Natural and Modified Montmorillonite Clays as Catalysts for Synthesis of Biolubricants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pillared Interlayered Clays (PILCs)
2.3. Acid Treatment
2.4. Catalyst Characterization
2.5. Catalytic Studies
2.6. Physicochemical Characterization
3. Results and Discussion
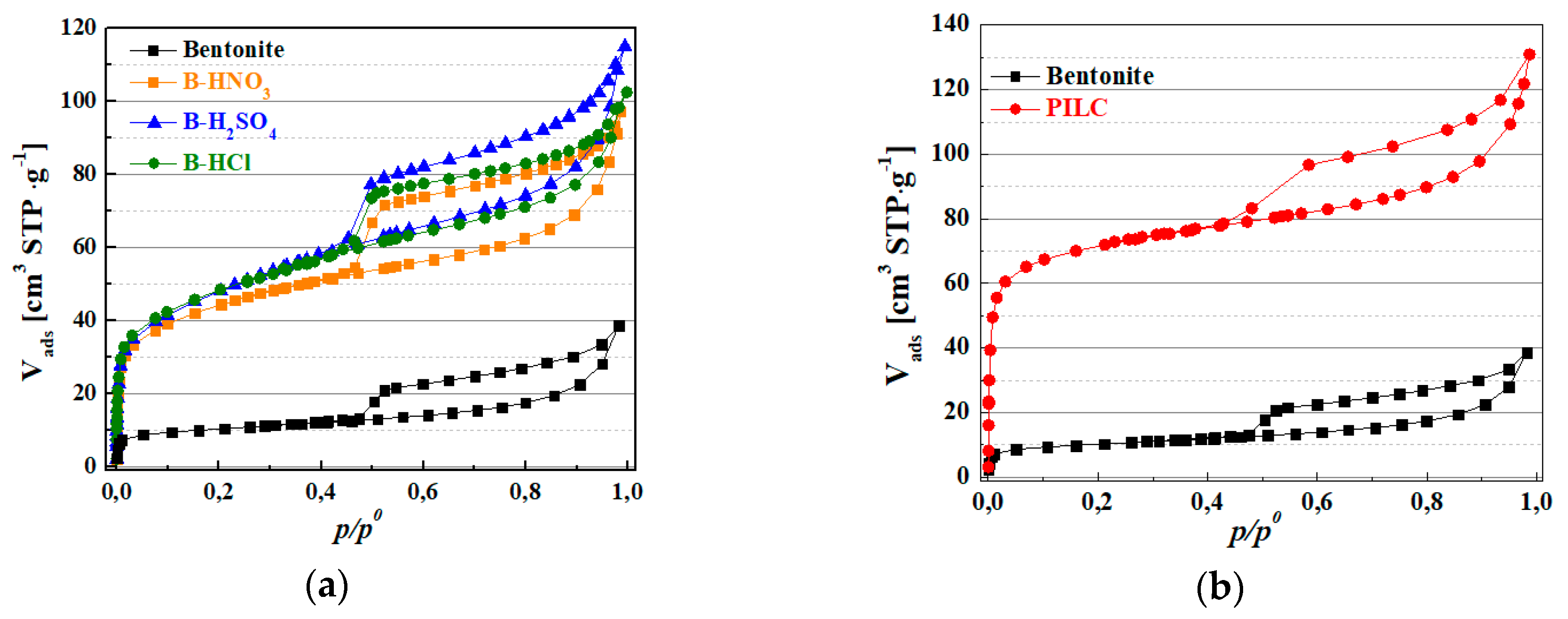
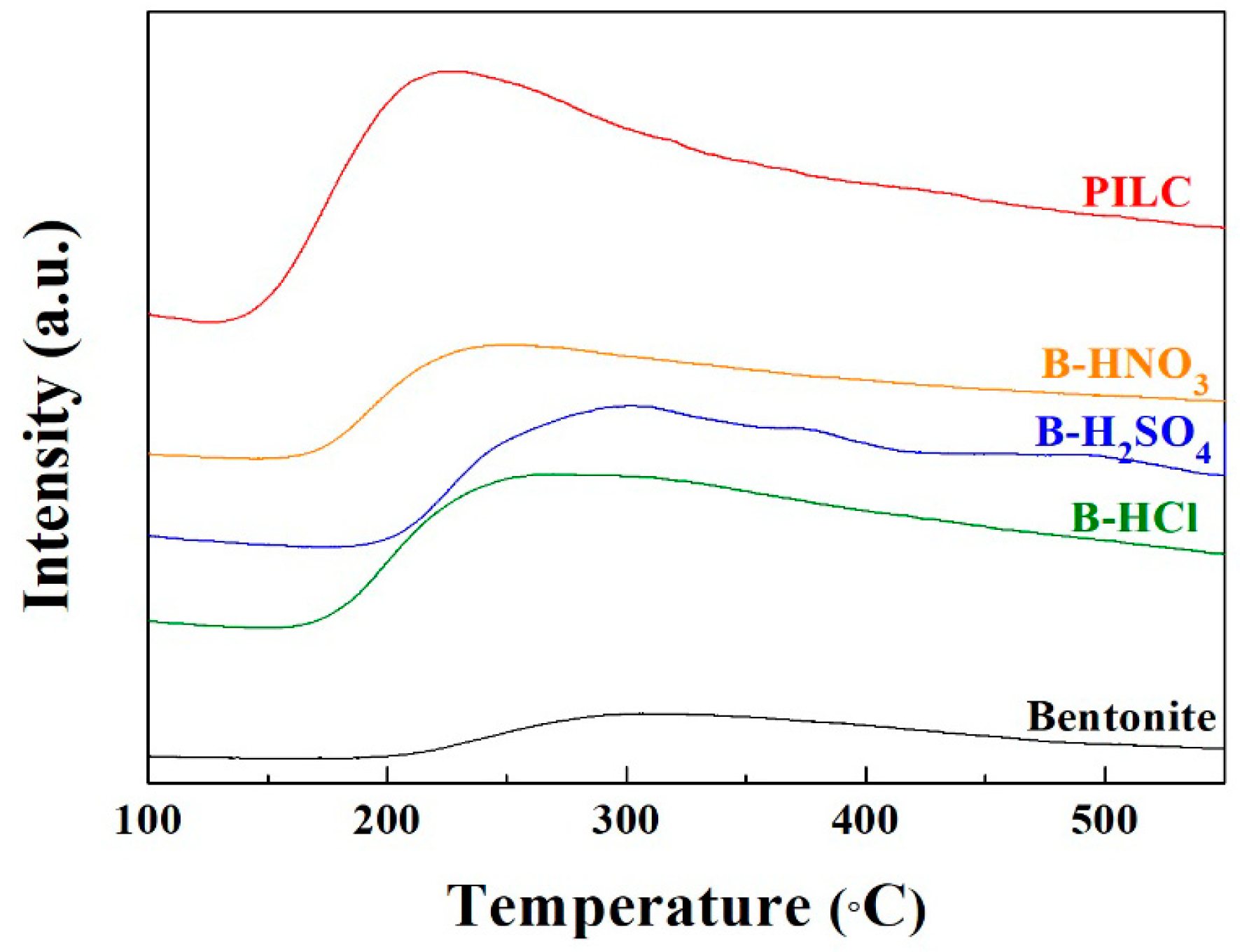
3.1. Characterization of the Natural and Modified Clays
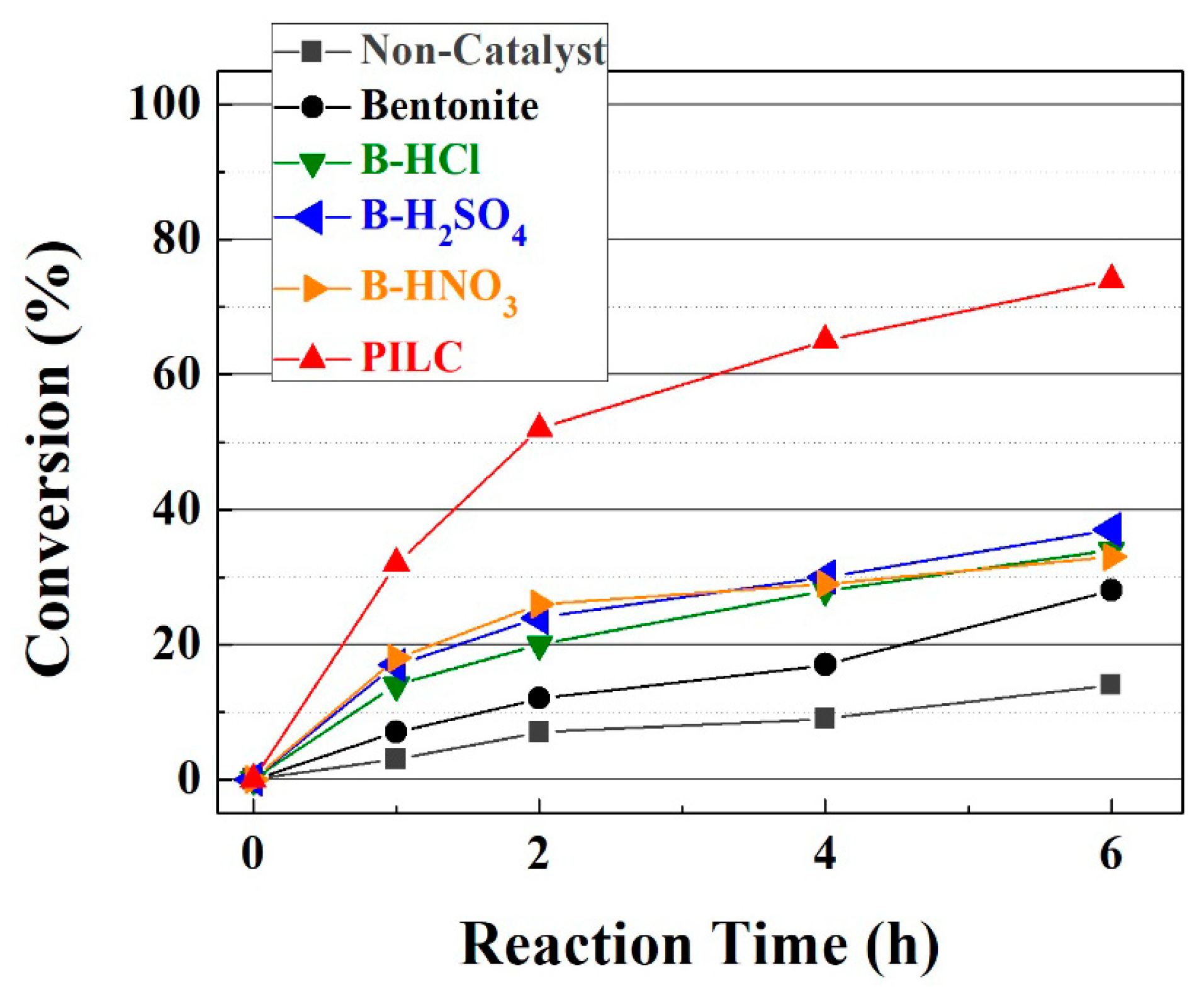
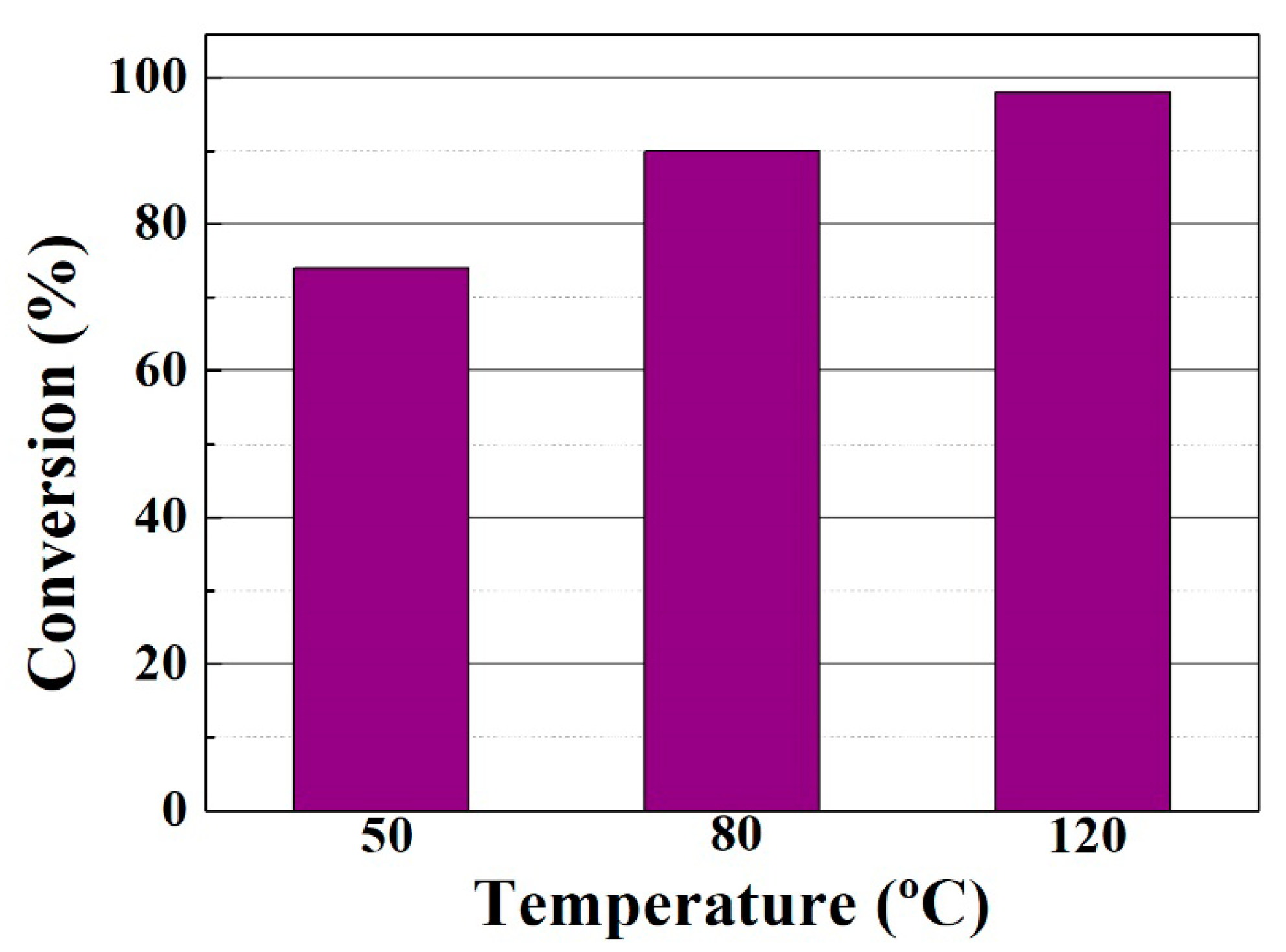
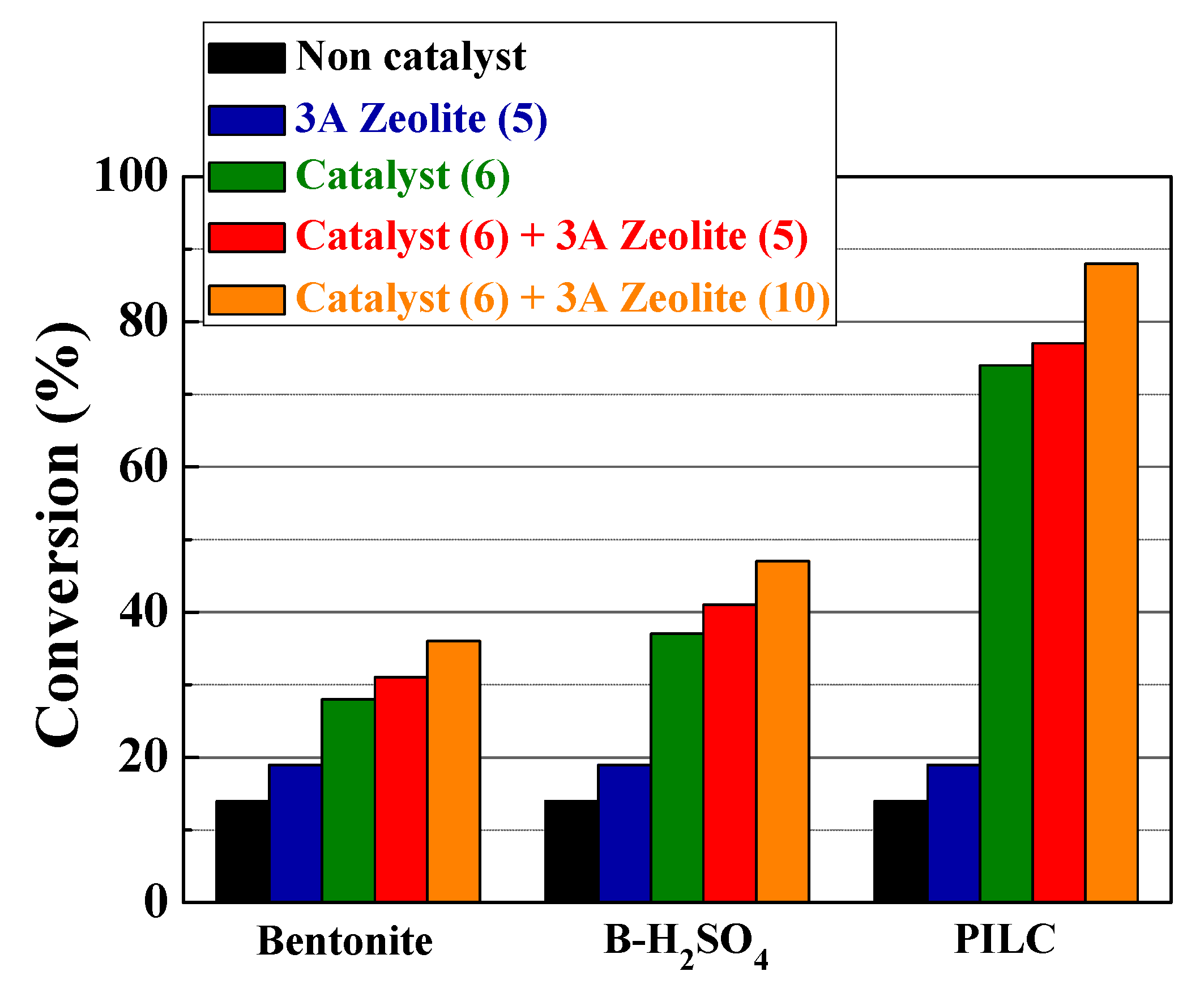
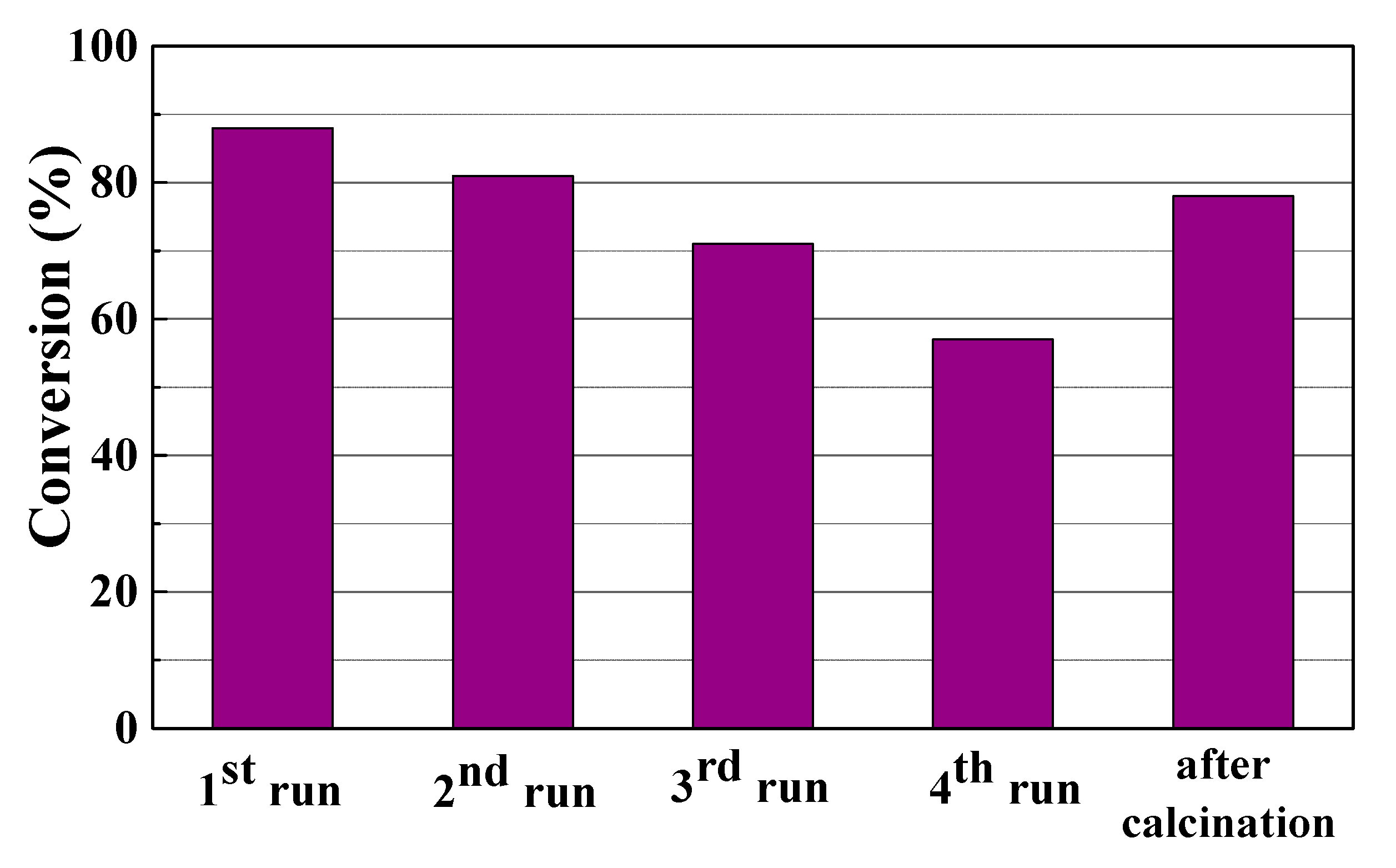
3.2. Catalytic Results
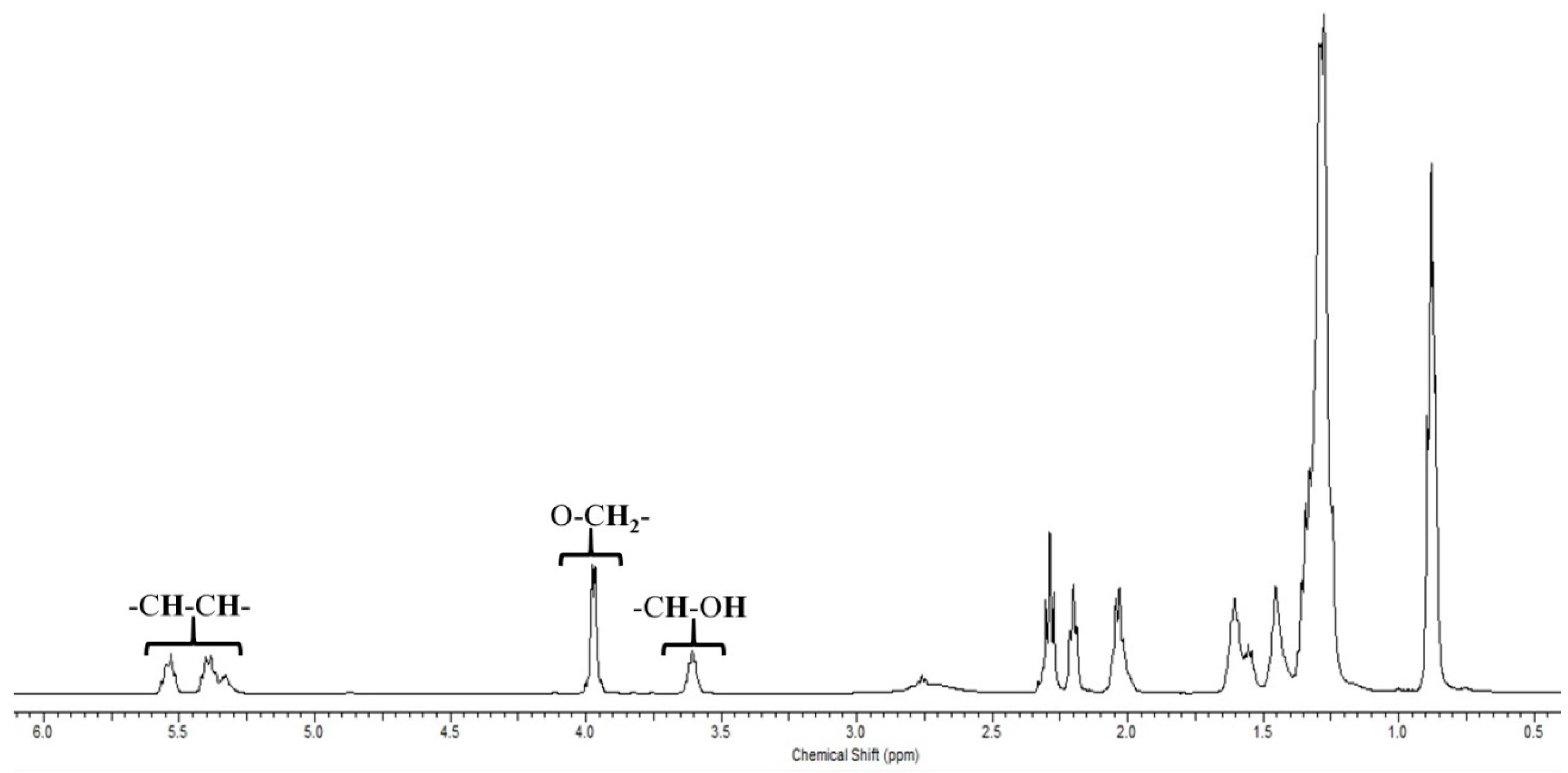
3.3. Physicochemical Properties and Chemical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nagendramma, P.; Kaul, S. Development of ecofriendly/biodegradable lubricants: An overview. Renew. Sustain. Energy Rev. 2012, 16, 764–774. [Google Scholar] [CrossRef]
- Rani, S.; Joy, M.L.; Nair, K.P. Evaluation of physiochemical and tribological properties of rice bran oil-biodegradable and potential base stoke for industrial lubricants. Ind. Crops Prod. 2015, 65, 328–333. [Google Scholar] [CrossRef]
- Urbanus, J.H.; Lobo, R.C.; Riley, A.J. European hazard classification advice for crude oil—Derived lubricant base oils compared with the proposed mineral oil mist TLV. Appl. Occup. Environ. Hyg. 2003, 18, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Luna, F.M.T.; Rocha, B.S.; Rola, E.M.; Albuquerque, M.C.G.; Azevedo, D.C.S.; Cavalcante, C.L., Jr. Assessment of biodegradability and oxidation stability of mineral, vegetable and synthetic oil samples. Ind. Crops Prod. 2011, 33, 579–583. [Google Scholar] [CrossRef]
- Soni, S.; Agarwal, M. Lubricants from renewable energy sources—A review. Green Chem. Lett. Rev. 2014, 7, 359–382. [Google Scholar] [CrossRef]
- Siniawski, M.T.; Saniei, N.; Stoyanov, P. Influence of humidity on the tribological performance of unmodified soybean and sunflower oils. Lubr. Sci. 2011, 23, 301–311. [Google Scholar] [CrossRef]
- Cermak, S.C.; Biresaw, G.; Isbell, T.A.; Evangelista, R.L.; Vaughn, S.F.; Murray, R. New crop oils—Properties as potential lubricants. Ind. Crops Prod. 2013, 44, 232–239. [Google Scholar] [CrossRef]
- Syahmllail, S.; Zubil, B.M.; Azwadi, C.S.N.; Ridzuan, M.J. Experimental evaluation of palm oil as lubricant in cold forward extrusion process. Int. J. Mech. Sci. 2011, 53, 549–555. [Google Scholar] [CrossRef]
- Mobarak, H.M.; Niza Mohamad, E.; Masjuki, H.H.; Kalam, M.A.; Al Mahmud, K.A.H.; Habibullah, M.; Ashraful, A.M. The prospects of biolubricants as alternatives in automotive applications. Renew. Sustain. Energy Rev. 2014, 33, 34–43. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. Potential non-edible oil resources as biodiesel feedstock: An Indian perspective. Renew. Sustain. Energy Rev. 2011, 15, 1791–1800. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.; Metzger, J.O.; Schäfer, H.J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef] [PubMed]
- Luna, F.M.T.; Cavalcante, J.B.; Silva, F.O.N.; Cavalcante, C.L., Jr. Studies on biodegradability of bio-based lubricants. Tribol. Int. 2015, 92, 301–306. [Google Scholar] [CrossRef]
- Isbell, A.T.; Kleiman, R.; Plattner, B.A. Acid-catalyzed condensation of oleic acid into estolides and polyestolides. J. Am. Oil Chem. Soc. 1994, 71, 169–174. [Google Scholar] [CrossRef]
- Isbell, A.T.; Frykman, H.B.; Abbott, T.P.; Lohr, J.E.; Drozd, J.C. Optimization of the sulfuric acid catalyzed estolide synthesis from oleic acid. J. Am. Oil Chem. Soc. 1997, 74, 473–476. [Google Scholar] [CrossRef]
- Cermak, S.C.; Isbell, A.T. Synthesis of estolides from oleic and saturated fatty acids. J. Am. Oil Chem. Soc. 2001, 78, 557–565. [Google Scholar] [CrossRef]
- Wagner, H.; Luther, R.; Mang, T. Lubricant base fluids based on renewable raw materials: Their catalytic manufacture and modification. Appl. Catal. A Gen. 2001, 221, 429–442. [Google Scholar] [CrossRef]
- Lilja, J.; Yu Murzin, D.; Salmi, T.; Aumo, J.; Maki-Arvela, P.; Sundell, M. Esterification of different acids over heterogeneous and homogeneous catalysts and correlation with the Taft equation. J. Mol. Catal. A Chem. 2002, 182, 555–563. [Google Scholar] [CrossRef]
- Suarez, P.A.Z.; Plentz Meneghetti, S.M.; Meneghetti, M.R.; Wolf, C.R. Transformação de triglicerídeos em combustíveis, materiais poliméricos e insumos químicos: algumas aplicações da catálise na oleoquímica. Quim. Nova 2007, 30, 667–676. [Google Scholar] [CrossRef]
- Vaccari, A. Clay and catalysis: A promising future. J. Appl. Clay Sci. 1999, 14, 161–198. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Frost, R.L. A review of the synthesis and characterization of pillared clays and related porous materials for cracking of vegetable oils to produce bilfuels. Environ. Geol. 2005, 47, 967–981. [Google Scholar] [CrossRef] [Green Version]
- Franco, F.; Pozo, M.; Cecilia, J.A.; Benítez-Guerrero, M.; Lorente, M. Effectiveness of microwave assisted acid treatment on dioctahedral and trioctahedral smectites. The influence of octahedral composition. Appl. Clay Sci. 2016, 120, 70–80. [Google Scholar] [CrossRef]
- Chitnis, S.R.; Sharma, M.M. Industrial applications of acid-treated clays as catalysts. React. Funct. Polym. 1997, 32, 93–115. [Google Scholar] [CrossRef]
- Flessner, U.; Jones, D.J.; Rozière, J.; Zajac, J.; Storaro, L.; Lenarda, M.; Pavan, M.; Jiménez-López, A.; Rodríguez-Castellón, E.; Trombetta, M.; et al. A study of the surface acidity of acid-treated montmorillonite clay catalysts. J. Mol. Catal. A Chem. 2001, 168, 247–256. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.K.; Lee, J.S. Esterification of free fatty acids using wáter-tolerable Amberlyst as a heterogeneous catalyst. Bioresour. Technol. 2010, 101, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Romero-Pérez, A.; Infantes-Molina, A.; Jiménez-López, A.; Roca Jalil, E.; Sapag, K.; Rodríguez-Castellón, E. Al-pillared montmorillonite as a support for catalysts based on ruthenium sulfide in HDS reactions. Catal. Today 2012, 187, 88–96. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Villarroel-Rocha, J.; Barrera, D.; García-Blanco, A.; Roca Jalil, M.E.; Sapag, K. Importance of the α-plot Method in the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2013, 31, 165–183. [Google Scholar] [CrossRef] [Green Version]
- Teo, W.K.; Ruthven, D.M. Adsorption of water from aqueous ethanol using 3-Å molecular sieves. Ind. Eng. Chem. Process Des. Dev. 1986, 25, 17–21. [Google Scholar] [CrossRef]
- ASTM International. Petroleum Products, Liquid Fuels, and Lubricants. In Proceedings of the ASTM Committees, Houston, TX, USA, 3–7 December 2017. [Google Scholar]
- Brindley, G.W. Order–disorder in clay mineral structures. In Clay Minerals; Brindley, G.W., Brown, G., Eds.; Elsevier Science: New York, NY, USA, 1985. [Google Scholar]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Zviagina, B.B.; McCarty, D.K.; Srodón, J.; Drits, V.A. Interpretation of infrared spectra of dioctahedral smectites in the region of OH-stretching vibrations. Clays Clay Miner. 2004, 52, 339–410. [Google Scholar] [CrossRef]
- Komadel, P.; Madejová, J. Acid activation of clay minerals. In Handbook of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 263–287. [Google Scholar]
- Vilarrasa-García, E.; Cecilia, J.A.; Ortigosa-Moya, E.M.; Cavalcante, C.L., Jr.; Azevedo, D.C.S.; Rodríguez-Castellón, E. “Low Cost” Pore Expanded SBA-15 Functionalized with Amine Groups Applied to CO2 Adsorption. Materials 2015, 8, 2495–2513. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Oliver, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Roca Jalil, M.E.; Baschini, M.; Rodríguez-Castellón, E.; Infantes-Molina, A.; Sapag, K. Effect of the Al/clay ratio on the thiabendazol removal by aluminum pillared clays. Appl. Clay Sci. 2014, 87, 245–253. [Google Scholar] [CrossRef]
- Occelli, M.L. Surface properties and cracking activity of delaminated clay catalysts. Catal. Today 1988, 2, 339–355. [Google Scholar] [CrossRef]
- Marchetti, J.; Miguel, V.U.; Errazu, A.F. Possible methods for biodiesel production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Altiokka, M.R.; Çitak, A. Kinetics study of the esterification of acetic acid with isobutanol in the presence of amberlite catalyst. Appl. Catal. A Gen. 2003, 239, 141–148. [Google Scholar] [CrossRef]
- Do Nascimento, L.A.S.; Tito, L.M.Z.; Angélica, R.S.; Da Costa, C.E.F.; Zamain, J.R.; Da Rocha Filho, G.N. Esterefication of oleic acid over solid acid catalysts prepared from Amazon flint kaolin. Appl. Catal. B Environ. 2011, 101, 495–503. [Google Scholar] [CrossRef]
- Saboya, R.M.A.; Cecilia, J.A.; García-Sancho, C.; Luna, F.M.T.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr. WO3-based catalysts supported on porous clay heterostructures (PCH) with Si-Zr pillars for synthetic esters production. Appl. Clay Sci. 2016, 124, 69–78. [Google Scholar] [CrossRef]
- Saboya, R.M.A.; Cecilia, J.A.; García-Sancho, C.; Sales, A.V.; Luna, F.M.T.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr. Assessment of commercial resins in the biolubricants production from free fatty acids of castor oil. Catal. Today 2017, 279, 274–285. [Google Scholar] [CrossRef]
- Schildhauer, T.J.; Hoek, I.; Kapteijn, F.; Moulijn, J.A. Zeolite BEA catalyzed esterification of hexanoic acid with 1-octanol: kinetics, side reactions and the role of water. Appl. Catal. A Gen. 2009, 358, 141–145. [Google Scholar] [CrossRef]
- Reddy, C.R.; Bhat, Y.S.; Nagendrappa, G.; Prakash, B.S.J. Brønsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy. Catal. Today 2009, 141, 157–160. [Google Scholar] [CrossRef] [Green Version]
- Saboya, R.M.A.; Cecilia, J.A.; García-Sancho, C.; Sales, A.V.; Luna, F.M.T.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr. Synthesis of biolubricants by the esterification of free fatty acids from castor oil with branched alcohols using cationic exchange resins as catalysts. Ind. Crops Prod. 2017, 104, 52–61. [Google Scholar] [CrossRef]
- Rat, M.; Zahedi-Niaki, M.H.; Kaliaguine, S.; Do, T.O. Sulfonic acid functionalized periodic mesoporous organosilicas as acetalization catalsts. Microporous Mesoporous Mater. 2008, 112, 26–31. [Google Scholar] [CrossRef]
- Borges, M.E.; Días, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: a review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Castor oil biodiesel production and optimization. Egypt. J. Petrol. 2018, in press. [Google Scholar] [CrossRef]




| Sample | Concentration (wt %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | CaO | MnO | P2O5 | TiO2 | |
| Bentonite | 66.85 | 20.51 | 4.06 | 4.11 | 1.65 | 0.60 | 1.67 | 0.03 | 0.08 | 0.15 |
| B-HNO3 | 68.13 | 22.29 | 4.03 | 3.91 | 0.49 | 0.42 | 0.35 | 0.02 | 0.02 | 0.15 |
| B-H2SO4 | 68.40 | 22.23 | 3.87 | 3.95 | 0.35 | 0.37 | 0.24 | 0.02 | 0.05 | 0.15 |
| B-HCl | 68.42 | 22.40 | 4.02 | 3.85 | 0.39 | 0.41 | 0.28 | 0.01 | 0.04 | 0.15 |
| PILC | 59.80 | 33.18 | 3.32 | 3.30 | 0.25 | 0.29 | 0.22 | 0.01 | 0.02 | 0.12 |
| Samples | SBET (m2·g−1) | VµP (cm3·g−1) | VTP (cm3·g−1) | Amount Acid Sites 1 (μmol·g−1) |
|---|---|---|---|---|
| Bentonite | 37 | 0.01 | 0.04 | 145 |
| B-H2SO4 | 171 | 0.05 | 0.14 | 357 |
| B-HNO3 | 158 | 0.05 | 0.12 | 320 |
| B-HCl | 172 | 0.05 | 0.13 | 374 |
| PILC | 270 | 0.10 | 0.17 | 522 |
| Samples | Density at 20 °C (g.cm−3) | Pour Point (°C) | Flash Point (°C) | TAN | Viscosity (cSt) | Viscosity Index | |
|---|---|---|---|---|---|---|---|
| (mg KOH.g−1) | 40 °C | 100 °C | |||||
| Castor Oil | 0.9582 | −15 | 145 | 1.19 | 261.3 | 19.60 | 84 |
| FACO | 0.9393 | −36 | 180 | 127 | 132.2 | 13.0 | 90 |
| Esters | 0.9084 | −39 | 205 | 0.61 | 38.4 | 7.29 | 157 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna, F.M.T.; Cecilia, J.A.; Saboya, R.M.A.; Barrera, D.; Sapag, K.; Rodríguez-Castellón, E.; Cavalcante, C.L. Natural and Modified Montmorillonite Clays as Catalysts for Synthesis of Biolubricants. Materials 2018, 11, 1764. https://doi.org/10.3390/ma11091764
Luna FMT, Cecilia JA, Saboya RMA, Barrera D, Sapag K, Rodríguez-Castellón E, Cavalcante CL. Natural and Modified Montmorillonite Clays as Catalysts for Synthesis of Biolubricants. Materials. 2018; 11(9):1764. https://doi.org/10.3390/ma11091764
Chicago/Turabian StyleLuna, Francisco Murilo Tavares, Juan Antonio Cecilia, Rosana Maria Alves Saboya, Deicy Barrera, Karim Sapag, Enrique Rodríguez-Castellón, and Célio Loureiro Cavalcante. 2018. "Natural and Modified Montmorillonite Clays as Catalysts for Synthesis of Biolubricants" Materials 11, no. 9: 1764. https://doi.org/10.3390/ma11091764
APA StyleLuna, F. M. T., Cecilia, J. A., Saboya, R. M. A., Barrera, D., Sapag, K., Rodríguez-Castellón, E., & Cavalcante, C. L. (2018). Natural and Modified Montmorillonite Clays as Catalysts for Synthesis of Biolubricants. Materials, 11(9), 1764. https://doi.org/10.3390/ma11091764








