The Impact of Iron Adsorption on the Electronic and Photocatalytic Properties of the Zinc Oxide (0001) Surface: A First-Principles Study
Abstract
:1. Introduction
2. Calculation Models and Methods
3. Results and Discussion
3.1. Geometries and Structural Stability
3.2. Electronic Structure
3.3. Optical Properties
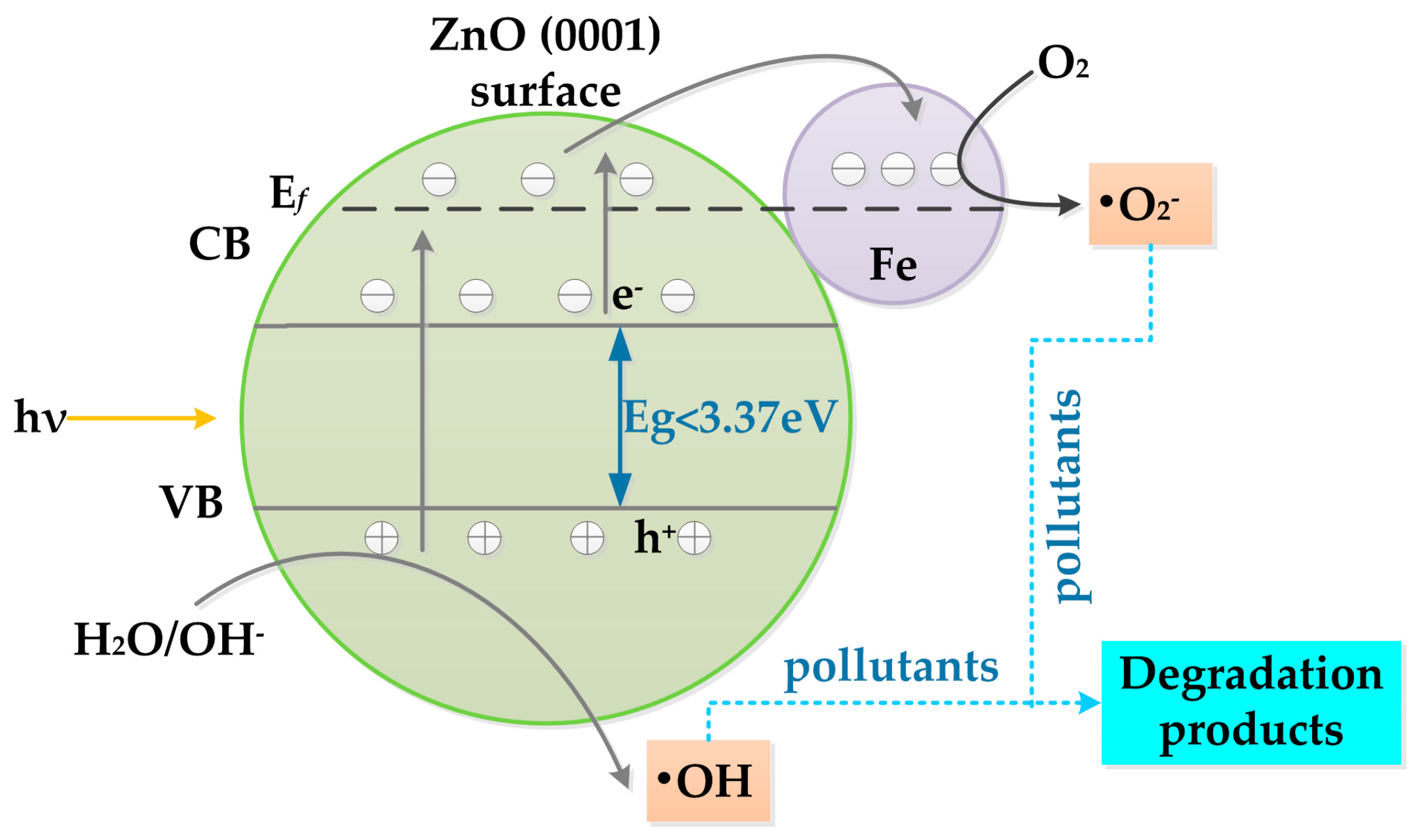
3.4. Photocatalytic Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muñoz-Fernandez, L.; Sierra-Fernandez, A.; Milošević, O. Solvothermal synthesis of Ag/ZnO and Pt/ZnO nanocomposites and comparison of their photocatalytic behaviors on dyes degradation. Adv. Powder Technol. 2016, 27, 983–993. [Google Scholar] [CrossRef]
- Wu, M.C.; Wu, P.Y.; Lin, T.H. Photocatalytic Performance of Cu-doped TiO2 Nanofibers Treated by the Hydrothermal Synthesis and Air-thermal Treatment. Appl. Surf. Sci. 2017, 430, 390–398. [Google Scholar] [CrossRef]
- Moradi, V.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Significant improvement in visible light photocatalytic activity of Fe doped TiO2 using an acid treatment process. Appl. Surf. Sci. 2017, 427, 791–799. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Martins, R. Photocatalytic TiO2 Nanorod Spheres and Arrays Compatible with Flexible Applications. Catalysts 2017, 7, 60. [Google Scholar] [CrossRef]
- Su, Y.; Ao, D.; Liu, H.; Wang, Y. MOF-derived yolk–shell CdS microcubes with enhanced visible-light photocatalytic activity and stability for hydrogen evolution. J. Mater. Chem. A 2017, 5, 8680–8689. [Google Scholar] [CrossRef]
- Garg, P.; Kumar, S.; Choudhuri, I.; Mahata, A.; Pathak, B. Hexagonal Planar CdS Monolayer Sheet for Visible Light Photocatalysis. J. Phys. Chem. C 2016, 120, 7052–7060. [Google Scholar] [CrossRef]
- Tabib, A.; Bouslama, W.; Sieber, B.; Addad, A.; Elhouichet, H.; Férid, M.; Boukherroub, R. Structural and optical properties of Na doped ZnO nanocrystals: Application to solar photocatalysis. Appl. Surf. Sci. 2017, 396, 1528–1538. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Jiao, W.; Li, Q.; Zhang, Y.; Li, S.; Li, D.; Che, R. Two hybrid Au-ZnO aggregates with different hierarchical structures: A comparable study in photocatalysis. J. Colloid Interface Sci. 2018, 509, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Liu, H.; Sun, J.; Tian, Y.; Chen, S.; Song, J.; Luo, R.; Li, D.; Chen, A.; Liu, C.C. Improvement of TiO2 photocatalytic properties under visible light by WO3/TiO2 and MoO3/TiO2 composites. Appl. Surf. Sci. 2015, 338, 61–68. [Google Scholar] [CrossRef]
- Arfaoui, A.; Touihri, S.; Mhamdi, A.; Labidi, A.; Manoubi, T. Structural, morphological, gas sensing and photocatalytic characterization of MoO3 and WO3 thin films prepared by the thermal vacuum evaporation technique. Appl. Surf. Sci. 2015, 357, 1089–1096. [Google Scholar] [CrossRef]
- Raji, R.; Sibi, K.S.; Gopchandran, K.G. ZnO:Ag nanorods as efficient photocatalyst: Sunlight driven photocatalytic degradation of sulforhodamine B. Appl. Surf. Sci. 2018, 427, 863–875. [Google Scholar] [CrossRef]
- Jiang, X.H.; Shi, J.J.; Zhang, M.; Zhong, H.X.; Huang, P.; Ding, Y.M.; Cao, X.; Wu, M. Modulation of electronic and optical properties of ZnO by inserting an ultrathin ZnX (X = S, Se and Te) layer to form short-period (ZnO)5/(ZnX)1 superlattice. J. Alloys Compd. 2017, 711, 581–591. [Google Scholar] [CrossRef]
- Razavi-Khosroshahi, H.; Edalati, K.; Wu, J.; Nakashima, Y.; Arita, M.; Ikoma, Y.; Sadakiyo, M.; Inagaki, Y.; Staykov, A.; Yamauchi, M.; et al. High-pressure zinc oxide phase as visible-light-active photocatalyst with narrow band gap. J. Mater. Chem. A 2017, 5, 20298–20303. [Google Scholar] [CrossRef]
- Bhatia, S.; Verma, N.; Bedi, R.K. Sn-doped ZnO Nanopetal networks for Efficient Photocatalytic Degradation of dye and Gas Sensing Applications. Appl. Surf. Sci. 2017, 407, 495–502. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, J.; Peng, T. New insight into the enhanced photocatalytic activity of N-, C- and S-doped ZnO photocatalysts. Appl. Catal. B-Environ. 2016, 181, 220–227. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Feng, Y.; Li, Z. Efficient photocatalytic performance enhancement in Co-doped ZnO nanowires coupled with CuS nanoparticles. Appl. Surf. Sci. 2017, 428, 154–164. [Google Scholar] [CrossRef]
- Harish, S.; Sabarinathan, M.; Archana, J.; Navaneethan, M.; Nisha, K.D.; Ponnusamy, S.; Gupta, V.; Muthamizhchelvan, C.; Aswal, D.K.; Ikeda, H.; et al. Synthesis of ZnO/SrO nanocomposites for enhanced photocatalytic activity under visible light irradiation. Appl. Surf. Sci. 2017, 418, 147–155. [Google Scholar] [CrossRef]
- Cheng, H.X.; Wang, X.X.; Hu, Y.W.; Song, H.Q.; Huo, J.R.; Li, L.; Qian, P.; Song, Y.J. Ag@ZnO core-shell nanoparticles study by first principle: The structural, magnetic and optical properties. J. Solid State Chem. 2016, 244, 181–186. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, T.; Wu, T.; Jin, C.; Qiao, R.; Qian, Y.; Tong, G. Polymorphous ZnO Nanostructures: Zn Polar Surface-Guided Size and Shape Evolution Mechanism and Enhanced Photocatalytic Activity. ChemCatChem 2017, 9, 3180–3190. [Google Scholar] [CrossRef]
- Abbas, K.N.; Bidin, N. Morphological driven photocatalytic activity of ZnO nanostructures. Appl. Surf. Sci. 2017, 394, 498–508. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, S.; Xu, W.; Yuan, F. First-principles study of electronic structures and photocatalytic activity of low-Miller-index surfaces of ZnO. J. Appl. Phys. 2013, 113, 034903. [Google Scholar] [CrossRef]
- Warschkow, O.; Chuasiripattana, K.; Lyle, M.J.; Delley, B.; Stampfl, C. Cu/ZnO (0001) under oxidating and reducing conditions: A first-principles survey of surface structures. Phys. Rev. B 2011, 84, 125311. [Google Scholar] [CrossRef]
- Lyle, M.J.; Warschkow, O.; Delley, B.; Stampfl, C. Molecular adsorption and methanol synthesis on the oxidized Cu/ZnO(0001) surface. Surf. Sci. 2015, 641, 97–104. [Google Scholar] [CrossRef]
- Nishidate, K.; Yoshizawa, M.; Hasegawa, M. Energetics of Mg and B adsorption on polar zinc oxide surfaces from first principles. Phys. Rev. B 2008, 77, 035330. [Google Scholar] [CrossRef]
- Chen, X.; Huang, D.; Deng, W.J.; Zhao, Y.J. Structural stability and magnetic properties of Co-doped or adsorbed polar-ZnO surface. Phys. Lett. A 2009, 373, 391–395. [Google Scholar] [CrossRef]
- Yang, Z.; Xiong, S.J. Adsorption of Ag and Au atoms on wurtzite ZnO (0001) surface. Surf. Sci. 2011, 605, 40–45. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, S.; Xu, W.; Yuan, F. First-principles study of Si atoms adsorbed on ZnO (0001) surface and the effect on electronic and optical properties. Surf. Sci. 2014, 625, 30–36. [Google Scholar] [CrossRef]
- Zhai, B.G.; Yang, L.; Ma, Q.L.; Huang, Y.M. Visible light driven photocatalytic activity of Fe-doped ZnO nanocrystals. Funct. Mater. Lett. 2016, 10, 1750002. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, Z.; Zhou, X.; Cao, J. Tunable electronic properties and optical properties of novel stanene/ZnO heterostructure: First-principles calculation. Comput. Mater. Sci. 2017, 139, 179–184. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, X.; Li, L.; Song, Q. First-principles calculations of electronic structure and optical properties of boron–phosphorus co-doped zinc oxide. Mater. Sci. Semicond. Proc. 2015, 30, 406–412. [Google Scholar] [CrossRef]
- Su, Y.L.; Zhang, Q.Y.; Zhou, N.; Ma, C.Y.; Liu, X.Z.; Zhao, J.J. Study on Co-doped ZnO comparatively by first-principles calculations and relevant experiments. Solid State Commun. 2017, 250, 123–128. [Google Scholar] [CrossRef]
- Abdel-Baset, T.A.; Fang, Y.W.; Anis, B.; Duan, C.G.; Abdel-Hafiez, M. Structural and Magnetic Properties of Transition-Metal-Doped Zn1−xFexO. Nanoscale Res. Lett. 2016, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Chung, Y.C. First-principles calculation of electronic structure and magnetic properties of copper adsorbed polar-ZnO surface. J. Vac. Sci. Technol. B 2007, 25, 2616–2618. [Google Scholar] [CrossRef]
- Xia, S.; Liu, L.; Kong, Y.; Wang, H.; Wang, M. Study of Cs adsorption on (100) surface of [001]-oriented GaN nanowires: A first principle research. Appl. Surf. Sci. 2016, 387, 1110–1115. [Google Scholar] [CrossRef]
- Sun, W.; Jha, J.K.; Shepherd, N.D.; Du, J. Interface structrues of ZnO/MoO3 and their effect on workfunction of ZnO surfaces from first principles calculations. Comput. Mater. Sci. 2018, 141, 162–169. [Google Scholar] [CrossRef]
- Sun, W.; Li, Y.; Jha, J.K.; Shepherd, N.D.; Du, J. Effect of surface adsorption and non-stoichiometry on the workfunction of ZnO surfaces: A first principles study. J. Appl. Phys. 2015, 117, 165304. [Google Scholar] [CrossRef]
- Strak, P.; Kempisty, P.; Sakowski, K.; Krukowski, S. Ab initio determination of electron affinity of polar nitride surfaces, clean and under Cs coverage. J. Vac. Sci. Technol. A Vac. Surf. Films 2017, 35, 021406. [Google Scholar] [CrossRef]
- Farooq, R.; Mahmood, T.; Anwar, A.W.; Abbasi, G.N. First-principles calculation of electronic and optical properties of graphene like ZnO (G-ZnO). Superlattices Microstruct. 2016, 90, 165–169. [Google Scholar] [CrossRef]
- Hou, Q.; Jia, X.; Xu, Z.; Zhao, C.; Qu, L. Effects of Li doping and point defect on the magnetism of ZnO. Ceram. Int. 2017, 44, 1376–1383. [Google Scholar] [CrossRef]
- Wu, M.; Sun, D.; Tan, C.; Tian, X.; Huang, Y. Al-Doped ZnO Monolayer as a Promising Transparent Electrode Material: A First-Principles Study. Materials 2017, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Sun, D.; Xu, D.; Tian, X.; Huang, Y. Tuning electronic structure and optical properties of ZnO monolayer by Cd doping. Ceram. Int. 2016, 42, 10997–11002. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.M. Structural, electronic, magnetic and optical properties of Zn1−xNixO from first-principles. J. Phys. Chem. Solids 2017, 104, 267–275. [Google Scholar] [CrossRef]
- Xie, L.Y.; Zhang, J.M. The structure, electronic, magnetic and optical properties of the Mn doped and Mn-X (X ¼ F, Cl, Br, I and At) co-doped monolayer WS2: A first-principles study. J. Alloys Compd. 2017, 702, 138–145. [Google Scholar] [CrossRef]
- Wróbel, J.; Piechota, J. On the structural stability of ZnO phases. Solid State Commun. 2008, 146, 324–329. [Google Scholar] [CrossRef]
- Decremps, F.; Datchi, F.; Saitta, A.M.; Polian, A. Local structure of condensed zinc oxide. Phys. Rev. B 2003, 68, 104101. [Google Scholar] [CrossRef]
- Wei, S.; Wang, Z.; Yang, Z. First-principles studies on the Au surfactant on polar ZnO surfaces. Phys. Lett. A 2007, 363, 327–331. [Google Scholar] [CrossRef]
- Strak, P.; Sakowski, K.; Kempisty, P.; Krukowski, S. Structural and electronic properties of AlN(0001) surface under partial N coverage as determined by ab initio approach. J. Appl. Phys. 2015, 118, 095705. [Google Scholar] [CrossRef]
- Kempisty, P.; Krukowski, S. Adsorption of ammonia at GaN(0001) surface in the mixed ammonia/hydrogen ambient—A summary of ab initio data. AIP Adv. 2014, 4, 117109. [Google Scholar] [CrossRef]
- Qiao, L.; Zeng, Y.; Qu, C.Q.; Zhang, H.Z.; Hu, X.Y.; Song, L.J.; Bi, D.M.; Liu, S.J. Adsorption of oxygen atom on Zn-terminated (0001) surface of wurtzite ZnO: A density-functional theory investigation. Physica E 2013, 48, 7–12. [Google Scholar] [CrossRef]
- Lahmer, M.A. Hydrogen sensing properties of the () surface enhanced by Be doping: A first principles study. Sens. Actuators B Chem. 2015, 221, 906–913. [Google Scholar] [CrossRef]
- Shaoqiang, G.; Qingyu, H.; Zhenchao, X.; Chunwang, Z. First principles study of magneto-optical properties of Fe-doped ZnO. Physica B 2016, 503, 93–99. [Google Scholar] [CrossRef]
- Pawar, R.C.; Choi, D.H.; Lee, J.S.; Lee, C.S. Formation of polar surfaces in microstructured ZnO by doping with Cu and applications in photocatalysis using visible light. Mater. Chem. Phys. 2015, 151, 167–180. [Google Scholar] [CrossRef]
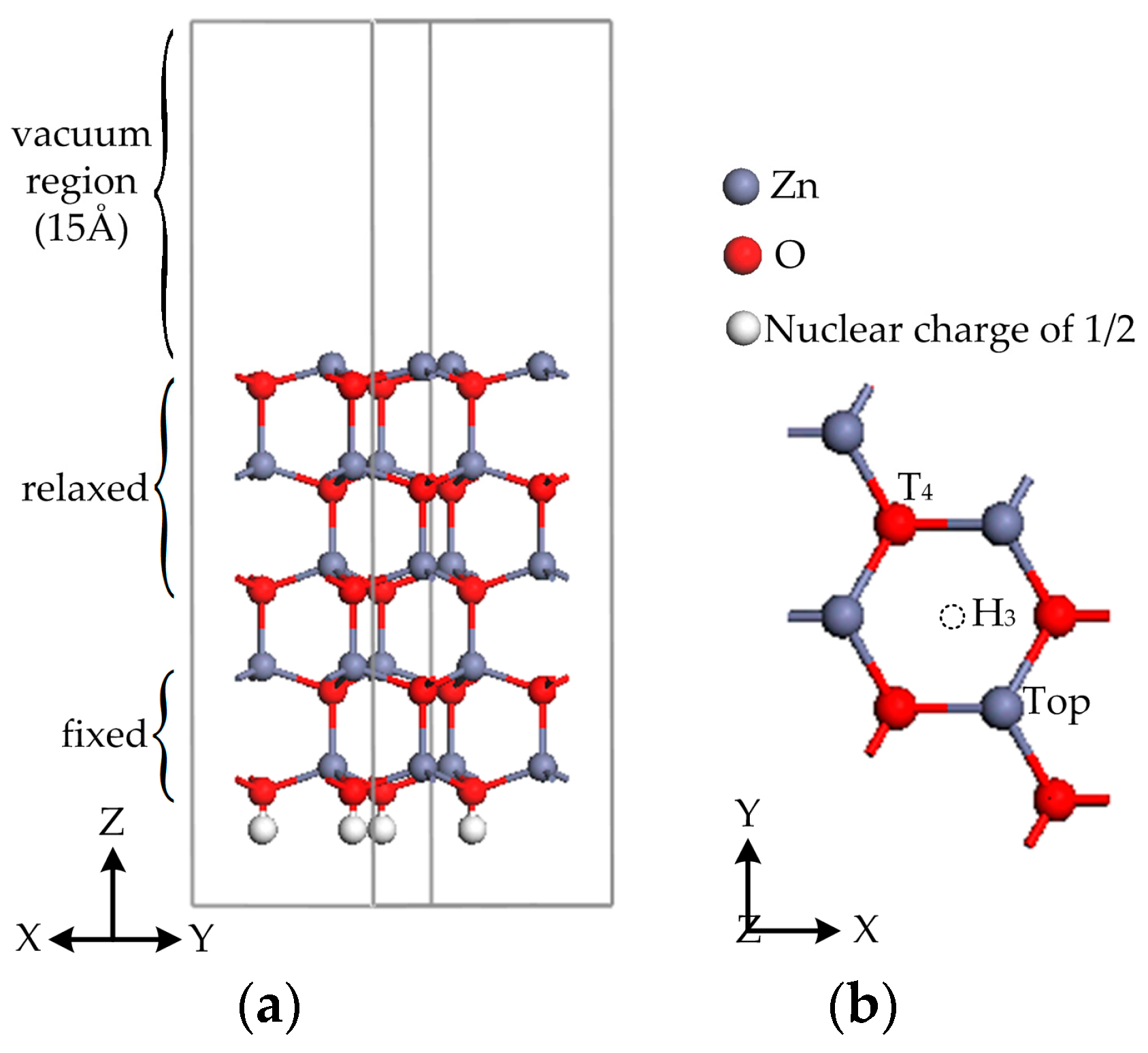
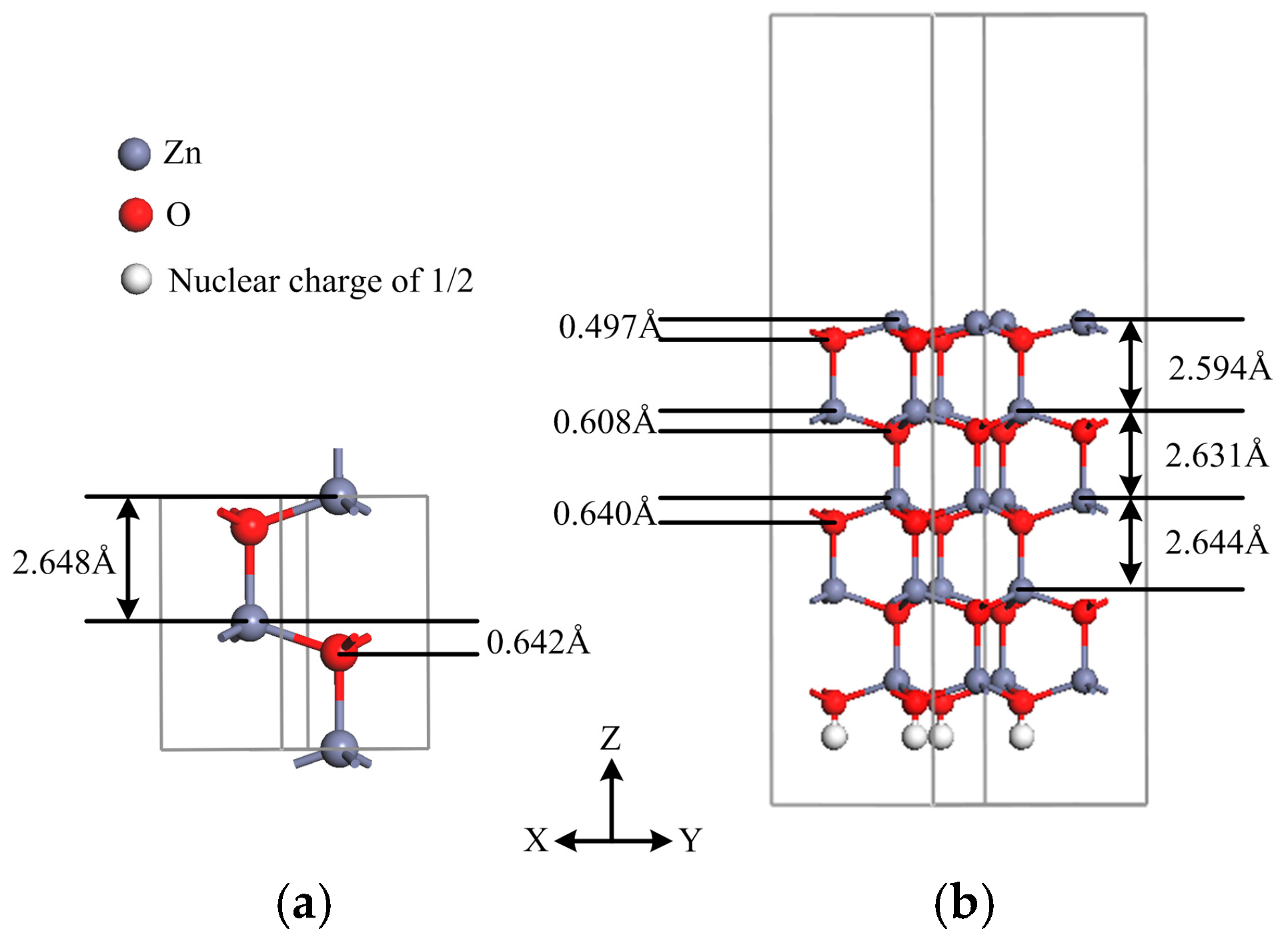






| Lattice Constants | Present Work | Previous Computation i | Experiment ii |
|---|---|---|---|
| a (Å) | 3.2820 | 3.2935 | 3.250 |
| b (Å) | 3.2820 | 3.2935 | 3.250 |
| c (Å) | 5.2951 | 5.2877 | 5.201 |
| c/a | 1.6134 | 1.6050 | 1.600 |
| Fe-Adsorbed Site | Etotal (eV) | Bond Length (Å) | Ea (eV) | |
|---|---|---|---|---|
| Fe-O | Fe-Zn | |||
| H3 | −43,845.903 | - | 2.482 | −5.665 |
| T4 | −43,844.674 | 1.703 | 2.318 | −4.436 |
| Top | −43,845.403 | - | 2.339 | −5.165 |
| Clean surface | −42,984.348 | - | - | - |
| Direction | Clean Surface | Fe(H3) | Fe(T4) | Fe(Top) | ||||
|---|---|---|---|---|---|---|---|---|
| Position (eV) | Process | Position (eV) | Process | Position (eV) | Process | Position (eV) | Process | |
| (100) | 1.72 | I | 1.12 | aI | 1.72 | bI | 1.17 | cI |
| 2.75 | aII | 2.30 | cII | |||||
| (001) | 1.92 | II | 2.11 | aIII | 2.12 | bII | 2.28 | cIII |
| 3.77 | aIV | 2.96 | cIV | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Wang, P.; Hua, C.; Yang, Y.; Zhang, Z. The Impact of Iron Adsorption on the Electronic and Photocatalytic Properties of the Zinc Oxide (0001) Surface: A First-Principles Study. Materials 2018, 11, 417. https://doi.org/10.3390/ma11030417
Cheng J, Wang P, Hua C, Yang Y, Zhang Z. The Impact of Iron Adsorption on the Electronic and Photocatalytic Properties of the Zinc Oxide (0001) Surface: A First-Principles Study. Materials. 2018; 11(3):417. https://doi.org/10.3390/ma11030417
Chicago/Turabian StyleCheng, Jingsi, Ping Wang, Chao Hua, Yintang Yang, and Zhiyong Zhang. 2018. "The Impact of Iron Adsorption on the Electronic and Photocatalytic Properties of the Zinc Oxide (0001) Surface: A First-Principles Study" Materials 11, no. 3: 417. https://doi.org/10.3390/ma11030417
APA StyleCheng, J., Wang, P., Hua, C., Yang, Y., & Zhang, Z. (2018). The Impact of Iron Adsorption on the Electronic and Photocatalytic Properties of the Zinc Oxide (0001) Surface: A First-Principles Study. Materials, 11(3), 417. https://doi.org/10.3390/ma11030417






