New Biocompatible Mesoporous Silica/Polysaccharide Hybrid Materials as Possible Drug Delivery Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Immobilization of Benzalkonium Chloride (BZC) to Mesoporous Silica Nanoparticles (MSN)
2.3. Synthesis of Chitosan (CS)-BZC and CS-MSN-BZC Hybrid Materials
2.4. Synthesis of Alginate (Al)-BZC and Al-MSN-BZC Hybrid Materials
2.5. Characterization
2.6. Adsorption Experiments
2.7. In Vitro Drug Release Studies
2.8. In Vitro Kinetic Evaluation
3. Results and Discussion
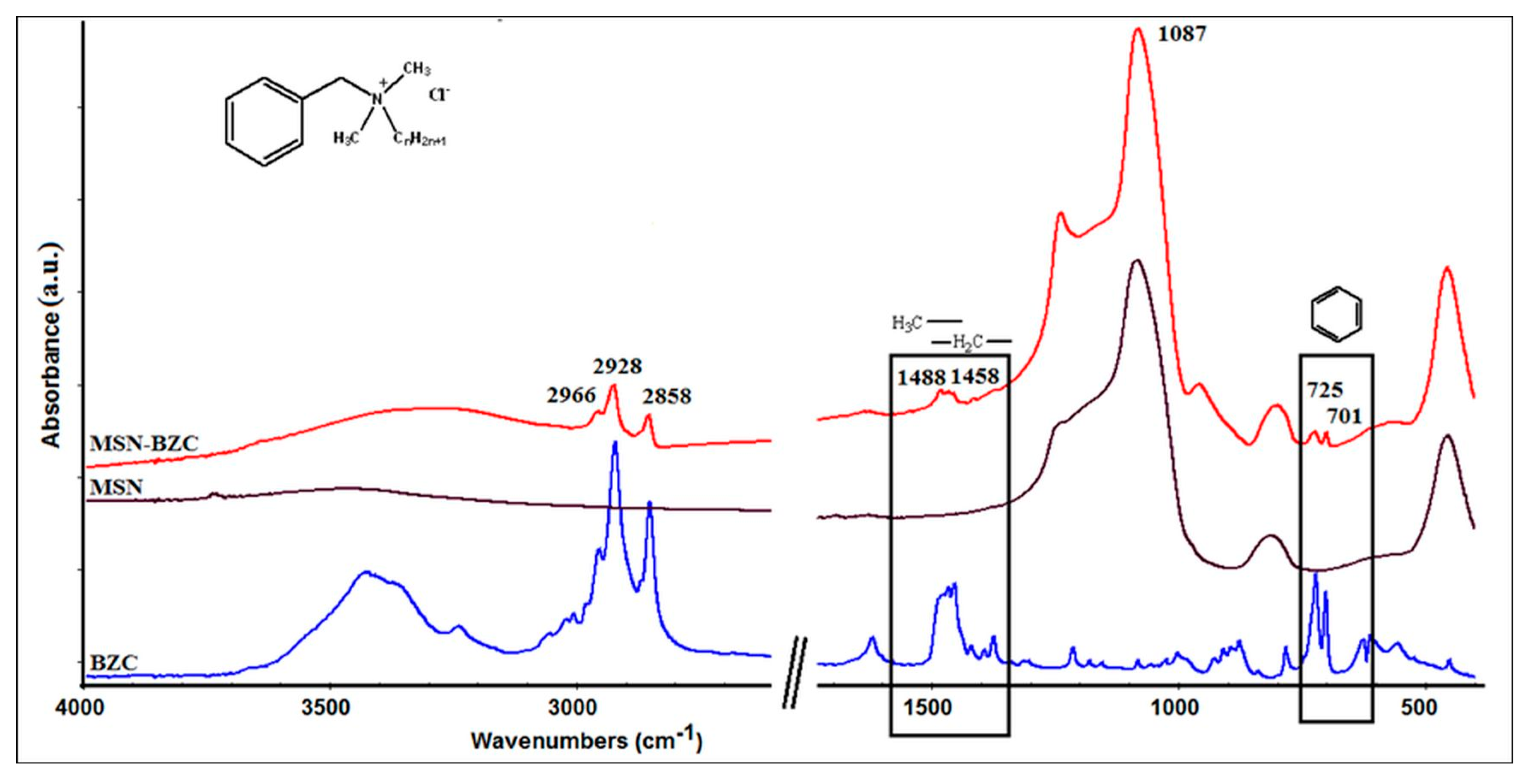
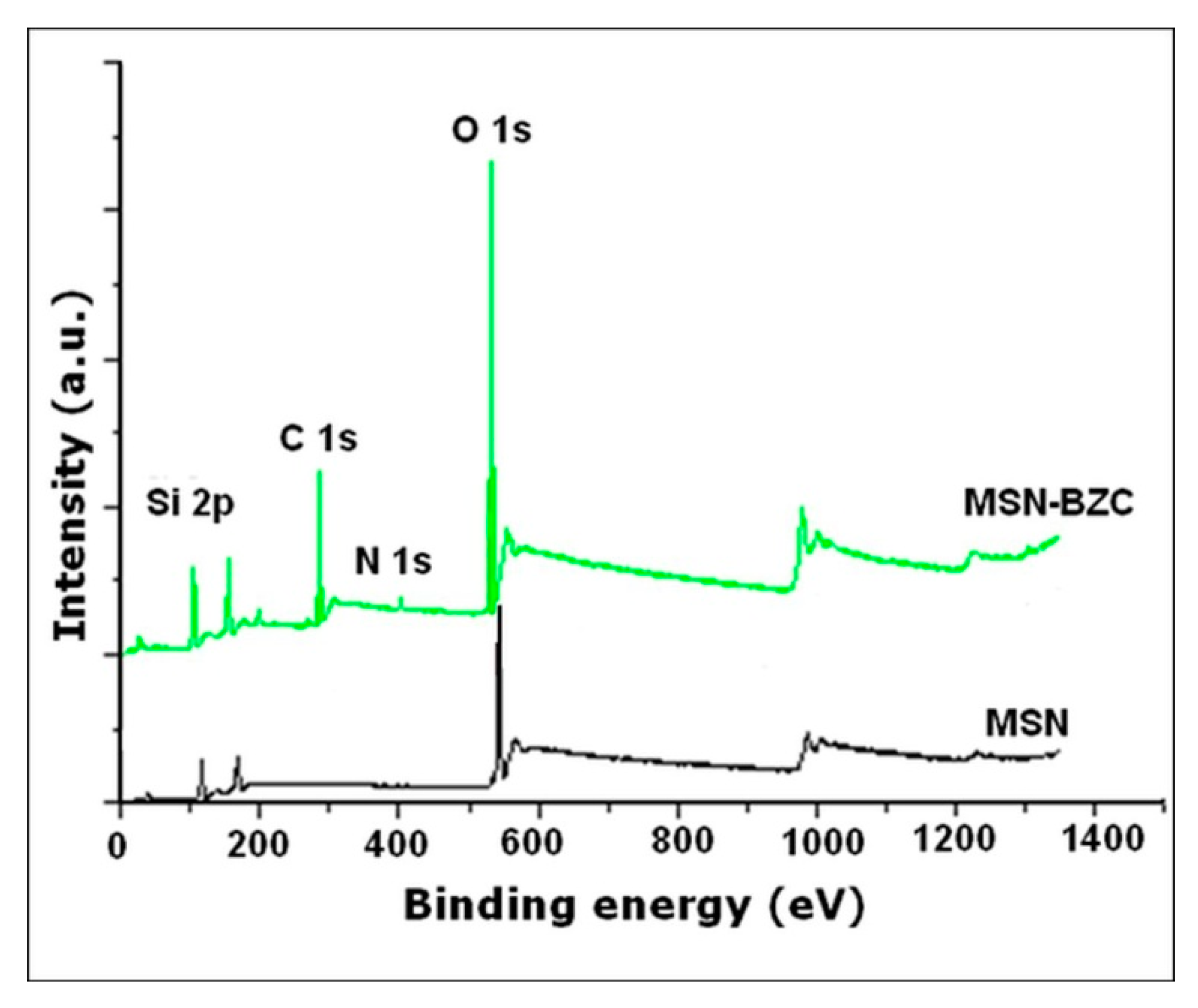
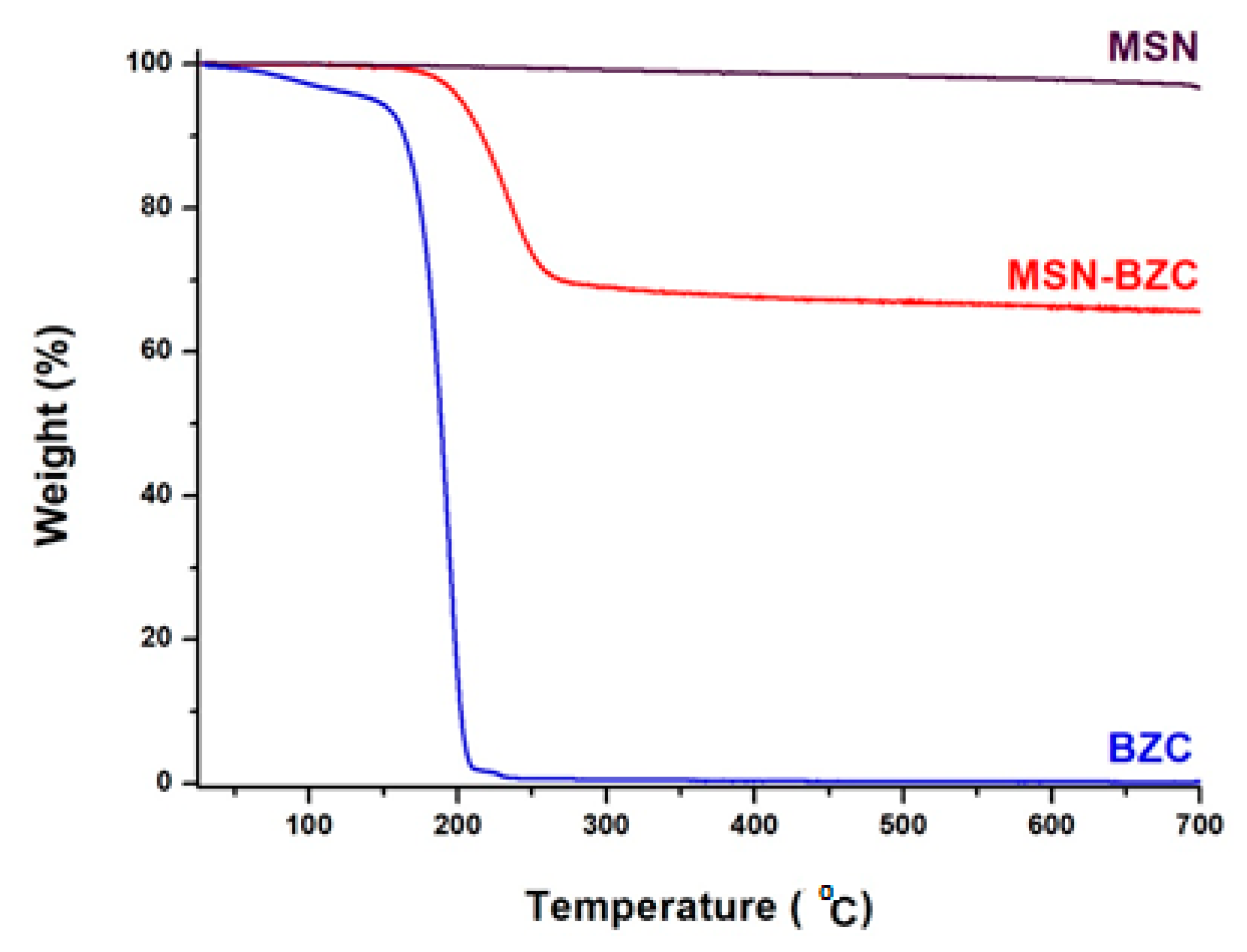
3.1. Characterization of the Modified MSN with BZC
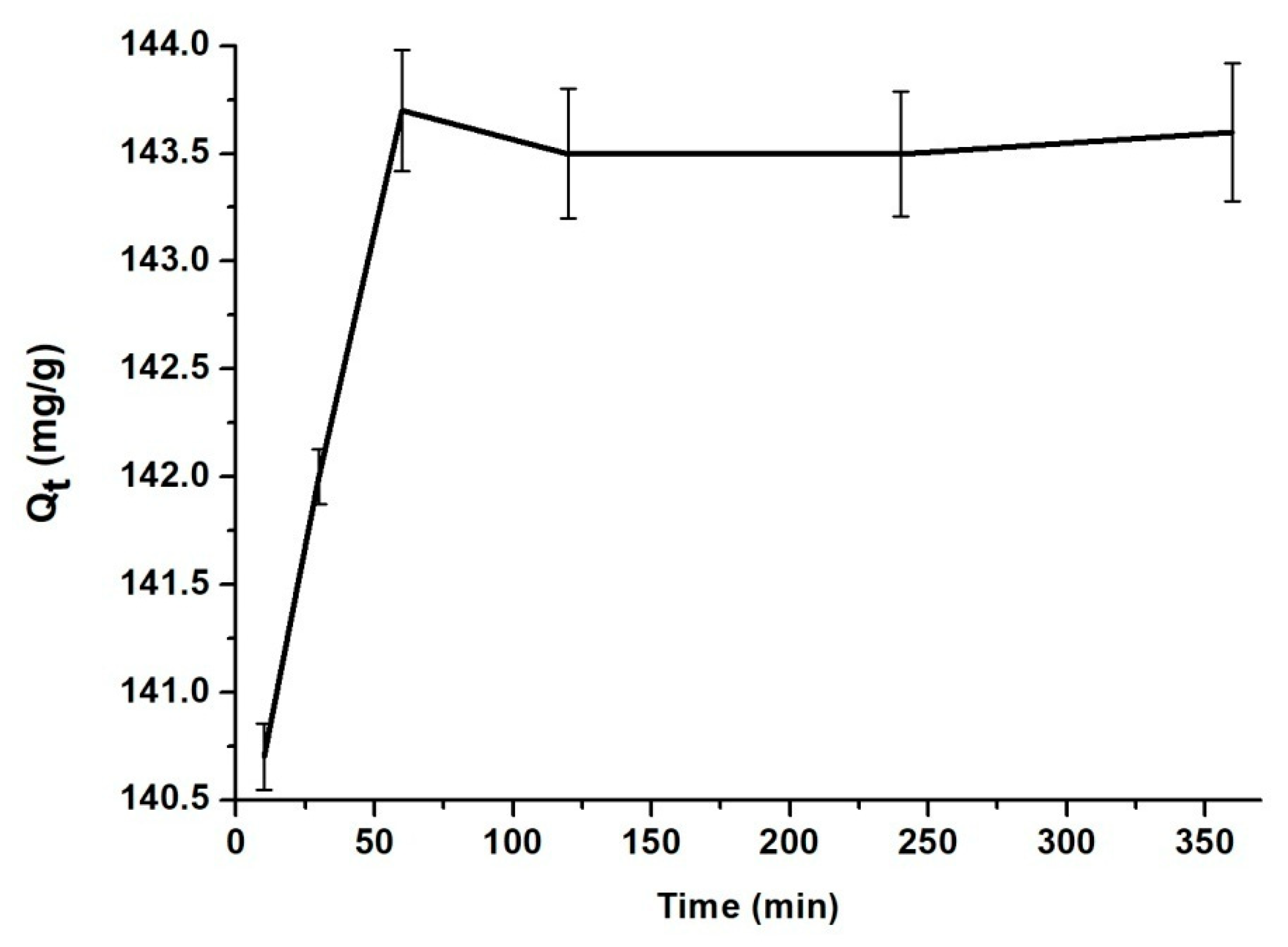
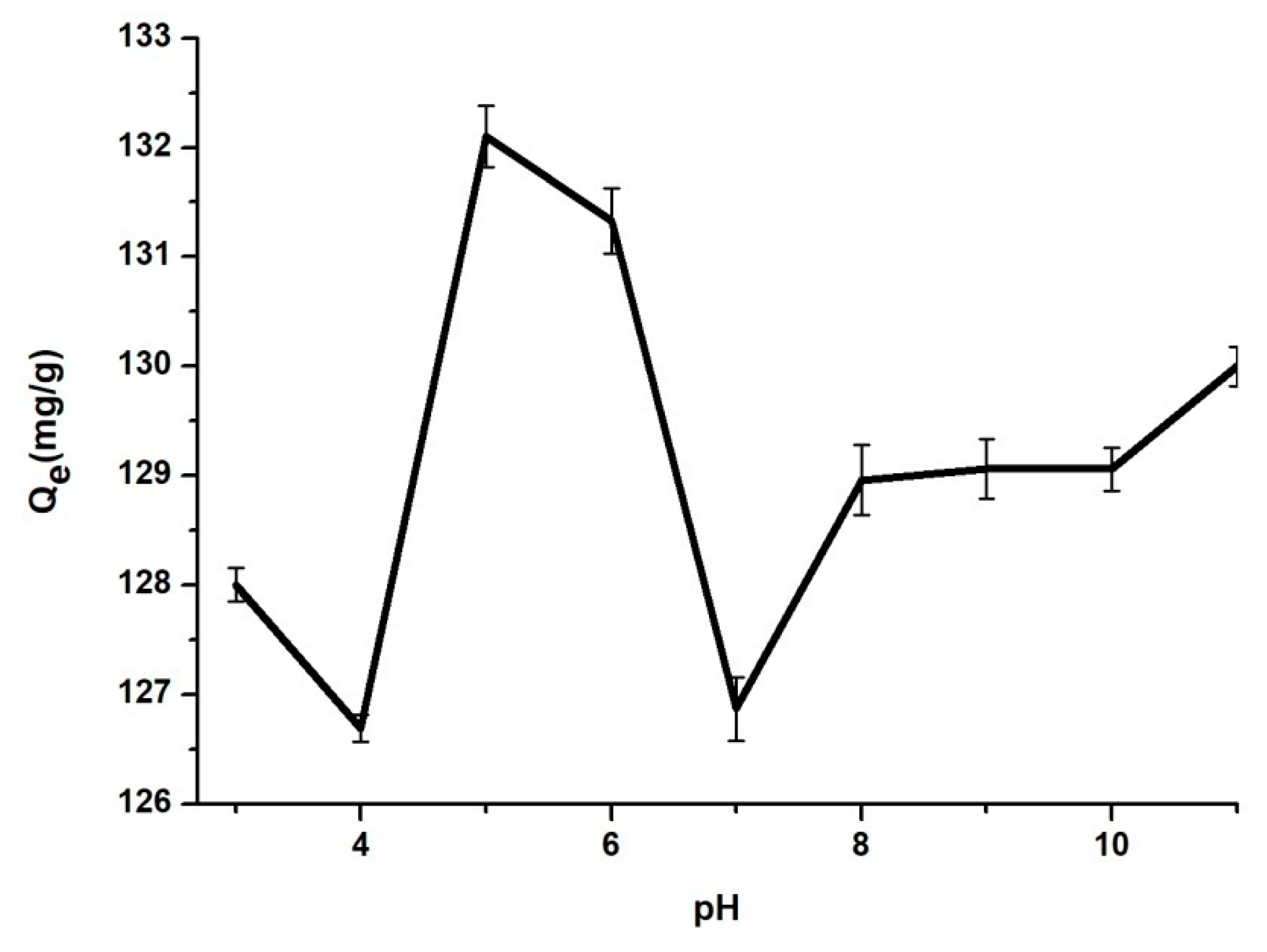
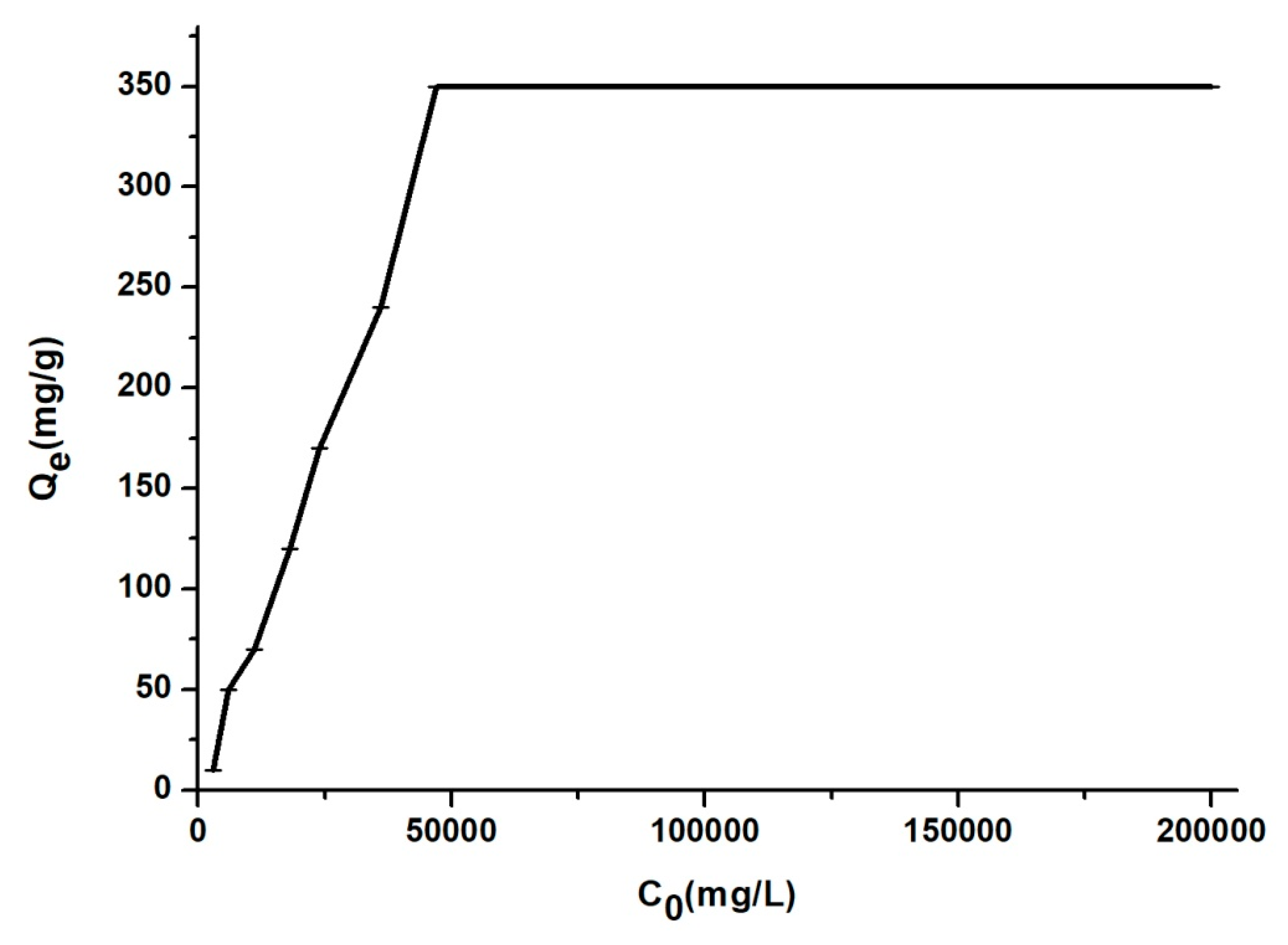
3.2. The Influence of Contact Time, pH, Temperature and Concentration of BZC
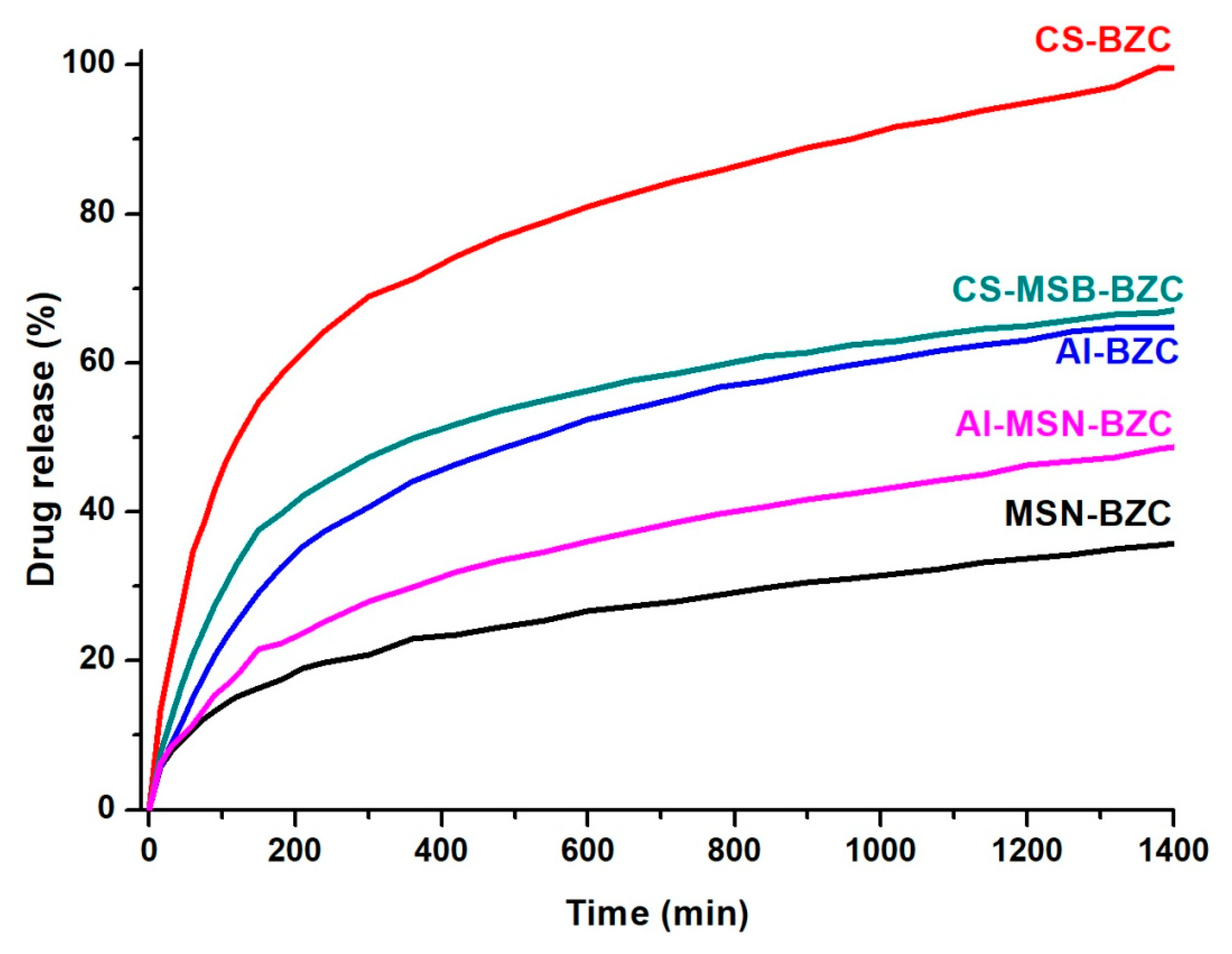
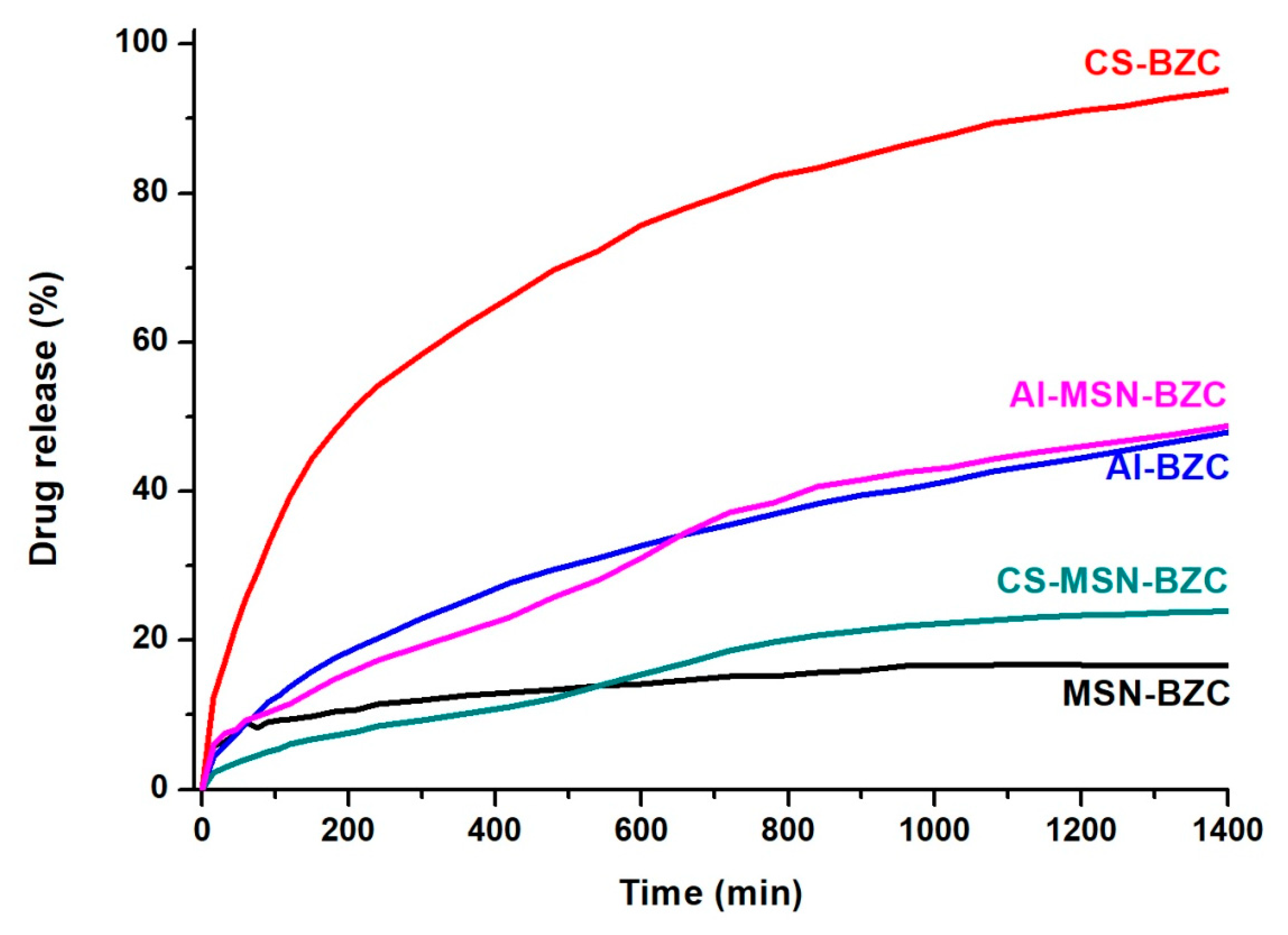
3.3. In Vitro Release Studies
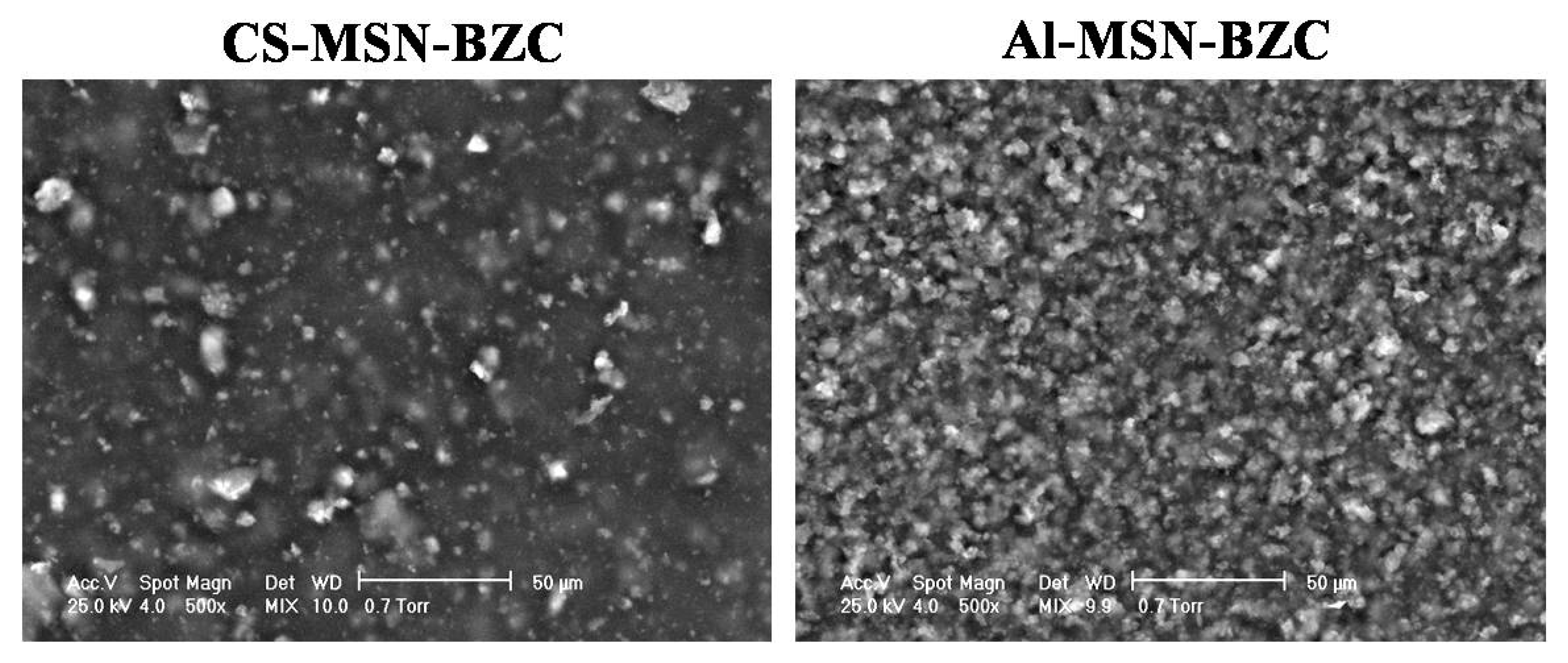
3.4. Scanning Electron Microscopy (SEM) Analysis
3.5. In Vitro Kinetic Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Digge, M.S.; Moon, R.S.; Gattani, S.G. Application of Carbon Nanotubes in Drug Delivery: A Review. Int. J. PharmTech Res. 2012, 4, 839–847. [Google Scholar]
- Alhamdi, J.; Jacobs, E.; Gronowicz, G.; Benkirane-Jessel, N.; Hurley, M.; Kuhn, L. Cell Type Influences Local Delivery of Biomolecules from a Bioinspired Apatite Drug Delivery System. Materials 2018, 11, 1703. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A. Nanocarrier systems for oral drug delivery: Do we really need them? Eur. J. Pharm. Sci. 2013, 49, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Rámila, A.; del Real, P.R.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Sun, C.Z.; Lu, C.T.; Dai, D.D.; Lv, H.F.; Wu, Y.; Wan, C.W.; Chen, L.J.; Lin, M.; Li, X.K. Characterization and anti-tumor activity of chemical conjugation of doxorubicin in polymeric micelles (DOX-P) in vitro. Cancer Lett. 2011, 311, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, G.; Ariana-Machado, J.; Enea, M.; Carmo, H.; Rodriguez-Gonzales, B.; Capelo, J.L.; Lodeiro, C.; Oliviera, E. Toxicological Evaluation of Luminescent Silica Nanoparticles as New Drug Nanocarriers in Different Cancer Cell Lines. Materials 2018, 11, 1310. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, F.; Mocanu, A.C.; Dascalu, C.A.; Maidaniuc, A.; Batalu, D.; Berbecaru, A.; Voicu, S.I.; Miculescu, M.; Thakur, V.K.; Ciocan, L.T. Facile synthesis and characterization of hydroxyapatite particles for high value nanocomposites and biomaterials. Vacuum 2017, 146, 614–622. [Google Scholar] [CrossRef]
- Chen, J.; Xia, N.; Zhou, T.; Tan, S.; Jiang, F. Mesoporous carbon spheres: Synthesis, characterization and supercapacitance. Int. J. Electrochem. Sci. 2009, 4, 1063–1073. [Google Scholar]
- Vallet-Regi, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Chem. Mater. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Swatantra, K.K.S.; Awani, R.K.; Satyawan, S. Chitosan: A Platform for Targeted Drug Delivery. Int. J. PharmTech Res. 2010, 2, 2271–2282. [Google Scholar]
- Tonnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Farías, T.; Charles de Ménorval, L. Benzalkonium chloride and sulfamethoxazole adsorption onto natural clinoptilolite: Effect of time, ionic strength, pH and temperature. J. Colloid Interface Sci. 2011, 363, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Kostić, D.A.; Mitić, S.S.; Nasković, D.C.; Zarubica, A.R.; Mitic, M.N. Determination of Benzalkonium Chloride in Nasal Drops by High-Performance Liquid Chromatography. E-J. Chem. 2012, 9, 1599–1604. [Google Scholar] [CrossRef]
- Ghebaur, A.; Garea, S.A.; Iovu, H. New polymer–halloysite hybrid materials—Potential controlled drug release system. Int. J. Pharm. 2012, 436, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Mhlanga, N.; Ray, S.S. Kinetic model for the release of the anticancer drug doxorubicin from biodegradable polylactide/metal oxide- based hybrid. Int. J. Biol. Macromol. 2015, 72, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Petropoulous, J.H.; Papadokostaki, K.G.; Sanopoulou, M. Higuchi’s equations and beyond: Overview of the formulation and application of generalized model of drug release from polymeric matrices. Int. J. Pharm. 2012, 437, 178–191. [Google Scholar] [CrossRef]
- Bruschi, M. (Ed.) Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Jose, S.; Fangueiro, J.F.; Smitha, J.; Cinu, T.A.; Chacko, A.J.; Premaletha, K.; Souto, E.B. Predictive modeling of insulin release profile from cross-linked chitosan microspheres. Eur. J. Med. Chem. 2013, 60, 249–253. [Google Scholar] [CrossRef]
- Lungan, M.A.; Popa, M.; Racovita, S.; Hitruc, G.; Doroftei, F.; Desbieres, J.; Vasiliu, S. Surface characterization and drug release from porous microparticles based on methacrylic monomers and xanthan. Carbohydr. Polym. 2015, 125, 323–333. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Tang, J.; Slowing, I.I.; Huang, Y.; Trewyn, B.G.; Hu, J.; Liu, H.; Lin, V.S.Y. poly(lactic acid)-coated mesoporous silica nanosphere for controlled release of venlafaxine. J. Colloid Interface Sci. 2011, 360, 488–496. [Google Scholar] [CrossRef]
- Li, Z.; Su, K.; Cheng, B.; Deng, Y. Organically modified MCM-type material preparation and its usage in controlled amoxicillin delivery. J. Colloid Interface Sci. 2010, 342, 607–613. [Google Scholar] [CrossRef]
- Havasi, F.; Ghorbani-Choghamarani, A.; Nikpour, F. Pd-Grafted functionalized mesoporous MCM-41: A novel, green and heterogeneous nanocatalyst for the selective synthesis of phenols and anilines from aryl halides in water. New J. Chem. 2015, 39, 6504–6512. [Google Scholar] [CrossRef]
- Najafi, M.; Yousefi, Y.; Rafati, A.A. Synthesis, characterization and adsorption studies of several heavy metal ions on amino-functionalized silica nano hollow sphere and silica gel. Sep. Purif. Technol. 2012, 85, 193–205. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, K.P.; Ou, H.D.; Chiang, Y.C.; Wang, C.F. Adsorption of cationic dyes onto mesoporous silica. Microporous Mesoporous Mater. 2011, 141, 102–109. [Google Scholar] [CrossRef]
- Kuo, C.Y. Comparison with as-grown and microwave modified carbon nanotubes to removal aqueous bisphenol A. Desalination 2009, 249, 976–982. [Google Scholar] [CrossRef]
- Mohammadi, N.; Khani, H.; Gupta, V.K.; Amereh, E.; Agarwal, S. Adsorption process of methyl orange dye onto mesoporous carbon material–kinetic and thermodynamic studies. J. Colloid Interface Sci. 2011, 362, 457–462. [Google Scholar] [CrossRef]
- Zanini, G.P.; Ovesen, R.G.; Hansen, H.C.B.; Strobel, B.W. Adsorption of the disinfectant benzalkonium chloride on montmorillonite. Syntergetic effect in mixture of molecules with different chain lengths. J. Environ. Manag. 2013, 128, 100–105. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, Y.; Lang, M. Micelles/sodium-alginate composite gel beads: A new matrix for oral drugdelivery of indomethacin. Carbohydr. Polym. 2012, 87, 790–798. [Google Scholar] [CrossRef]
- Miculescu, F.; Bojin, D.; Ciocan, L.T.; Antoniac, I.A.; Miculescu, M.; Niculescu, N. Experimental Research on Biomaterial-Tissue Interface Interactions. J. Optoelectron. Adv. Mater. 2007, 9, 3303–3306. [Google Scholar]
- Martin, A.; Morales, V.; Ortiz-Bustos, J.; Perez-Garnes, J.; Bautista, L.F.; Garcia-Munoz, R.A.; Sanz, R. Modelling the adsorption and controlled release of drug from the pure and amino surface-functionalized mesoporous silica host. Microporous Mesoporous Mater. 2018, 262, 23–34. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]

| n | Transport mechanism |
| 0.5 | Fickian diffusion |
| 0.5 < n < 1 | Anomalous Transport |
| 1 | Case II transport |
| 1 < n | Super case II transport |
| Kinetic Model | |
|---|---|
| Pseudo-Second Order | |
| qe, calc (mg/g) | 142.85 |
| k2 (g/mg min) | 0.037692 |
| R2 | 1 |
| Intr-Particle Diffusion | |
| kp (mg/g min) | 0.6245 |
| R2 | 0.9914 |
| Temperature (K) | 298 | 313 | 333 | 353 |
|---|---|---|---|---|
| ΔG0 (kJ/mol) | −2.3 | −2.77 | −3.4 | −4.02 |
| ΔH0 (kJ/mol) | 7.04 | |||
| ΔS0 (kJ/molK) | 0.031 | |||
| Sample | First Order | Higuchi | Korsmayer-Peppas | Weibull | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C0, mg/g | K, h−1 | R2 | KH, h−1/2 | R2 | K (min−n) | n | R2 | a | b | R2 | ||
| MSN-BZC | pH 1.2 | 94.8 | 0.00069 | 0.89652 | 0.138 | 0.9339 | 0.4428 | 0.3848 | 0.9923 | 0.001 | 0.0024 | 0.9754 |
| pH 7.4 | 93.22 | 1.15 × 10−6 | 0.9007 | 0.093 | 0.8327 | 0.4203 | 0.2916 | 0.9337 | 0.0005 | 0.0011 | 0.8017 | |
| AL-BZC | pH 1.2 | 98.81 | 0.00184 | 0.9796 | 0.2925 | 0.9456 | 0.4913 | 0.5 | 0.9594 | 0.0023 | 0.0067 | 0.9875 |
| pH 7.4 | 90.78 | 0.00046 | 0.9681 | 0.1951 | 0.9976 | 0.9806 | 0.5379 | 0.9964 | 0.0017 | 0.0054 | 0.9466 | |
| CS-BZC | pH 1.2 | 83.69 | 0.00276 | 0.885 | 0.526 | 0.8266 | 0.1418 | 0.3782 | 0.9419 | 0.0034 | 0.0079 | 0.9974 |
| pH 7.4 | 96.74 | 0.00184 | 0.991 | 0.433 | 0.9442 | 0.2283 | 0.4401 | 0.9801 | 0.0031 | 0.0085 | 0.9845 | |
| AL-MSN-BZC | pH 1.2 | 96.83 | 0.001152 | 0.9407 | 0.2026 | 0.9795 | 0.5025 | 0.45 | 0.9892 | 0.0016 | 0.0046 | 0.9736 |
| pH 7.4 | 96.6 | 0.00046 | 0.88 | 0.1684 | 0.8716 | 1.7196 | 0.5924 | 0.9559 | 0.0015 | 0.0055 | 0.7707 | |
| CS-MSN-BZC | pH 1.2 | 74.1 | 0.00069 | 0.8743 | 0.6255 | 0.8451 | 0.2614 | 0.4146 | 0.9304 | 0.0043 | 0.0106 | 0.9949 |
| pH 7.4 | 97.19 | 1.15 × 10−9 | 0.977 | 0.9418 | 0.9555 | 3.8884 | 0.6314 | 0.9911 | 0.0098 | 0.0376 | 0.8927 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandele, A.M.; Andronescu, C.; Ghebaur, A.; Garea, S.A.; Iovu, H. New Biocompatible Mesoporous Silica/Polysaccharide Hybrid Materials as Possible Drug Delivery Systems. Materials 2019, 12, 15. https://doi.org/10.3390/ma12010015
Pandele AM, Andronescu C, Ghebaur A, Garea SA, Iovu H. New Biocompatible Mesoporous Silica/Polysaccharide Hybrid Materials as Possible Drug Delivery Systems. Materials. 2019; 12(1):15. https://doi.org/10.3390/ma12010015
Chicago/Turabian StylePandele, Andreea Madalina, Corina Andronescu, Adi Ghebaur, Sorina Alexandra Garea, and Horia Iovu. 2019. "New Biocompatible Mesoporous Silica/Polysaccharide Hybrid Materials as Possible Drug Delivery Systems" Materials 12, no. 1: 15. https://doi.org/10.3390/ma12010015
APA StylePandele, A. M., Andronescu, C., Ghebaur, A., Garea, S. A., & Iovu, H. (2019). New Biocompatible Mesoporous Silica/Polysaccharide Hybrid Materials as Possible Drug Delivery Systems. Materials, 12(1), 15. https://doi.org/10.3390/ma12010015







