Controlled Synthesis and Microstructure of Metastable Flower-Like Vaterite
Abstract
:1. Introduction
2. Experimental Section
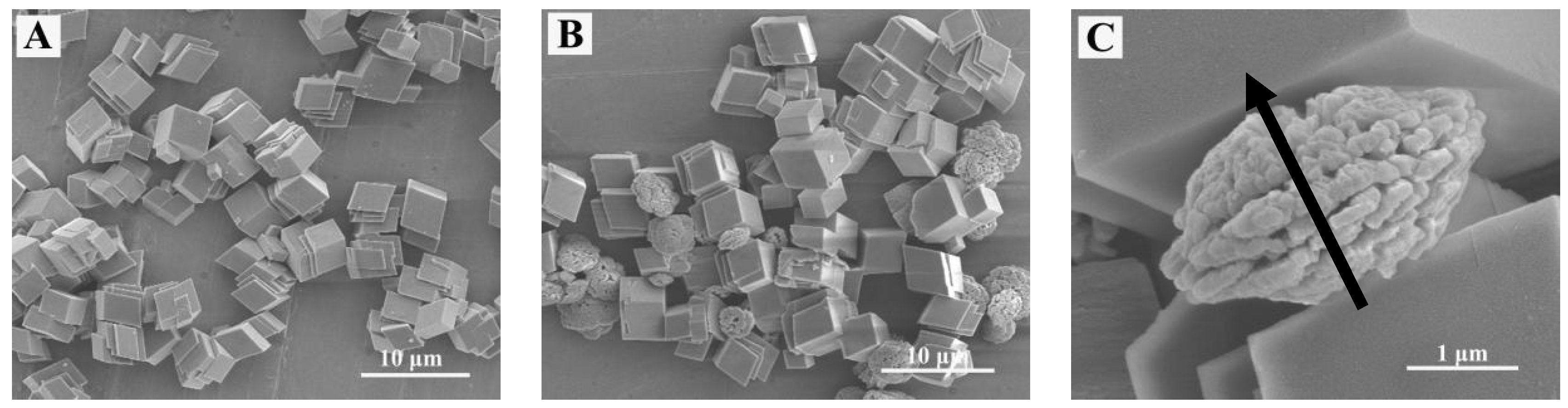
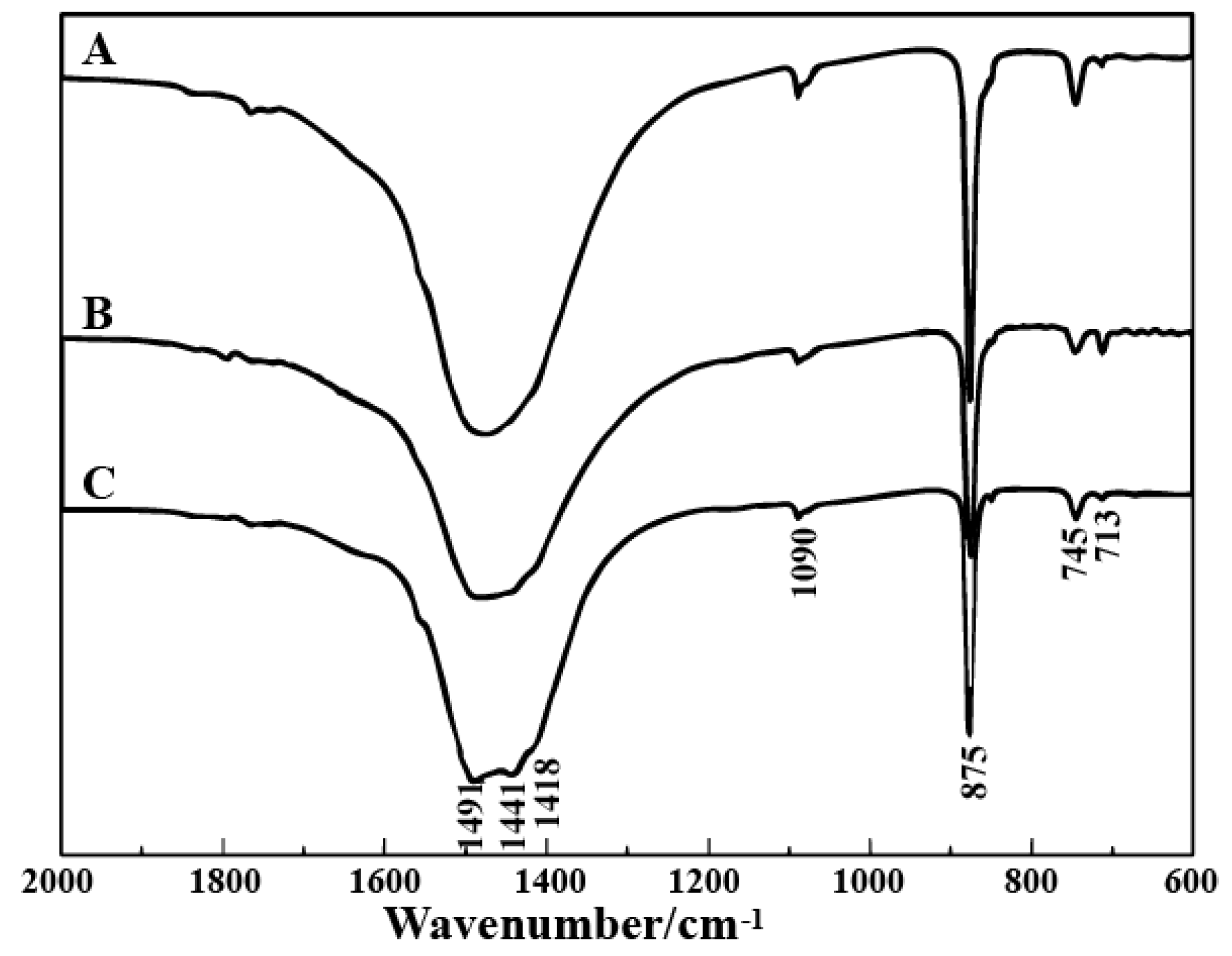
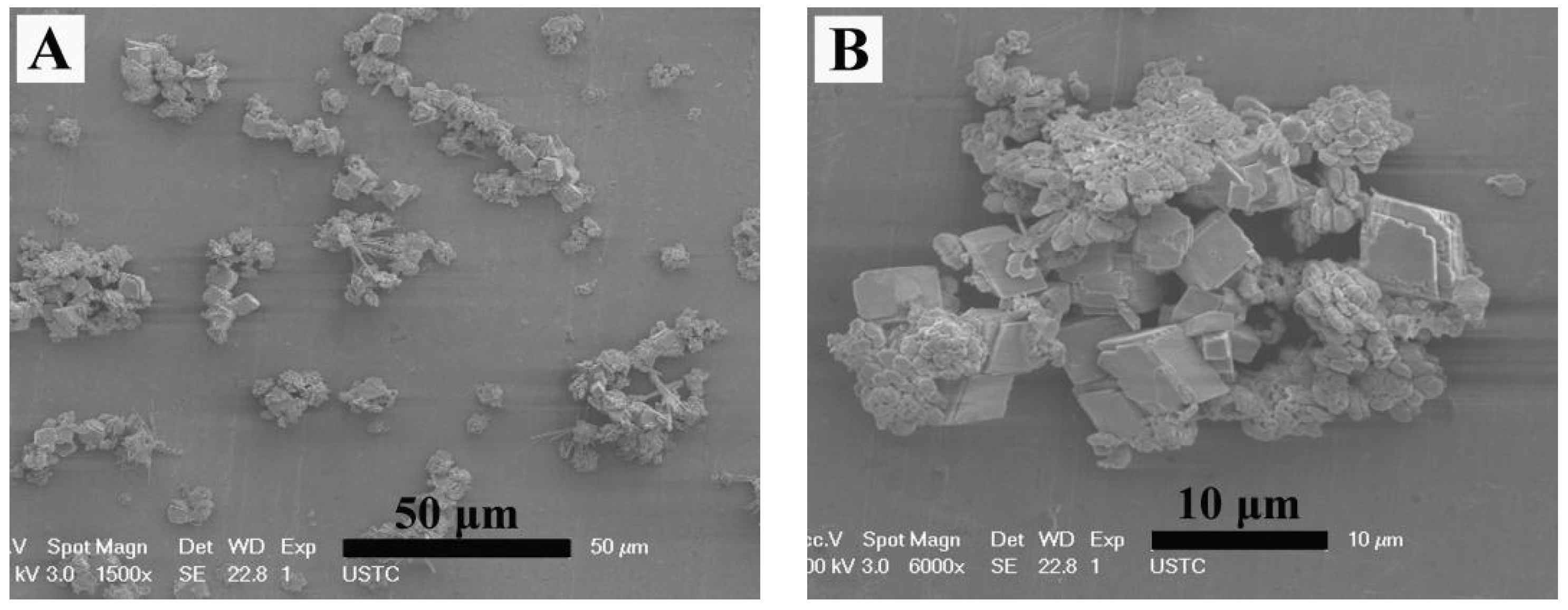
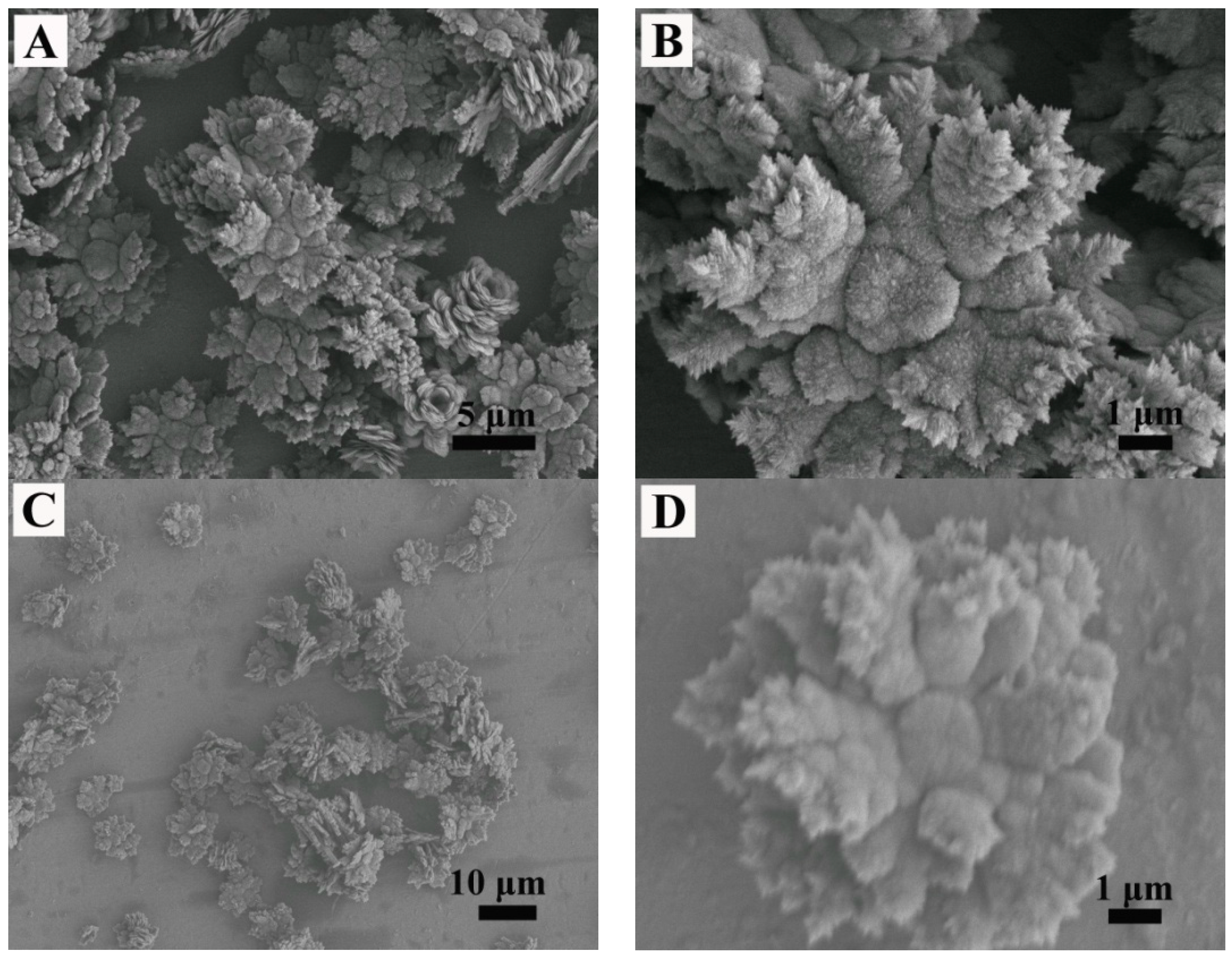
3. Results
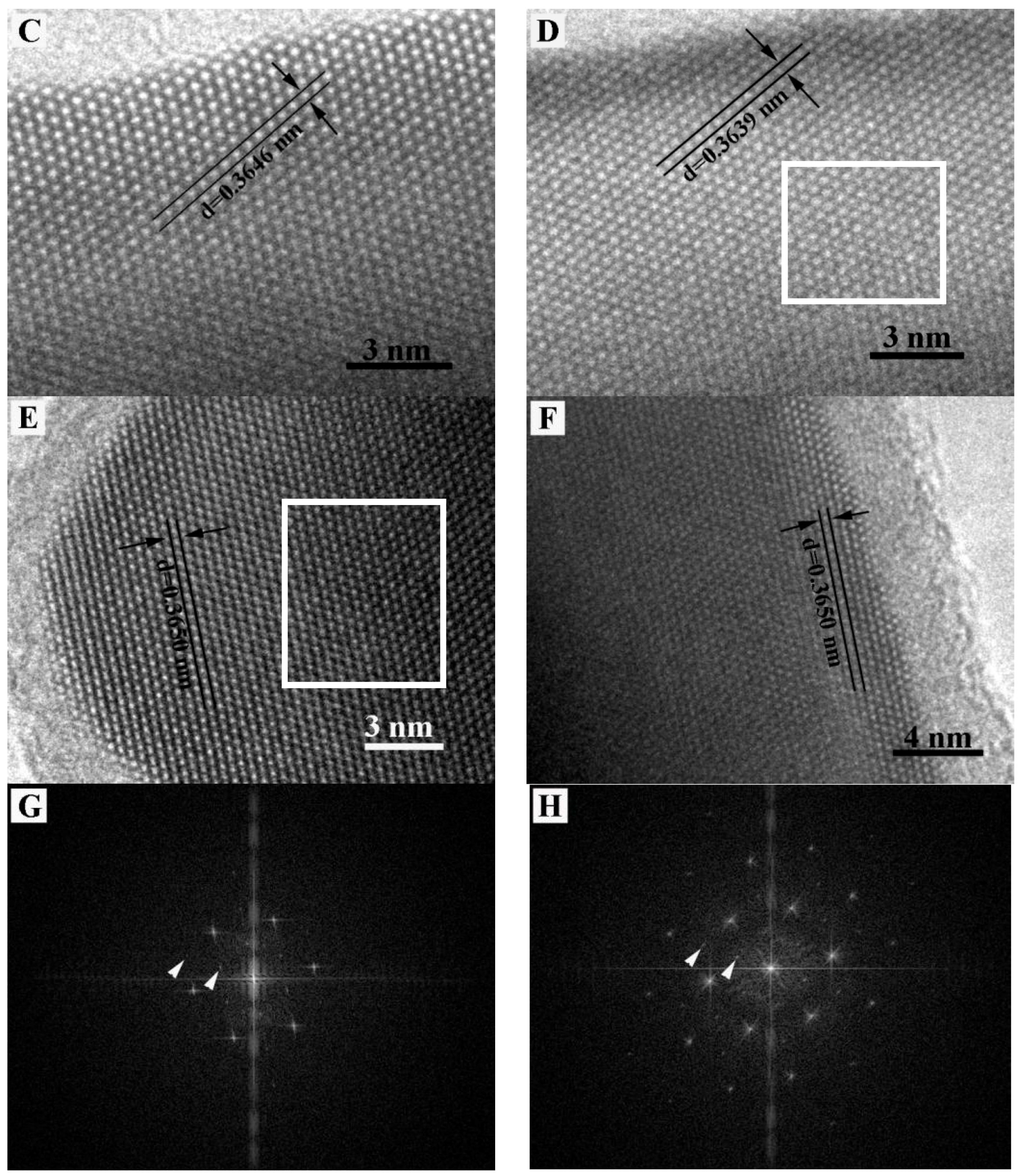
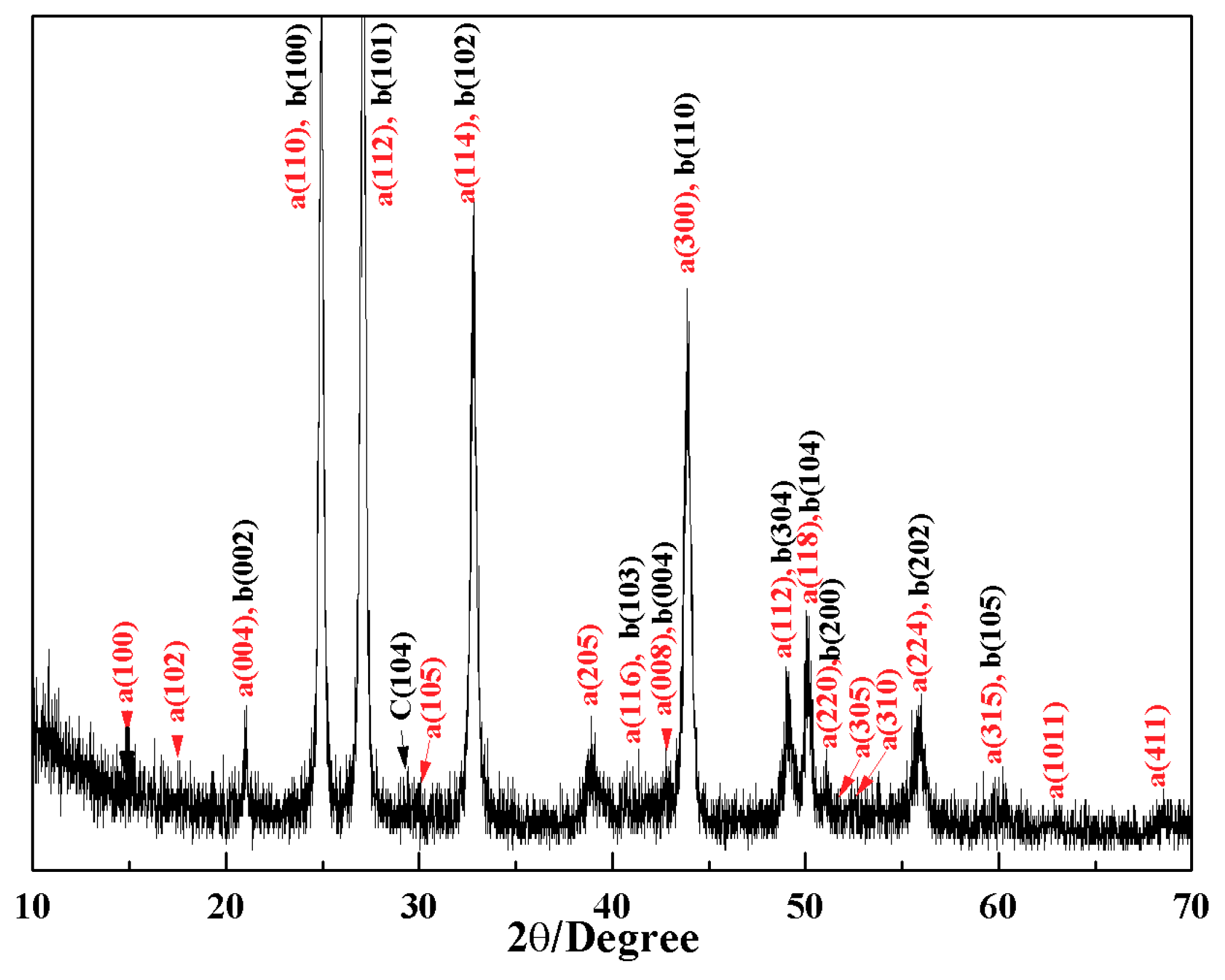
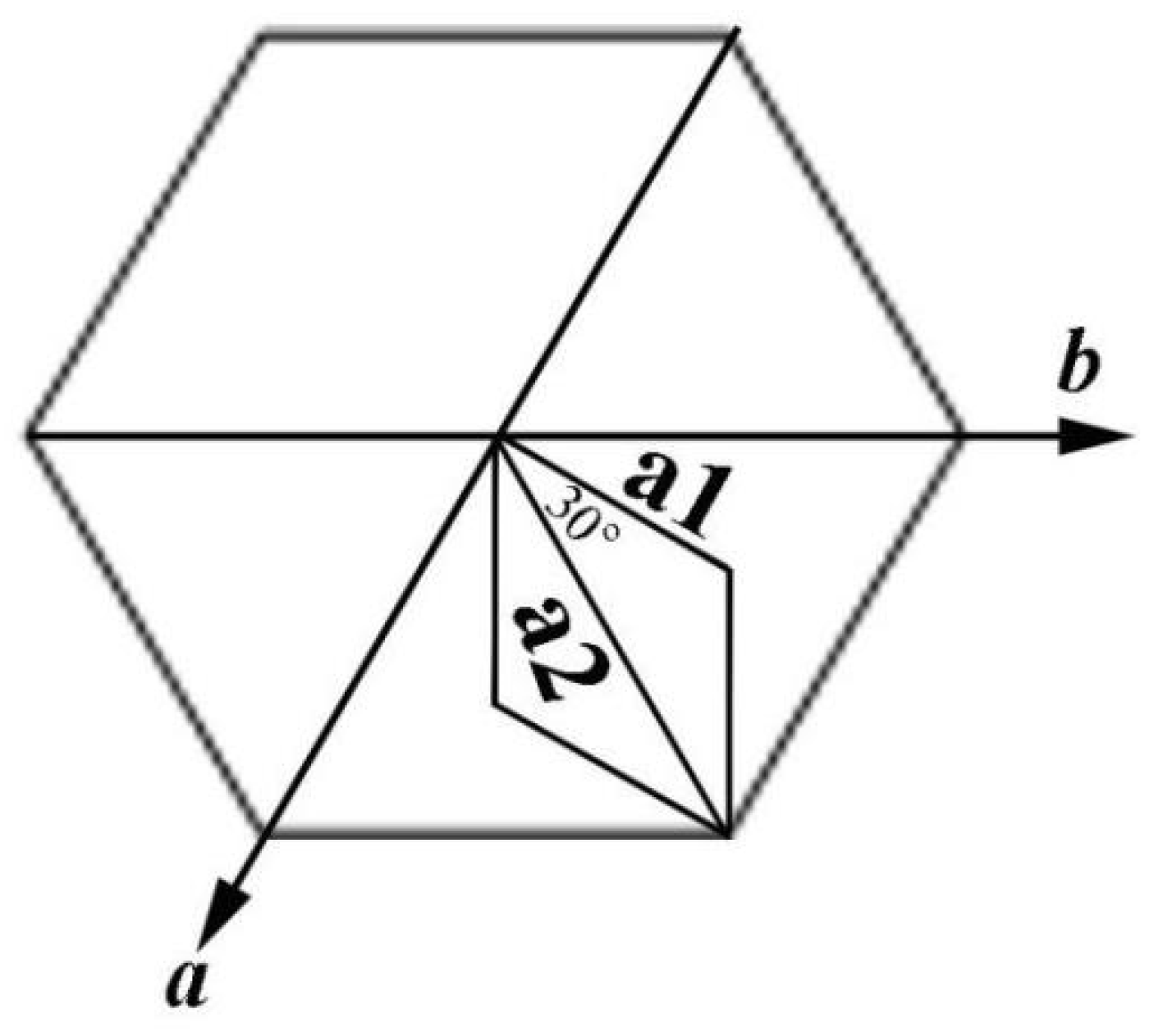
4. Microstructure Analysis of Flower-Like Vaterite Crystals
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boettcher, A.L.; Wyllie, P.J. The calcite-aragonite transition measured in the system CaO-CO2-H2O. J. Geol. 1968, 76, 314–330. [Google Scholar] [CrossRef]
- Johnanesss, W.; Puhan, D. The calcite-aragonite transition, reinvestigated. Contrib. Mineral. Petrol. 1971, 31, 28–38. [Google Scholar] [CrossRef]
- Turnbull, A.G. A thermodynamic study of vaterite. Geochim. Cosmochim. Acta 1973, 37, 1593–1601. [Google Scholar] [CrossRef]
- Lakshminarayanan, R.; Chi-Jin, E.O.; Loh, X.J.; Kini, R.M.; Valiyaveettil, S. Purification and characterization of a vaterite-Inducing peptide, pelovaterin, from the eggshells of pelodiscus sinensis (Chinese Soft-Shelled Turtle). Biomacromolecules 2005, 6, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Wehrmeister, U.; Jacob, D.E.; Soldati, A.L.; Loges, N.; Häger, T.; Hofmeister, W. Amorphous, nanocrystalline and crystalline calcium carbonates in biological materials. J. Raman Spectrosc. 2011, 42, 926–935. [Google Scholar] [CrossRef]
- Isaure, M.P.; Sarret, G.; Harada, E.; Choi, Y.E.; Marcus, M.A.; Fakra, S.C.; Geoffroy, N.; Pairis, S.; Susini, J.; Clemens, S.; et al. Calcium promotes cadmium elimination as vaterite grains by tobacco trichomes. Geochim. Cosmochim. Acta 2010, 74, 5817–5834. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.L.; Nishimura, T.; Kato, T. Morphology tuning in the formation of vaterite crystal thin films with thermoresponsive poly(N-isopropylacrylamide) brush matrices. CrystEngComm 2014, 16, 3540–3547. [Google Scholar] [CrossRef]
- Yao, H.B.; Ge, J.; Mao, L.B.; Yan, Y.X.; Yu, S.H. Artificial carbonate nanocrystals and layered structural nanocomposites inspired by nacre: Synthesis, fabrication and applications. Adv. Mater. 2014, 26, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Brečević, L.; Noethig-Laslo, V.; Kralk, D.; Popevic, S. Effect of divalent cations on the formation and structure of calcium carbonate polymorphs. J. Chem. Soc. Faraday Trans. 1996, 92, 1017–1022. [Google Scholar] [CrossRef]
- Andreassen, J.P.; Hounslow, M.J. Growth and aggregation of vaterite in seeded-batch experiments. AIChE J. 2004, 50, 2772–2782. [Google Scholar] [CrossRef]
- Flaten, E.M.; Seiersten, M.; Andreassen, J.P. Growth of the calcium carbonate polymorph vaterite in mixtures of water and ethylene glycol at conditions of gas processing. J. Cryst. Growth 2010, 312, 953–960. [Google Scholar] [CrossRef]
- Chen, Y.X.; Ji, X.B.; Wang, X.B. Microwave-assisted synthesis of spheroidal vaterite CaCO3 in ethylene glycol-water mixed solvents without surfactants. J. Cryst. Growth 2010, 312, 3191–3197. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Antipina, M.N. Size-controlled synthesis of vaterite calcium carbonate by the mixing method: Aiming for nanosized particles. Cryst. Growth Des. 2016, 16, 1311–1319. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, J.; Xu, J.; Yang, L.; Song, T.; Zhang, P. Synthesis of template-free hollow vaterite CaCO3 microspheres in the H2O/EG system. Mater. Lett. 2013, 104, 28–30. [Google Scholar] [CrossRef]
- Falini, G.; Albeck, S.; Weiner, S.; Addadi, L. Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 1996, 271, 67–69. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.M.; Cao, Z.N.; Xu, Y.; Gong, Y.H.; Shi, X.T. Fabrication of uniform casein/CaCO3 vaterite microspheres and investigation of its formation mechanism. Cryst. Growth Des. 2017, 17, 6178–6188. [Google Scholar] [CrossRef]
- Xu, Y.F.; Ma, G.B.; Wang, M. Fabrication and growth mechanism of pumpkin-shaped vaterite hierarchical structures. Cryst. Growth Des. 2014, 14, 6166–6171. [Google Scholar] [CrossRef]
- Arita, Y.; Richards, J.M.; Mazilu, M.; Spalding, G.C.; Spesyvtseva, S.E.S.; Craig, D.; Dholakia, K. Rotational dynamics and heating of trapped nanovaterite particles. ACS Nano 2016, 10, 11505–11510. [Google Scholar] [CrossRef] [PubMed]
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Dovesi, R. A new structural model for disorder in vaterite from first-principles calculations. CrystEngComm 2012, 14, 44–47. [Google Scholar] [CrossRef]
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Dovesi, R. The multiple structures of vaterite. Cryst. Growth Des. 2013, 13, 2247–2251. [Google Scholar] [CrossRef]
- Kabalah-Amitai, L.; Mayzel, B.; Kauffmann, Y.; Fitch, A.N.; Bloch, L.; Gilbert, P.U.P.A.; Pokroy, B. Vaterite crystals contain two interspersed crystal structures. Science 2013, 340, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ding, Y.; Li, F.Q.; Xie, B.; Qian, Y.T. Solvothermal growth of vaterite in the presence of ethylene glycol, 1,2-propanediol and glycerin. J. Cryst. Growth 2002, 236, 357–362. [Google Scholar] [CrossRef]
- Zhou, G.T.; Yao, Q.Z.; Fu, S.Q.; Guan, Y.B. Controlled crystallization of unstable vaterite with distinct morphologies and their polymorphic transition to stable calcite. Eur. J. Mineral. 2010, 22, 259–269. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Haase, A.; Antolini, R. Sub-micrometer vaterite containers: Synthesis, substance loading, and release. Angew. Chem. 2012, 124, 1221–1223. [Google Scholar] [CrossRef]
- Ševčík, R.; Pérez-Estébanez, M.; Viani, A.; Šašek, P.; Mácová, P. Characterization of vaterite synthesized at various temperatures and stirring velocities without use of additives. Powder Technol. 2015, 284, 265–271. [Google Scholar] [CrossRef]
- Donnelly, F.C.; Purcell-Milton, F.; Framont, V.; Cleary, O.; Dunne, P.W.; Gun’ko, Y.K. Synthesis of CaCO3 nano- and micro-particles by dry ice carbonation. Chem. Commun. 2017, 53, 6657–6660. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.B.; Xu, A.W.; Fang, Y.P.; Liu, H.Q.; Frommen, C.; Fenske, D. Surfactant-assisted growth of novel PbS dendritic nanostructures via facile hydrothermal process. Adv. Mater. 2003, 15, 1747–1750. [Google Scholar] [CrossRef]
- Cao, M.H.; Liu, T.F.; Gao, S.; Sun, G.B.; Wu, X.L.; Hu, C.W.; Wang, Z.L. Single-crystal dendritic micro-pines of magnetic α-Fe2O3: Large-scale synthesis, formation mechanism, and properties. Angew. Chem. Int. Ed. 2005, 44, 4197–4201. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yamauchi, Y. Block copolymer mediated synthesis of dendritic platinum nanoparticles. J. Am. Chem. Soc. 2009, 131, 9152–9153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.P.; Xie, Y.; Tang, R.; Chen, M.; Tian, X.B. Novel ultrasonically assisted templated synthesis of palladium and silver dendritic nanostructures. Adv. Mater. 2001, 13, 1887–1891. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Wang, X.; Cao, W.; Zhou, G. Controlled Synthesis and Microstructure of Metastable Flower-Like Vaterite. Materials 2018, 11, 2300. https://doi.org/10.3390/ma11112300
Guan Y, Wang X, Cao W, Zhou G. Controlled Synthesis and Microstructure of Metastable Flower-Like Vaterite. Materials. 2018; 11(11):2300. https://doi.org/10.3390/ma11112300
Chicago/Turabian StyleGuan, Yebin, Xiaohong Wang, Weicheng Cao, and Gentao Zhou. 2018. "Controlled Synthesis and Microstructure of Metastable Flower-Like Vaterite" Materials 11, no. 11: 2300. https://doi.org/10.3390/ma11112300
APA StyleGuan, Y., Wang, X., Cao, W., & Zhou, G. (2018). Controlled Synthesis and Microstructure of Metastable Flower-Like Vaterite. Materials, 11(11), 2300. https://doi.org/10.3390/ma11112300




