Preparation and Properties Analysis of Chlorinated Butyl Rubber (CIIR)/Organic Diatomite Damping Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
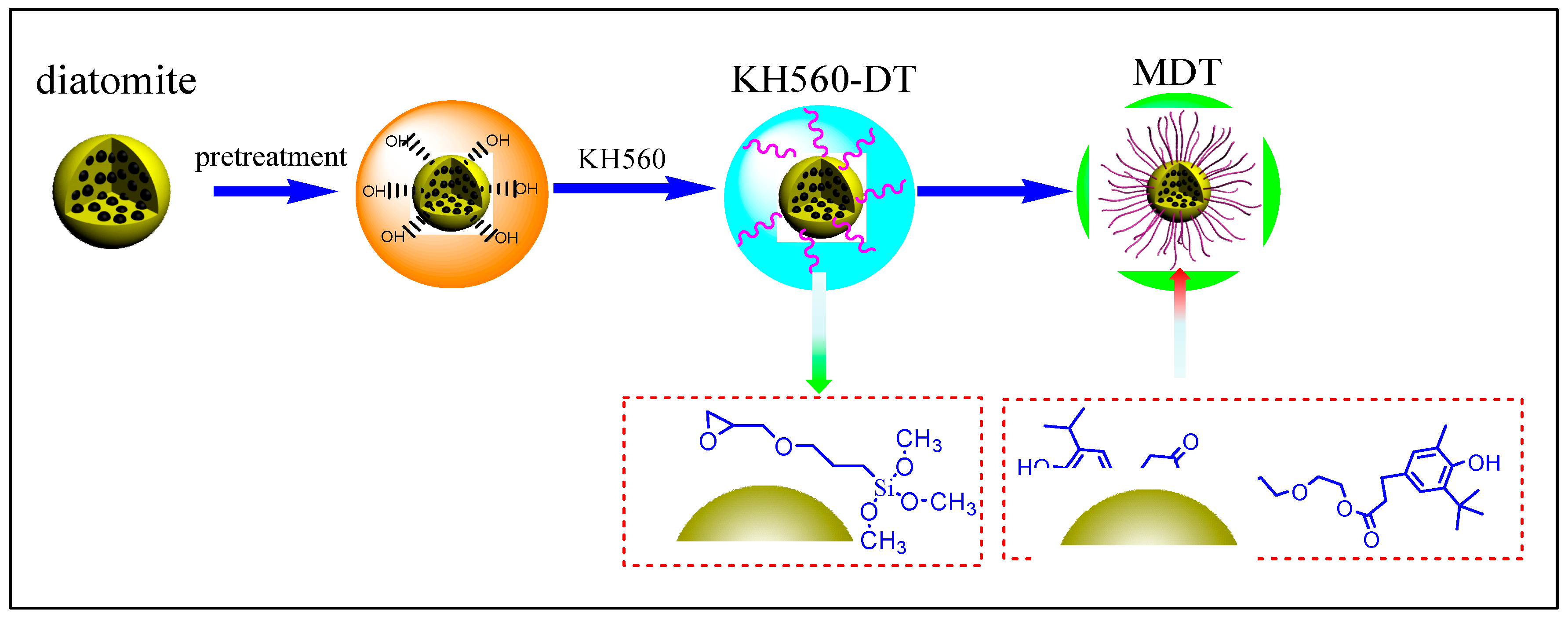
2.2. Preparation of Modified Diatomite (MDT)
2.3. Preparation of CIIR Composites
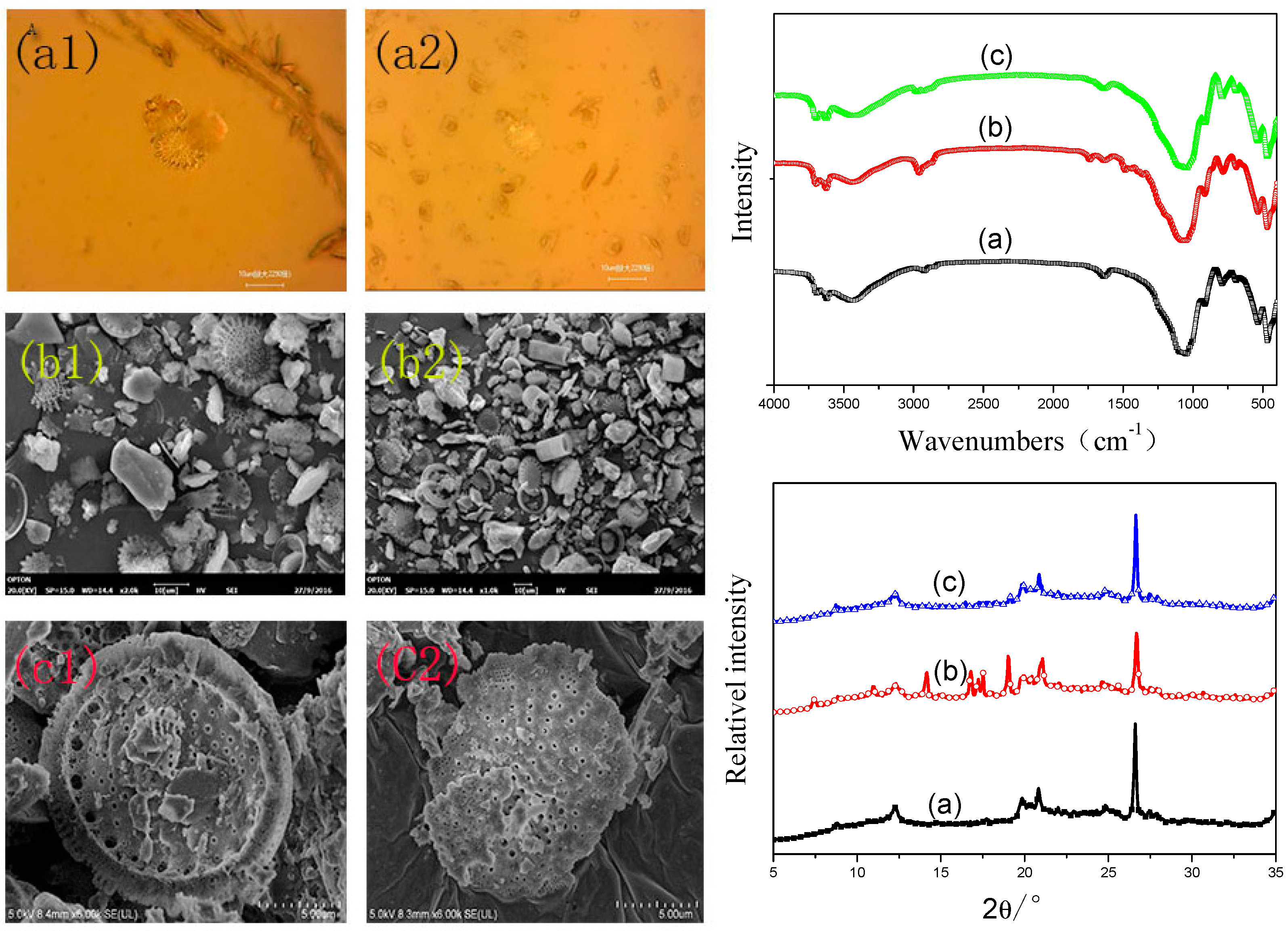
2.4. Characterization
3. Results and Discussion
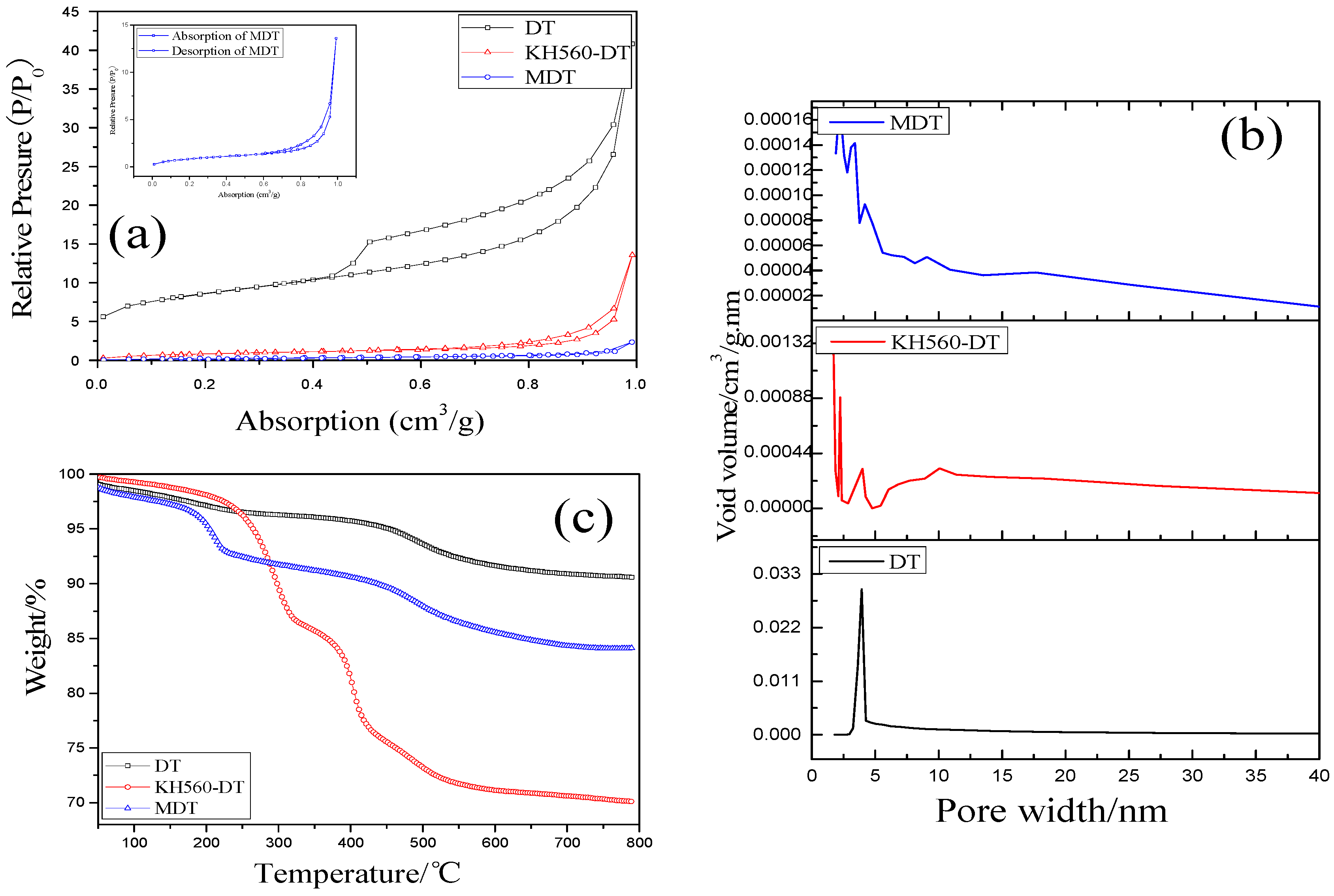
3.1. Analysis of Modified Diatomite (MDT)
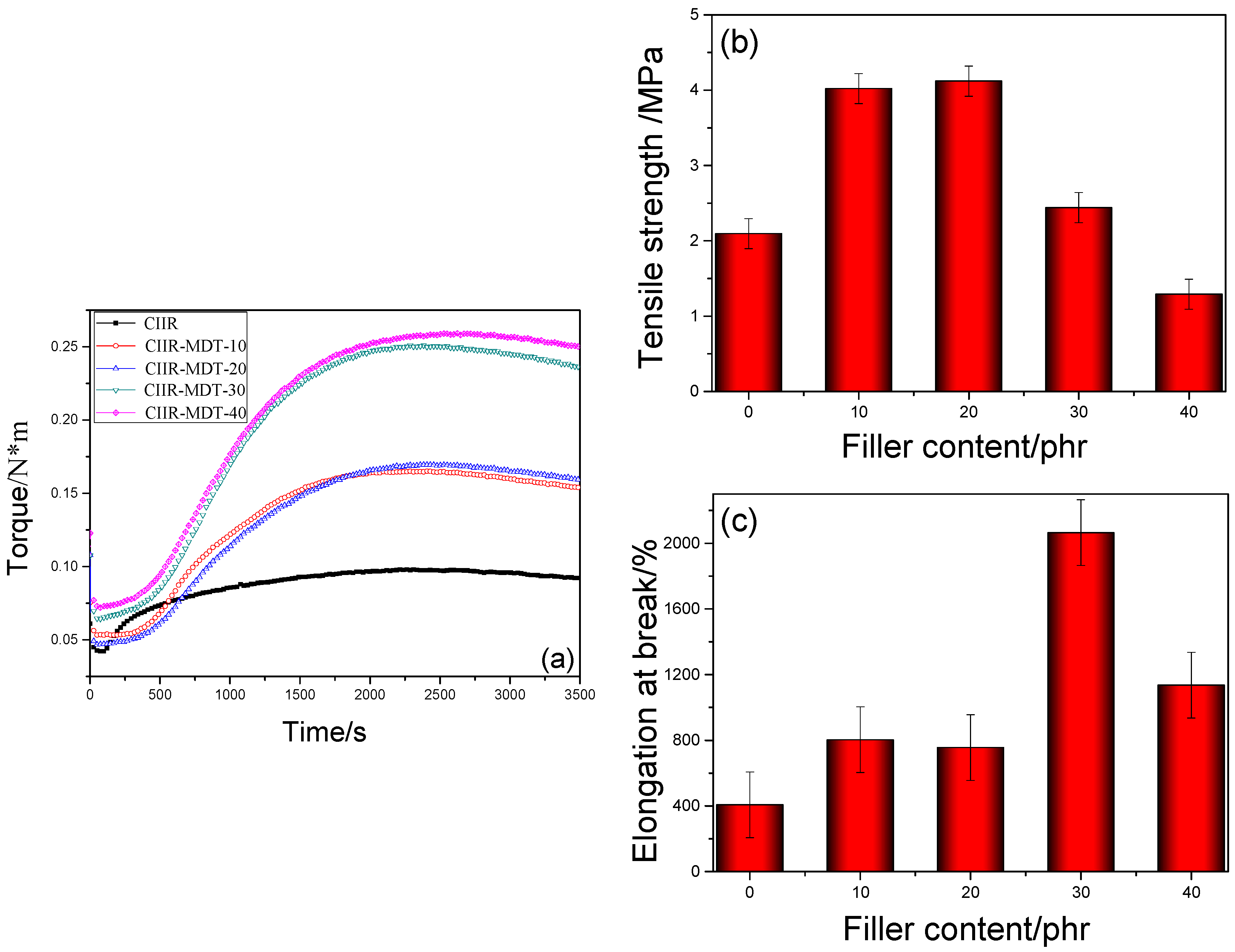
3.2. Cure and Tensile Properties of CIIR Composites
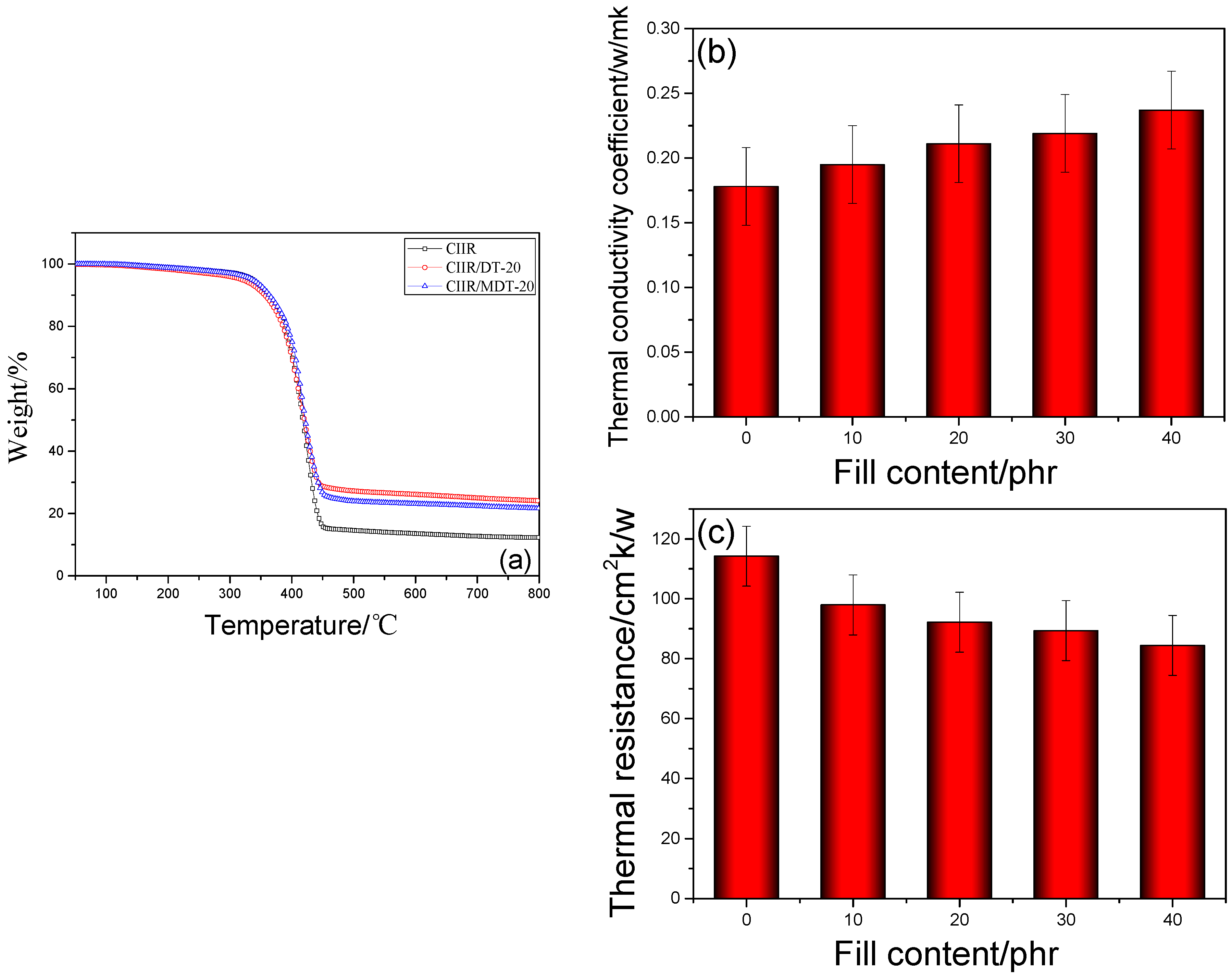
3.3. Thermal Properties of CIIR Composites
3.4. Dynamic Mechanical Properties of CIIR Composites
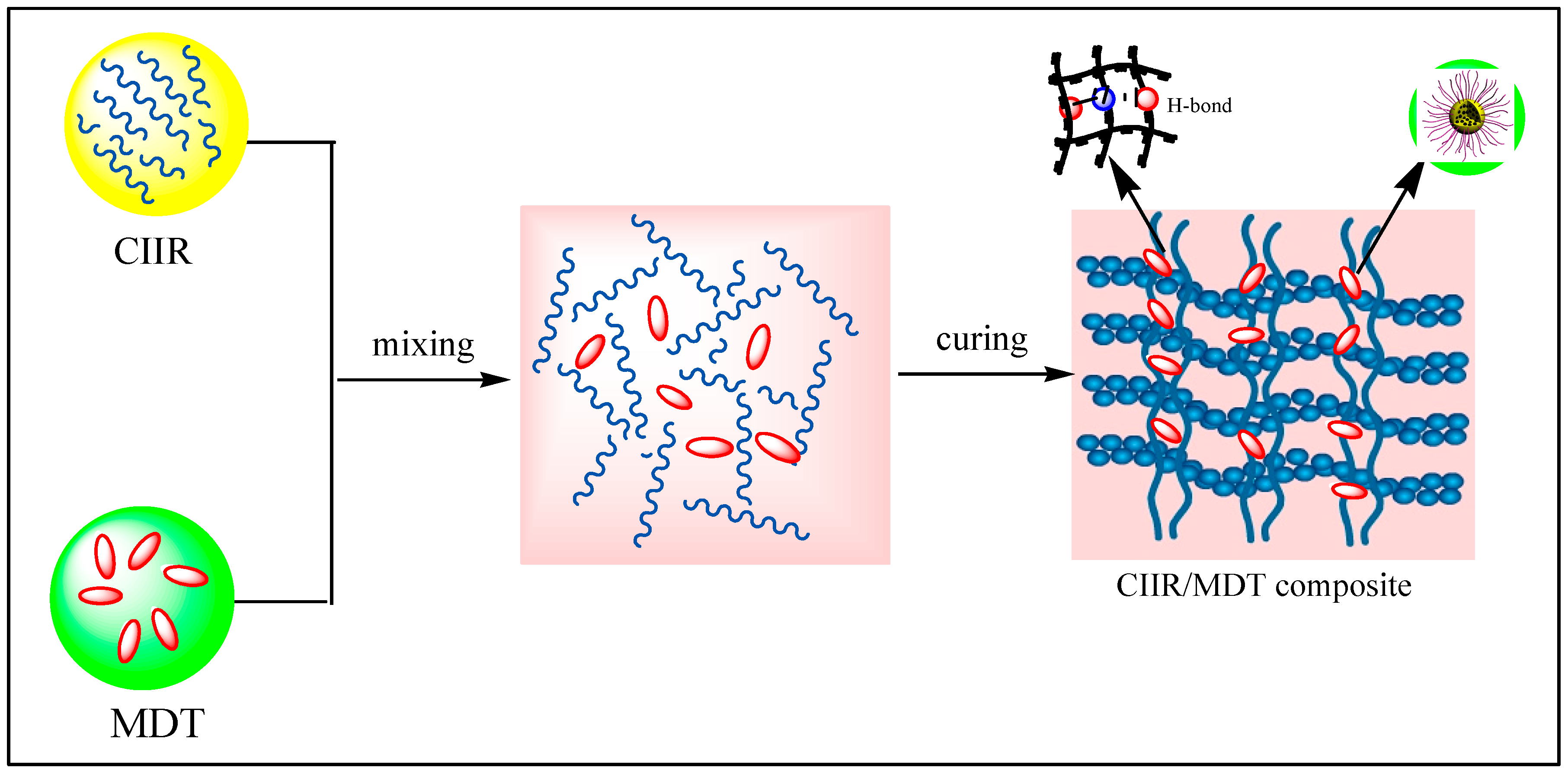
3.5. Reinforcing and Damping Mechanisms of MDT in CIIR Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Remillat, C. Damping mechanism of polymers filled with elastic particles. Mech. Mater. 2007, 39, 525–537. [Google Scholar] [CrossRef]
- Zhang, J.; Richards, C.M. Parameter identification of analytical and experimental rubber isolators represented by Maxwell models. Mech. Syst. Signal Process. 2007, 21, 2814–2832. [Google Scholar] [CrossRef]
- Urayama, K.; Miki, T.; Takigawa, A.; Kohjiya, S. Damping Elastomer Based on Model Irregular Networks of End-Linked Poly (Dimethylsiloxane). Chem. Mater. 2004, 16, 173–178. [Google Scholar] [CrossRef]
- Liu, B.; Gao, X.; Zhao, Y.; Dai, L.; Xie, Z.; Zhang, Z. 9,10-Dihydro-9-oxa-10-phosphaphenanthrene 10-oxide-based oligosiloxane as a promising damping additive for methyl vinyl silicone rubber (VMQ). J. Mater. Sci. 2017, 52, 8603–8617. [Google Scholar] [CrossRef]
- Zhang, F.; He, G.; Xu, K.; Wu, H.; Guo, S. The damping and flame-retardant properties of poly (vinyl chloride)/chlorinated butyl rubber multilayered composites. J. Appl. Polym. Sci. 2015, 132, 41259. [Google Scholar] [CrossRef]
- Cao, F.; Wang, J. Preparation and characterization of hyperbranched polymer modified montmorillonite/chlorinated butyl rubber damping composites. J. Appl. Polym. Sci. 2016, 133, 43653. [Google Scholar]
- Ding, G.; Fang, H.; Shi, Y.; Wang, X.; Luo, S. Preparation technology and mechanical damping property of high-performance chlorinated butyl rubber compound damping material. Mater. Rev. 2015, 29, 57–61. [Google Scholar]
- Li, Z.; Wang, D.; Wang, Z.; Wang, H.; Li, C. Chlorinated butyl rubber and its research status. J. Shananxi Univ. Technol. Nat. Sci. Ed. 2013, 29, 6–11. [Google Scholar]
- Wu, C.; Yamagishi, T.A.; Nakamoto, Y.; Ishida, S.; Nitta, K. Organic hybrid of chlorinated polyethylene and hindered phenol. II. Influence of the chemical structure of small molecules on viscoelastic properties. J. Polym. Sci. Part B Polym. Phys. 2015, 38, 1496–1503. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, C.; Zhang, D.; Tian, M.; Zhang, L. Preparation and damping properties of hindered phenol AO-80/chlorobutyl rubber/butadiene rubber composites. China Synth. Rubber Ind. 2014, 37, 42–46. [Google Scholar]
- Saritha, A.; Joseph, K. Effect of nano clay on the constrained polymer volume of chlorobutyl rubber nanocomposites. Polym. Compos. 2015, 36, 2135–2139. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Huang, G.; Wu, J. Damping characteristics of chlorobutyl rubber/poly(ethyl acrylate)/piezoelectric ceramic/carbon black composites. J. Appl. Polym. Sci. 2008, 108, 3670–3676. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, H.; Huang, Z.; Zhang, T. Mechanical, Dynamic cechanical and electrical properties of conductive carbon black/piezoelectric ceramic/chlorobutyl rubber composites. J. Macromol. Sci. Part D Rev. Polym. Process. 2012, 51, 105–110. [Google Scholar]
- Lu, X.; Li, X.; Tian, M. Preparation of high damping elastomer with broad temperature and frequency ranges based on ternary rubber blends. Polym. Adv. Technol. 2014, 25, 21–28. [Google Scholar] [CrossRef]
- Tao, G.; Lu, X.; Guo, J.; Tian, M. Preparation and properties of high-damping rubber with wide temperature range and frequency range. Polym. Mater. Sci. Eng. 2013, 29, 114–118. [Google Scholar]
- Wang, Y.; Zhou, C.; Yan, H.; Huang, Z. Dynamic mechanical properties of phenolic resin/chlorinated butyl rubber composites. J. Macromol. Sci. Part B 2014, 53, 813–819. [Google Scholar] [CrossRef]
- Zhang, F.; He, G.; Xu, K.; Wu, H.; Guo, S.; Zhang, C. Damping mechanism and different modes of molecular motion through the glass transition of chlorinated butyl rubber and petroleum resin blends. J. Appl. Polym. Sci. 2014, 131, 8–387. [Google Scholar] [CrossRef]
- Johnson, L.K.; Stefan Mecking, A.; Brookhart, M. Copolymerization of ythylene and propylene with functionalized vinyl monomers by palladium (II) catalysts. J. Am. Chem. Soc. 1996, 18, 267–268. [Google Scholar] [CrossRef]
- Liu, K.; Lv, Q.; Hua, J. Study on damping properties of HVBR/EVM blends prepared by in situ polymerization. Polym. Test. 2017, 60, 321–325. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Huang, G. The preparation and characterization of chlorinate butyl rubber/poly (methyl) acrylate IPN camping materials. J. Southwest Petroleum Univ. 2007, 29, 134–137. [Google Scholar]
- Ping, D.; Wang, Y. The dynamic mechanical properties of chlorobutyl rubber/polybutyl methacrylate sequential interpenetrating networks. J. Macromol. Sci. Part D Rev. Polym. Process. 2010, 49, 1310–1314. [Google Scholar]
- Zhang, F.; Guo, M.; Xu, K.; He, G.; Wu, H.; Guo, S. Multilayered damping composites with damping layer/constraining layer prepared by a novel method. Compos. Sci. Tech. 2014, 101, 167–172. [Google Scholar] [CrossRef]
- Pechurai, W.; Nakason, C.; Sahakaro, K. Thermoplastic natural rubber based on oil extended NR and HDPE blends: Blend compatibilizer, phase inversion composition and mechanical properties. Polym. Test. 2008, 27, 621–631. [Google Scholar] [CrossRef]
- Oka, T.; Nishihara, S.; Yasuda, H. Polymer Blend Fibers Having Phase Separation Structure and Method for Producing the Same. U.S. Patent US5869183A, 9 February 1999. [Google Scholar]
- Yin, X.; Liu, C.; Lin, Y.; Guan, A.; Wu, G. Influence of hydrogen bonding interaction on the damping properties of poly(n-butyl methacrylate)/small molecule hybrids. J. Appl. Polym. Sci. 2015, 132, 41954. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, F.; Zhang, X.; Guo, J.; Wu, H.; Guo, S. Molecular insights into the damping mechanism of poly(vinyl acetate)/hindered phenol hybrids by a combination of experiment and molecular dynamics simulation. RSC Adv. 2015, 5, 4200–4209. [Google Scholar] [CrossRef]
- Jiang, S.; Jie, L.M. Damping properties and micro-morphology of textile waste rubber powder-AO 2246 composites. J. Compos. Mater. 2016, 50, 963–970. [Google Scholar]
- Garcia, G.; Cardenas, E.; Cabrera, S.; Hedlund, J.; Mouzon, J. Synthesis of zeolite Y from diatomite as silica source. Microporous Mesoporous Mater. 2016, 219, 29–37. [Google Scholar] [CrossRef]
- Vu, D.H.; Wang, K.S.; Bac, B.H.; Nam, B.X. Humidity control materials prepared from diatomite and volcanic ash. Constr. Build. Mater. 2013, 38, 1066–1072. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Z. Modified-diatomite reinforced rubbers. Mater. Lett. 2017, 209, 159–162. [Google Scholar] [CrossRef]
- Liao, J.; Du, G.; Xue, Q.; Ding, H. Surface modification of diatomite by steatic acid and it’s effects on reinforcing for natural rubber/styrene butadiene rubber blend. J. Chin. Ceram. Soc. 2011, 39, 641–645. [Google Scholar]
- Hu, Z.; Zheng, S.; Tan, Y.; Jia, M. Preparation and Characterization of Diatomite/Silica Composite Humidity Control Material by Partial Alkali Dissolution. Mater. Lett. 2017, 196, 234–237. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Oh, J.; Chung, K. Fabrication and properties of magnetorheological elastomers based on CR/ENR self-crosslinking blends. Smart Mater. Struct. 2015, 24, 095006. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Cheminform. 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, M.; Zaborski, M. Thermal analysis and mechanical methods applied for studying properties of SBR compounds containing ionic liquids. Polym. Test. 2017, 61, 349–363. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Liu, M.; Xi, X.; Zhang, X.; Jia, D. Effect of montmorillonite on carboxylated styrene butadiene rubber/hindered phenol damping material with improved extraction resistance. Mater. Des. 2014, 58, 316–323. [Google Scholar] [CrossRef]
- Tan, J.H.; Wang, X.P.; Luo, Y.F.; Jia, D.M. Rubber/clay nanocomposites by combined latex compounding and melt mixing: A masterbatch process. Mater. Des. 2012, 34, 325–331. [Google Scholar] [CrossRef]
- Liu, B.; Gao, X.; Zhao, Y.; Dai, L.; Xie, Z.; Zhang, Z. Prospect of 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide-based oligosiloxane in the preparation of high damping methyl phenyl vinyl silicone rubbers with broad temperature range. J. Mater. Sci. 2017, 52, 13307–13317. [Google Scholar] [CrossRef]
- Razzaq, M.Y.; Frormann, L. Thermomechanical studies of aluminum nitride filled shape memory polymer composites. Polym. Compos. 2007, 28, 287–293. [Google Scholar] [CrossRef]
- Li, T.L.; Hsu, L.C. Enhanced thermal conductivity of polyimide films via a hybrid of micro- and nano-sized boron nitride. J. Phys. Chem. B 2010, 114, 6825–6829. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Mohanty, S.; Nayak, S. Toughened bio-based epoxy blend network modified with transesterified epoxidized soybean oil: synthesis and characterization. RSC Adv. 2015, 5, 13674–13691. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, G.; Lu, F.; Zhang, L.; Wu, S. A molecular-level insight of hindered phenol AO-70/nitrile-butadiene rubber damping composites through a combination of molecular dynamics simulation and experimental method. RSC Adv. 2016, 6, 85994–86005. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Feng, L.; Chao, X.; Zhao, K.; Feng, Y. Nano-graphite controlling properties of novel composites with damping-absorption functions and storage-loss behaviors: Nano-graphite/PZT-PMN-PNN/RTV. Curr. Appl. Phys. 2017, 17, 130–136. [Google Scholar] [CrossRef]
- Shi, X.; Li, Q.; Fu, G.; Jia, L. The effects of a polyol on the damping properties of EVA/PLA blends. Polym. Test. 2014, 33, 1–6. [Google Scholar] [CrossRef]



| Component | phr (Parts per Hundred of Rubber) |
|---|---|
| CIIR | 100 |
| zinc oxide | 3 |
| tetramethyl thiuram disulfide | 1.5 |
| stearic acid | 1.5 |
| N-phenyl-β-naphthylamine | 2 |
| diben zothiazole disulfide | 2 |
| sulfur | 3 |
| DT/MDT | 0, 5, 10, 15, 20 |
| Samples | Specific Surface Area (cm3/g) | Average Pore Size (nm) |
|---|---|---|
| Original DT | 26.37 | 9.15 |
| KH560-DT | 3.67 | 22.90 |
| MDT | 1.04 | 13.52 |
| Samples | T10/min | T90/min | ML/N·m | MH/N·m | Cure Rate, VC1 |
|---|---|---|---|---|---|
| CIIR | 2.40 | 26.23 | 0.04 | 0.10 | 4.20 |
| CIIR/MDT-10 | 7.57 | 25.72 | 0.05 | 0.17 | 5.51 |
| CIIR/MDT-20 | 7.82 | 30.00 | 0.05 | 0.17 | 4.48 |
| CIIR/MDT-30 | 8.00 | 27.23 | 0.06 | 0.25 | 5.20 |
| CIIR/MDT-40 | 7.80 | 28.17 | 0.07 | 0.26 | 4.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Z.; Yang, S.; Wang, J.; Lu, Y.; Tang, K.; Song, S. Preparation and Properties Analysis of Chlorinated Butyl Rubber (CIIR)/Organic Diatomite Damping Composites. Materials 2018, 11, 2172. https://doi.org/10.3390/ma11112172
Sheng Z, Yang S, Wang J, Lu Y, Tang K, Song S. Preparation and Properties Analysis of Chlorinated Butyl Rubber (CIIR)/Organic Diatomite Damping Composites. Materials. 2018; 11(11):2172. https://doi.org/10.3390/ma11112172
Chicago/Turabian StyleSheng, Zeyuan, Siyuan Yang, Jincheng Wang, Yao Lu, Keya Tang, and Shiqiang Song. 2018. "Preparation and Properties Analysis of Chlorinated Butyl Rubber (CIIR)/Organic Diatomite Damping Composites" Materials 11, no. 11: 2172. https://doi.org/10.3390/ma11112172
APA StyleSheng, Z., Yang, S., Wang, J., Lu, Y., Tang, K., & Song, S. (2018). Preparation and Properties Analysis of Chlorinated Butyl Rubber (CIIR)/Organic Diatomite Damping Composites. Materials, 11(11), 2172. https://doi.org/10.3390/ma11112172




