In Vitro Comparison of the Efficacy of Peri-Implantitis Treatments on the Removal and Recolonization of Streptococcus gordonii Biofilm on Titanium Disks
Abstract
1. Introduction
2. Materials and Methods
2.1. Decontamination Assay
2.2. Recolonization Assay
3. Results
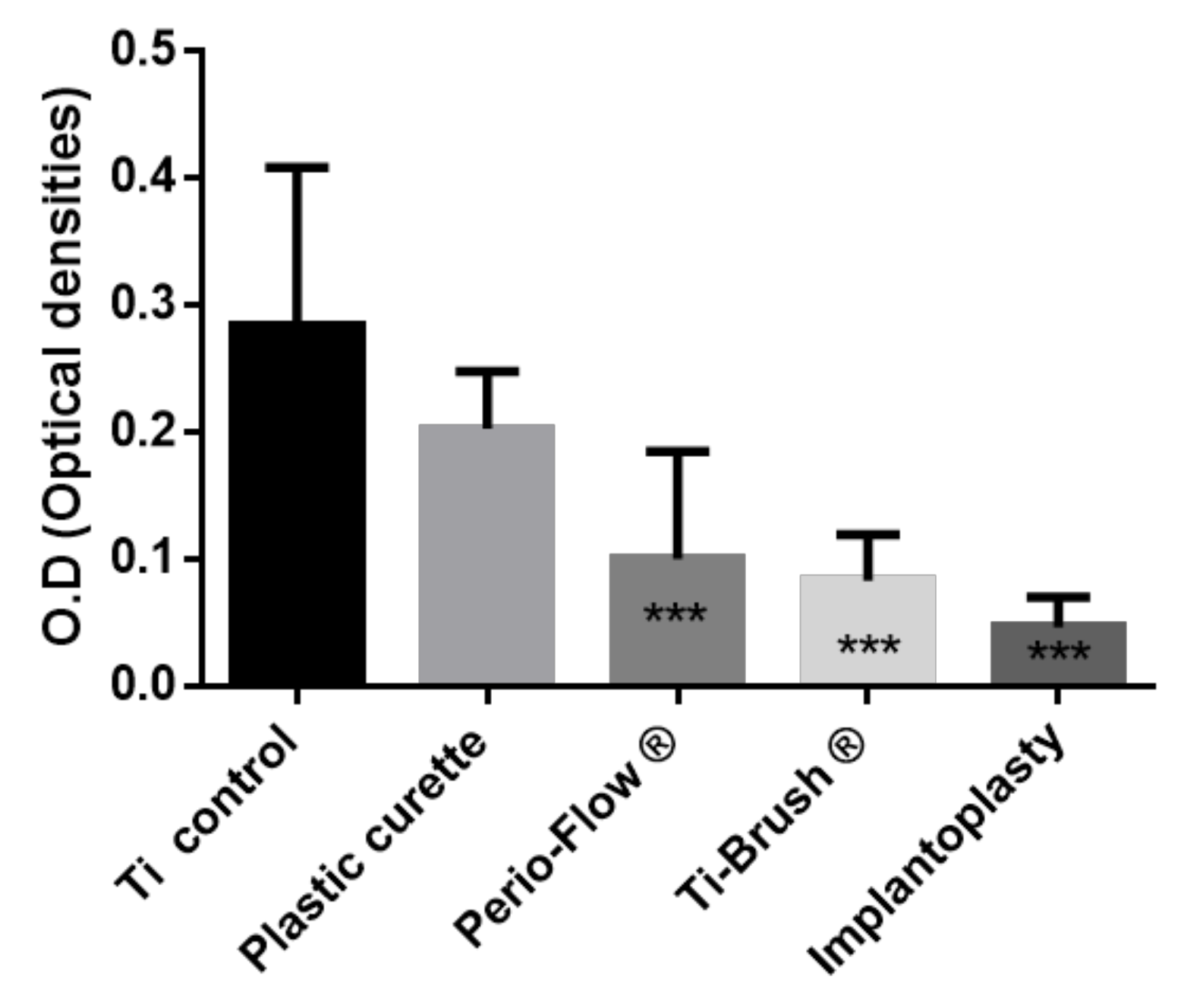
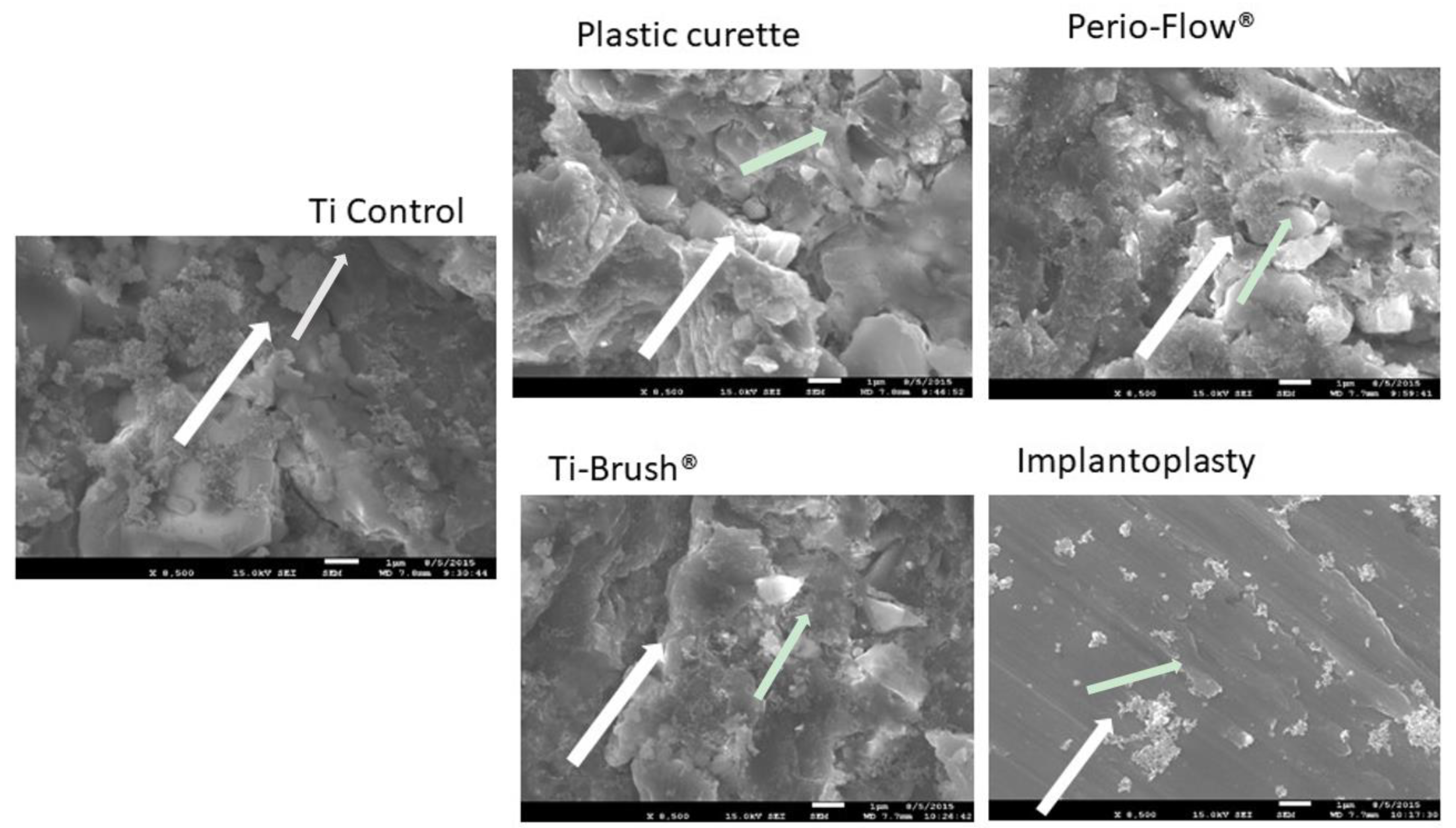
3.1. Bacterial Elimination After Surface Decontamination
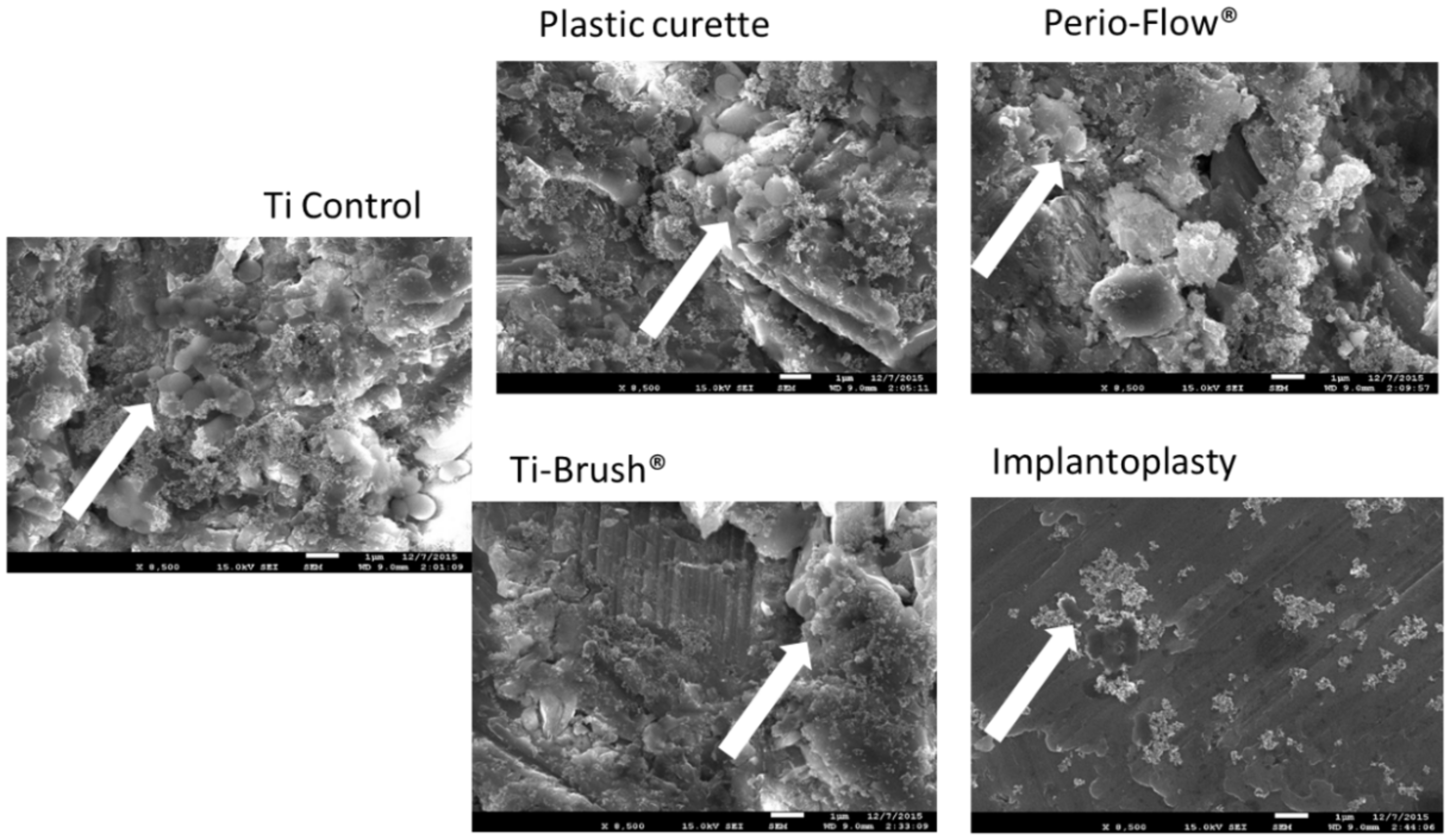
3.2. Bacterial Recolonization After Surface Treatment
3.3. Surface Roughness
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29, 197–212. [Google Scholar] [CrossRef]
- Mombelli, A.; Muller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implants Res. 2012, 23, 67–76. [Google Scholar] [CrossRef]
- Roos-Jansaker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine- to fourteen-year follow-up of implant treatment. Part I: Implant loss and associations to various factors. J. Clin. Periodontol. 2006, 33, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Marrone, A.; Lasserre, J.; Bercy, P.; Brecx, M.C. Prevalence and risk factors for peri-implant disease in Belgian adults. Clin. Oral Implants Res. 2013, 24, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontology 2010, 53, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Bonino, F.; Aglietta, M.; Dalmasso, P. Ten-year results of a three arms prospective cohort study on implants in periodontally compromised patients. Part 2: Clinical results. Clin. Oral Implants Res. 2012, 23, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; De Angelis, N.; Bonino, L.; Aglietta, M. Ten-year results of a three-arm prospective cohort study on implants in periodontally compromised patients. Part 1: Implant loss and radiographic bone loss. Clin. Oral Implants Res. 2010, 21, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Quirynen, M. Risk indicators for peri-implantitis. A narrative review. Clin. Oral Implants Res. 2015, 26, 15–44. [Google Scholar] [CrossRef] [PubMed]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: A systematic review. Clin. Oral Implants Res. 2014, 25, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F.A. Titanium surface alterations following the use of different mechanical instruments: A systematic review. Clin. Oral Implants Res. 2012, 23, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Interventions for replacing missing teeth: Treatment of peri-implantitis. Cochrane Database Syst. Rev. 2012, 1, Cd004970. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Polyzois, I.; Claffey, N. Surgical therapy for the control of peri-implantitis. Clin. Oral Implants Res. 2012, 23, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Tastepe, C.S.; van Waas, R.; Liu, Y.; Wismeijer, D. Air powder abrasive treatment as an implant surface cleaning method: A literature review. Int. J. Oral Maxillofac. Implants 2012, 27, 1461–1473. [Google Scholar] [PubMed]
- Sahrmann, P.; Ronay, V.; Hofer, D.; Attin, T.; Jung, R.E.; Schmidlin, P.R. In vitro cleaning potential of three different implant debridement methods. Clin. Oral Implants Res. 2015, 26, 314–319. [Google Scholar] [CrossRef] [PubMed]
- John, G.; Becker, J.; Schwarz, F. Rotating titanium brush for plaque removal from rough titanium surfaces—An in vitro study. Clin. Oral Implants Res. 2014, 25, 838–842. [Google Scholar] [CrossRef]
- Romeo, E.; Ghisolfi, M.; Murgolo, N.; Chiapasco, M.; Lops, D.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part I: Clinical outcome. Clin. Oral Implants Res. 2005, 16, 9–18. [Google Scholar] [CrossRef]
- Geng, H.; Yuan, Y.; Adayi, A.; Zhang, X.; Song, X.; Gong, L.; Zhang, X.; Gao, P. Engineered chimeric peptides with antimicrobial and titanium-binding functions to inhibit biofilm formation on Ti implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 141–154. [Google Scholar] [CrossRef]
- Ota-Tsuzuki, C.; Martins, F.L.; Giorgetti, A.P.; de Freitas, P.M.; Duarte, P.M. In vitro adhesion of Streptococcus sanguinis to dentine root surface after treatment with Er:YAG laser, ultrasonic system, or manual curette. Photomed. Laser Surg. 2009, 27, 735–741. [Google Scholar] [CrossRef]
- Toma, S.; Lasserre, J.; Brecx, M.C.; Nyssen-Behets, C. In vitro evaluation of peri-implantitis treatment modalities on Saos-2osteoblasts. Clin. Oral Implants Res. 2016, 27, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Augthun, M.; Tinschert, J.; Huber, A. In vitro studies on the effect of cleaning methods on different implant surfaces. J. Periodontol. 1998, 69, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Mengel, R.; Buns, C.E.; Mengel, C.; Flores-de-Jacoby, L. An in vitro study of the treatment of implant surfaces with different instruments. Int. J. Oral Maxillofac. Implants 1998, 13, 91–96. [Google Scholar] [PubMed]
- Ruhling, A.; Kocher, T.; Kreusch, J.; Plagmann, H.C. Treatment of subgingival implant surfaces with Teflon-coated sonic and ultrasonic scaler tips and various implant curettes. An in vitro study. Clin. Oral Implants Res. 1994, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Parham, P.L., Jr.; Cobb, C.M.; French, A.A.; Love, J.W.; Drisko, C.L.; Killoy, W.J. Effects of an air-powder abrasive system on plasma-sprayed titanium implant surfaces: An in vitro evaluation. J. Oral Implantol. 1989, 15, 78–86. [Google Scholar] [PubMed]
- Duarte, P.M.; Reis, A.F.; de Freitas, P.M.; Ota-Tsuzuki, C. Bacterial adhesion on smooth and rough titanium surfaces after treatment with different instruments. J. Periodontol. 2009, 80, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Chairay, J.P.; Boulekbache, H.; Jean, A.; Soyer, A.; Bouchard, P. Scanning electron microscopic evaluation of the effects of an air-abrasive system on dental implants: A comparative in vitro study between machined and plasma-sprayed titanium surfaces. J. Periodontol. 1997, 68, 1215–1222. [Google Scholar] [CrossRef]
- Menini, M.; Piccardo, P.; Baldi, D.; Dellepiane, E.; Pera, P. Morphological and chemical characteristics of different titanium surfaces treated by bicarbonate and glycine powder air abrasive systems. Implant Dent. 2015, 24, 47–56. [Google Scholar] [CrossRef]
- Park, J.B.; Jang, Y.J.; Koh, M.; Choi, B.K.; Kim, K.K.; Ko, Y. In vitro analysis of the efficacy of ultrasonic scalers and a toothbrush for removing bacteria from resorbable blast material titanium disks. J. Periodontol. 2013, 84, 1191–1198. [Google Scholar] [CrossRef]
- Park, J.B.; Kim, N.; Ko, Y. Effects of ultrasonic scaler tips and toothbrush on titanium disc surfaces evaluated with confocal microscopy. J. Craniofac. Surg. 2012, 23, 1552–1558. [Google Scholar] [CrossRef]
- Widodo, A.; Spratt, D.; Sousa, V.; Petrie, A.; Donos, N. An in vitro study on disinfection of titanium surfaces. Clin. Oral Implants Res. 2016, 27, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, S.; Iorio Siciliano, V.; Aglietta, M.; Andreuccetti, G.; Salvi, G.E. Clinical and radiographic outcomes of a combined resective and regenerative approach in the treatment of peri-implantitis: A prospective case series. Clin. Oral Implants Res. 2014, 25, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Lops, D.; Storelli, S.; Ghisolfi, M. Clinical peri-implant sounding accuracy in the presence of chronic inflammation of peri-implant tissues. Clinical observation study. Miner. Stomatol. 2009, 58, 81–91. [Google Scholar]
- Schwarz, F.; John, G.; Schmucker, A.; Sahm, N.; Becker, J. Combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination: A 7-year follow-up observation. J. Clin. Periodontol. 2017, 44, 337–342. [Google Scholar] [CrossRef]
- Ramel, C.F.; Lussi, A.; Ozcan, M.; Jung, R.E.; Hammerle, C.H.; Thoma, D.S. Surface roughness of dental implants and treatment time using six different implantoplasty procedures. Clin. Oral Implants Res. 2016, 27, 776–781. [Google Scholar] [CrossRef]
- Schwarz, F.; John, G.; Becker, J. The influence of implantoplasty on the diameter, chemical surface composition, and biocompatibility of titanium implants. Clin. Oral Investig. 2017, 21, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Subramani, K.; Wismeijer, D. Decontamination of titanium implant surface and re-osseointegration to treat peri-implantitis: A literature review. Int. J. Oral Maxillofac. Implants 2012, 27, 1043–1054. [Google Scholar]
- Busscher, H.J.; van der Mei, H.C. Physico-chemical interactions in initial microbial adhesion and relevance for biofilm formation. Adv. Dent. Res. 1997, 11, 24–32. [Google Scholar] [CrossRef]
- Carlen, A.; Nikdel, K.; Wennerberg, A.; Holmberg, K.; Olsson, J. Surface characteristics and in vitro biofilm formation on glass ionomer and composite resin. Biomaterials 2001, 22, 481–487. [Google Scholar] [CrossRef]
- Rimondini, L.; Fare, S.; Brambilla, E.; Felloni, A.; Consonni, C.; Brossa, F.; Carrassi, A. The effect of surface roughness on early in vivo plaque colonization on titanium. J. Periodontol. 1997, 68, 556–562. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Ge, J.; Catt, D.M.; Gregory, R.L. Streptococcus mutans surface alpha-enolase binds salivary mucin MG2 and human plasminogen. Infect. Immun. 2004, 72, 6748–6752. [Google Scholar] [CrossRef]
- Okada, A.; Nikaido, T.; Ikeda, M.; Okada, K.; Yamauchi, J.; Foxton, R.M.; Sawada, H.; Tagami, J.; Matin, K. Inhibition of biofilm formation using newly developed coating materials with self-cleaning properties. Dent. Mater. J. 2008, 27, 565–572. [Google Scholar] [CrossRef]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: Radiographic outcome. Clin. Oral Implants Res. 2007, 18, 179–187. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wunder, A.; Auschill, T.M.; Follo, M.; Braun, G.; Hellwig, E.; Arweiler, N.B. The in vivo dynamics of Streptococcus spp., Actinomyces naeslundii, Fusobacterium nucleatum and Veillonella spp. in dental plaque biofilm as analysed by five-colour multiplex fluorescence in situ hybridization. J. Med. Microbiol. 2007, 56, 681–687. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. The oral cavity—A key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; London, J. Adhere today, here tomorrow: Oral bacterial adherence. J. Bacteriol. 1993, 175, 3247–3252. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.C.; Norde, W.; Zehnder, A.J. Physical chemical description of bacterial adhesion. J. Biomater. Appl. 1990, 5, 91–106. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef]
- Giaouris, E.; Chapot-Chartier, M.P.; Briandet, R. Surface physicochemical analysis of natural Lactococcus lactis strains reveals the existence of hydrophobic and low charged strains with altered adhesive properties. Int. J. Food Microbiol. 2009, 131, 2–9. [Google Scholar] [CrossRef]
- Conserva, E.; Generali, L.; Bandieri, A.; Cavani, F.; Borghi, F.; Consolo, U. Plaque accumulation on titanium disks with different surface treatments: An in vivo investigation. Odontology 2018, 106, 145–153. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Wang, H.L.; Carossa, S.; Mussano, F. Plasma of Argon Increases Cell Attachment and Bacterial Decontamination on Different Implant Surfaces. Int. J. Oral Maxillofac. Implants 2017, 32, 1315–1323. [Google Scholar] [CrossRef]

| Treatment | Ra (Mean ± SD) | Rz (Mean ± SD) | p Value |
|---|---|---|---|
| Control group | 1.65 ± 0.107 | 9.79 ± 0.34 | ns |
| Plastic curette | 1.61 ± 0.17 | 9.98 ± 0.24 | ns |
| Perio-Flow® | 1.31 ± 0.14 | 9.22 ± 0.27 | ns |
| Ti-Brush® | 1.22 ± 0.31 | 8.76 ± 0.15 | ns |
| Implantoplasty | 0.98 ± 0.12 | 7.26 ± 0.022 | ** p < 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, S.; Behets, C.; Brecx, M.C.; Lasserre, J.F. In Vitro Comparison of the Efficacy of Peri-Implantitis Treatments on the Removal and Recolonization of Streptococcus gordonii Biofilm on Titanium Disks. Materials 2018, 11, 2484. https://doi.org/10.3390/ma11122484
Toma S, Behets C, Brecx MC, Lasserre JF. In Vitro Comparison of the Efficacy of Peri-Implantitis Treatments on the Removal and Recolonization of Streptococcus gordonii Biofilm on Titanium Disks. Materials. 2018; 11(12):2484. https://doi.org/10.3390/ma11122484
Chicago/Turabian StyleToma, Selena, Catherine Behets, Michel C. Brecx, and Jerome F. Lasserre. 2018. "In Vitro Comparison of the Efficacy of Peri-Implantitis Treatments on the Removal and Recolonization of Streptococcus gordonii Biofilm on Titanium Disks" Materials 11, no. 12: 2484. https://doi.org/10.3390/ma11122484
APA StyleToma, S., Behets, C., Brecx, M. C., & Lasserre, J. F. (2018). In Vitro Comparison of the Efficacy of Peri-Implantitis Treatments on the Removal and Recolonization of Streptococcus gordonii Biofilm on Titanium Disks. Materials, 11(12), 2484. https://doi.org/10.3390/ma11122484






