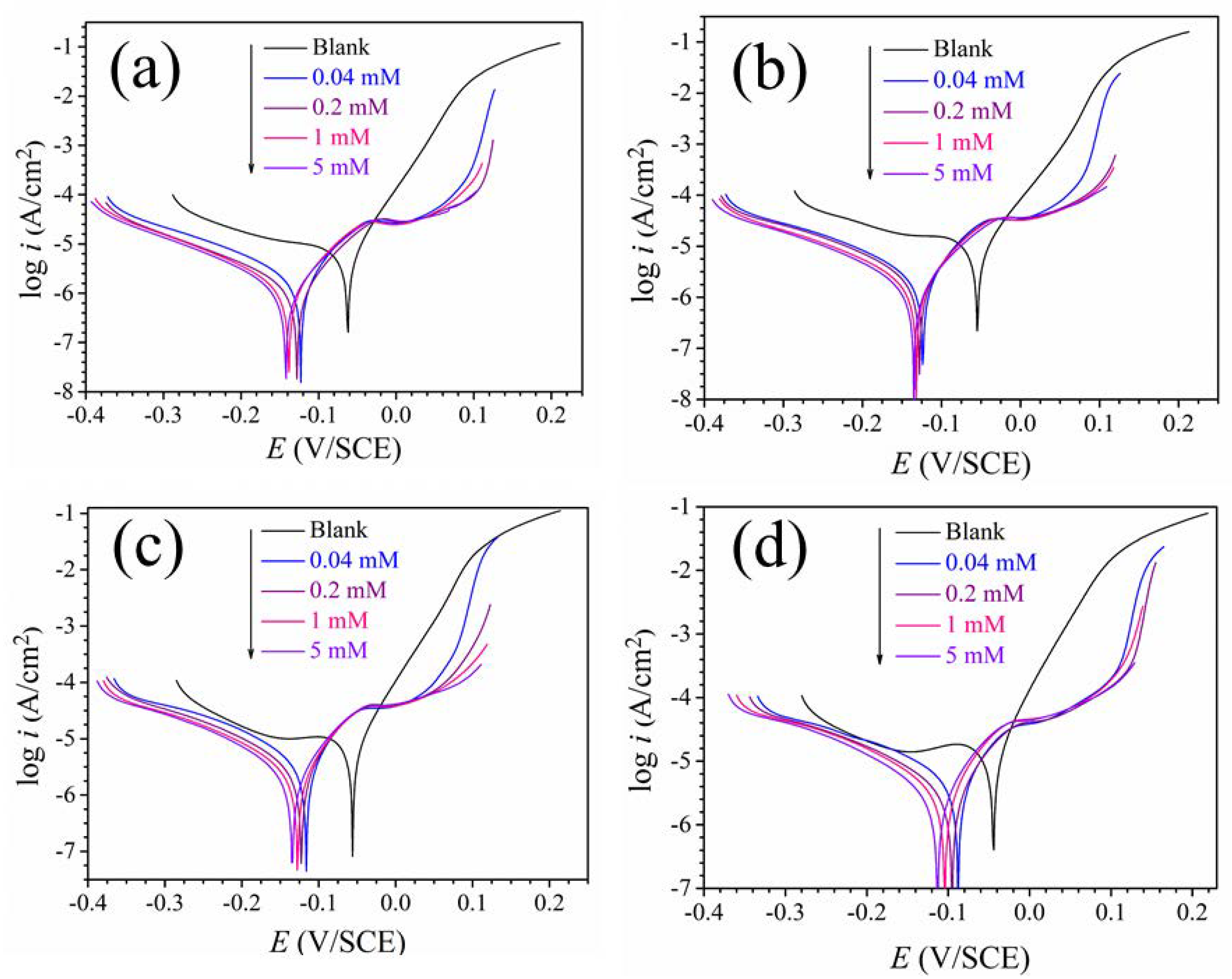
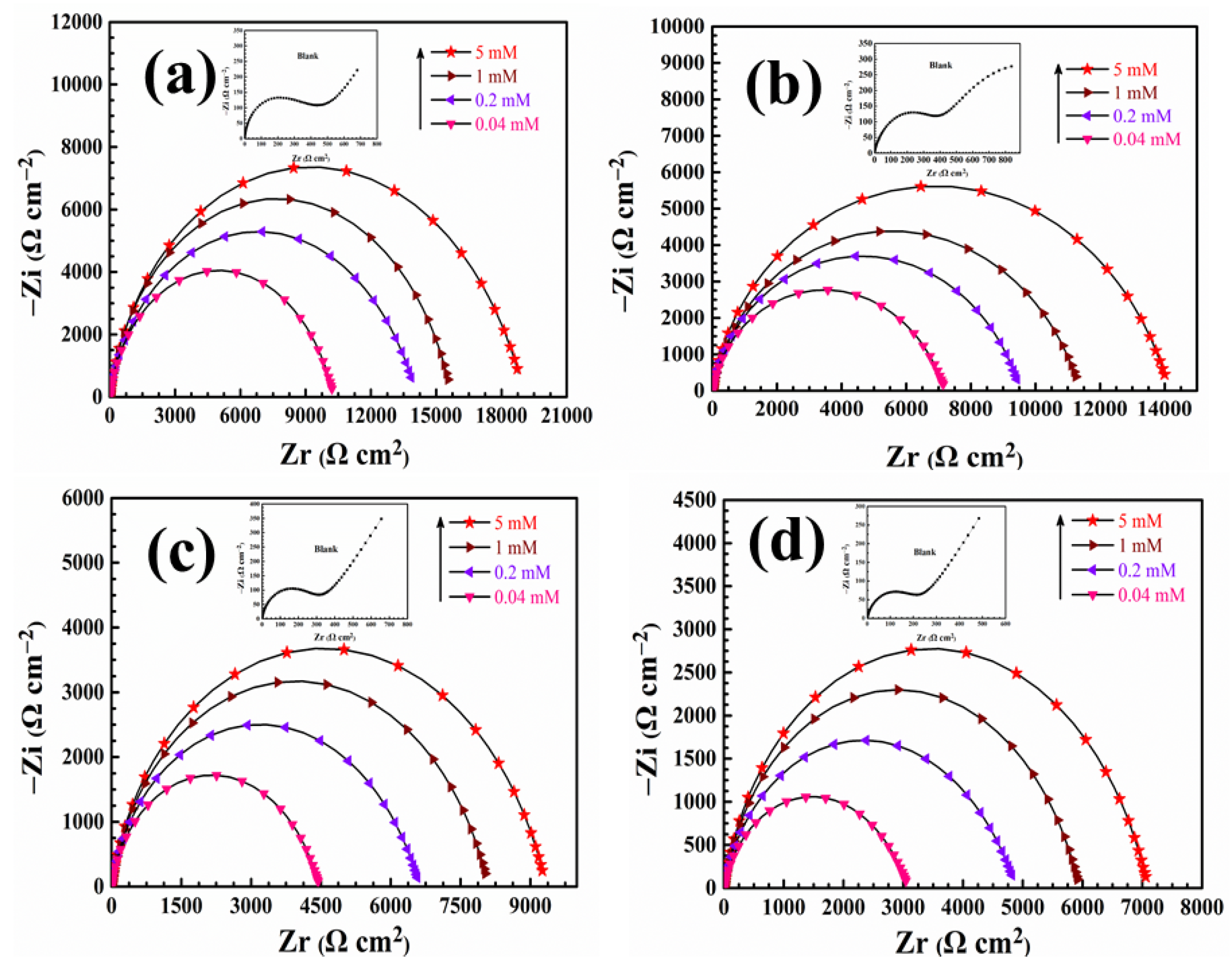

| T (K) | C (mM) | Ecorr (mV/SCE) | Icorr (μA·cm−2) | βc (mV·dec−1) | βa (mV·dec−1) | η (%) |
|---|---|---|---|---|---|---|
| Blank | 62 | 18.20 | 121.4 | 41.4 | - | |
| 0.04 | 123 | 1.91 | 136.8 | 61.4 | 89.5 | |
| 298 | 0.2 | 128 | 1.36 | 159.1 | 62.6 | 92.5 |
| 1 | 138 | 1.09 | 127.6 | 50.2 | 94.0 | |
| 5 | 142 | 0.78 | 112.5 | 62.4 | 95.7 | |
| Blank | 55 | 21.71 | 209.5 | 49.1 | - | |
| 0.04 | 124 | 2.15 | 154.4 | 57.8 | 90.1 | |
| 303 | 0.2 | 128 | 1.76 | 156.2 | 55.7 | 91.9 |
| 1 | 132 | 1.26 | 135.3 | 66.4 | 92.2 | |
| 5 | 135 | 1.01 | 106.8 | 63.7 | 95.3 | |
| Blank | 56 | 25.31 | 109.6 | 47.0 | - | |
| 0.04 | 116 | 2.68 | 163.3 | 51.9 | 89.4 | |
| 308 | 0.2 | 123 | 2.12 | 157.2 | 56.9 | 91.6 |
| 1 | 128 | 1.86 | 157.5 | 63.6 | 92.6 | |
| 5 | 135 | 1.23 | 150.8 | 66.2 | 95.1 | |
| Blank | 44 | 28.64 | 113.8 | 46.9 | - | |
| 0.04 | 88 | 3.65 | 200.8 | 70.9 | 87.3 | |
| 313 | 0.2 | 95 | 2.98 | 164.1 | 72.9 | 89.6 |
| 1 | 104 | 2.15 | 156.9 | 61.9 | 92.5 | |
| 5 | 113 | 1.82 | 151.1 | 54.1 | 93.6 |
| T (K) | C (mM) | Rs (Ω·cm2) | Rf (Ω·cm2) | Rct (kΩ·cm2) | Cf (μF·cm−2) | n1 | Cdl (μF·cm−2) | n2 | W (×10−2 Ω·cm2·s1/2) | η (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Blank | 1.31 | 0.9 | 0.55 | 60.4 | 0.49 | 68.9 | 1 | 1.19 | - | |
| 0.04 | 0.89 | 150.1 | 10.14 | 49.8 | 0.97 | 32.7 | 0.73 | - | 94.6 | |
| 298 | 0.2 | 0.76 | 170.6 | 13.90 | 40.2 | 1 | 31.5 | 0.72 | - | 96.0 |
| 1 | 1.21 | 232.3 | 15.40 | 35.7 | 0.96 | 29.7 | 0.76 | - | 96.4 | |
| 5 | 1.35 | 329.4 | 18.94 | 28.9 | 0.98 | 27.8 | 0.74 | - | 97.1 | |
| Blank | 1.24 | 8.3 | 0.37 | 65.2 | 0.41 | 71.5 | 0.59 | 4.10 | - | |
| 0.04 | 1.76 | 71.94 | 7.01 | 51.3 | 0.95 | 34.5 | 0.59 | - | 94.7 | |
| 303 | 0.2 | 2.12 | 112.5 | 9.42 | 46.5 | 1 | 31.9 | 0.74 | - | 96.1 |
| 1 | 3.15 | 140.2 | 11.13 | 38.9 | 0.97 | 28.8 | 0.75 | - | 96.7 | |
| 5 | 2.49 | 231.2 | 13.62 | 27.8 | 0.99 | 26.5 | 0.76 | - | 97.3 | |
| Blank | 1.39 | 13.2 | 0.31 | 68.9 | 0.54 | 74.2 | 0.65 | 8.02 | - | |
| 0.04 | 1.61 | 96.13 | 4.37 | 59.7 | 0.97 | 36.5 | 0.71 | - | 92.9 | |
| 308 | 0.2 | 1.87 | 153.4 | 6.47 | 51.2 | 0.94 | 33.1 | 0.70 | - | 95.2 |
| 1 | 1.65 | 161.1 | 7.95 | 43.7 | 1 | 27.9 | 0.75 | - | 96.1 | |
| 5 | 1.76 | 180.8 | 9.16 | 31.3 | 0.98 | 24.3 | 0.76 | - | 96.6 | |
| Blank | 1.27 | 25.2 | 0.23 | 72.2 | 0.49 | 77.6 | 0.55 | 10.37 | - | |
| 0.04 | 2.07 | 31.72 | 3.05 | 60.3 | 1 | 34.5 | 0.68 | - | 92.4 | |
| 313 | 0.2 | 1.89 | 80.28 | 4.82 | 56.8 | 0.98 | 29.6 | 0.67 | - | 95.2 |
| 1 | 2.45 | 120.1 | 5.83 | 48.7 | 0.96 | 26.5 | 0.75 | - | 95.7 | |
| 5 | 1.88 | 174.6 | 6.92 | 34.2 | 1 | 22.8 | 0.76 | - | 96.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Peng, S.; Huang, X.; Gao, L. Investigation the Corrosion Inhibition Effect of Itraconazole on Copper in H2SO4 at Different Temperatures: Combining Experimental and Theoretical Studies. Materials 2018, 11, 2107. https://doi.org/10.3390/ma11112107
Gong Z, Peng S, Huang X, Gao L. Investigation the Corrosion Inhibition Effect of Itraconazole on Copper in H2SO4 at Different Temperatures: Combining Experimental and Theoretical Studies. Materials. 2018; 11(11):2107. https://doi.org/10.3390/ma11112107
Chicago/Turabian StyleGong, Zhili, Shini Peng, Xiaomei Huang, and Lanzhou Gao. 2018. "Investigation the Corrosion Inhibition Effect of Itraconazole on Copper in H2SO4 at Different Temperatures: Combining Experimental and Theoretical Studies" Materials 11, no. 11: 2107. https://doi.org/10.3390/ma11112107
APA StyleGong, Z., Peng, S., Huang, X., & Gao, L. (2018). Investigation the Corrosion Inhibition Effect of Itraconazole on Copper in H2SO4 at Different Temperatures: Combining Experimental and Theoretical Studies. Materials, 11(11), 2107. https://doi.org/10.3390/ma11112107





