The Use of Cashew Nut Shell Liquid (CNSL) in PP/HIPS Blends: Morphological, Thermal, Mechanical and Rheological Properties
Abstract
:1. Introduction
2. Materials and Methods
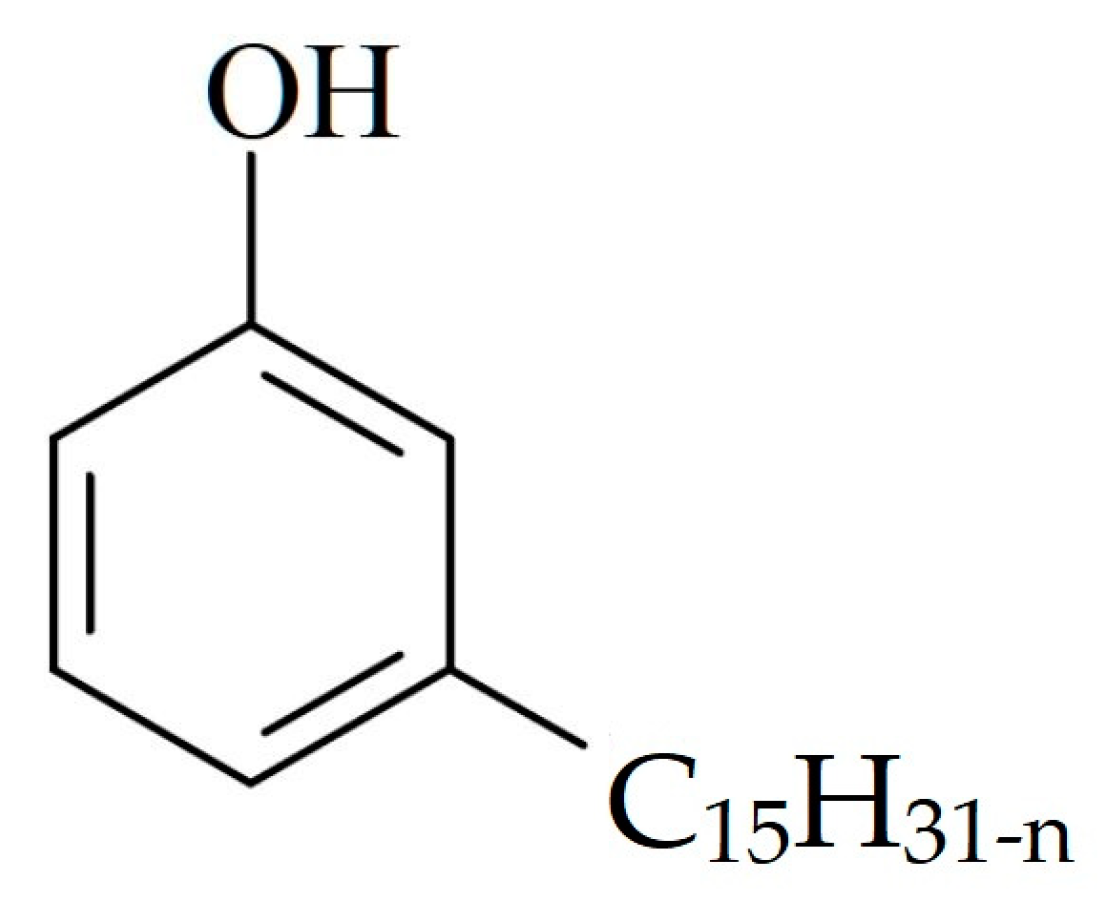
2.1. Materials
2.2. Preparation and Injection Molding of the Blends
2.3. Scanning Electron Microscopy (SEM)
2.4. Thermal Analysis by Differential Scanning Calorimetry (DSC)
2.5. Mechanical Tests
2.6. Rheological Measurements
3. Results
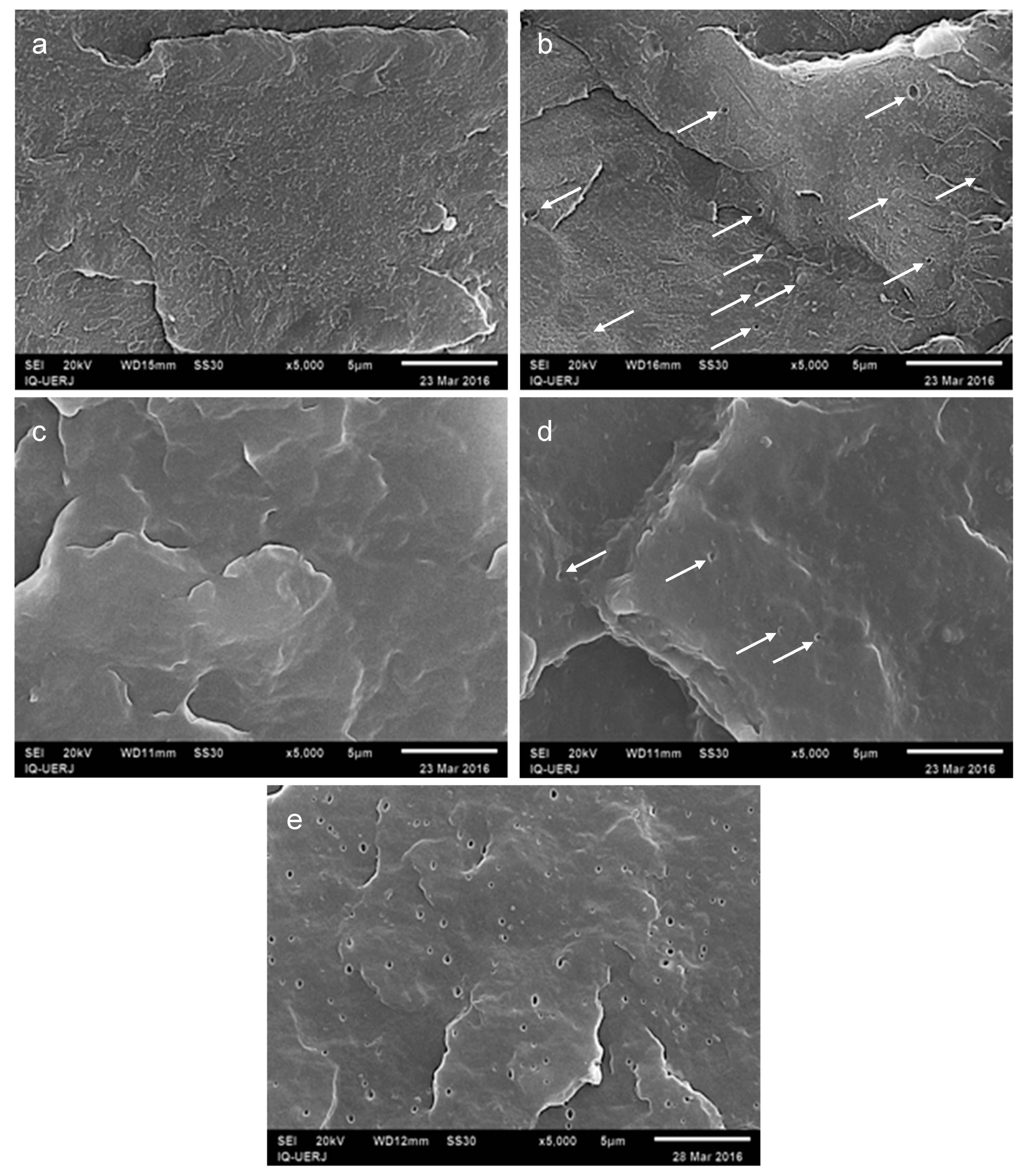
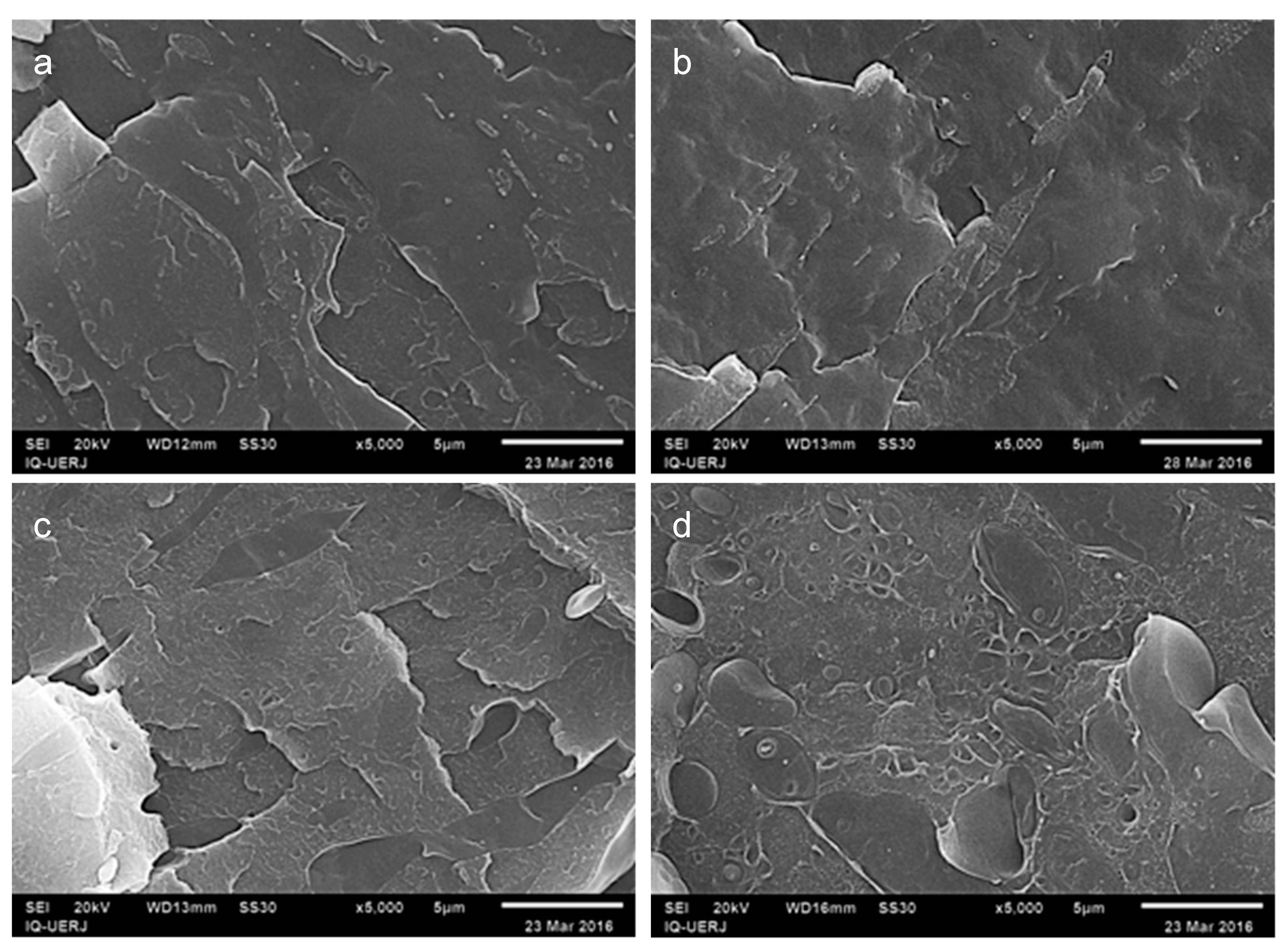
3.1. Morphology
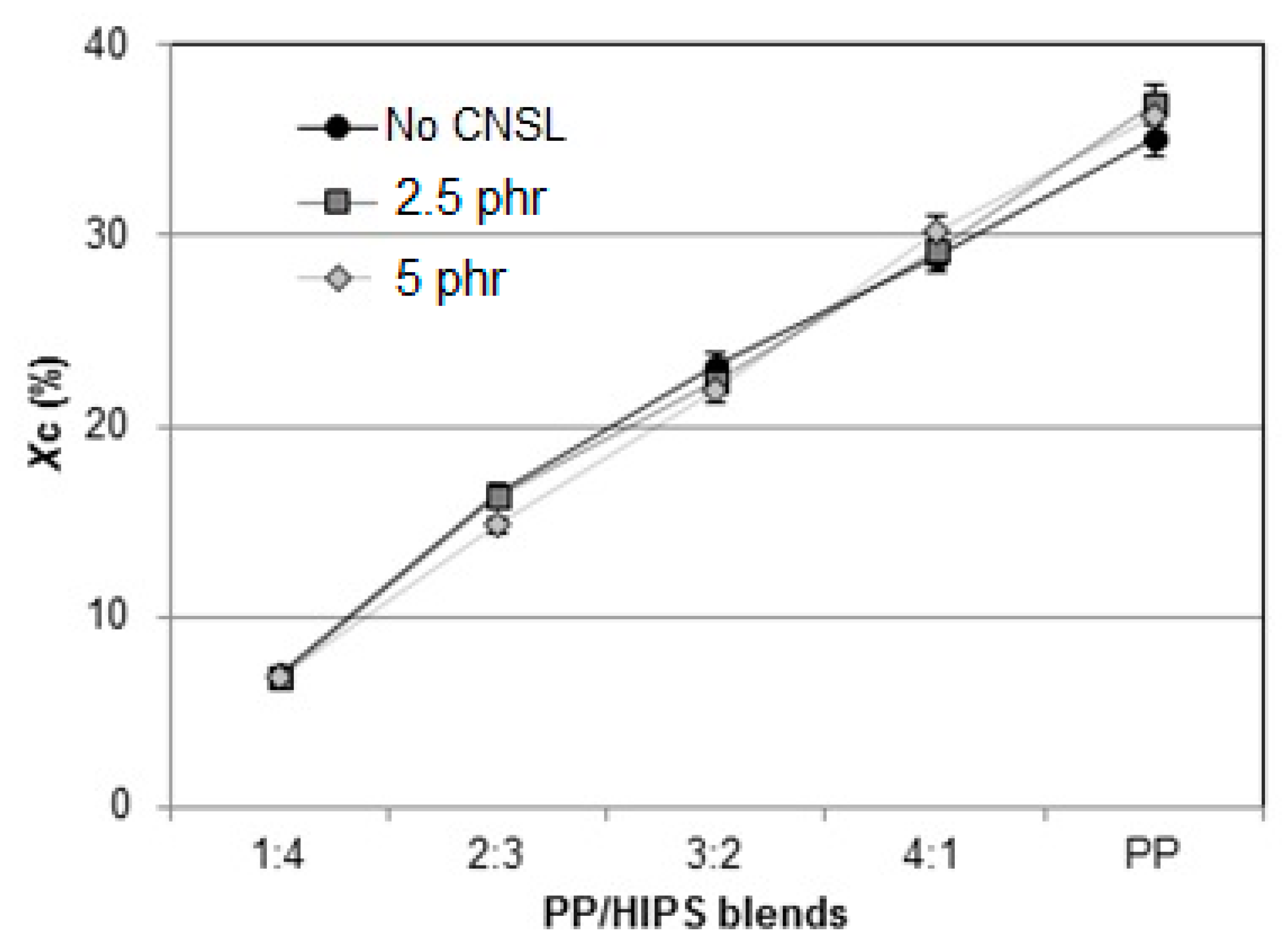
3.2. Thermal Behavior
3.3. Mechanical Tests
3.3.1. Impact Strength Analysis
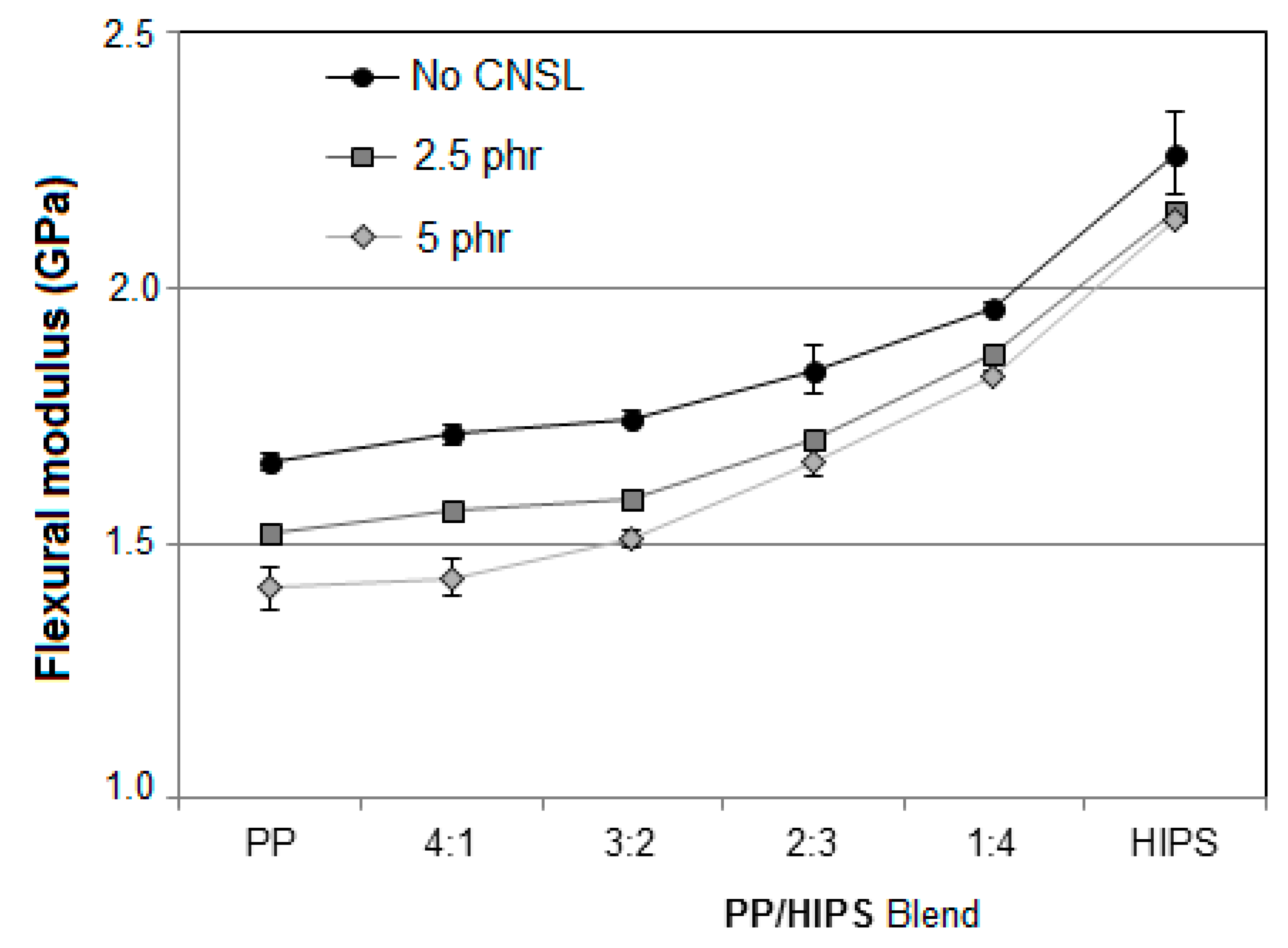
3.3.2. Flexural Analysis
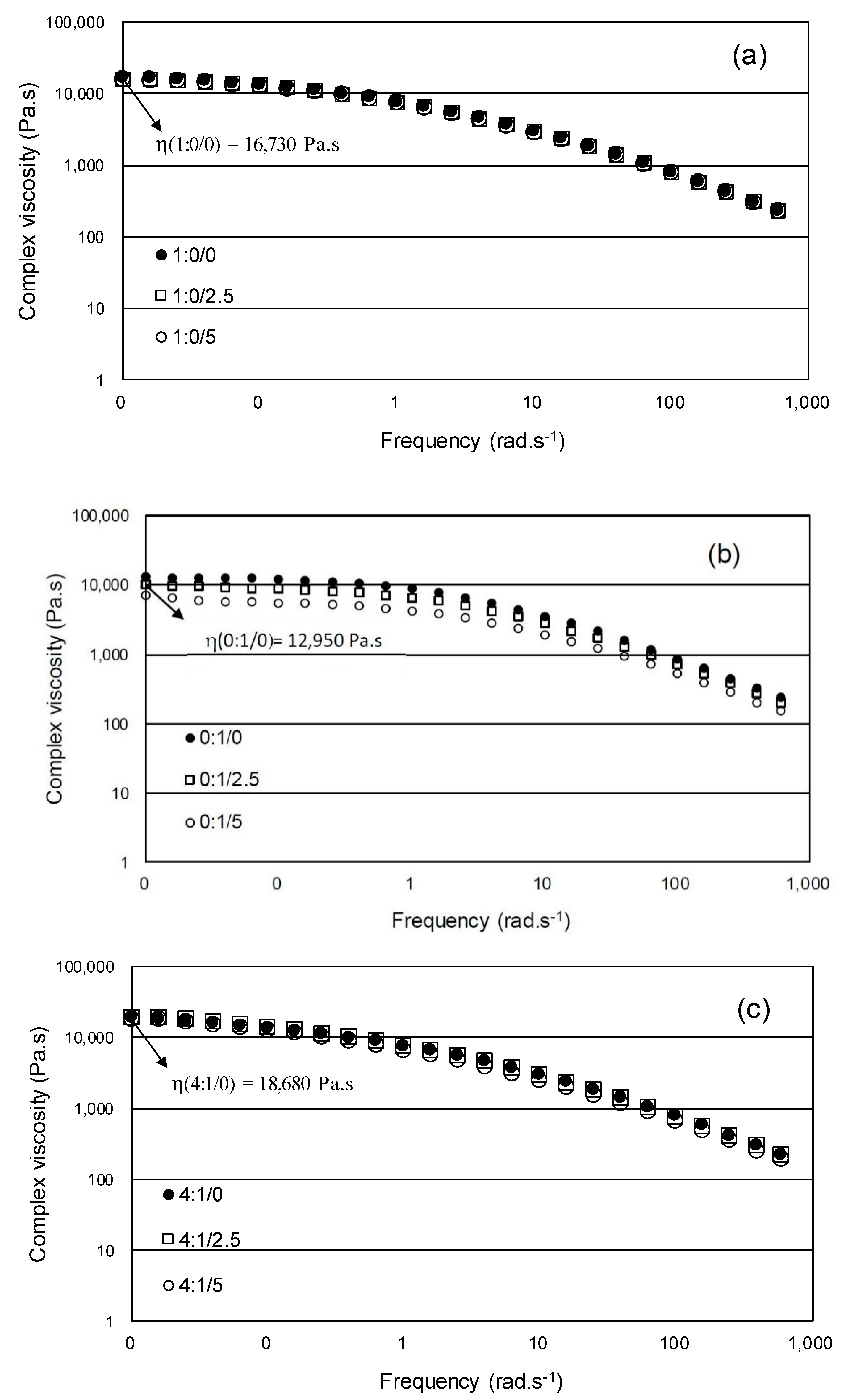
3.3.3. Dynamic Melt Rheological Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Godecke, M.V.; Naime, R.H.; Figueiredo, J.A.S.O. Consumismo e a geração de resíduos sólidos urbanos no Brasil (Consumerism and the generation of solid urban waste in Brazil). Revista Eletrônica em Gestão Educação e Tecnologia Ambiental 2012, 8, 1700–1712. Available online: http://cascavel.ufsm.br/revistas/ojs-2.2.2/index.php/reget (accessed on 23 April 2019).
- Huysman, S.; Debaveye, S.; Schaubroeck, T.; De Meester, S.; Ardente, F.; Mathieux, F.; Dewulf, J. The recyclability benefit rate of closed-loop and open-loop systems: A case study on plastic recycling in Flanders. Resour. Conserv. Recycl. 2015, 101, 53–60. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Theis, V.; Schreiber, D. A inovação e as alternativas de realizar as atividades em P&D: Estudo de caso da Braskem (The innovation and the alternatives to carry out the activities in R&D: Braskem case study). In Inovação e Aprendizagem Organizacional; Universidade Feevale: Rio Grande do sul, Brazil, 2013; pp. 141–164. ISBN 978-85-7717-163-7. [Google Scholar]
- Mélo, T.J.A.; Carvalho, L.H.; Calumby, R.B.; Brito, K.G.Q.; D’Almeida, J.R.M.; Spieth, E. Mechanical properties and morphology of a PP/HIPS polymer blend compatibilized with SEBS. Polímeros Ciência e Tecnologia 2000, 10, 82–89. [Google Scholar] [CrossRef]
- Santana, R.M.C.; Manrich, S. Morphology and mechanical properties of polypropylene/high-impact polystyrene blends from postconsumer plastic waste. J. Appl. Polym. Sci. 2003, 88, 2861–2867. [Google Scholar] [CrossRef]
- Fernandes, L.L.; Freitas, C.A.; Demarquette, N.R.; Fechine, G.J.M. Photodegradation of thermodegraded polypropylene/high-impact polystyrene blends: Mechanical properties. J. Appl. Polym. Sci. 2011, 120, 770–779. [Google Scholar] [CrossRef]
- Fernandes, L.L.; Freitas, C.A.; Demarquette, N.R.; Fechine, G.J.M. Influence of the type of polypropylene on the photodegradation of blends of polypropylene/high impact polystyrene. Polímeros 2012, 22, 61–68. [Google Scholar] [CrossRef]
- Parres, F.; Balart, R.; López, J.; García, D. Changes in the mechanical and thermal properties of high impact polystyrene (HIPS) in the presence of low polypropylene (PP) contents. J. Mater. Sci. 2008, 43, 3203–3209. [Google Scholar] [CrossRef]
- Paiva, F.F.A.; Garrutti, D.S.; Silva Neto, R.M. Cashew Industrial Exploitation, 1st ed.; EMBRAPA Agroindústria Tropical—SEBRAE: Fortaleza, Brazil, 2000; 88p. [Google Scholar]
- Mazzetto, S.E.; Lomonaco, D.; Mele, G. Cashew nut oil: Opportunities and challenges in the context of sustainable industrial development. Química Nova 2009, 32, 732–741. [Google Scholar] [CrossRef]
- Gedam, P.H.; Sampathkumaran, P.S. Cashew nut shell liquid: Extraction, chemistry and applications. Prog. Org. Coat. 1986, 14, 115–157. [Google Scholar] [CrossRef]
- Alexander, M.; Thachil, E.T. The effectiveness of cardanol as plasticiser, activator, and antioxidant for natural rubber processing. Prog. Rubber Plast. Recycl. Technol. 2010, 26, 107–123. [Google Scholar] [CrossRef]
- Lomonaco, D.; Cangane, F.Y.; Mazzetto, S.E. Thiophosphate esters of cashew nutshell liquid derivatives as new antioxidants for poly(methyl methacrylate). J. Therm. Anal. Calorim. 2011, 104, 1177–1183. [Google Scholar] [CrossRef]
- Balgude, D.; Sabnis, A.S. CNSL: An environment friendly alternative for the modern coating industry. J. Coat. Technol. Res. 2014, 11, 169–183. [Google Scholar] [CrossRef]
- Oliveira, L.D.M. Synthesis, Characterization and Functionality of Lubricity Additives Derived from the LCC; Universidade Federal do Ceará: Fortaleza, Brazil, 2007; Available online: http://www.repositorio.ufc.br/bitstream/riufc/2102/1/2007_dis_Lin_Oliveira.pdf (accessed on 23 April 2019).
- Grassi, V.G.; Forte, M.M.C.; Dal Pizzol, M.F. Morphologic Aspects and Structure-Properties Relations of High Impact Polystyrene. Polímeros Ciência e Tecnologia 2001, 11, 158–168. [Google Scholar] [CrossRef]
- Omonov, T.S.; Harrats, C.; Moldenaers, P.; Groeninckx, G. Phase continuity detection and phase inversion phenomena in immiscible polypropylene/polystyrene blends with different viscosity ratios. Polymer 2007, 48, 5917–5927. [Google Scholar] [CrossRef]
- Lucas, E.F.; Soares, B.G.; Monteiro, E. Caracterização de Polímeros. Determinação de Peso Molecular e Análise Térmica (Characterization of Polymers. Determination of Molecular Weight and Thermal Analysis), 1st ed.; Editora e-papers: Rio de Janeiro, Brazil, 2001. [Google Scholar]
- Wunderlich, B. Thermal Analysis of Polymeric Materials; Springer Science + Business Media: Berlim, Germany, 2005. [Google Scholar]
- Rosen, S.L. Fundamental Principles of Polymeric Materials, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Rahman, M.; Brazel, C.S. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Almeida, J.F.M.; Silva, A.L.N.; Silva, A.H.M.F.T.; Sousa, A.M.F.; Nascimento, C.R.; Bertolino, L.C. Rheological, mechanical and morphological behavior of polylactide/nano-sized calcium carbonate composites. Polym. Bull. 2016, 73, 3531–3545. [Google Scholar] [CrossRef]
- Kiss, A.; Fekete, E.; Pukánszky, B. Aggregation of CaCO3 particles in PP composites: Effect of surface coating. Compos. Sci. Technol. 2007, 67, 1574–1583. [Google Scholar] [CrossRef]


















| Component | Natural CNSL (%) | Technical CNSL (%) |
|---|---|---|
| Anacardic acid | 71–82 | 0–1.8 |
| Cardanol | 1.6–9.2 | 63–95 |
| Cardol | 13.8–20.3 | 3.8–18.9 |
| 2-Methyl-cardol | 1.6–3.9 | 1.2–5.2 |
| Polymeric material | - | 0–21.63 |
| Minor components | 0–2.2 | 0–4 |
| Zone | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Die |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 90 | 140 | 150 | 150 | 160 | 165 | 170 | 175 | 180 | 180 |
| PP/HIPS Ratio (%, w/w) | CNSL (phr) | Sample |
|---|---|---|
| 1:0 | 0 | 1:0/0 |
| 2.5 | 1:0/2.5 | |
| 5.0 | 1:0/5 | |
| 4:1 | 0 | 1:0/0 |
| 2.5 | 1:0/2.5 | |
| 5.0 | 1:0/5 | |
| 3:2 | 0 | 4:1/0 |
| 2.5 | 4:1/2.5 | |
| 5.0 | 4:1/5 | |
| 2:3 | 0 | 3:2/0 |
| 2.5 | 3:2/2.5 | |
| 5.0 | 3:2/5 | |
| 1:4 | 0 | 2:3/0 |
| 2.5 | 2:3/2.5 | |
| 5.0 | 2:3/5 | |
| 0:1 | 0 | 1:4/0 |
| 2.5 | 1:4/2.5 | |
| 5.0 | 0:1/5 |
| Parameter | Zone | Die | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Temperature (°C) | 170 | 175 | 180 | 190 | 200 |
| Injection pressure (bar) | 1000 | ||||
| Back pressure (bar) | 300 | ||||
| PP/HIPS Ratio (%, w/w) | CNSL (phr) | Sample | Modulus at Crossover Point G’ = G” (Pa) | Crossover Point ωc (rad/s) |
|---|---|---|---|---|
| 1:0 | 0 | 1:0/0 | 20,670 | 10 |
| 2.5 | 1:0/2.5 | 21,080 | 10 | |
| 5.0 | 1:0/5 | 20,780 | 10 | |
| 4:1 | 0 | 4:1/0 | 21,010 | 10 |
| 2.5 | 4:1/2.5 | 20,730 | 10 | |
| 5.0 | 4:1/5 | 17,700 | 10 | |
| 3:2 | 0 | 3:2/0 | 22,120 | 10 |
| 2.5 | 3:2/2.5 | 21,900 | 10 | |
| 5.0 | 3:2/5 | 19,910 | 10 | |
| 2:3 | 0 | 2:3/0 | 21,600 | 10 |
| 2.5 | 2:3/2.5 | 22,940 | 10 | |
| 5.0 | 2:3/5 | 18,260 | 10 | |
| 1:4 | 0 | 1:4/0 | 25,130 | 10 |
| 2.5 | 1:4/2.5 | 21,620 | 10 | |
| 5.0 | 1:4/5 | 18,030 | 10 | |
| 0:1 | 0 | 0:1/0 | 25,280 | 10 |
| 2.5 | 0:1/2.5 | 20,250 | 10 | |
| 5.0 | 0:1/5 | 17,270 | 16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, M.N.; Visconte, L.L.Y.; Barreto, D.W.; Escócio, V.A.; Silva, A.L.N.d.; Sousa, A.M.F.d.; Pacheco, E.B.A.V. The Use of Cashew Nut Shell Liquid (CNSL) in PP/HIPS Blends: Morphological, Thermal, Mechanical and Rheological Properties. Materials 2019, 12, 1904. https://doi.org/10.3390/ma12121904
Araújo MN, Visconte LLY, Barreto DW, Escócio VA, Silva ALNd, Sousa AMFd, Pacheco EBAV. The Use of Cashew Nut Shell Liquid (CNSL) in PP/HIPS Blends: Morphological, Thermal, Mechanical and Rheological Properties. Materials. 2019; 12(12):1904. https://doi.org/10.3390/ma12121904
Chicago/Turabian StyleAraújo, Mirna Nunes, Leila Lea Yuan Visconte, Daniel Weingart Barreto, Viviane Alves Escócio, Ana Lucia Nazareth da Silva, Ana Maria Furtado de Sousa, and Elen Beatriz Acordi Vasques Pacheco. 2019. "The Use of Cashew Nut Shell Liquid (CNSL) in PP/HIPS Blends: Morphological, Thermal, Mechanical and Rheological Properties" Materials 12, no. 12: 1904. https://doi.org/10.3390/ma12121904





