Investigation on Water Vapor Adsorption of Silica-Phosphonium Ionic Liquids Hybrid Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of grafted silica gel
2.3. Characterization
2.3.1. Chemical Analysis
2.3.2. Pore Structure Characteristics
2.4. Adsorption Studies
3. Results and Discussion
3.1. Characterization of the Samples
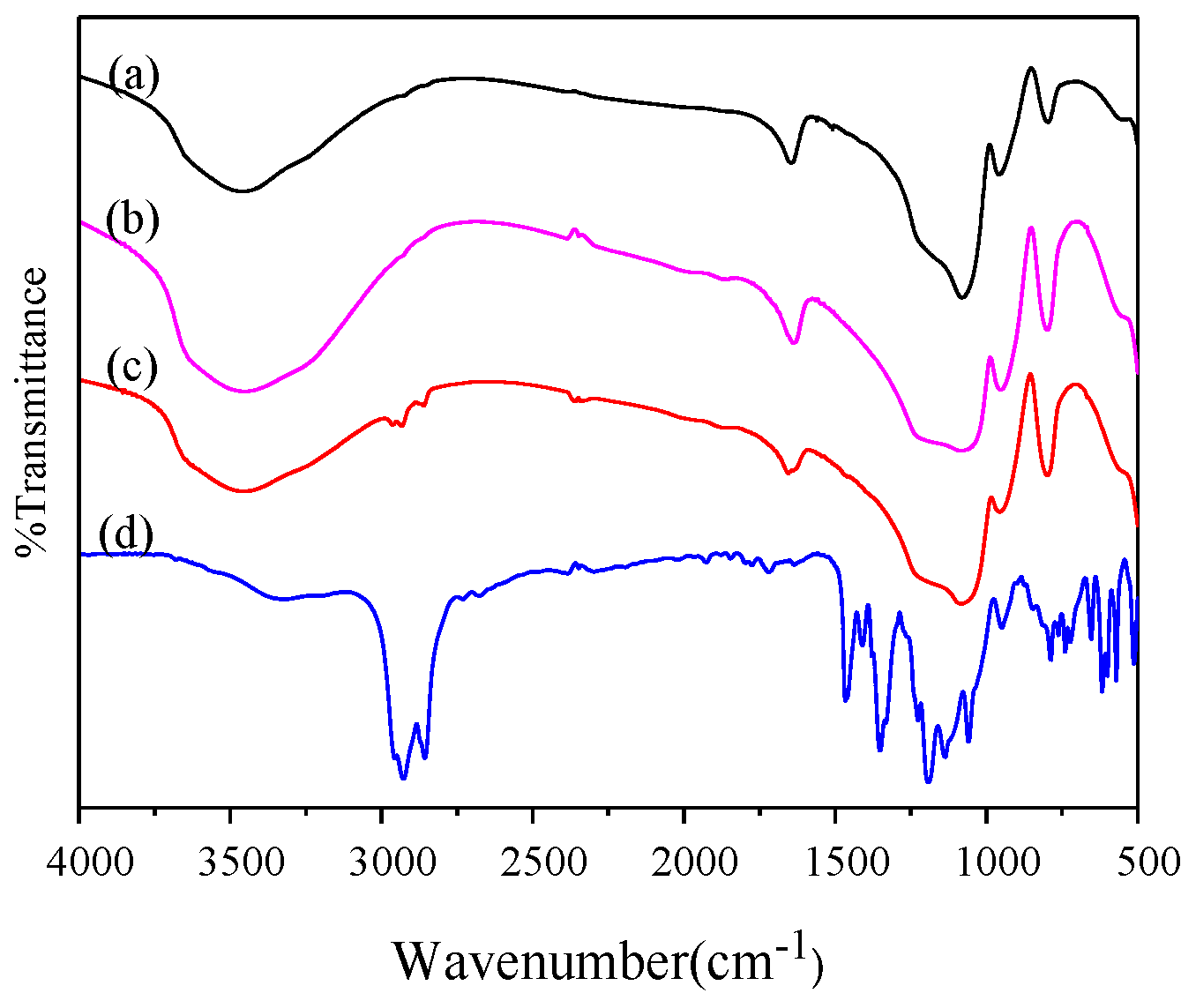
3.1.1. FTIR Analysis
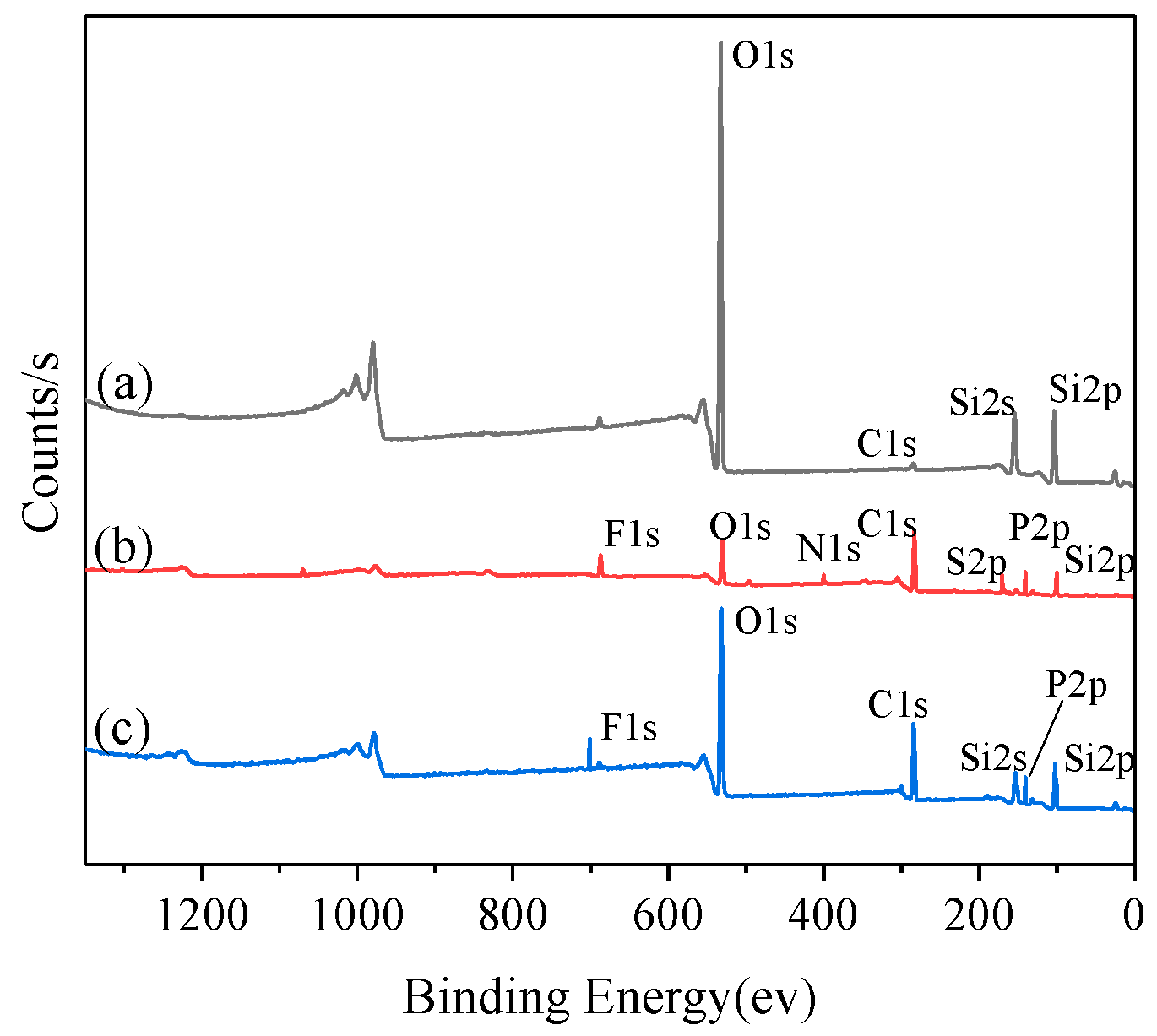
3.1.2. XPS Analysis
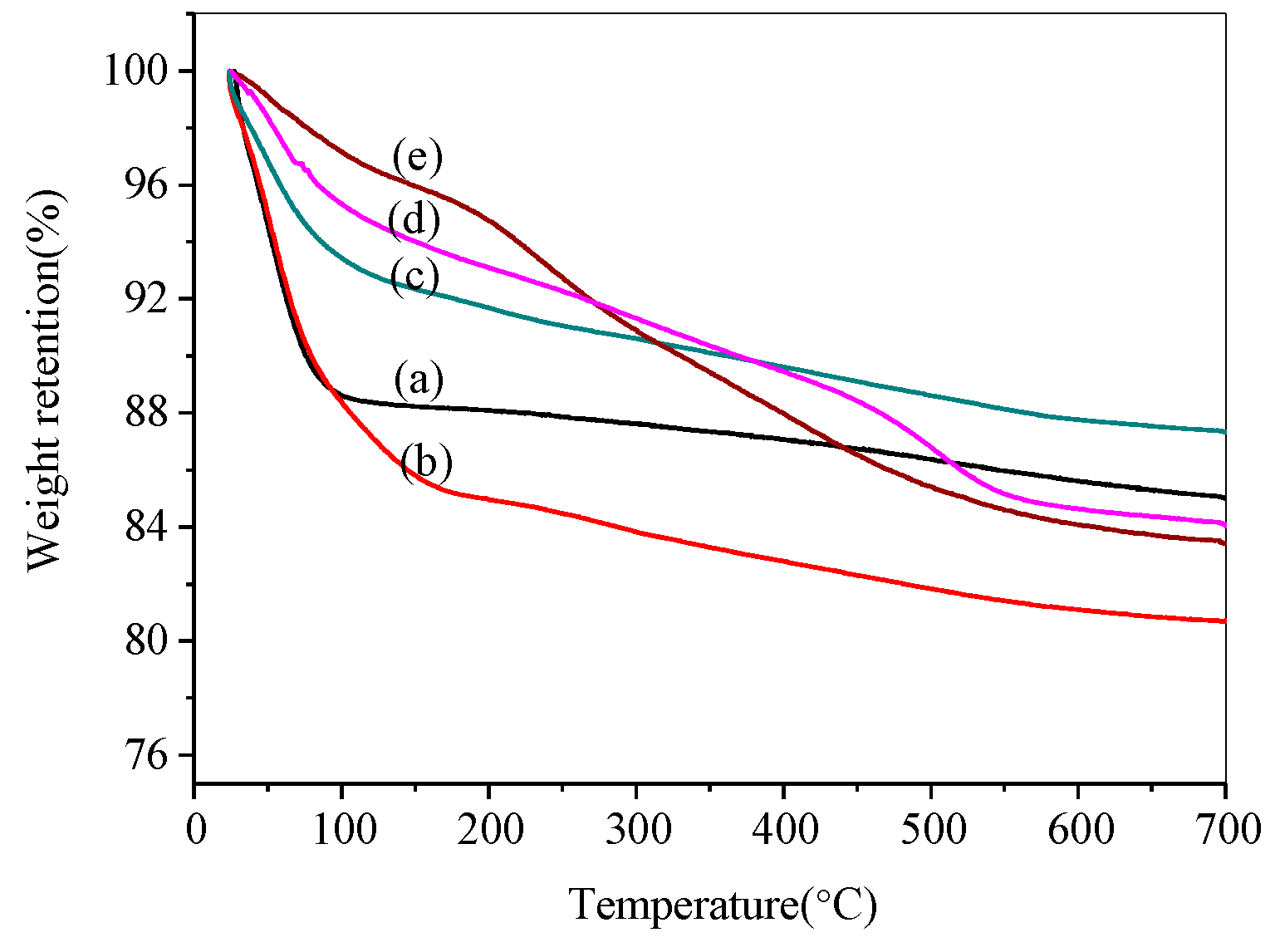
3.1.3. Thermogravimetric Analysis
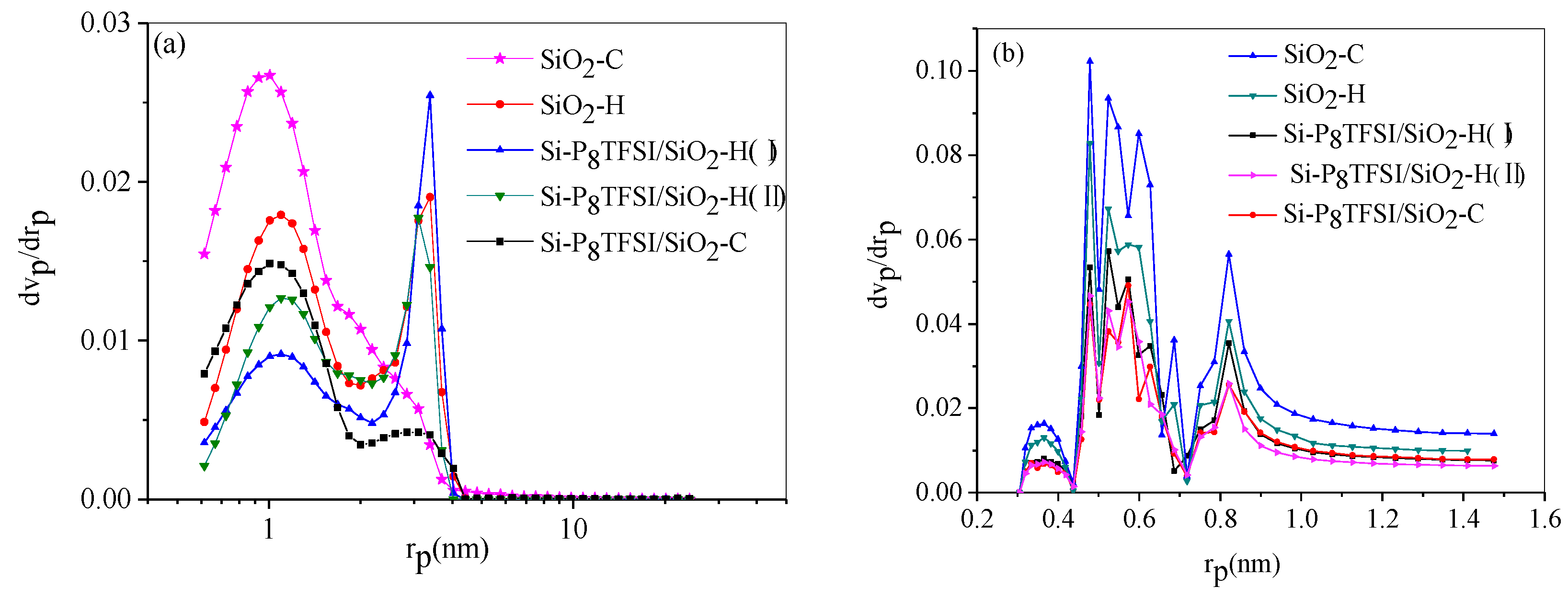
3.1.4. Pore size distribution
3.2. Water Vapor Adsorption
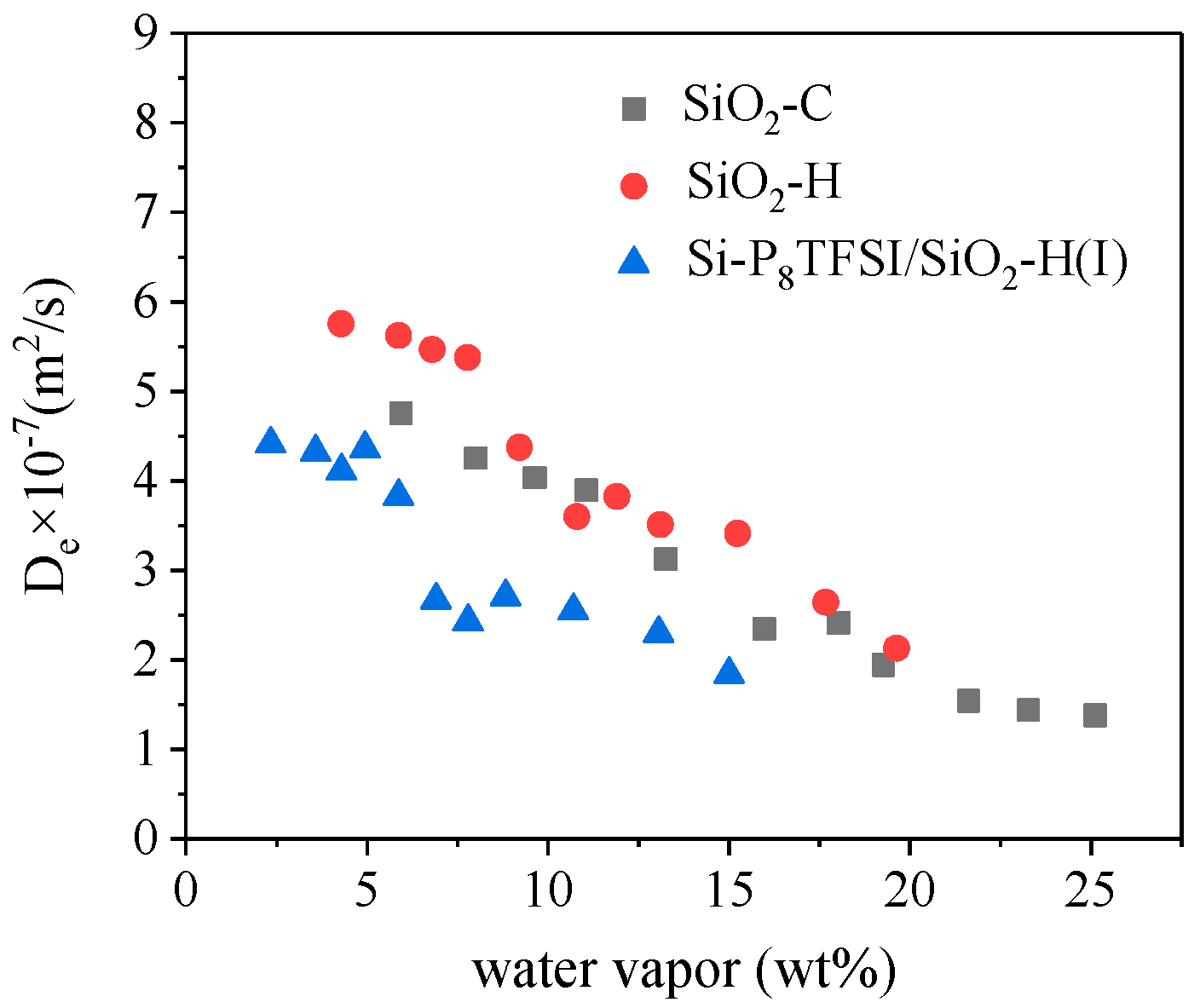
3.3. Water Diffusion Coefficient
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramdin, M.; Amplianitis, A.; Bazhenov, S.; Volkov, A.; Volkov, V.; Vlugt Thijs, J.H. Solubility of CO2 and CH4 in ionic liquids: Ideal CO2/CH4 selectivity. Ind. Eng. Chem. Res. 2015, 53, 15427–15435. [Google Scholar] [CrossRef]
- WlazlO, M.; Karpińska, M.; Domańska, U. Separation of water/butan-1-ol mixtures based on limiting activity coefficients with phosphonium-based ionic liquid. J. Chem. Thermodyn. 2017, 113, 183–191. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Pan, M.G.; Kang, X.J.; Tu, W.H.; Gao, H.S.; Zhang, X.P. Gas separation by ionic liquids: A theoretical study. Chem. Eng. Sci. 2018, 189, 43–55. [Google Scholar] [CrossRef]
- Brennecke, J.F.; Gurkan, B.E. Ionic liquids for CO2 capture and emission reduction. J. Phys. Chem. Lett. 2010, 1, 3459–3464. [Google Scholar] [CrossRef]
- Makino, T.; Kanakubo, M. Absorption of n-Butane in imidazolium and phosphonium ionic liquids and application to separation of hydrocarbon gases. Sep. Purif. Technol. 2019, 214, 139–147. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Fan, J.; Shang, X.M. CO2 capture and separation properties in the ionic liquid 1-n-butyl-3-methylimidazolium nonafluorobutylsulfonate. Materials 2014, 7, 3867–3880. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Gumerova, O.R.; Atlaskin, A.A.; Petukhov, A.N.; Vorotyntsev, I.V. Permeability and selectivity of acid gases in supported conventional and novel imidazolium-based ionic liquid membranes. Sep. Purif. Technol. 2016, 176, 92–106. [Google Scholar] [CrossRef]
- Wang, S.B.; Wang, X. Imidazolium Ionic liquids, imidazolylidene heterocyclic carbenes and zeolitic imidazolate frameworks for CO2 capture and photochemical reduction. Angew. Chem. Int. Edit. 2016, 55, 2308–2320. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Simoes, P.N.; Ferreira, A.G.M. Quaternary phosphonium-based ionic liquids: Thermal stability and heat capacity of the liquid phase. J. Chem. Thermodyn. 2012, 45, 16–27. [Google Scholar] [CrossRef]
- Oster, K.; Goodrich, P.; Jacquemin, J.; Hardacre, C.; Ribeiro, A.P.C.; Elsinawi, A. A new insight into pure and water-saturated quaternary phosphonium-based carboxylate ionic liquids: Density, heat capacity, ionic conductivity, thermogravimetric analysis, thermal conductivity and viscosity. J. Chem. Thermodyn. 2018, 121, 97–111. [Google Scholar] [CrossRef]
- Keglevich, G.; Grun, A.; Hermecz, I.; Odinets, I.L. Quaternary phosphonium salt and 1,3–dialkylimidazolium hexafluorophosphate ionic liquids as green chemical tools in organic syntheses. Curr. Org. Chem. 2011, 15, 3824–3848. [Google Scholar] [CrossRef]
- Tsunashima, K.; Ono, Y.; Sugiya, M. Physical and electrochemical characterization of ionic liquids based on quaternary phosphonium cations containing a carbon-carbon double bond. Electrochim. Acta. 2011, 56, 4351–4355. [Google Scholar] [CrossRef]
- Bradaric, C.J.; Downard, A.; Kennedy, C.; Robertson, A.J.; Zhou, Y.H. Industrial preparation of phosphonium ionic liquids. Green Chem. 2003, 5, 143–152. [Google Scholar] [CrossRef]
- Rzelewska, M.; Baczyńska, M.; Wiśniewski, M.; Regelrosocka, M. Phosphonium ionic liquids as extractants for recovery of ruthenium(III) from acidic aqueous solutions. Chem. Pap. 2017, 71, 1065–1072. [Google Scholar] [CrossRef]
- Deferm, C.; Malaquias, J.C.; Onghena, B.; Banerjee, D.; Luyten, J.; Oosterhof, H.; Fransaer, J.; Binnemans, K. Electrodeposition of indium from the ionic liquid trihexyl(tetradecyl)phosphonium chloride. Green Chem. 2019, 21, 1517–1530. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, S.; Lu, X.; Zhou, Q.; Fan, W.; Zhang, X.P. Dual amino-functionalised phosphonium ionic liquids for CO2 capture. Chem. Eur. J. 2010, 15, 3003–3011. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, M.; Erto, A.; Lancia, A.; Totarella, G.; Montagnaro, F.; Turco, R. Post-combustion CO2 capture: On the potentiality of amino acid ionic liquid as modifying agent of mesoporous solids. Fuel. 2018, 218, 155–161. [Google Scholar] [CrossRef]
- Ansaloni, L.; Nykaza, J.R.; Ye, Y.S.; Elabd, Y.A.; Baschetti, M.G. Influence of water vapor on the gas permeability of polymerized ionic liquids membranes. J. Membran. Sci. 2015, 487, 199–208. [Google Scholar] [CrossRef]
- Chanut, N.; Bourrelly, S.; Kuchta, B.; Serre, C.; Chang, J.S.; Wright, P.A.; Llewellyn, P.L. Screening the effect of water vapour on gas adsorption performance: Application to CO2 capture from flue gas in metal-organic frameworks. ChemSusChem 2017, 10, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Badiei, A.R.; Mokhtarani, B.; Sharifi, A. Experimental study on CO2, sorption capacity of the neat and porous silica supported ionic liquids and the effect of water content of flue gas. J. Mol. Liq. 2017, 232, 462–470. [Google Scholar] [CrossRef]
- Li, G.; Xiao, P.; Webley, P. Binary adsorption equilibrium of carbon dioxide and water vapor on activated alumina. Langmuir 2009, 25, 10666–10675. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xiao, P.; Zhang, J.; Li, G.; Xiao, G.K.; Webley, P.A.; Zhai, Y.C. Effects of water vapour on CO2 capture with vacuum swing adsorption using activated carbon. Chem. Eng. J. 2013, 230, 64–72. [Google Scholar] [CrossRef]
- Uehara, Y.; Karami, D.; Mahinpey, N. Effect of water vapor on CO2 sorption-desorption behaviors of supported amino acid ionic liquid sorbents on porous microspheres. Ind. Eng. Chem. Res. 2017, 56, 14316–14323. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Pérez-Cadenas, M.; Materials, D. Influence of pore size distribution on water adsorption on silica gels. J. Porous. Mat. 2010, 17, 409–416. [Google Scholar] [CrossRef]
- Saliba, S.; Ruch, P.; Volksen, W.; Magbitang, T.P.; Dubois, G.; Michel, B. Combined influence of pore size distribution and surface hydrophilicity on the water adsorption characteristics of micro-and mesoporous silica. Micropor. Mesopor. Mat. 2016, 226, 221–228. [Google Scholar] [CrossRef]
- Zhu, J.M.; He, B.T.; Huang, J.H.; Li, C.C.; Ren, T. Effect of immobilization methods and the pore structure on CO2 separation performance in silica-supported ionic liquids. Micropor. Mesopor. Mat. 2018, 260, 190–200. [Google Scholar] [CrossRef]
- Kim, J.M.; Chang, S.M.; Kong, S.M.; Kim, K.S.; Kim, J.; Kim, W.S. Control of hydroxyl group content in silica particle synthesized by the sol-precipitation process. Ceram. Int. 2009, 35, 1015–1019. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Yokozek, A. Solubilities and Diffusivities of carbon dioxide in ionic liquids: [bmim][PF6] and [bmim][BF4]. Ind. Eng. Chem. Res. 2005, 44, 4453–4464. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.; Pendleton, P. Experimental and modelling study of CO2 absorption in ionic liquids containing Zn (II) ions. Energy Procedia. 2011, 4, 59–66. [Google Scholar] [CrossRef]
- Detallante, V.; Langevin, D.; Chappey, M.; Métayer, M.; Mercier, R.; Pinéri, M. Kinetics of water vapor sorption in sulfonated polyimide membranes. Desalination 2002, 148, 333–339. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, S.L. A two-parameter stretched exponential function for dynamic water vapor sorption of cement-based porous materials. Mater. Struct. 2017, 50, 128–141. [Google Scholar] [CrossRef]
- Yang, H.; Wang, D.K.; Motuzas, J.; Diniz, da.; Costa, J.C. Hybrid vinyl silane and P123 template sol−gel derived carbon silica membrane for desalination. J. Sol-Gel. Sci. Technol. 2018, 85, 280–289. [Google Scholar] [CrossRef]
- Firoozmandan, M.; Moghaddas, J.; Yasrebi, N. Performance of water glass-based silica aerogel for adsorption of phenol from aqueous solution. J. Sol-Gel. Sci. Technol. 2016, 79, 67–75. [Google Scholar] [CrossRef]
- Mcdaniel, J.G.; Son, C.Y.; Yethiraj, A. Ab initio force fields for organic anions: Properties of [BMIM][TFSI], [BMIM][FSI], and [BMIM][OTF] ionic liquids. J. Phys. Chem. B 2018, 122, 4101–4114. [Google Scholar] [CrossRef] [PubMed]
- Akbari, Z.; Ghiaci, M. Heterogenization of a green homogeneous catalyst: Synthesis and characterization of imidazolium ionene/Br–Cl–@SiO2 as an efficient catalyst for the cycloaddition of CO2 with epoxides. Ind. Eng. Chem. Res. 2017, 56, 9045–9053. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Ma, Y.C.; Foster, A.S.; Nieminen, R.M. Reactions and clustering of water with silica surface. J. Chem. Phys. 2005, 122, 144709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahadevan, T.S.; Du, J.C. Evaluating Water Reactivity at Silica Surfaces Using Reactive Potentials. J. Phys. Chem. C 2018, 122, 9875–9885. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975. [Google Scholar]
- Oehler, A.; Tomozawa, M. Water diffusion into silica glass at a low temperature under high water vapor pressure. J. Non-Cryst. Solids. 2004, 347, 211–219. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Tokarev, M.M.; Freni, A.; Glaznev, I.S.; Restuccia, G. Kinetics of water adsorption on silica Fuji Davison RD. Micropor. Mesopor. Mater. 2006, 96, 65–71. [Google Scholar] [CrossRef]
- Mohammed, R.H.; Mesalhy, O.; Elsayed, M.L.; Hou, S.; Su, M.; Chow, L.C. Physical properties and adsorption kinetics of silica-gel/water for adsorption chillers. Appl. Therm. Eng. 2018, 137, 368–376. [Google Scholar] [CrossRef]

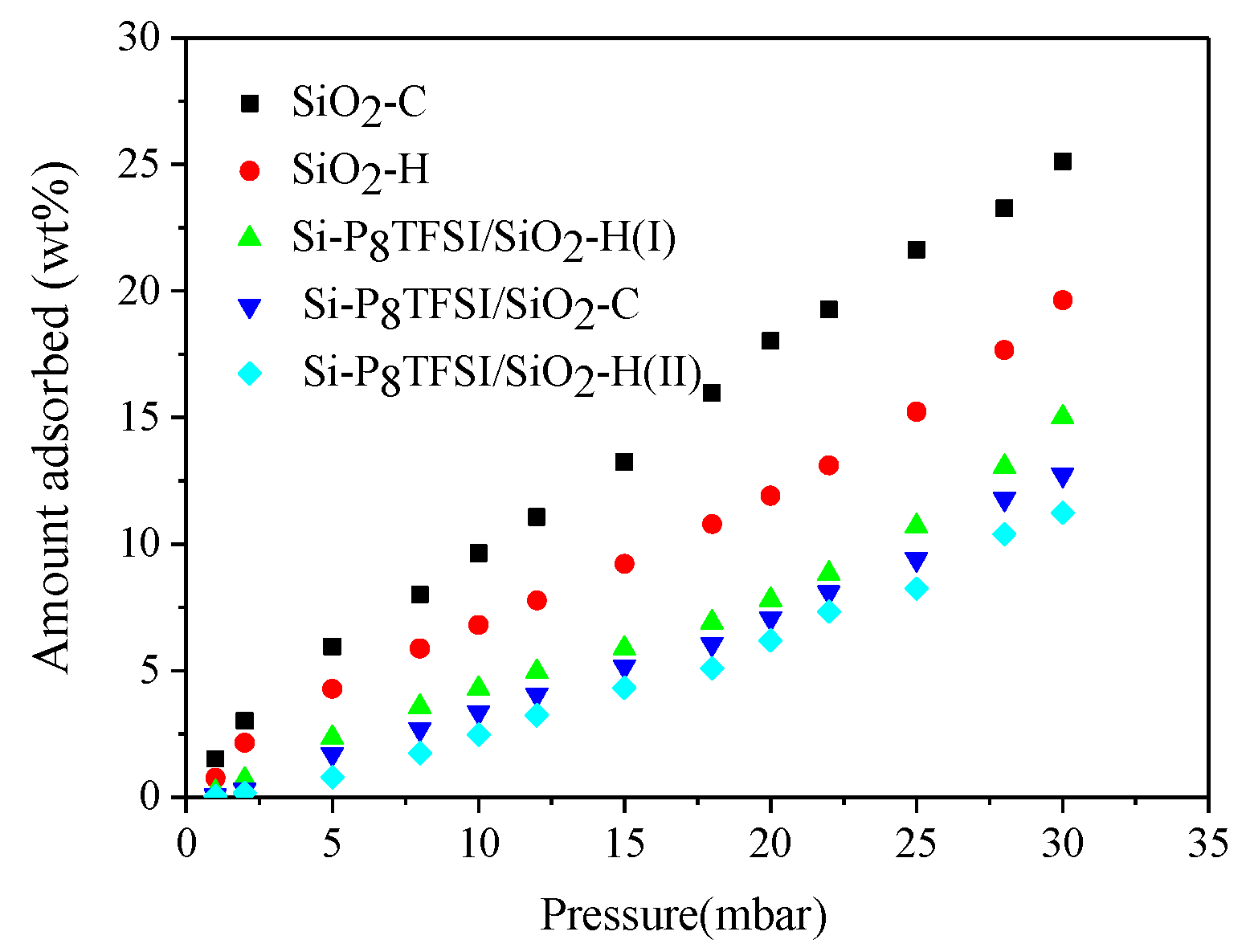
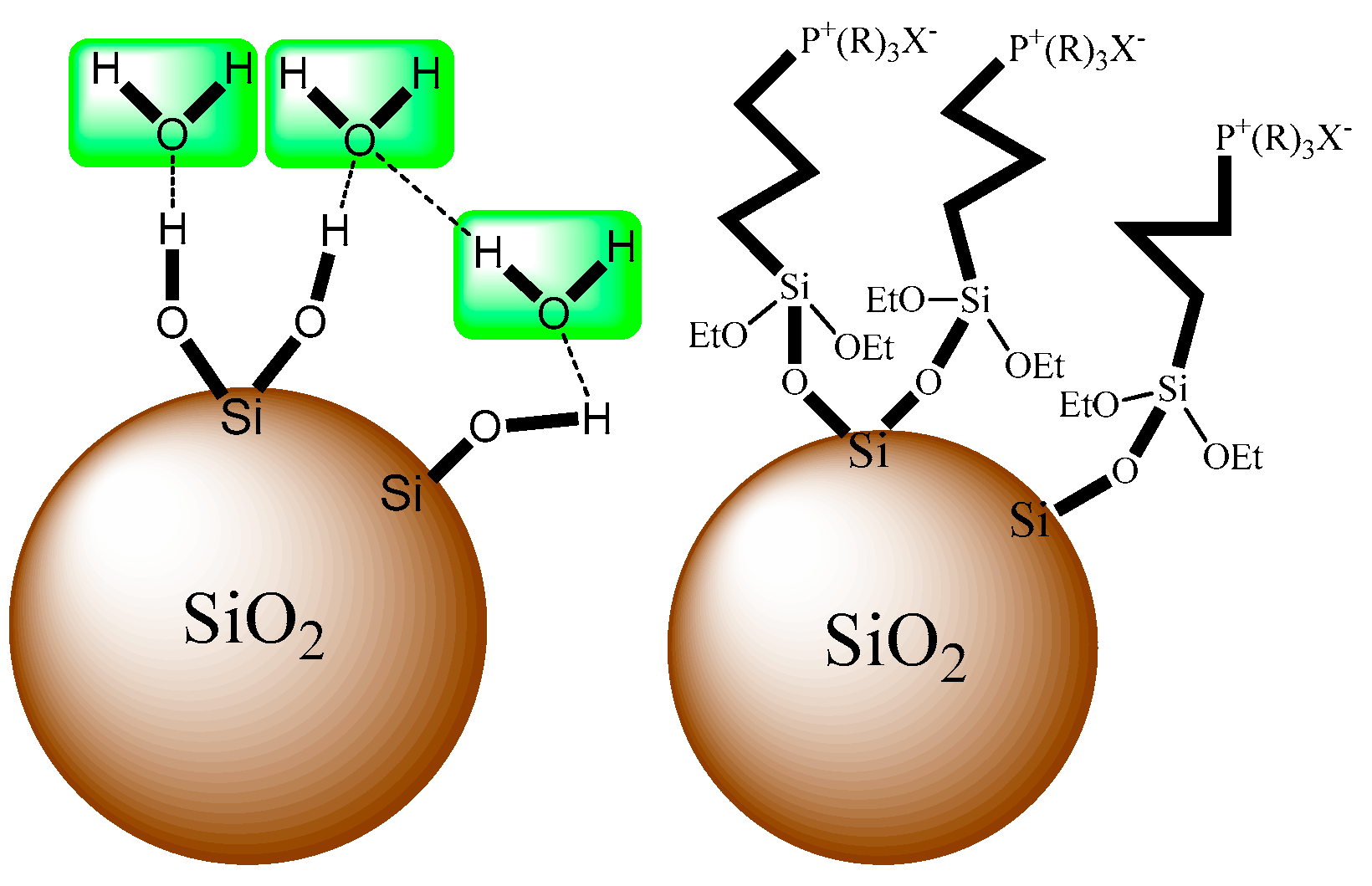
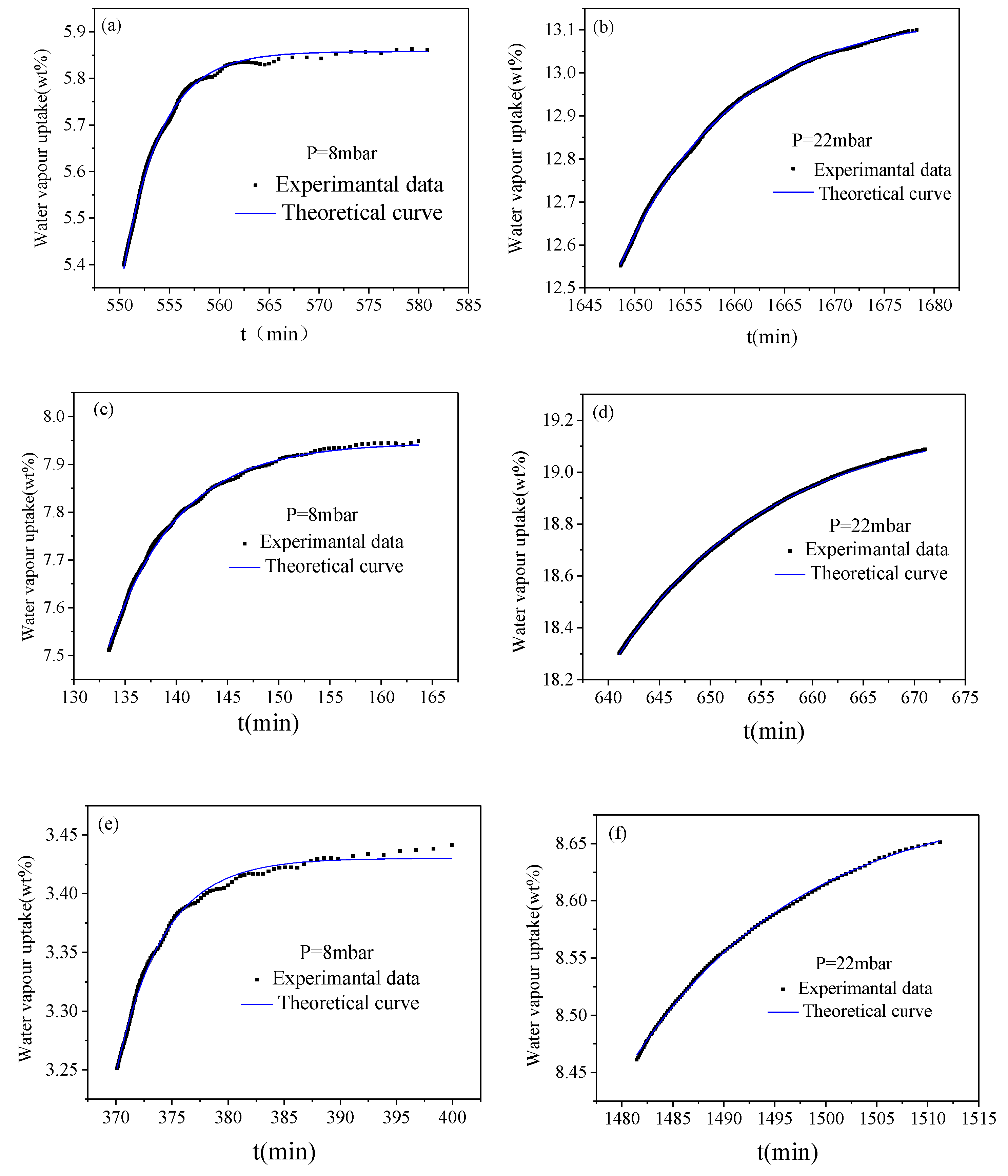
| Sample | OH Group Content(mmol/g) | Amount of Grafting (wt%) |
|---|---|---|
| SiO2-H | 3.867 | - |
| SiO2-C | 5.479 | - |
| Si-P8TFSI/SiO2-H(I) | - | 4.92 |
| Si-P8TFSI/SiO2-H(II) | - | 10.62 |
| Si-P8TFSI/SiO2-C | - | 10.79 |
| Sample | N2 Sorption Isotherms | CO2 Sorption Isotherms | V (CO2) (wt%) | ||
|---|---|---|---|---|---|
| CPV × 10−1 (cm3·g−1) | CSA (m2·g−1) | CPV × 10−1 (cm3·g−1) | CSA (m2·g−1) | ||
| SiO2-H | 4.001 | 525.3 | 1.058 | 306.8 | 5.51 |
| SiO2-C | 4.149 | 635.6 | 1.241 | 362.3 | 6.47 |
| Si-P8TFSI/SiO2-H(I) | 3.303 | 425.8 | 0.860 | 263.2 | 4.78 |
| Si-P8TFSI/SiO2-H(II) | 3.263 | 373.1 | 0.801 | 238.7 | 4.27 |
| Si-P8TFSI/SiO2-C | 3.347 | 455.4 | 0.980 | 283.9 | 4.96 |
| Pressure (mbar) | De × 10−7 (m2/s) SiO2-C | De × 10−7 (m2/s) SiO2-H | De × 10−7 (m2/s) Si-P8TFSI/SiO2vH(I) |
|---|---|---|---|
| 5 | 4.76 | 5.75 | 4.41 |
| 8 | 4.25 | 5.62 | 4.32 |
| 10 | 4.03 | 5.46 | 4.11 |
| 12 | 3.89 | 5.37 | 4.36 |
| 15 | 3.12 | 4.37 | 3.82 |
| 18 | 2.38 | 3.61 | 2.67 |
| 20 | 2.41 | 3.82 | 2.43 |
| 22 | 1.94 | 3.51 | 2.71 |
| 25 | 1.54 | 3.42 | 2.55 |
| 28 | 1.44 | 2.64 | 2.98 |
| 30 | 1.38 | 2.13 | 1.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhu, J.; Zhou, M.; Zhang, S.; He, X. Investigation on Water Vapor Adsorption of Silica-Phosphonium Ionic Liquids Hybrid Material. Materials 2019, 12, 1782. https://doi.org/10.3390/ma12111782
Li C, Zhu J, Zhou M, Zhang S, He X. Investigation on Water Vapor Adsorption of Silica-Phosphonium Ionic Liquids Hybrid Material. Materials. 2019; 12(11):1782. https://doi.org/10.3390/ma12111782
Chicago/Turabian StyleLi, Cancan, Jiamei Zhu, Min Zhou, Shuangquan Zhang, and Xiaodong He. 2019. "Investigation on Water Vapor Adsorption of Silica-Phosphonium Ionic Liquids Hybrid Material" Materials 12, no. 11: 1782. https://doi.org/10.3390/ma12111782
APA StyleLi, C., Zhu, J., Zhou, M., Zhang, S., & He, X. (2019). Investigation on Water Vapor Adsorption of Silica-Phosphonium Ionic Liquids Hybrid Material. Materials, 12(11), 1782. https://doi.org/10.3390/ma12111782




