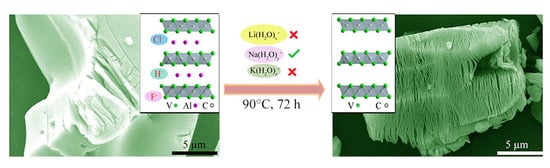
The Synthesis Process and Thermal Stability of V2C MXene
Abstract
:1. Introduction
2. Experimental
2.1. Syntheses of V2C MXene
2.2. Characterization
3. Results and Discussion
3.1. Synthesis Process Analysis
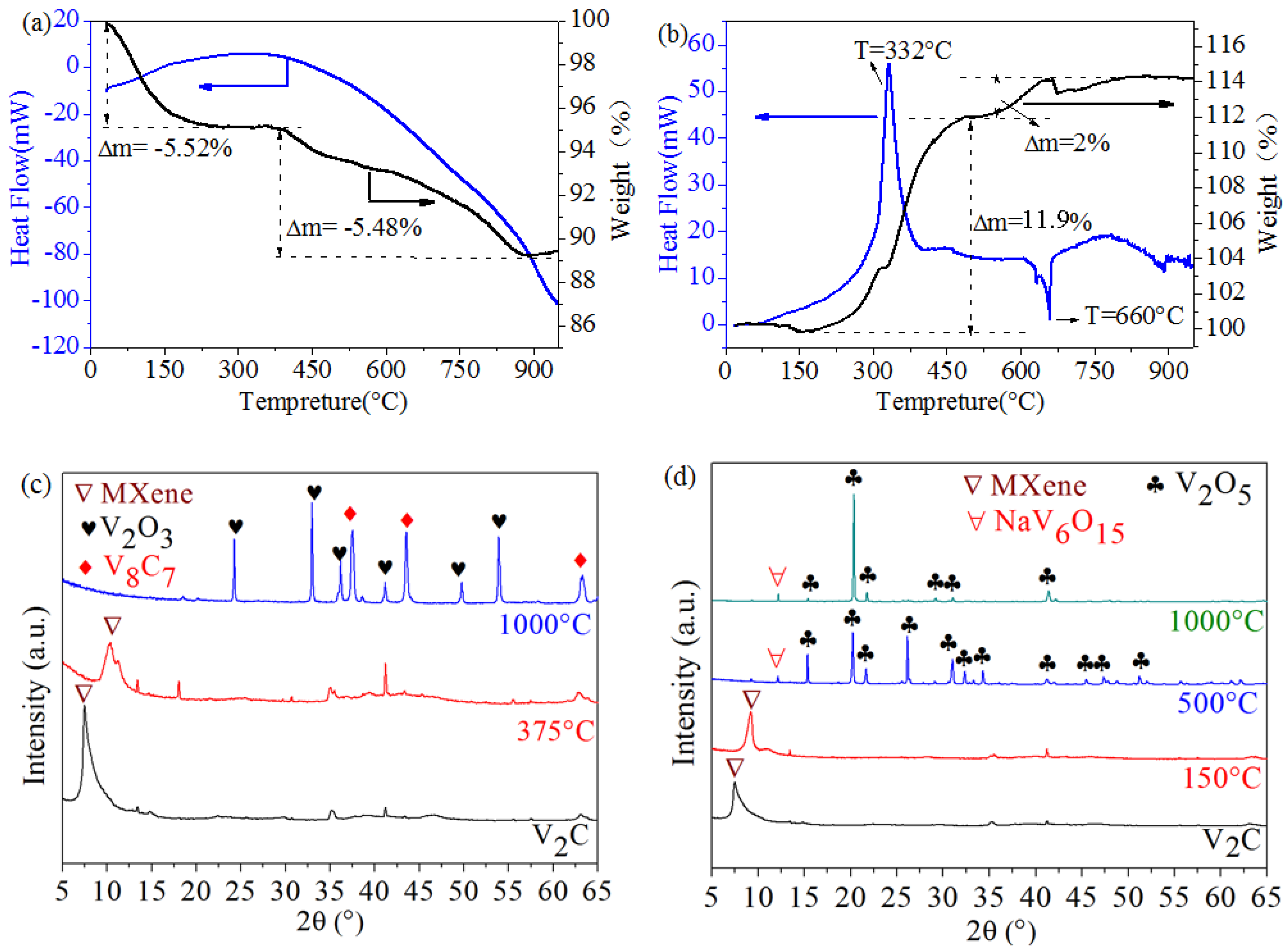
3.2. Thermal Stability Analysis
(1) Thermal Stability in Ar
(2) Thermal Stability in Air
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Min, H.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4207. [Google Scholar] [CrossRef]
- Ying, G.; Dillon, A.D.; Fafarman, A.T.; Barsoum, M.W. Transparent, conductive solution processed spincast 2D Ti3AlC2. (MXene) films. Mate. Res. Lett. 2017, 5, 391–398. [Google Scholar] [CrossRef]
- Zhi, W.S.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, M.; Yang, X.; Wang, Z.; Luo, G.; Huang, Z.; Sui, X.; Li, C. Preparation and tribological properties of Ti3C2(OH)2 nanosheets as additives in base oil. Rsc. Adv. 2014, 5, 2762–2767. [Google Scholar] [CrossRef]
- Ling, C.; Li, S.; Ouyang, Y.; Chen, Q.; Wang, J. Transition metal-promoted V2CO2(MXenes): A new and highly active catalyst for hydrogen evolution reaction. Adv. Sci. 2016, 3, 1600180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Dong, S.; Ye, Z.; Wei, Y. Synthesis and tribological property of Ti3C2Tx nanosheets. J. Mater. Sci. 2017, 52, 2200–2209. [Google Scholar] [CrossRef]
- Wu, Y.; Nie, P.; Jiang, J.; Ding, B.; Dou, H.; Zhang, X. MoS2-Nanosheet- Decorated 2D titanium carbide (MXene) as high-performance anodes for sodium-ion batteries. Chem. Electro. Chem. 2017, 4, 1560–1565. [Google Scholar] [CrossRef]
- Li, X.; Qian, Y.; Liu, T.; Cao, F.; Zang, Z.; Sun, X.; Sun, S.; Niu, Q.; Wu, J. Enhanced lithium and electron diffusion of LiFePO4 cathode with two- dimensional Ti3C2 MXene nanosheets. J. Mater. Sci. 2018, 53, 1–13. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Zhu, J.; Yang, H.; Chen, X.; Wang, L.; Yang, C. Facile synthesis SnO2 nanoparticle-modified Ti3C2 MXene nanocomposites for enhanced lithium storage application. J. Mater. Sci. 2016, 52, 1–10. [Google Scholar] [CrossRef]
- Sun, D.; Wang, M.; Li, Z.; Fan, G.; Fan, L.Z.; Zhou, A. Two-dimensional Ti3C2 as anode material for Li-ion batteries. Electrochem. Commun. 2014, 47, 80–83. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.T.; Ma, T.Y.; Du, A.; Qiao, S.Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhou, J.; Zhu, L.; Sun, Z. MXene: A promising photocatalyst for water splitting. J. Mater. Chem. A 2016, 4, 11446–11452. [Google Scholar] [CrossRef]
- Sun, D.; Hu, Q.; Chen, J.; Zhang, X.; Wang, L.; Wu, Q.; Zhou, A. Structural Transformation of MXene (V2C, Cr2C, and Ta2C) with O Groups during Lithiation: A First-Principles Investigation. ACS Appl. Mater. Interfaces 2016, 8, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Dall’Agnese, Y.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Two-dimensional vanadium carbide (MXene) as positive electrode for sodium-ion capacitors. J. Phys. Chem. Lett. 2015, 6, 2305–2309. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, A.; Liu, F.; Cao, J.; Wang, L.; Hu, Q. Carbon dioxide adsorption of two-dimensioanl carbide MXenes. J. Adv. Ceram. 2018, 7, 237–245. [Google Scholar] [CrossRef]
- Sun, D.D.; Hu, Q.K.; Chen, J.F.; Zhou, A.G. First principles calculations of the relative stability, structure and electronic properties of two dimensional metal carbides and nitrides. Key Eng. Mater. 2014, 602–603, 527–531. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.; Song, H.; Tang, H.; Li, C. Synthesis, characterization, and tribological properties of two-dimensional Ti3C2. Cryst. Res. Technol. 2014, 49, 926–932. [Google Scholar] [CrossRef]
- Chen, J.; Chen, K.; Tong, D.; Huang, Y.; Zhang, J.; Xue, J.; Huang, Q.; Chen, T. CO2 and temperature dual responsive “Smart” MXene phases. Chem. Commun. 2015, 51, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, S.; Guo, Z.; Sun, Z. Ti-enhanced exfoliation of V2AlC into V2C MXene for lithium-ion battery anodes. Ceram. Int. 2017, 43, 11450–11454. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, J.; Wang, S.; Wang, B.; Shen, C.; Wang, L.; Hu, Q.; Huang, Q.; Zhou, A. Preparation of high-purity V2C MXene and electrochemical properties as li-ion batteries. J. Electrochem. Soc. 2017, 164, A709–A713. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Sun, D.; Zhang, Y.; Liu, B.; Hu, Q.; Zhou, A. Synthesis and thermal stability of two-dimensional carbide MXene Ti3C2. Mater. Sci. Eng. B. 2015, 191, 33–40. [Google Scholar] [CrossRef]
- Zhou, J.; Zha, X.; Chen, F.Y.; Ye, Q.; Eklund, P.; Du, S.; Huang, Q. A Two-Dimensional Zirconium Carbide by Selective Etching of Al3C3 from Nanolaminated Zr3Al3C5. Angew. Chem. 2016, 55, 5008–5013. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, A.; Hu, Q.; Wang, L. Synthesis and oxidation resistance of V2AlC powders by molten salt method. Int. J. Appl. Ceram. Tec. 2017, 14, 873–879. [Google Scholar] [CrossRef]
- Duan, X.; Wu, C.; Xiang, S.; Zhou, W.; Yildirim, T.; Cui, Y.; Yang, Y.; Chen, B.; Qian, G. Novel microporous metal-organic framework exhibiting high acetylene and methane storage capacities. Inorg. Chem. 2015, 54, 4377–4381. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano. 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Spanier, J.E.; Gupta, S.; Amer, M.; Barsoum, M.W. Vibrational behavior of the Mn+1AXn phases from first-order raman scattering (M = Ti, V, Cr, A = Si, X = C, N). Phys. Rev. B 2005, 71, 012103. [Google Scholar] [CrossRef]
- Champagne, A.; Lu, S.; Ouisse, T.; Hackens, B.; Charlier, J.C. Electronic and vibrational properties of V2C-based MXenes: From experiments to first-principles modeling. Phys. Rev. B 2017, 97, 115439. [Google Scholar] [CrossRef]
- Wu, Y.P.; Ong, C.K.; Li, Z.W.; Chen, L.; Lin, G.Q.; Wang, S.J. Microstructural and high-frequency magnetic characteristics of W-type barium ferrites doped with V2O5. J. Appl. Phys. 2005, 97, 1294. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Shen, C.; Qin, G.; Hu, Q.; Zhou, A. Synthesis of NaV6O15 nanorods via thermal oxidation of sodium-intercalated 2d V2CTx and their electrochemical properties as anode for lithium-ion batteries. Electrochim. Acta 2017, 248, 178–187. [Google Scholar] [CrossRef]



| Abbreviation | Full Name |
|---|---|
| V2C MXene | two-dimensional vanadium carbide |
| V2AlC | vanadium aluminum carbide |
| V2O3 | vanadium trioxide |
| V2O5 | vanadium pentoxide |
| V8C7 | vanadium carbide |
| NaV6O15 | sodium vanadium oxide |
| LiF | lithium fluoride |
| NaF | sodium fluoride |
| KF | potassium fluoride |
| HCl | hydrochloric acid |
| HF | hydrofluoric acid |
| NaCl | sodium chloride |
| Etching Solution | 2θ (°) of (002) Peak | IMXene/IMAX |
|---|---|---|
| LiF + HCl | 9.13 | 0.66 |
| NaF + HCl | 8.03 | 18.11 |
| KF + HCl | - | - |
| NaF + HF | 7.8 | 0.18 |
| 40% HF [19] | 7.33 | 1.00 |
| 50% HF [4] | 8.96 | 0.22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Wang, B.; Hu, Q.; Wang, L.; Zhou, A. The Synthesis Process and Thermal Stability of V2C MXene. Materials 2018, 11, 2112. https://doi.org/10.3390/ma11112112
Wu M, Wang B, Hu Q, Wang L, Zhou A. The Synthesis Process and Thermal Stability of V2C MXene. Materials. 2018; 11(11):2112. https://doi.org/10.3390/ma11112112
Chicago/Turabian StyleWu, Meng, Bingxin Wang, Qianku Hu, Libo Wang, and Aiguo Zhou. 2018. "The Synthesis Process and Thermal Stability of V2C MXene" Materials 11, no. 11: 2112. https://doi.org/10.3390/ma11112112
APA StyleWu, M., Wang, B., Hu, Q., Wang, L., & Zhou, A. (2018). The Synthesis Process and Thermal Stability of V2C MXene. Materials, 11(11), 2112. https://doi.org/10.3390/ma11112112