Carbon Redistribution and Microstructural Evolution Study during Two-Stage Quenching and Partitioning Process of High-Strength Steels by Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbon Diffusion at the Interface
2.2. Interface Migration
2.3. Martensite Formation in Quenching Processes
2.4. Interaction between Carbon Diffusion and Interface Migration
2.5. Carbon Diffusion in Martensite and Austenite
2.6. Simulation Conditions
3. Results and Discussion
3.1. One-Stage Q-P Process
3.1.1. Carbon Redistribution during the First Partitioning Process in the One-Stage Q-P Process
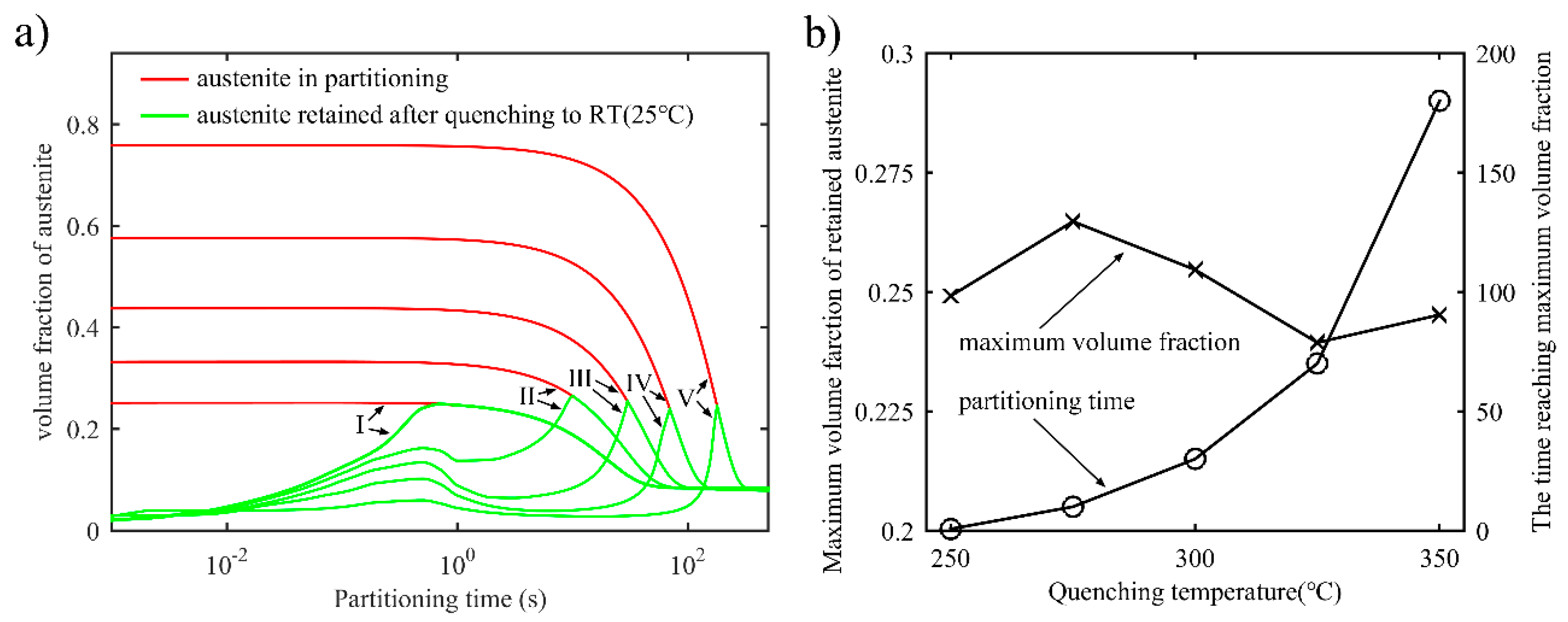
3.1.2. Retained Austenite after the Final Quenching in the One-Stage Q-P Process
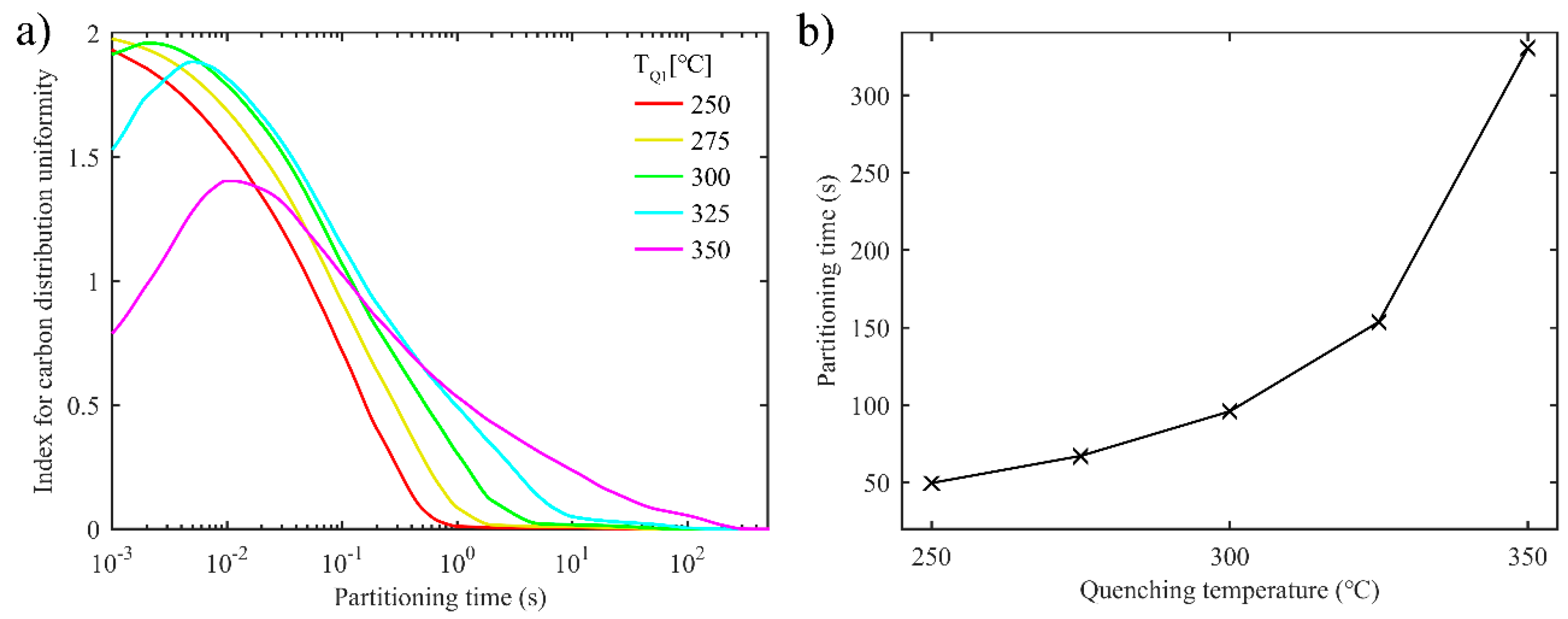
3.1.3. Uniformity of Carbon Distribution in Austenite during the First Partitioning Process in the One-Stage Q-P Process
3.2. Two-Stage Q-P Process
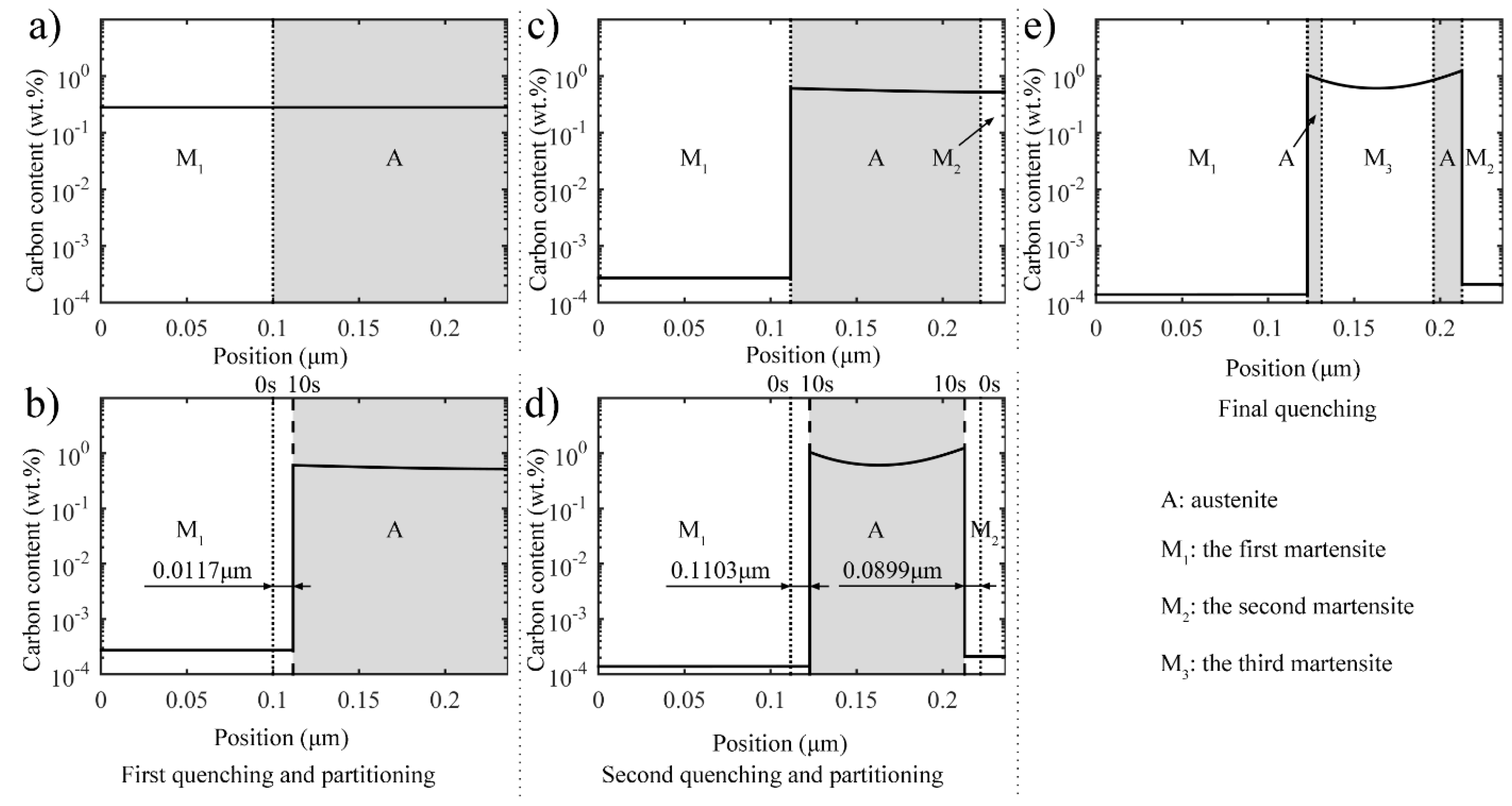
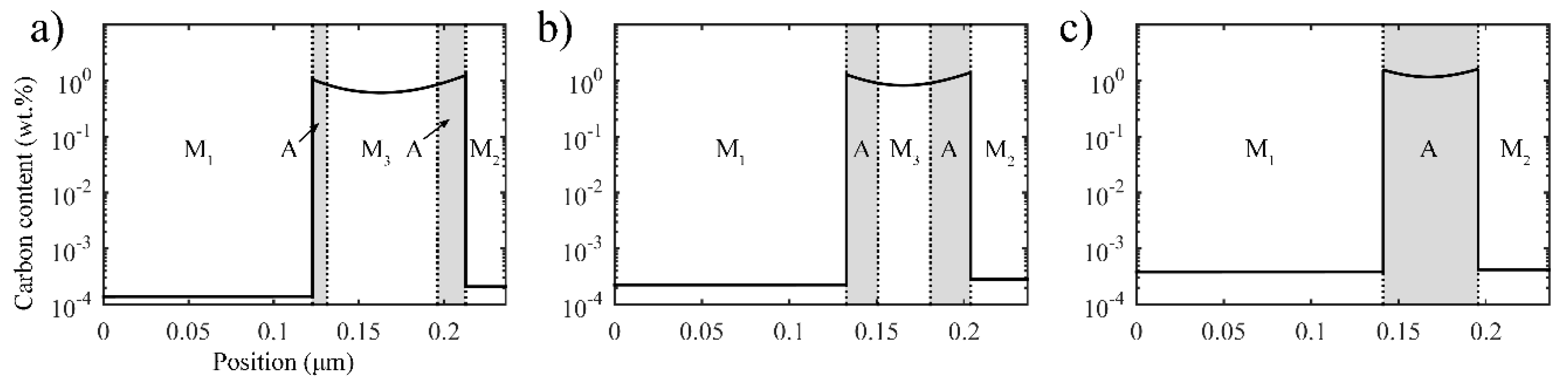
3.2.1. Carbon Redistribution during the Second Partitioning Process in the Two-Stage Q-P Process
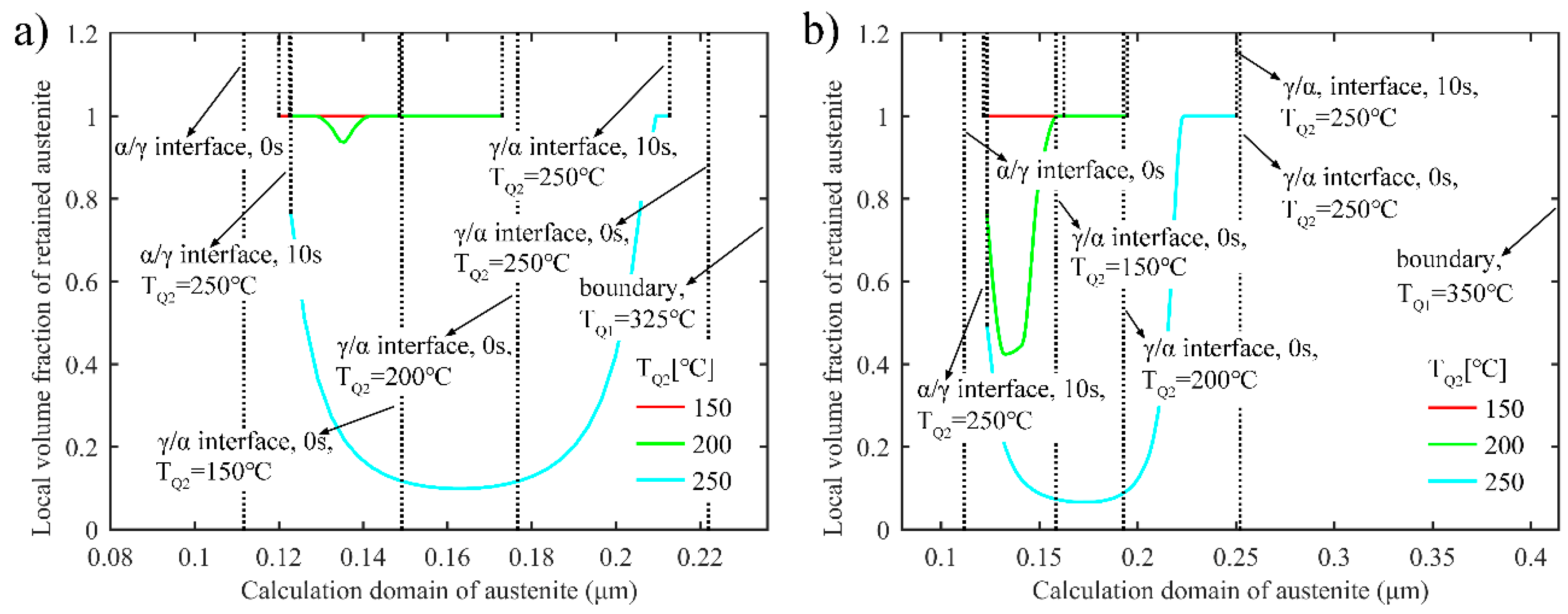
3.2.2. Retained Austenite after Final Quenching in the Two-Stage Q-P Process
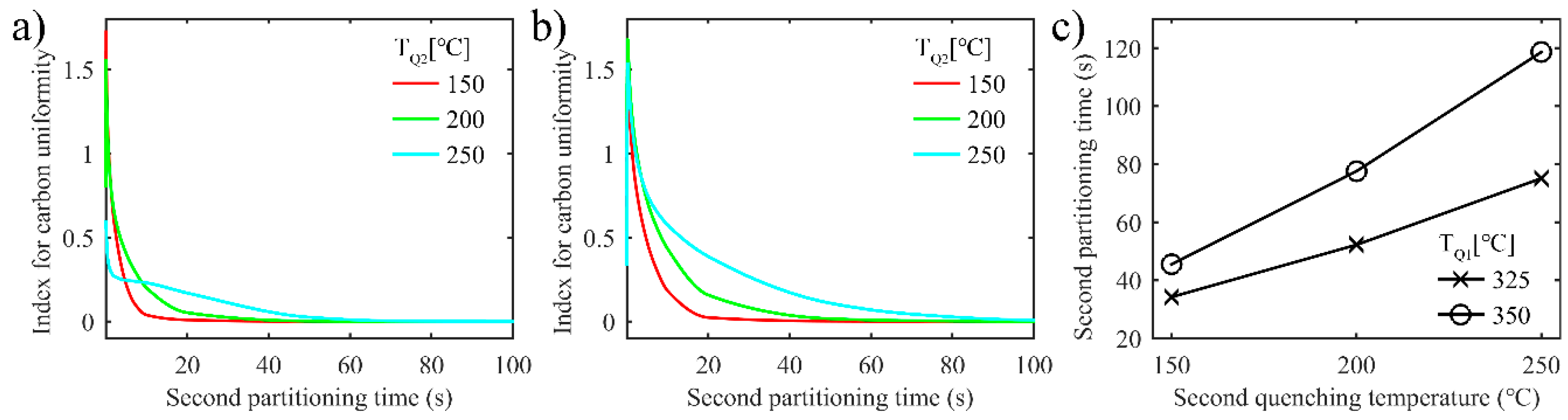
3.2.3. Uniformity of Carbon Distribution in Austenite during the Second Partitioning in the Two-Stage Q-P Process
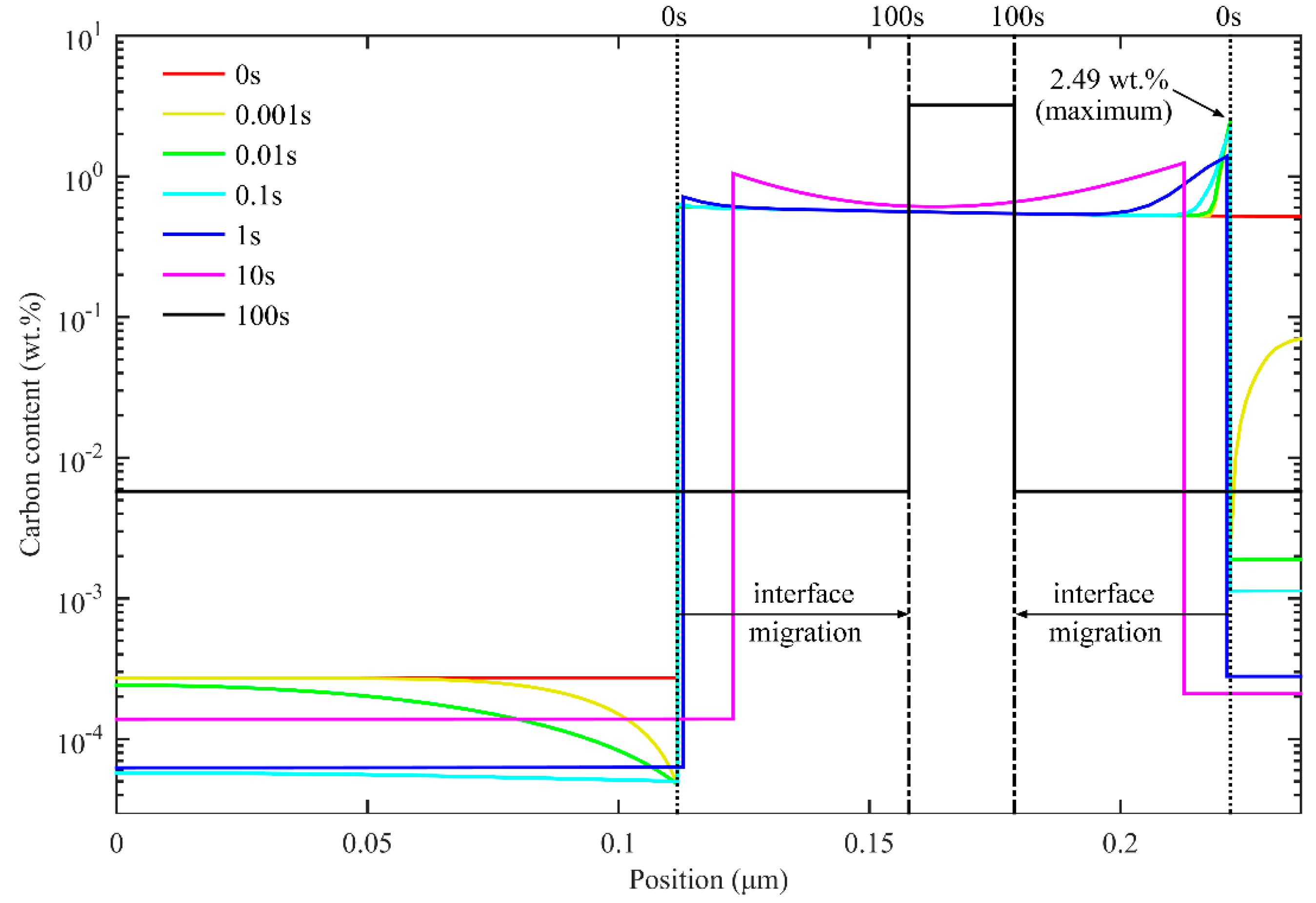
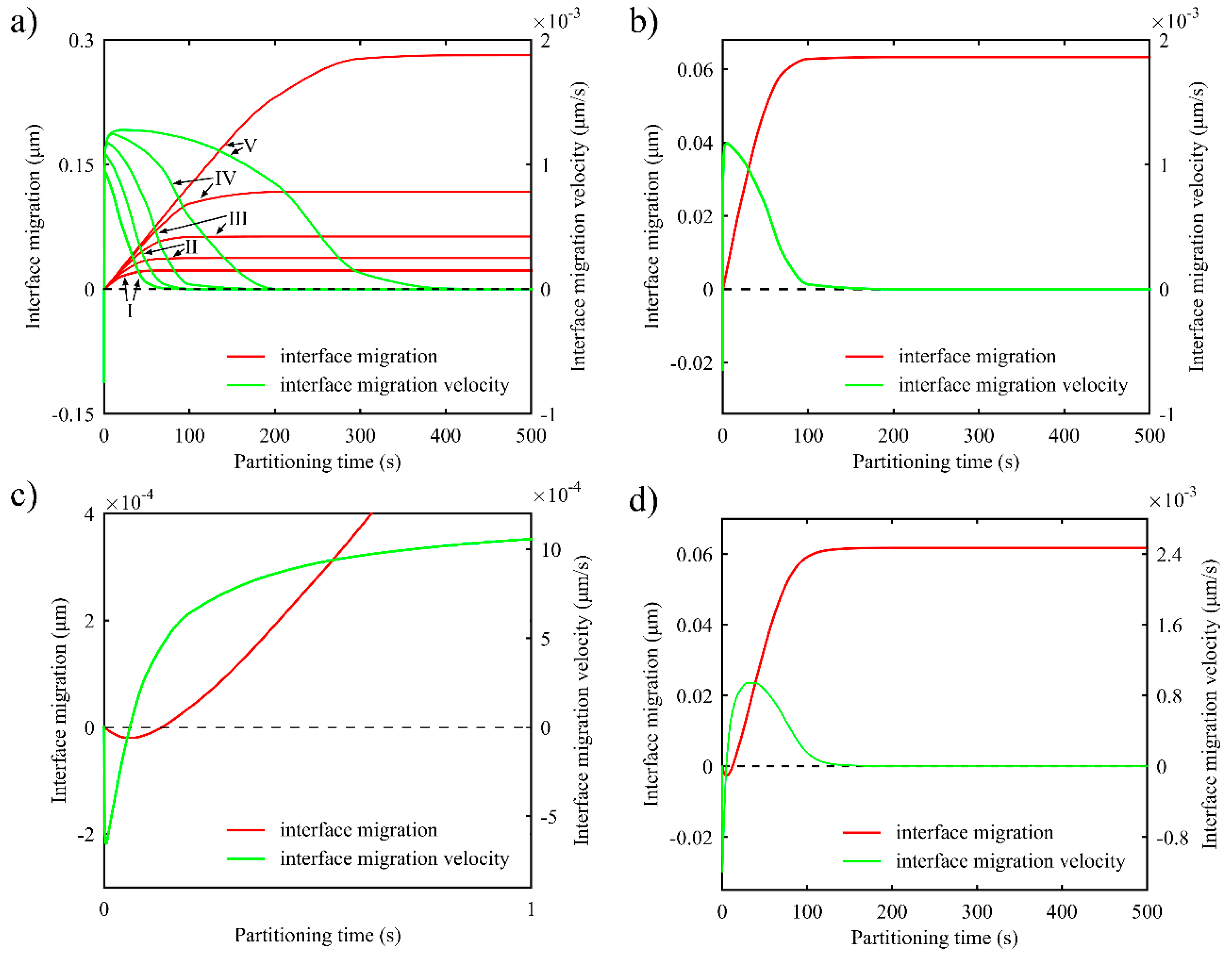
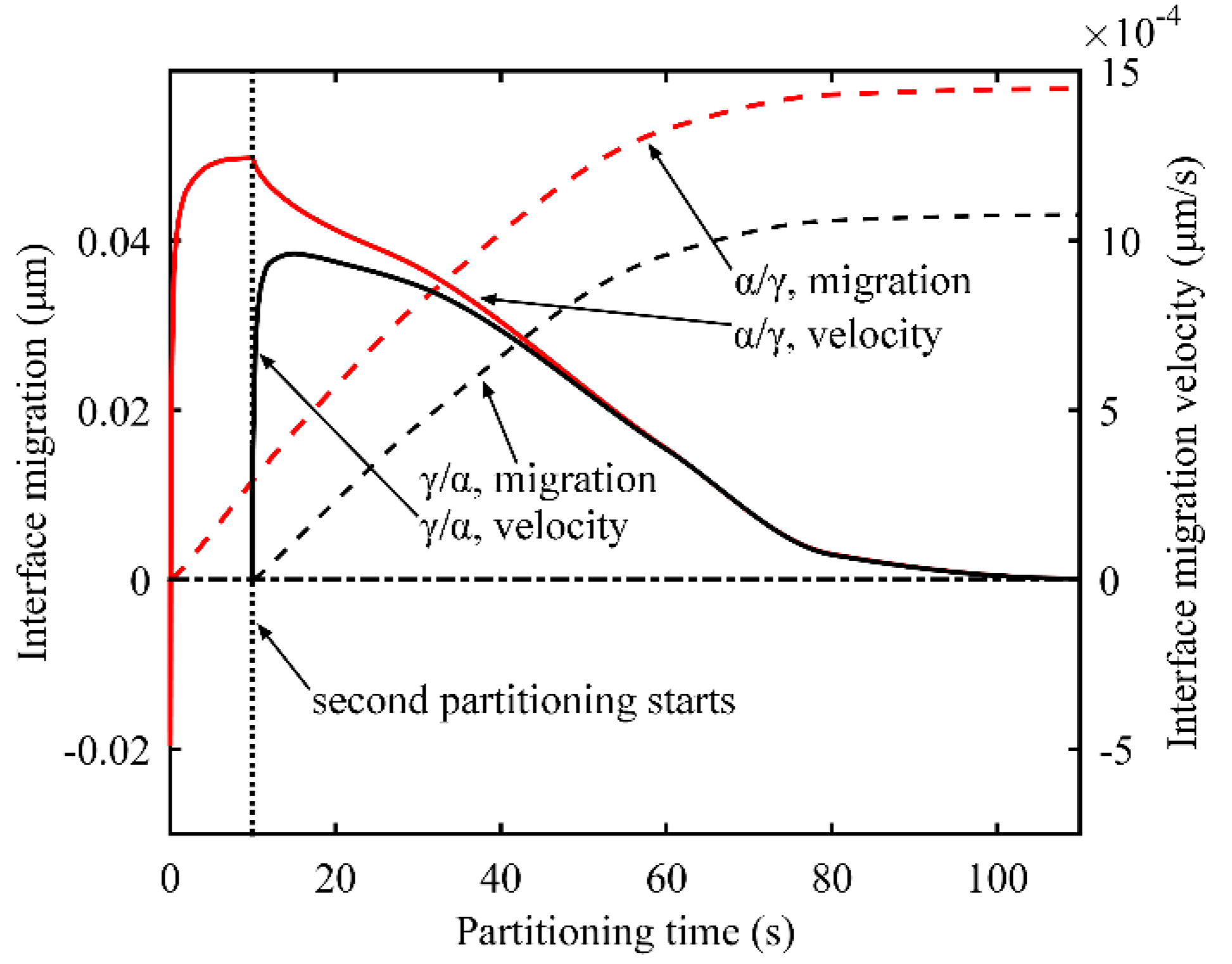
3.3. Interface Migration and Migration Velocity
3.3.1. One-Stage Q-P Process
3.3.2. Two-Stage Q-P process
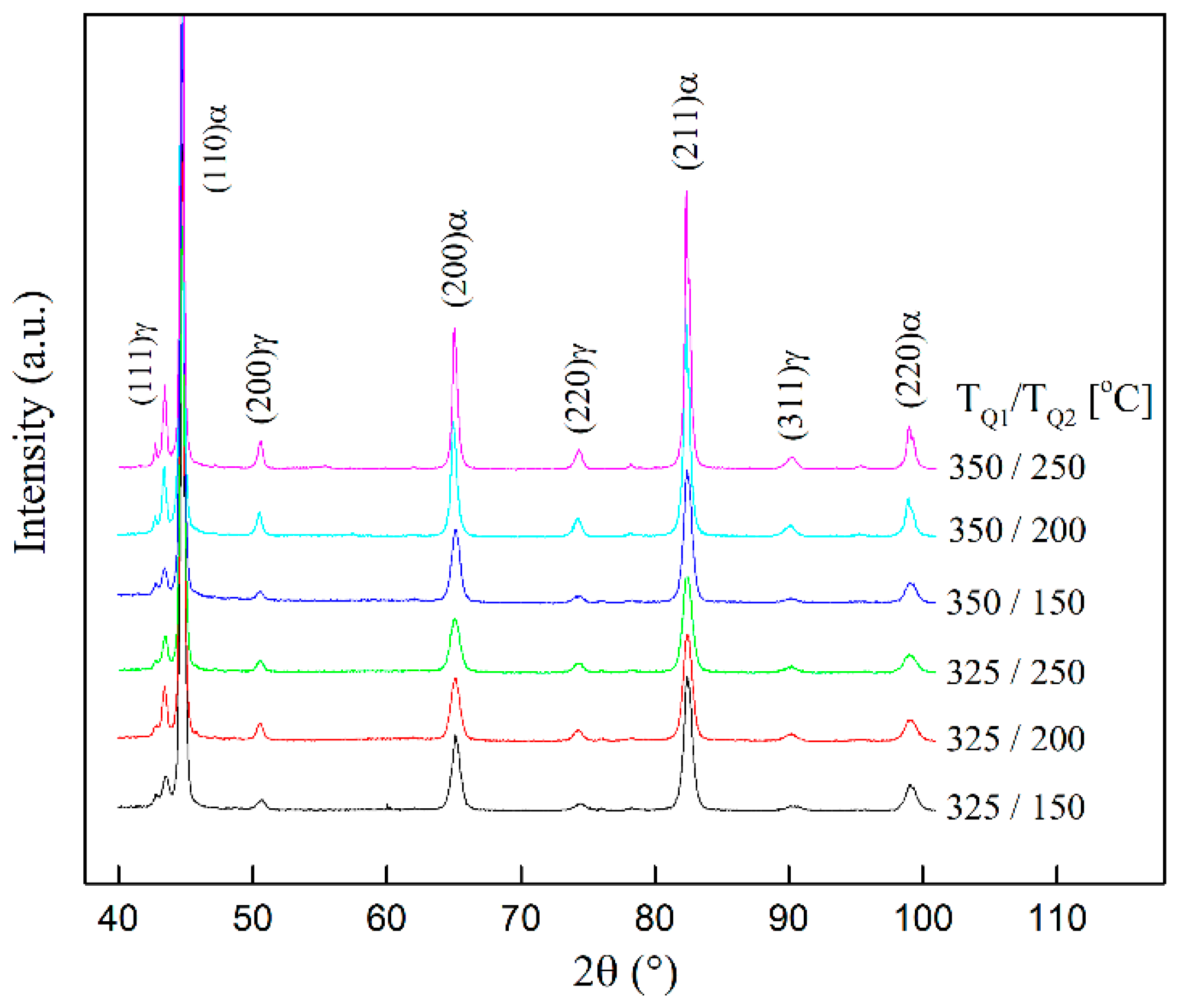
3.4. XRD Tests to Obtain the Volume Fraction of Austenite and Carbon Content in Austenite
4. Conclusions
- The two-stage Q-P process involves two quenching processes, with the second quenching temperature being lower than the first one, and both of which are between Ms and Mf, with each quenching process being followed by an incomplete partitioning process. The refined microstructure with low-carbon martensite laths, small-sized low-carbon martensite plates, retained austenite, and high-carbon martensite plates can be obtained using the two-stage Q-P process.
- In both the one-stage Q-P process and the two-stage Q-P process, the peak value of the volume fraction of stable retained austenite can be detected as the partitioning time increases. For example, a peak value of 25.5% can be obtained in the one-stage Q-P process when TQ1 = 300 °C, TP1 = 425 °C and tP1 = 30 s, while a peak volume fraction of 23.0% can be obtained in the two-stage Q-P process when TQ1 = 325 °C, TP1 = 425 °C and tP1 = 10 s, TQ2 = 250 °C, TP2 = 350 °C and tP2 = 30 s. Although the peak value of the volume fraction of retained austenite may be less in the two-stage Q-P process, the peak value is still considerable.
- In the two-stage Q-P process, a combination of suitable refined microstructure and higher retained austenite content can be achieved by using appropriate quenching temperatures and partitioning times.
- Partitioning temperature has great influence on interface migration at the beginning of partitioning process. With lower partitioning temperature, the carbon concentration in the austenite near the interface takes a longer time to drop below the equilibrium concentration, intensifying the tendency of interface migration to the martensite. Different heating processes in the partitioning process affect interface migration, but will not change the final conditions in partitioning process.
- During the second partitioning process in the two-stage Q-P process, as the carbon concentration in the austenite grows symmetrically to the middle of austenite, the interface migration velocity of the two interfaces tends to coincide in the second partitioning process.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santofimia, M.J.; Zhao, L.; Petrov, R.; Kwakernaak, C.; Sloof, W.G.; Sietsma, J. Microstructural development during the quenching and partitioning process in a newly designed low-carbon steel. Acta Mater. 2011, 59, 6059–6068. [Google Scholar] [CrossRef]
- Speer, J.G.; De Moor, E.; Clarke, A.J. Critical assessment 7: Quenching and partitioning. Mater. Sci. Technol. Lond. 2015, 31, 3–9. [Google Scholar] [CrossRef]
- Xie, Z.J.; Han, G.; Zhou, W.H.; Zeng, C.Y.; Shang, C.J. Study of retained austenite and nano-scale precipitation and their effects on properties of a low alloyed multi-phase steel by the two-step intercritical treatment. Mater. Charact. 2016, 113, 60–66. [Google Scholar] [CrossRef]
- Arlazarov, A.; Ollat, M.; Masse, J.P.; Bouzat, M. Influence of partitioning on mechanical behavior of q&p steels. Mater. Sci. Eng. A 2016, 661, 79–86. [Google Scholar] [CrossRef]
- Yan, S.; Liu, X.; Liu, W.J.; Lan, H.; Wu, H. Microstructural evolution and mechanical properties of low-carbon steel treated by a two-step quenching and partitioning process. Mater. Sci. Eng. A 2015, 640, 137–146. [Google Scholar] [CrossRef]
- De Moor, E.; Lacroix, S.; Clarke, A.J.; Penning, J.; Speer, J.G. Effect of retained austenite stabilized via quench and partitioning on the strain hardening of martensitic steels. Metall. Mater. Trans. A 2008, 39, 2586–2595. [Google Scholar] [CrossRef]
- Seo, E.J.; Cho, L.; Estrin, Y.; De Cooman, B.C. Microstructure-mechanical properties relationships for quenching and partitioning (q&p) processed steel. Acta Mater. 2016, 113, 124–139. [Google Scholar] [CrossRef]
- Liu, H.; Jin, X.; Dong, H.; Shi, J. Martensitic microstructural transformations from the hot stamping, quenching and partitioning process. Mater. Charact. 2011, 62, 223–227. [Google Scholar] [CrossRef]
- Yan, S.; Liu, X.; Liu, W.J.; Lan, H.; Wu, H. Comparison on mechanical properties and microstructure of a c–mn–si steel treated by quenching and partitioning (q&p) and quenching and tempering (q&t) processes. Mater. Sci. Eng. A 2015, 620, 58–66. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, P.; Li, W.; Guo, Z.; Rong, Y. Ultrahigh strength-ductility steel treated by a novel quenching–partitioning–tempering process. Mater. Sci. Eng. A 2014, 619, 205–211. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, K.; Wang, Y.; Gu, J.F.; Rong, Y.H. High strength-elongation product of Nb-microalloyed low-carbon steel by a novel quenching–partitioning–tempering process. Mater. Sci. Eng. A 2011, 528, 8006–8012. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Zhou, H.; Jiang, B.; Wang, G. A novel microstructural design and heat treatment technique based on gradient control of carbon partitioning between austenite and martensite for high strength steels. Sci. China Technol. Sci. 2013, 56, 1847–1857. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, F.; Zhou, H.; Wang, G.; Jiang, B. Stepping-quenching-partitioning treatment of 20simn2mova steel and effects of carbon and carbide forming elements. Sci. China Technol. Sci. 2012, 55, 1838–1843. [Google Scholar] [CrossRef]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Sietsma, J. Model for the interaction between interface migration and carbon diffusion during annealing of martensite–austenite microstructures in steels. Scr. Mater. 2008, 59, 159–162. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Speer, J.G.; Clarke, A.J.; Zhao, L.; Sietsma, J. Influence of interface mobility on the evolution of austenite–martensite grain assemblies during annealing. Acta Mater. 2009, 57, 4548–4557. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Barlat, F.; Lee, M. Transformation kinetics and density models of quenching and partitioning (q&p) steels. Acta Mater. 2016, 109, 394–404. [Google Scholar] [CrossRef]
- Dai, Z.; Ding, R.; Yang, Z.; Zhang, C.; Chen, H. Elucidating the effect of mn partitioning on interface migration and carbon partitioning during quenching and partitioning of the Fe-C-Mn-Si steels: Modeling and experiments. Acta Mater. 2018, 144, 666–678. [Google Scholar] [CrossRef]
- Hajyakbary, F.; Sietsma, J.; Miyamoto, G.; Furuhara, T.; Santofimia, M.J. Interaction of carbon partitioning, carbide precipitation and bainite formation during the q&p process in a low c steel. Acta Mater. 2016, 104, 72–83. [Google Scholar] [CrossRef]
- Toji, Y.; Matsuda, H.; Raabe, D. Effect of Si on the acceleration of bainite transformation by pre-existing martensite. Acta Mater. 2016, 116, 250–262. [Google Scholar] [CrossRef]
- Toji, Y.; Miyamoto, G.; Raabe, D. Carbon partitioning during quenching and partitioning heat treatment accompanied by carbide precipitation. Acta Mater. 2015, 86, 137–147. [Google Scholar] [CrossRef]
- Pierce, D.T.; Coughlin, D.R.; Williamson, D.L.; Clarke, K.D.; Clarke, A.J.; Speer, J.G.; De Moor, E. Characterization of transition carbides in quench and partitioned steel microstructures by mössbauer spectroscopy and complementary techniques. Acta Mater. 2015, 90, 417–430. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, Z.; Wang, Y.; Rolfe, B.; Wang, L.; Zhang, Y. Application of a model for quenching and partitioning in hot stamping of high-strength steel. Metall. Mater. Trans. A 2018, 49, 1304–1312. [Google Scholar] [CrossRef]
- Mecozzi, M.G.; Eiken, J.; Santofimia, M.J.; Sietsma, J. Phase field modelling of microstructural evolution during the quenching and partitioning treatment in low-alloy steels. Comp. Mater. Sci. 2016, 112, 245–256. [Google Scholar] [CrossRef]
- Takahama, Y.; Santofimia, M.J.; Mecozzi, M.G.; Zhao, L.; Sietsma, J. Phase field simulation of the carbon redistribution during the quenching and partitioning process in a low-carbon steel. Acta Mater. 2012, 60, 2916–2926. [Google Scholar] [CrossRef]
- Seo, E.J.; Cho, L.; De Cooman, B.C. Kinetics of the partitioning of carbon and substitutional alloying elements during quenching and partitioning (q&p) processing of medium mn steel. Acta Mater. 2016, 107, 354–365. [Google Scholar] [CrossRef]
- Kim, B.; Sietsma, J.; Santofimia, M.J. The role of silicon in carbon partitioning processes in martensite/austenite microstructures. Mater. Des. 2017, 127, 336–345. [Google Scholar] [CrossRef]
- Nishikawa, A.S.; Santofimia, M.J.; Sietsma, J.; Goldenstein, H. Influence of bainite reaction on the kinetics of carbon redistribution during the quenching and partitioning process. Acta Mater. 2018, 142, 142–151. [Google Scholar] [CrossRef]
- Wilson, E.A.; Medina, S.F. Application of koistinen and marburger’s athermal equation for volume fraction of martensite to diffusional transformations obtained on continuous cooling 0·13%c high strength low alloy steel. Mater. Sci. Technol. Lond. 2000, 16, 630–633. [Google Scholar] [CrossRef]
- Mahieu, J.; De Cooman, B.C.; Maki, J. Phase transformation and mechanical properties of si-free cmnal transformation-induced plasticity-aided steel. Metall. Mater. Trans. A 2002, 33, 2573–2580. [Google Scholar] [CrossRef]
- De Knijf, D.; Santofimia, M.J.; Shi, H.; Bliznuk, V.; Föjer, C.; Petrov, R.; Xu, W. In situ austenite–martensite interface mobility study during annealing. Acta Mater. 2015, 90, 161–168. [Google Scholar] [CrossRef]





| C | Si | Mn | P | S | Cr | Nb |
|---|---|---|---|---|---|---|
| 0.28 | 1.60 | 1.10 | 0.012 | 0.005 | 0.99 | 0.029 |
| A (J·mol−1) | B (J·mol−1·K−1) | C (J·mol−1·wt.%−1) | D (J·mol−1·K−1·wt.%−1) |
|---|---|---|---|
| −90566 | 60.5 | 9776 | −5.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Geng, H.; Zhu, B.; Wang, Z.; Zhang, Y. Carbon Redistribution and Microstructural Evolution Study during Two-Stage Quenching and Partitioning Process of High-Strength Steels by Modeling. Materials 2018, 11, 2302. https://doi.org/10.3390/ma11112302
Wang Y, Geng H, Zhu B, Wang Z, Zhang Y. Carbon Redistribution and Microstructural Evolution Study during Two-Stage Quenching and Partitioning Process of High-Strength Steels by Modeling. Materials. 2018; 11(11):2302. https://doi.org/10.3390/ma11112302
Chicago/Turabian StyleWang, Yilin, Huicheng Geng, Bin Zhu, Zijian Wang, and Yisheng Zhang. 2018. "Carbon Redistribution and Microstructural Evolution Study during Two-Stage Quenching and Partitioning Process of High-Strength Steels by Modeling" Materials 11, no. 11: 2302. https://doi.org/10.3390/ma11112302
APA StyleWang, Y., Geng, H., Zhu, B., Wang, Z., & Zhang, Y. (2018). Carbon Redistribution and Microstructural Evolution Study during Two-Stage Quenching and Partitioning Process of High-Strength Steels by Modeling. Materials, 11(11), 2302. https://doi.org/10.3390/ma11112302




