Vehiculation of Active Principles as a Way to Create Smart and Biofunctional Textiles
Abstract
:1. Introduction
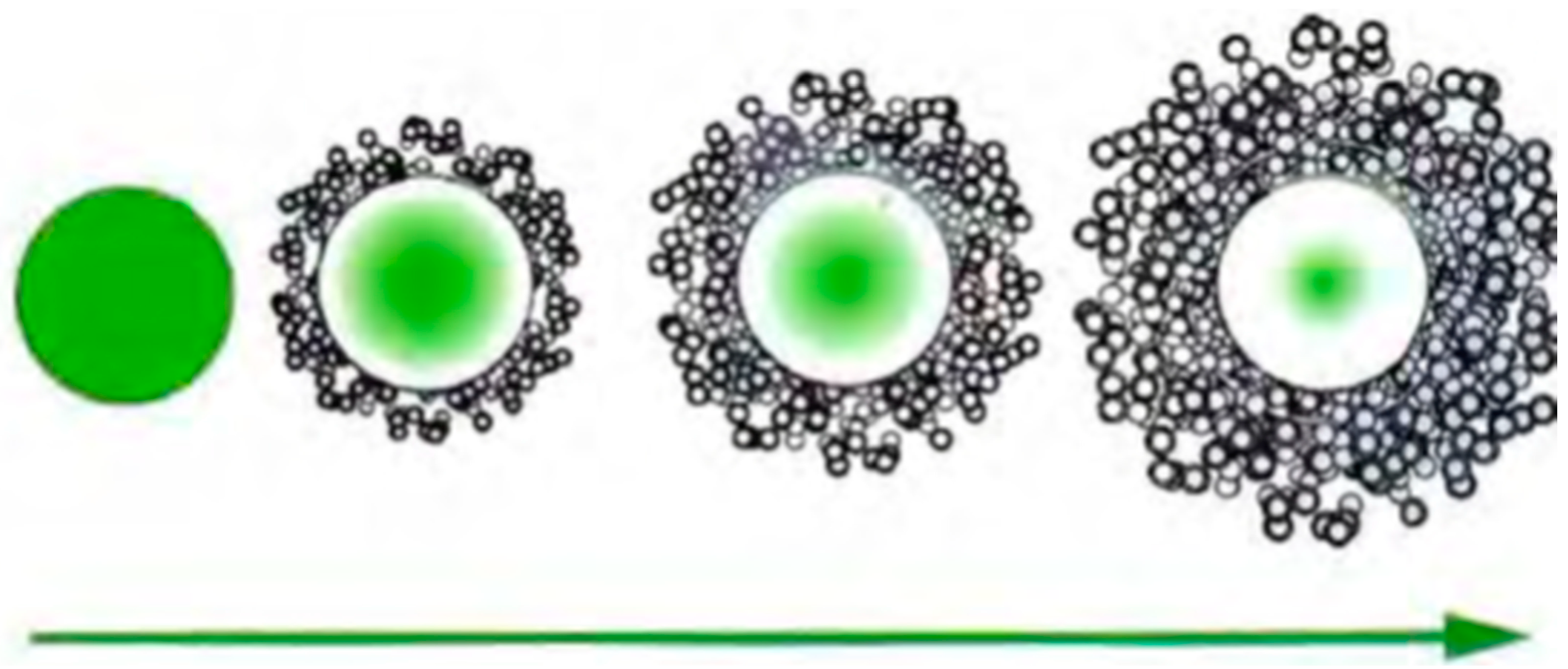
1.1. Microcapsules
1.2. Cyclodextrines (CDs)
1.3. Liposomes
2. Materials and Methods
2.1. Microcapsules
2.1.1. Materials
2.1.2. Methodology
2.2. Cyclodextrin Complexes
2.2.1. Materials
2.2.2. Methodology
2.3. Liposomes
2.3.1. Materials
2.3.2. Methodology
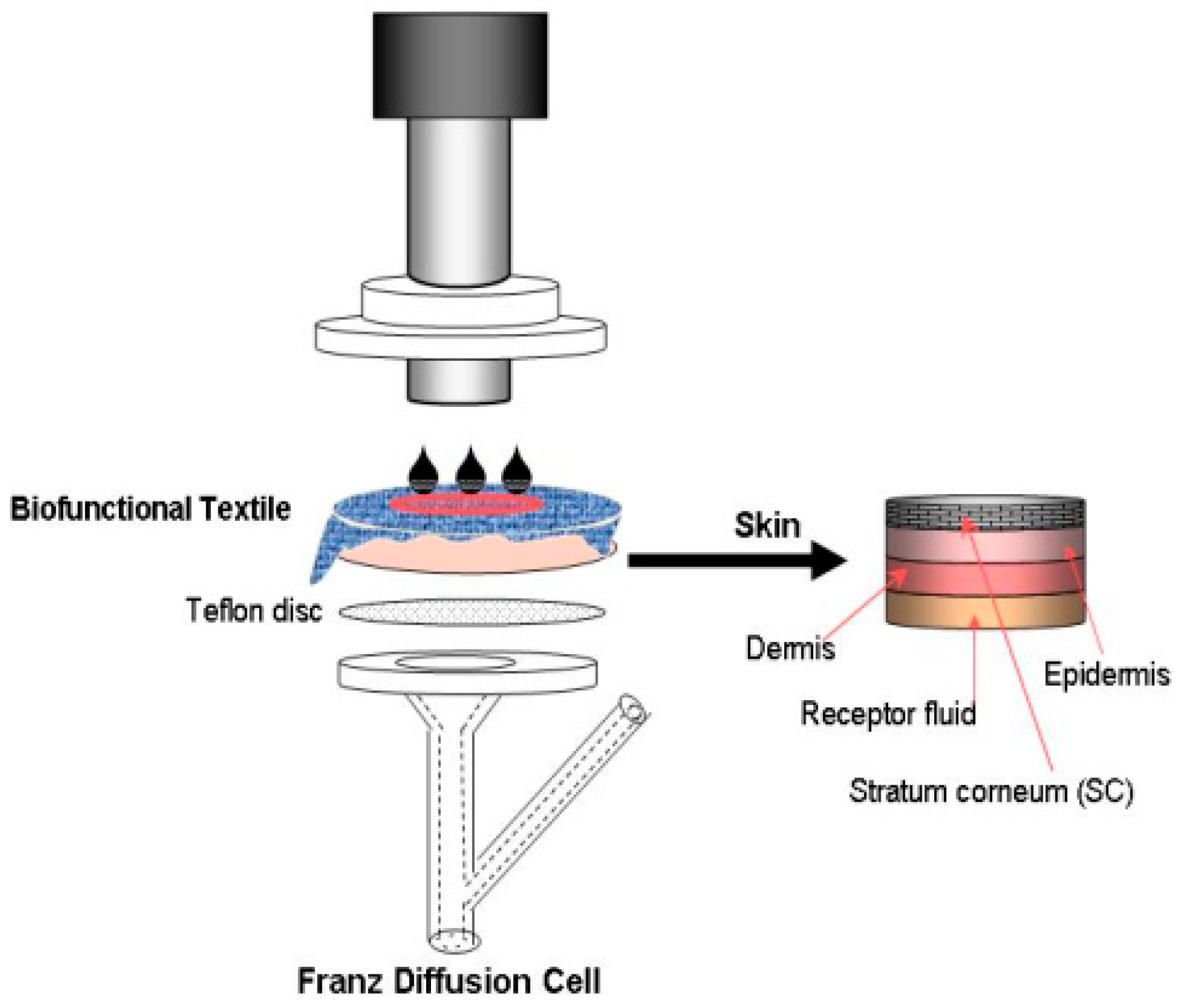
2.3.3. Assessment of the Absorption of Skin
In Vivo Methodologies
3. Results and Discussion
3.1. Microcapsules
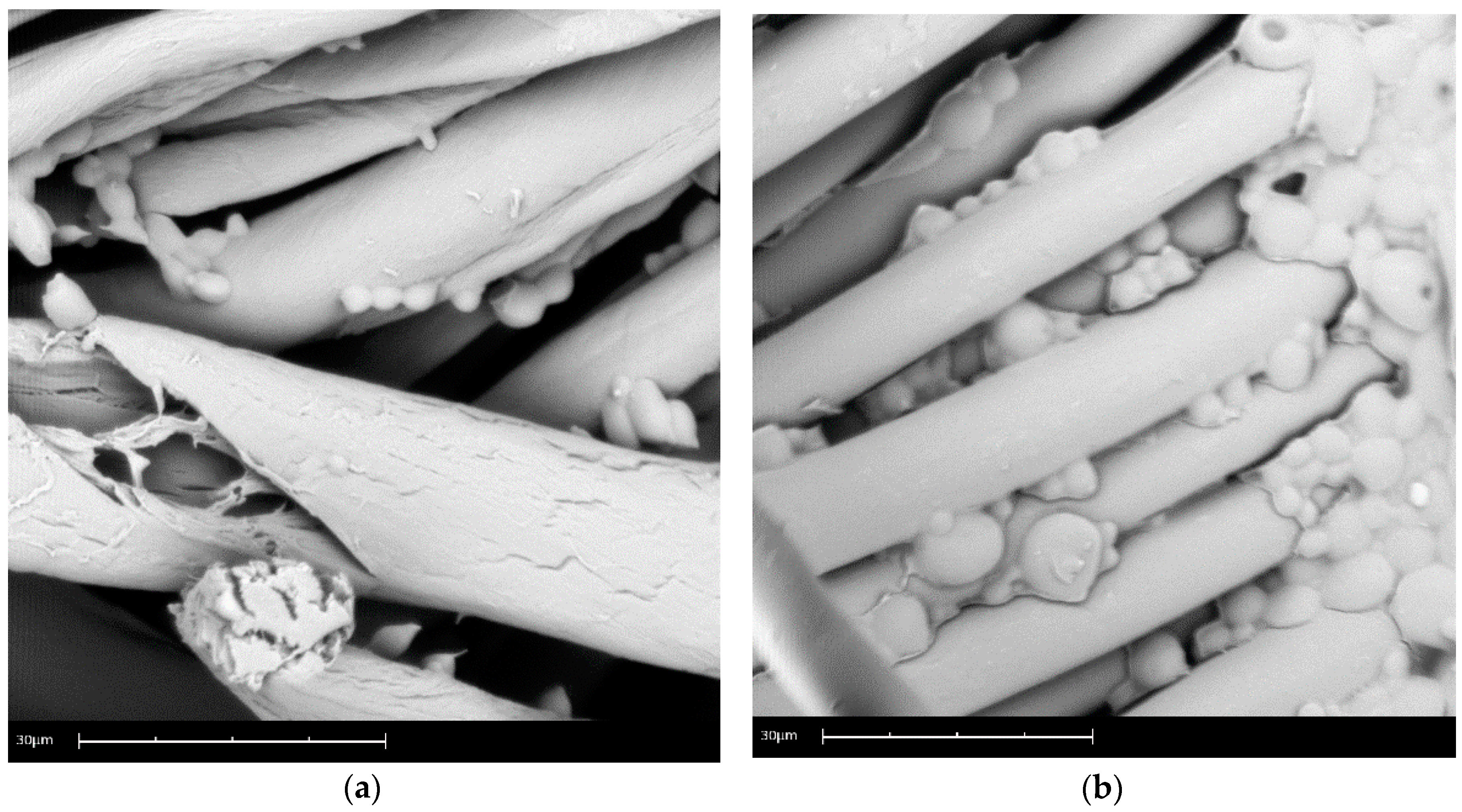
3.1.1. Microscopy: Optical and SEM
3.1.2. TGA and DTGA
3.1.3. Application to PES and COT Fabrics Using Pad-Dry
3.1.4. Drug Delivery Kinetics
3.2. Cyclodextrin Complexes
3.2.1. Microscopy: Optical and SEM
3.2.2. Drug Delivery Kinetics
3.3. Liposomes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lewis, R.W. Foreword. In Smart Fibres, Fabrics and Clothing; Tao, X., Ed.; Woodhead Publishing Limited: Cambridge, UK; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Wachter, R.; Weuthen, M.; Panzer, C.; Paff, E. Liposomes Are Used as Textile Finishes Which Not Only Improve Elasticity and Hand but Can Also Be Transferred to Skin Contact. Patent No. EP1510619-A2; DE10339358-A1; US2005058700-A1, 2 March 2005. [Google Scholar]
- Guarducci, M. Product Having Particular Functional Properties for the Skin and Process for the Preparation Thereof. Patent No. WO/2006/106546, 13 March 2006. [Google Scholar]
- Lei, M.; Jiang, F.; Cai, F.; Hu, S.; Zhou, R.; Li, G.; Wang, Y.; Wang, H.; He, J.; Xiong, X. Facile microencapsulation of olive oil in porous starch granules: Fabrication, characterization, and oxidative stability. Int. J. Biol. Macromol. 2018, 111, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, A.; Pandit, N.; Badgujar, M.; Bhaskar, C.; Rao, M. Encapsulation of endoglucanase using a biopolymer Gum Arabic for its controlled release. Bioresour. Technol. 2007, 98, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Reshetnikov, I.S.; Zubkova, N.S.; Antonov, V.A.; Potapova, E.V.; Svistunov, V.S.; Tuganova, M.A.; Khalturinskij, N.K. Microencapsulated fire retardants for polyolefins. Mater. Chem. Phys. 1998, 52, 78–82. [Google Scholar] [CrossRef]
- Fadinia, A.L.; Alvim, I.D.; Ribeiro, I.P.; Ruzene, L.G.; Silva, L.B.; Queiroz, M.B.; Miguel, A.M.R.O.; Chaves, F.C.M.; Rodrigues, R.A.F. Innovative strategy based on combined microencapsulation technologies for food application and the influence of wall material composition. LWT 2018, 91, 345–352. [Google Scholar] [CrossRef]
- Lamoudi, L.; Chaumeil, J.C.; Daoud, K. Effet des paramètres du procédé demicroencapsulation du piroxicam par coacervation complexe. Ann. Pharm. Fr. 2014, 1, 1–6. [Google Scholar]
- Skeie, S. Developments in microencapsulation science applicable to cheese research and development. A review. Int. Dairy J. 1994, 4, 573–595. [Google Scholar] [CrossRef]
- Souza, F.N.; Gebara, C.; Ribeiro, M.C.E.; Chaves, K.S.; Gigante, M.L.; Grosso, C.R.F. Production and characterization of microparticles containing pectin and whey proteins. Food Res. Int. 2012, 49, 560–566. [Google Scholar] [CrossRef]
- Ren, P.W.; Ju, X.J.; Xie, R.; Chu, L.Y. Monodisperse alginate microcapsules with oil core generated from microfluidic device. J. Colloid Interface Sci. 2010, 343, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Ré, M.I. Microencapsulação—Em busca de produtos ‘Inteligente’. Ciência Hoje 2000, 27, 24–29. [Google Scholar]
- Yoshizawa, H. Trends in microencapsulation research. KONA Powder Part. 2004, 22, 23–31. [Google Scholar] [CrossRef]
- Donbrow, M. Microcapsules and Nanoparticles in Medicine and Pharmacy; CRC Press: Boca Raton, FL, USA, 1992; 360p. [Google Scholar]
- Song, X.; Zhao, Y.; Hou, S.; Xu, F.; Zhao, R.; He, J.; Cai, Z.; Li, Y.; Chen, Q. Dual agents loaded PLGA nanoparticles: Systematic study of particle size and drug entrapment efficiency. Eur. J. Pharm. Biopharm. 2008, 69, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.; Merkle, H.P.; Gander, B. Microencapsulation by solvent extraction/evaporation: Reviewing the state of the art of microsphere preparation process technology. J. Control. Release 2005, 102, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Selmi, G.A.; Bozza, F.T.; Thomazini, M.; Bolini, H.M.A.; Fávaro-Trindade, C.S. Microencapsulation of aspartame by double emulsion followed by complex coacervation to provide protection and prolong sweetness. Food Chem. 2013, 139, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Nagao, H. Microencapsulation of oil droplets using freezing-induced gelatin-acacia complex coacervation. Colloids Surf. A Physicochem. Eng. Asp. 2012, 411, 129–139. [Google Scholar] [CrossRef]
- Jun-Xia, X.; Hai-Yan, Y.; Jian, Y. Microencapsulation of sweet orange oil by complex coacervation with soybean protein isolate/gum Arabic. Food Chem. 2011, 125, 1267–1272. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Moschakis, T.; Biliaderis, C.G. Complex coacervation as a novel microencapsulation technique to improve viability of probiotics under different stress. Food Bioprocess Technol. 2014, 7, 2767–2781. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Farid, M.; Al-Muhtaseb, S.; Kurdi, J. Emulsion stability and cross-linking of PMMA microcapsules containing phase change materials. Sol. Energy Mater. Sol. Cells 2015, 132, 311–318. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Hua, T.; Au, W.M.; Wong, K.S. Characteristics of weaving parameters in microcapsule fabrics and their influence on loading capability. Text. Res. J. 2013, 83, 113–121. [Google Scholar] [CrossRef]
- Semyonov, D.; Ramon, O.; Kaplun, Z.; Levin-Brener, L.; Gurevich, N.; Shimoni, E. Microencapsulation of Lactobacillus paracasei by spray freeze drying. Food Res. Int. 2010, 43, 193–202. [Google Scholar] [CrossRef]
- Shalaka, D.S.R.; Amruta, N.A.; Parimal, K. Vitamin and loaded pectin alginate microspheres for cosmetic application. J. Pharm. Res. 2009, 6, 1098–1102. [Google Scholar]
- Suave, J.; Dall’agnol, E.C.; Pezzini, A.P.T.; Silva, D.A.K.; Meier, M.M.; Soldi, V. Microencapsulação: Inovação em diferentes áreas. Rev. Saúde Ambiente 2006, 7, 12–20. [Google Scholar]
- Santos, A.B.; Ferreira, V.P.; Grosso, C.R.F. Microesferas—Uma alternativa viável. Biotecnol. Ciênc. Desenvolv. 2000, 16, 26–30. [Google Scholar]
- Wang, J.M.; Zheng, W.; Song, Q.W.; Zhu, H.; Zhou, Y. Preparation and characterization of natural fragrant microcapsules. J. Fiber Bioeng. Inform. 2009, 4, 293–300. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, Z.; Li, J.; Li, S.; Kong, L.; Li, P.; Wang, Q. Development and evaluation of novel flavor microcapsules containing vanilla oil using complex coacervation approach. Food Chem. 2014, 145, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Sharipova, A.A.; Aidarova, S.B.; Grigoriev, D.; Mutalieva, B.; Madibekova, G.; Tleuova, A.; Miller, R. Polymer-surfactant complexes for microencapsulation of vitamin E and its release. Colloids Surf. B Biointerfaces 2016, 137, 152–157. [Google Scholar] [CrossRef] [PubMed]
- França, D.; Medina, A.F.; Messa, L.L.; Souza, C.F.; Faez, R. Chitosan spray-dried microcapsule and microsphere as fertilizer host for swellable—Controlled release materials. Carbohydr. Polym. 2018, 196, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, D. Microencapsulation: Fundamentals, methods and applications. In Surface Chemistry in Biomedical and Environmental Science; Blitz, J., Gunoko, V., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 23–34. [Google Scholar]
- Jamekhorshida, A.; Sadramelia, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Tsuda, N.; Ohtsubo, T.; Fuji, M. Preparation of self-bursting microcapsules by interfacial polymerization. Adv. Powder Technol. 2012, 23, 724–730. [Google Scholar] [CrossRef]
- Alcázar, A.; Borreguero, A.M.; Lucas, A.; Rodríguez, J.F.; Carmona, M. Microencapsulation of TOMAC by suspension polymerisation: Process optimisation. Chem. Eng. Res. Des. 2017, 117, 1–10. [Google Scholar] [CrossRef]
- Zuo, M.; Liu, T.; Han, J.; Tang, Y.; Yao, F.; Yuan, Y.; Qian, Z. Preparation and characterization of microcapsules containing ammonium persulfate as core by in situ polymerization. Chem. Eng. J. 2014, 249, 27–33. [Google Scholar] [CrossRef]
- Bezerra, F.M.; Carmona, O.G.; Carmona, C.G.; Lis, M.J.; Moraes, F.F. Controlled release of microencapsulated citronella essential oil on cotton and polyester matrices. Cellulose 2016, 23, 1459–1470. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, C.; Wu, T.; Wang, L.; Chen, S.; Ding, T.; Hu, Y. Preparation and characterization of citrus essential oils loaded in chitosan microcapsules by using different emulsifiers. J. Food Eng. 2018, 217, 108–114. [Google Scholar] [CrossRef]
- Bae, J. Fabrication of carbon microcapsules containing silicon nanoparticles–carbon nanotubes nanocomposite by sol-gel method for anode in lithium ion battery. J. Solid State Chem. 2011, 184, 1749–1755. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders—Physical Properties, Processing, and Functionality; Springer: New York, NY, USA, 2005; 372p. [Google Scholar]
- Ulrich, K.; Eppinger, S. Product Design and Development; McGraw Hill: New York, NY, USA, 2003; 432p. [Google Scholar]
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62. [Google Scholar] [CrossRef]
- Tekin, R.; Bac, N.; Erdogmus, H. Microencapsulation of fragrance and natural volatile oils application in cosmetics, and household cleaning products. Macromol. Symp. 2013, 333, 35–40. [Google Scholar] [CrossRef]
- Zimet, P.; Livney, Y.D. Beta-lactoglobulin and its nanocomplexes with pectin as vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocoll. 2009, 23, 1120–1126. [Google Scholar] [CrossRef]
- Annan, N.T.; Borza, A.D.; Hansen, L.T. Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703T during exposure to simulated gastro-intestinal conditions. Food Res. Int. 2008, 41, 184–193. [Google Scholar] [CrossRef]
- Prata, A.S.; Grosso, C.R.F. Production of microparticles with gelatina and chitosan. Carbohydr. Polym. 2015, 116, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, M.B.; Azizi, N.; Baffoun, A.; Chevalier, Y.; Majdoub, M. New microcapsules based on isosorbide for cosmetotextile: Preparation and characterization. Ind. Crops Prod. 2018, 123, 591–599. [Google Scholar] [CrossRef]
- Rubio, L.; Alonso, C.; Coderch, L.; Parra, J.L.; Martí, M.; Cebrián, J.; Navarro, J.A.; Lis, M.; Valldeperas, J. Skin delivery of caffeine contained in biofunctional textiles. Text. Res. J. 2010, 80, 1214–1221. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Yuen, M.C.W.; Kan, C.W.; Cheuk, K.K.L.; Chui, C.H.; Lam, K.H. Cosmetic textiles with biological benefits: Gelatin microcapsules containing Vitamin C. Int. J. Mol. Med. 2009, 24, 411–419. [Google Scholar] [PubMed]
- Meyer, A. Perfume microencapsulation by complex coacervation. CHIMIA 1992, 46, 101–102. [Google Scholar]
- Chinta, S.K.; Pooja, P.W. Use of microencapsulation in textiles. Indian J. Eng. 2013, 3, 37–40. [Google Scholar]
- Sanchez, P.; Sanchez-Fernandez, M.V.; Romero, A. Development of thermoregulating textiles using paraffin wax microcapsules. Thermochim. Acta 2010, 498, 16–21. [Google Scholar] [CrossRef]
- Ma, Z.H.; Yu, D.G.; Branford-White, C.J. Microencapsulation of tamoxifen: Application to cotton fabric. Colloids Surf. B 2009, 69, 85–90. [Google Scholar] [CrossRef] [PubMed]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Aït Addi, E.H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yang, F.; Li, X.; Zhang, X.; Abbas, S. Formation of heat-resistant nanocapsules of jasmine essential oil via gelatin/gum Arabic based complex coacervation. Food Hydrocoll. 2014, 35, 305–314. [Google Scholar] [CrossRef]
- Wang, B.; Adhikari, B.; Barrow, C.J. Optimisation of the microencapsulation of tuna oil in gelatin-sodium hexametaphosphate using complex coacervation. Food Chem. 2014, 158, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Patrick, K.E.; Abbas, S.; Lv, Y.; Ntsama, I.S.B.; Zhang, X. Microencapsulation by complex coacervation of fish oil using gelatin/SDS/NaCMC. Pak. J. Food Sci. 2013, 23, 17–25. [Google Scholar]
- Piacentini, E.; Giorno, L.; Dragosavac, M.M.; Vladisavljevic, G.T.; Holdich, R.G. Microencapsulation of oil droplets using cold water fish gelatine/gum arabic complex coacervation by membrane emulsification. Food Res. Int. 2013, 53, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Thilagavathi, G.; Kannaian, T. Combined antimicrobial and aroma finishing treatment for cotton, using micro encapsulated geranium (Pelargonium graveolens L’Herit. Ex Ait.) leaves extract. Indian J. Nat. Prod. Resour. 2010, 3, 348–352. [Google Scholar]
- Wang, C.X.; Chen, S.L. Aromachology and its application in the textile field. Fibres Text. East. Eur. 2005, 13, 41–44. [Google Scholar]
- Solomon, B.; Sahle, F.F.; Gebre-Mariam, T.; Asres, K.; Neubert, R.H.H.H. Microencapsulation of citronela oil for mosquito-repellent application: Formulation and in vitro permeation studies. Eur. J. Pharm. Biopharm. 2012, 80, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Specos, M.M.M.; Garcia, J.J.; Tornesello, J.; Marino, P.; Della Vecchia, M.; Defain Tesoriero, M.V.; Hermida, L.G. Microencapsulated citronella oil for mosquito repelente finishing of cotton textiles. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, J.K.M.C.; Costa, A.M.B.; Sousa, D.P.; Cavalcanti, S.C.H.; Nunes, R.S. Microencapsulação do óleo essencial de Citrus sinensis (L.) Osbeck pelo método da coacervação simples. Sci. Plena 2009, 5, 1–8. [Google Scholar]
- Tawatsin, A.; Wratten, S.D.; Scott, R.R.; Thavara, U.; Techadamrongsin, Y. Reppelency of volatile oils from plants against three mosquito vectors. J. Vector Ecol. 2001, 26, 76–82. [Google Scholar] [PubMed]
- Chatterjee, S.; Salaün, F.; Campagne, C. Development of multilayer microcapsules by a phase coacervation method based on ionic interations for textile applications. Pharmaceutics 2014, 6, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Duchêne, D. Cyclodextrins and Their Industrial Uses; Edition Santé: Paris, France, 1987; 300p. [Google Scholar]
- Bender, H. Production, characterization and application of CDs. Adv. Biotechnol. Processs. 1986, 6, 31–71. [Google Scholar]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Matioli, G.; Moraes, F.F.; Zanin, G.M. Ciclodextrinas e suas Aplicações em: Alimentos, Fármacos, Cosméticos, Agricultura, Biotecnologia, Química Analítica e Produtos Gerais; Eduem: Maringá, Brazil, 2000; p. 124. [Google Scholar]
- Bhaskara-Amrit, U.R.; Pramod, B.A.; Warmoeskerken, M.C.G. Applications of β-cyclodextrins in textiles. AUTEX Res. J. 2011, 11, 94–101. [Google Scholar]
- Shlar, I.; Droby, S.; Rodov, V. Antimicrobial Coatings on Polyethylene Terephthalate Based on Curcumin/Cyclodextrin Complex Embedded in a Multilayer Polyelectrolyte Architecture. Colloids Surf. B Biointerfaces 2018, 164, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Lucio, D.; Irache, J.M.; Font, M.; Martíinez-Oharriz, M.C. Supramolecular structure of glibenclamide and cyclodextrins complexes. Int. J. Pharm. 2017, 530, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of phytochemicals with cyclodextrin derivatives—An insight. Biomed. Pharm. 2017, 88, 1122–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Man, S.; Qiu, H.; Liu, Z.; Zhang, M.; Ma, L.; Gao, W. Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ. Toxicol. Pharmacol. 2016, 48, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Sali, N.; Csepregi, R.; Kőszegi, T.; Kunsági-Máté, S.; Szente, L.; Poór, M. Complex formation of flavonoids fisetin and geraldol with β-cyclodextrins. J. Lumin. 2018, 194, 82–90. [Google Scholar] [CrossRef]
- Lo Nostro, P.; Fratoni, L.; Ridi, F.; Baglioni, P. Surface treatments on Tencel fabric: Grafting with β-cyclodextrin. J. Appl. Polym. Sci. 2003, 88, 706–715. [Google Scholar] [CrossRef]
- Partanen, R.; Ahro, M.; Hakala, M.; Kallio, H.; Forssell, P. Microencapsulation of caraway extract in β-cyclodextrin and modified starches. Eur. Food Res. Technol. 2002, 214, 242–247. [Google Scholar] [CrossRef]
- Yildiz, Z.A.; Celebioglu, A.; Kilic, M.E.; Durgun, E.; Uyar, T. Menthol/cyclodextrin inclusion complex nanofibers: Enhanced water-solubility and high-temperature stability of menthol. J. Food Eng. 2018, 224, 27–36. [Google Scholar] [CrossRef]
- Ciobanu, A.; Mallard, I.; Landy, D.; Brabie, G.; Nistor, S.; Fourmentin, S. Retention of aroma compounds from menthe piperita essential oil by cyclodextrins and crosslinked cyclodextrin polymers. Food Chem. 2013, 138, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Peila, R.; Migliavacca, G.; Aimone, F.; Ferri, A.; Sicardi, S. A comparison of analytical methods for the quantification of a reactive β-cyclodextrin fixed onto cotton yarns. Cellulose 2012, 19, 1097–1105. [Google Scholar] [CrossRef]
- Teschke, O.; de Souza, E.F. Liposome structure imaging by atomic force microscopy: Verification of improved liposome stability during absorption of multiple aggregated vesicles. Langmuir 2002, 18, 6513–6520. [Google Scholar] [CrossRef]
- Lian, T.; Ho, R.J.Y. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Betz, G.; Aeppli, A.; Menshutina, N.; Leuenberge, H. In vivo comparison of various liposome formulations for cosmetic application. Int. J. Pharm. 2005, 296, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Dayal, A.; Kumar, N. Microencapsulation processes and application in textile processing. Colourage 1998, 45, 15–24. [Google Scholar]
- Martí, M.; de la Maza, A.; Parra, J.L.; Coderch, L. Dyeing wool at low temperatures: New method using liposomes. Text. Res. J. 2001, 71, 678–682. [Google Scholar] [CrossRef]
- Montazer, M.; Validi, M.; Toliyat, T. Influence of temperature on stability of multilamellar liposomes in wool dyeing. J. Liposome Res. 2008, 16, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.; Martí, M.; Manich, A.M.; Parra, J.L.; Coderch, L. Ceramides Extracted from Wool: Pilot Plant Solvent. Text. Res. J. 2008, 78, 73–80. [Google Scholar] [CrossRef]
- Coderch, L.; Fonollosa, J.; Martí, M.; Garde, F.; de la Maza, A.; Parra, J.L. Extraction and Análisis of Ceramides from Internal Wool Lipids. J. Am. Oil Chem. Soc. 2002, 79, 1215–1220. [Google Scholar] [CrossRef]
- de Pera, M.; Coderch, L.; Fonollosa, J.; de la Maza, A.; Parra, J.L. Effect of Internal Wool Lipid liposomes on Skin Repair. Skin Pharmacol. Appl. Physiol. 2000, 13, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; de Pera, M.; Fonollosa, J.; de la Maza, A.; Parra, J.L. Efficacy of stratum corneum lipid supplementation on human skin. Contact Dermat. 2002, 47, 139–146. [Google Scholar] [CrossRef]
- Ramírez, R.; Martí, M.; Barba, C.; Méndez, S.; Parra, J.L.; Coderch, L. Skin Efficacy of liposomes composed of internal wool lipids rich in ceramides. J. Cosmet. Sci. 2010, 61, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; Fonollosa, J.; de Pera, M.; de la Maza, A.; Parra, J.L.; Martí, M. Compositions of Internal Lipid Extract of Wool and Use Thereof in the Preparation of Products for Skin Care and Treatment. Patent No. WO/2001/004244, 30 January 2001. [Google Scholar]
- Ramón, E.; Alonso, C.; Coderch, L.; De la Maza, A.; López, O.; Parra, J.L.; Notario, J. Liposomes as Alternative Vesicles for Sun Filter Formulations. Drug Deliv. 2005, 12, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C. Transdermal and Topical Drug Delivery: From Theory to Clinical Practice; Pharmaceutical Press: London, UK, 2003. [Google Scholar]
- Pinkus, H. Examination of the epidermis by the strip method of removing horny layers. I. Observation on thickness of the horny layer, and on mitotic activity after stripping. J. Investig. Dermatol. 1951, 16, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.; Botelho, G.; Alves, N.; Mano, J.F. Inclusion complexes of alfa-cyclodextrines with poly(d,l-Lactic acid): Structural characterization, and glass transition dynamics. Colloid Polym. Sci. 2014, 293, 863–871. [Google Scholar] [CrossRef]
- Dehabadi, V.A.; Buschmann, H.; Gutmann, J.S. A novel approach for fixation of β-cyclodextrin on cotton fabrics. J. Incl. Phenom. Macrocycl. Chem. 2013, 79, 459–464. [Google Scholar] [CrossRef]
- Martí, M.; Martínez, V.; Rubio, L.; Coderch, L.; Parra, J.L. Biofunctional Textiles prepared with Liposomes: In Vivo and in Vitro assessment. J. Microencapsul. 2011, 28, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from catos fruit (Opuntia ficus-indica) by spray-drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar]
- Vahabzadeh, F.; Zivdar, M.; Najafi, A. Microencapsulation of orange oil by complex coacervation and its release behavior. IJE Trans. B Appl. 2004, 17, 333–342. [Google Scholar]
- Mihailiasa, M.; Caldera, F.; Li, J.; Peila, R.; Ferri, A.; Trotta, F. Preparation of functionalized cotton fabrics by means of melatonin loaded cyclodextrin nanosponges. Carbohydr. Polym. 2016, 142, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Soares-Latour, E.M.; Bernard, J.; Chambert, S.; Fleury, E.; Sintes-Zydowicz, N. Environmentally benign 100% bio-based oligoamide microcapsules. Colloids Surf. A 2017, 524, 193–203. [Google Scholar] [CrossRef]
- Ghaheh, F.S.; Khoddami, A.; Alihosseini, F.; Jing, S.; Ribeiro, A.; Cavaco-Paulo, A.; Silva, C. Antioxidant cosmetotextiles: Cotton coating with nanoparticles containing vitamin E. Process Biochem. 2017, 59, 46–51. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; El-Ghany, N.A.A.; Eid, B.M.; Mabrouk, E.M. Green options for imparting antibacterial functionality to cotton fabrics. Int. J. Biol. Macromol. 2018, 111, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Carreras, N.; Acuña, V.; Martí, M.; Lis, M.J. Drug reléase system of ibuprofen in PCL-microespheres. Colloid Polym. Sci. 2013, 291, 157–165. [Google Scholar] [CrossRef]
- Bezerra, F.M.; Croscato, G.S.; Valldeperas, J.; Lis, M.J.; Carreras, C.; Acuna, V. Aplicação de microesferas para desenvolvimento de novos acabamentos têxteis. Química Têxtil 2011, 105, 49–58. [Google Scholar]
- Bird, R.B.; Stewart, W.E.; Lightfood, E.N. Fenômenos de Transporte; LTC Editora: Sao Paulo, Brasil, 2004; p. 838. [Google Scholar]
- Manadas, R.; Pina, M.E.; Veiga, F. A dissolução in vitro na previsão da absorção oral de fármacos em formas farmacêuticas de liberação modificada. Braz. J. Pharm. Sci. 2002, 38, 375–399. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Wu, J.; Li, S. Development of thermosensitive microgel-loaded cotton fabric for controlled drug release. Appl. Surf. Sci. 2017, 403, 509–518. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, L.; Wang, D.; West, J.L.; Fu, S. Preparation of thermochromic liquid crystal microcapsules for intelligent functional fiber. Mater. Des. 2018, 147, 28–34. [Google Scholar] [CrossRef]
- Mocanu, G.; Nichifor, M.; Mihai, D.; Oproiu, L.C. Bioactive cotton fabrics containing chitosan and biologically active substances extracted from plants. Mater. Sci. Eng. C 2013, 33, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Todorova, S.B.; Silva, C.J.S.; Simeonov, M.P.; Cavaco-Paulo, A. Cotton fabric: A natural matrix suitable for controlled release systems. Enzym. Microb. Technol. 2007, 40, 1646–1650. [Google Scholar] [CrossRef] [Green Version]
- Radu, C.D.; Parteni, O.; Popa, M.; Muresan, I.E.; Ochiuz, L.; Bulgariu, L.; Munteanu, C.; Istrate, B.; Ule, E. Comparative Study of a Drug Release from a Textile to Skin. J. Pharm. Drug Deliv. Res. 2015, 4, 2–8. [Google Scholar] [CrossRef]
- Martin, A.; Tabary, N.; Leclercq, L.; Junthip, J.; Degoutin, S.; Aubert-Viard, F.; Cazaux, F.; Lyskawa, J.; Janus, L.; Bria, M.; et al. Multilayered textile coating based on a cyclodextrin polyelectrolyte for the controlled release of drugs. Carbohydr. Polym. 2013, 93, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.; Martínez, V.; Lis, M.J.; Valldeperas, J.; de la Maza, A.; Parra, J.L.; Coderch, L. Gallic Acid vehiculized through liposomes or mixed micelles in biofunctional textiles. J. Text. Inst. 2014, 105, 175–186. [Google Scholar] [CrossRef]
- Martí, M.; Alonso, C.; Lis, V.M.J.; de la Maza, A.; Parra, J.L.; Coderch, L. Cosmetotextiles with Gallic Acid: Skin reservoir effect. J. Drug Release 2013, 2013, 456248. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.; Rodríguez, R.; Carreras, N.; Lis, M.J.; Valldeperas, J.; de la Maza, A.; Coderch, L.; Parra, J.L. Monitoring of the microcapsule/liposome application on textile fabrics. J. Text. Inst. 2012, 103, 19–27. [Google Scholar] [CrossRef]








| Source | Polymer |
|---|---|
| Natural Polysaccharides | Starch, cellulose, chitosan, gum arabic, and alginate |
| Modified Polysaccharides | Dextrins, carboxymethylcellulose, ethylcellulose, methylcellulose, acetylcellulose, and nitrocellulose |
| Proteins | Gluten, casein, gelatin, and albumin |
| Waxes and lipids | Paraffin, tristearine, stearic acid, monoacyl, and diacyl |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis Arias, M.J.; Coderch, L.; Martí, M.; Alonso, C.; García Carmona, O.; García Carmona, C.; Maesta, F. Vehiculation of Active Principles as a Way to Create Smart and Biofunctional Textiles. Materials 2018, 11, 2152. https://doi.org/10.3390/ma11112152
Lis Arias MJ, Coderch L, Martí M, Alonso C, García Carmona O, García Carmona C, Maesta F. Vehiculation of Active Principles as a Way to Create Smart and Biofunctional Textiles. Materials. 2018; 11(11):2152. https://doi.org/10.3390/ma11112152
Chicago/Turabian StyleLis Arias, Manuel J., Luisa Coderch, Meritxell Martí, Cristina Alonso, Oscar García Carmona, Carlos García Carmona, and Fabricio Maesta. 2018. "Vehiculation of Active Principles as a Way to Create Smart and Biofunctional Textiles" Materials 11, no. 11: 2152. https://doi.org/10.3390/ma11112152
APA StyleLis Arias, M. J., Coderch, L., Martí, M., Alonso, C., García Carmona, O., García Carmona, C., & Maesta, F. (2018). Vehiculation of Active Principles as a Way to Create Smart and Biofunctional Textiles. Materials, 11(11), 2152. https://doi.org/10.3390/ma11112152








