Lignin Nanoparticles as A Promising Way for Enhancing Lignin Flame Retardant Effect in Polylactide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lignin Nanoparticle Preparation
2.3. Lignin Treatment
2.4. Composites Preparation
2.5. Lignin and Composites Characterization
3. Results
3.1. Lignin Particles Structural and Thermal Properties
3.1.1. Unmodified Lignin
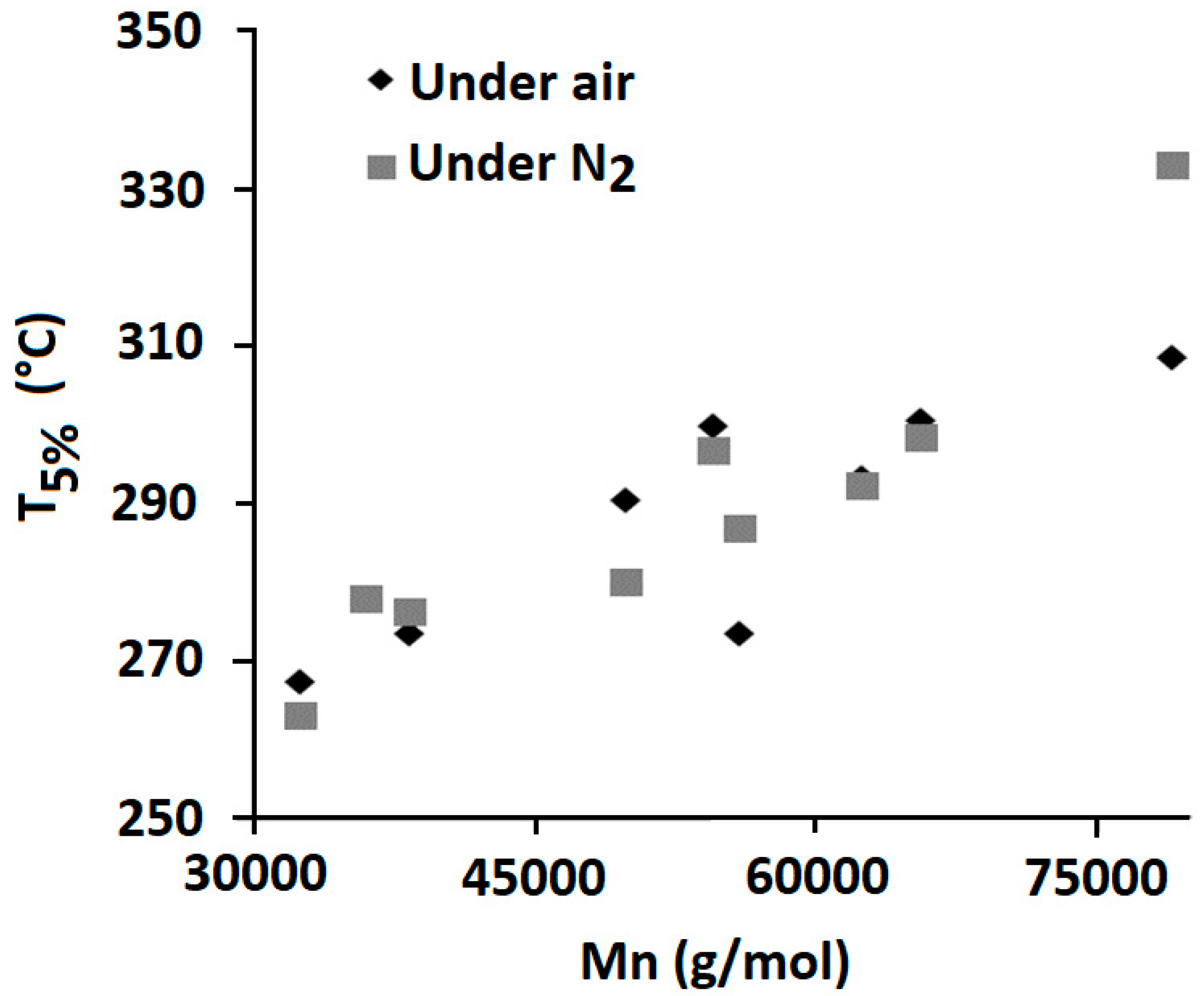
3.1.2. Phosphorylated Lignin
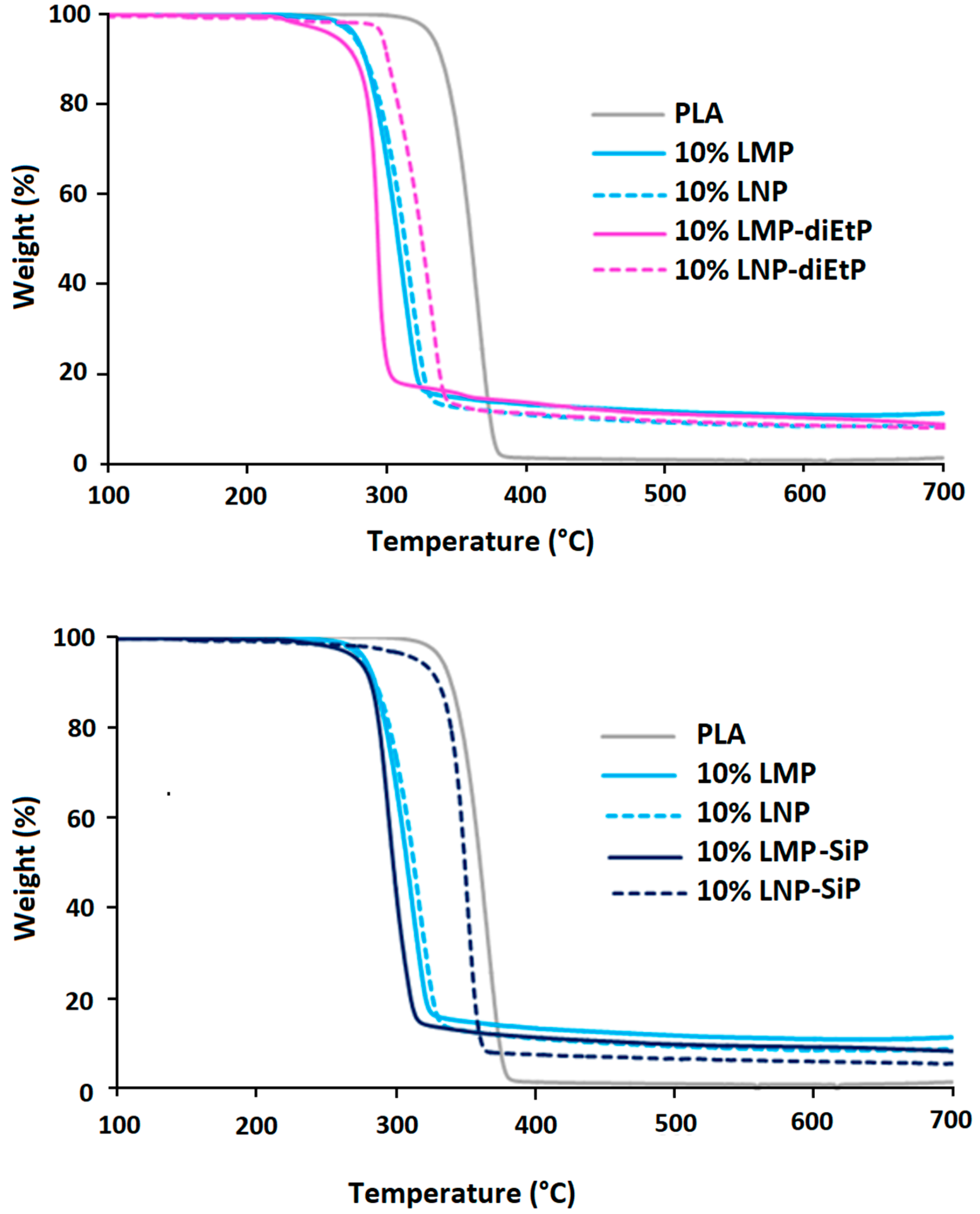
3.2. Composites Thermal Degradation and Fire Behavior
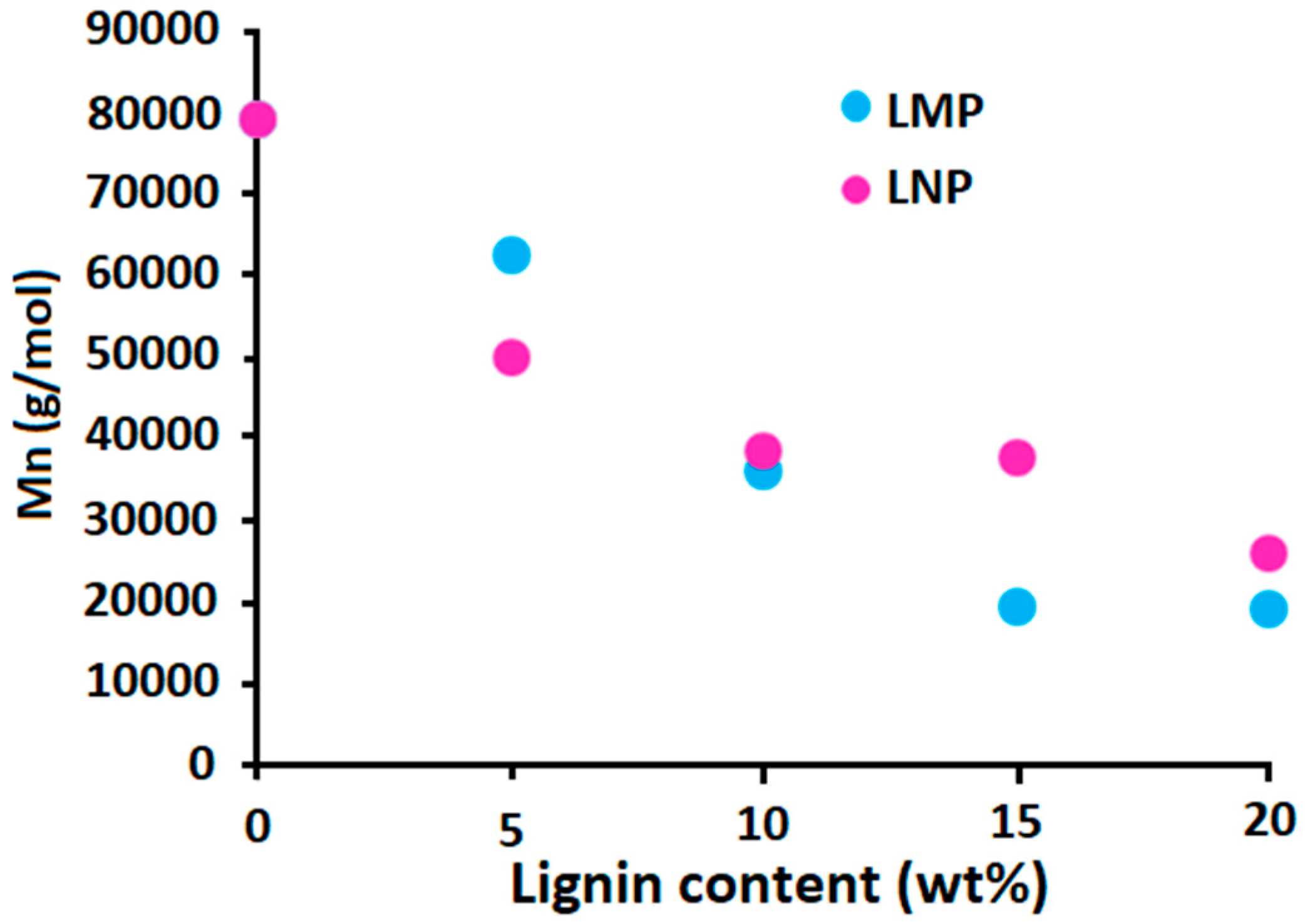
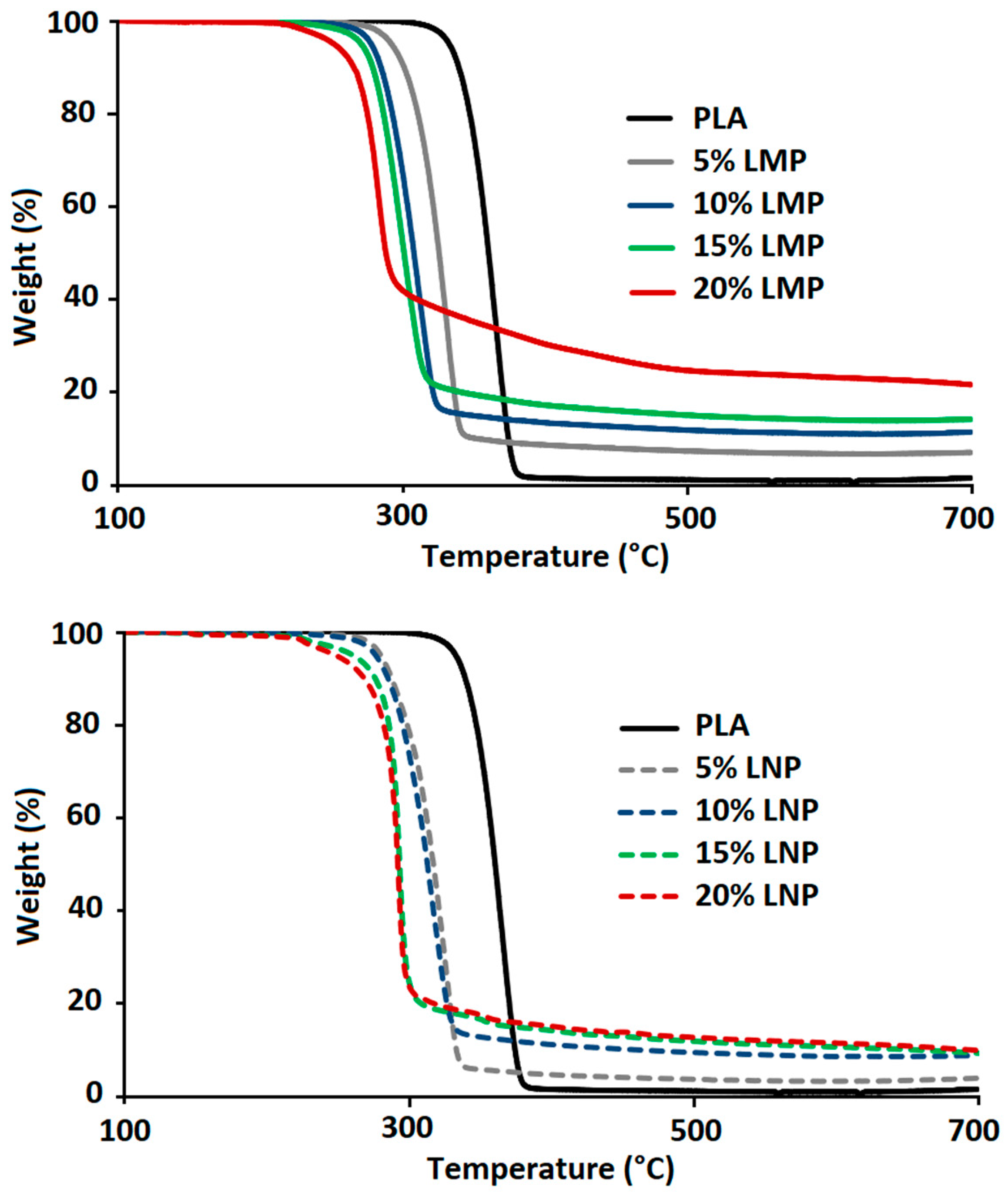
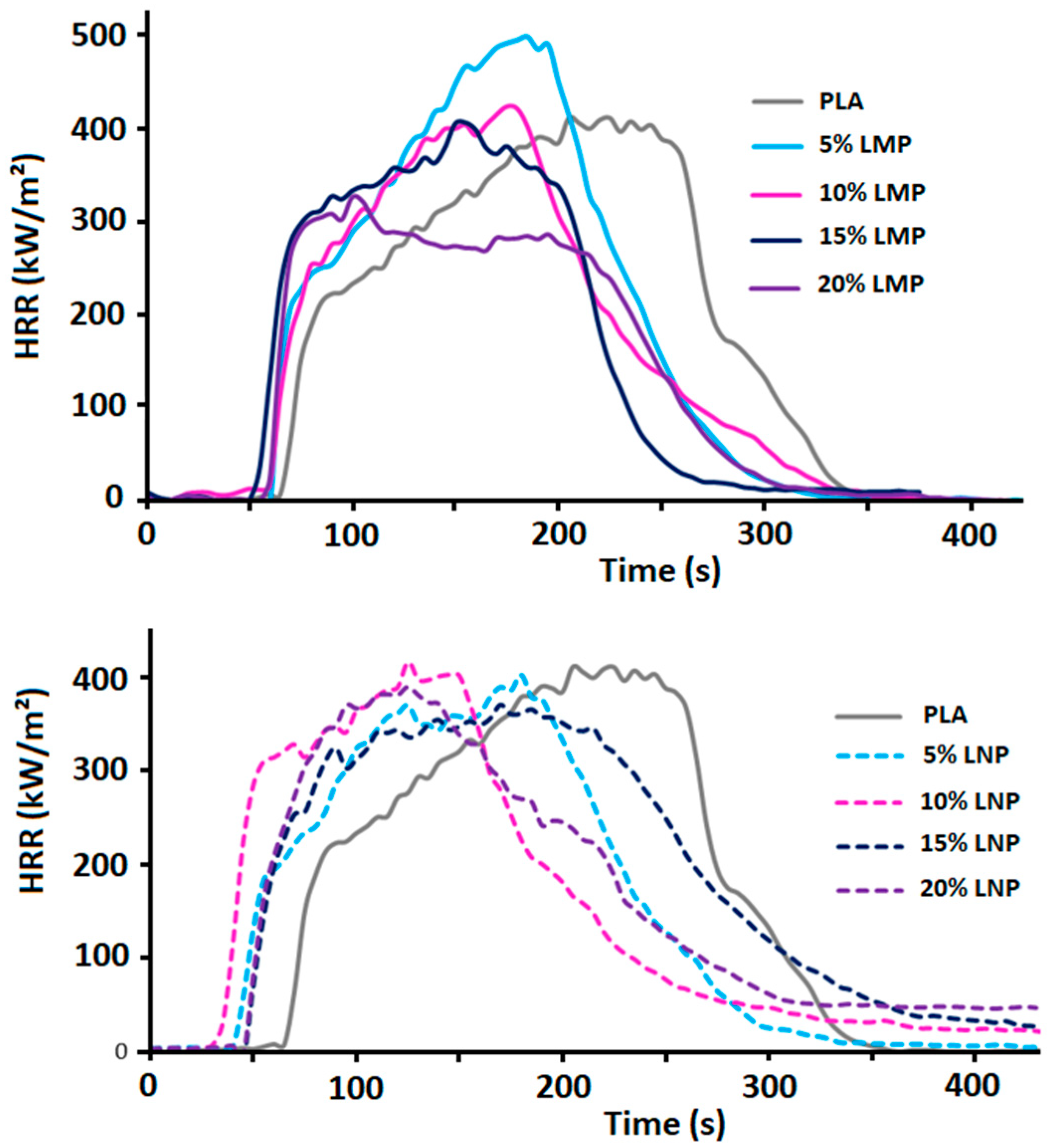
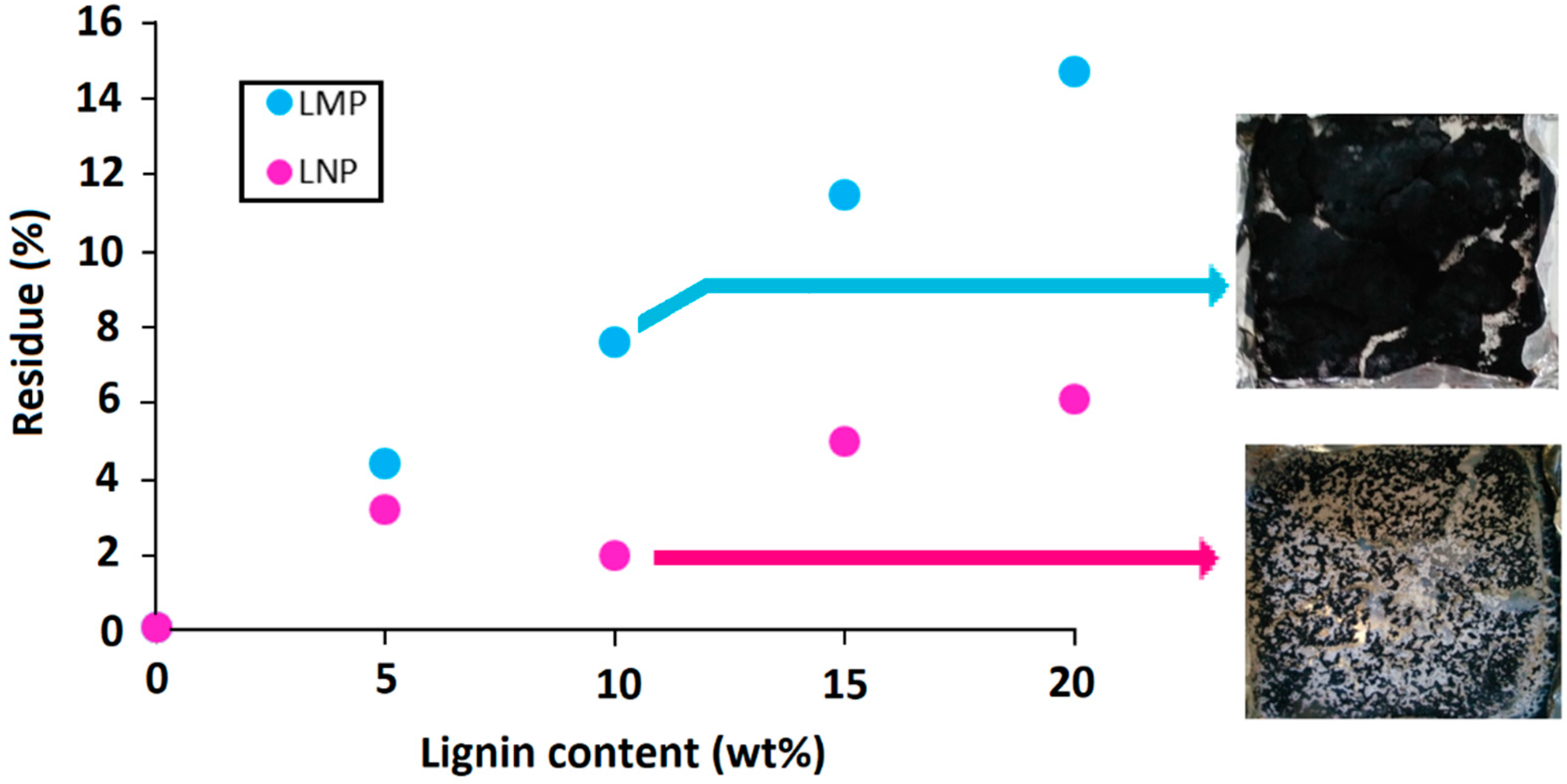
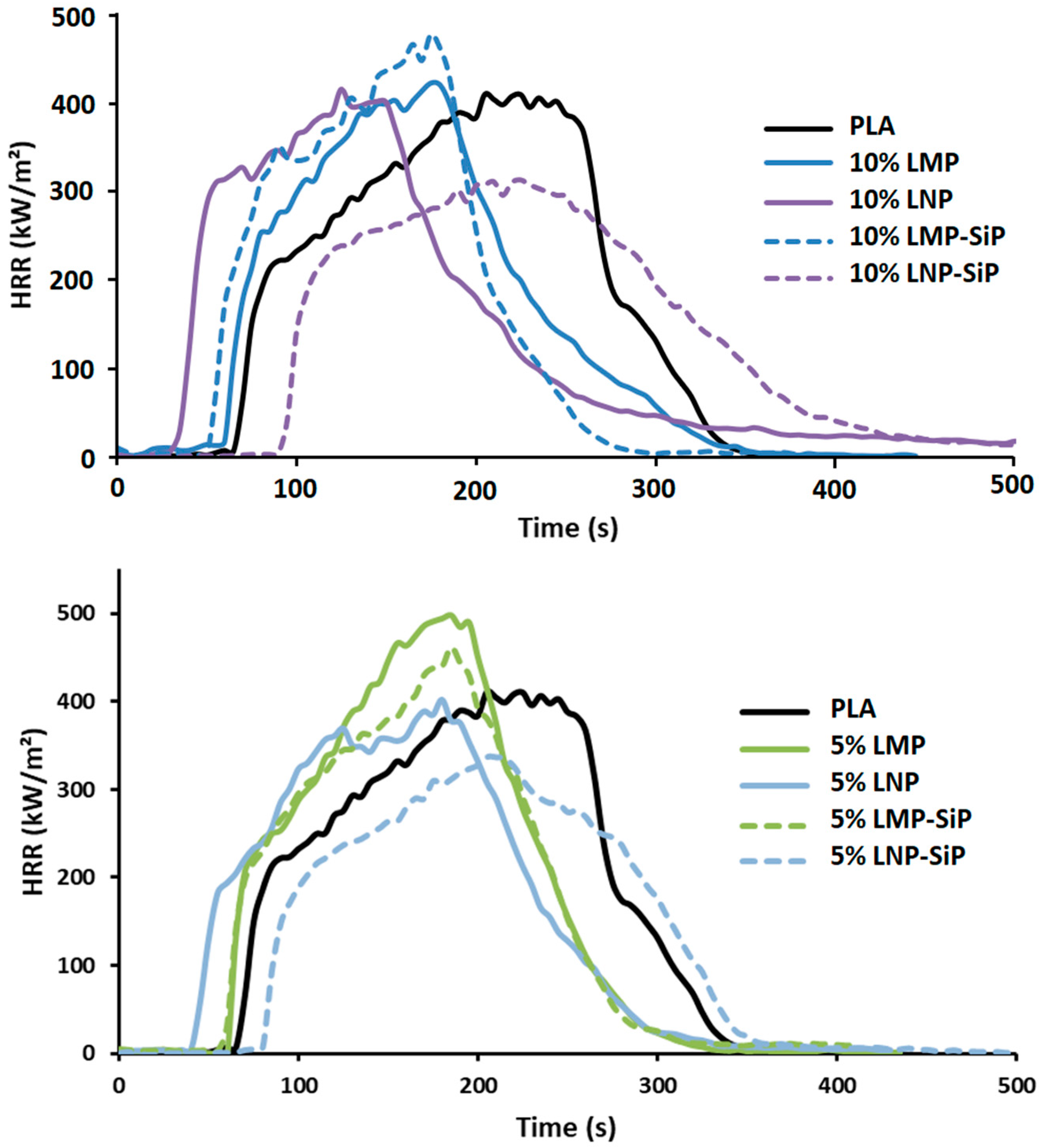
3.2.1. Effect of Untreated Lignin Content and Particle Size
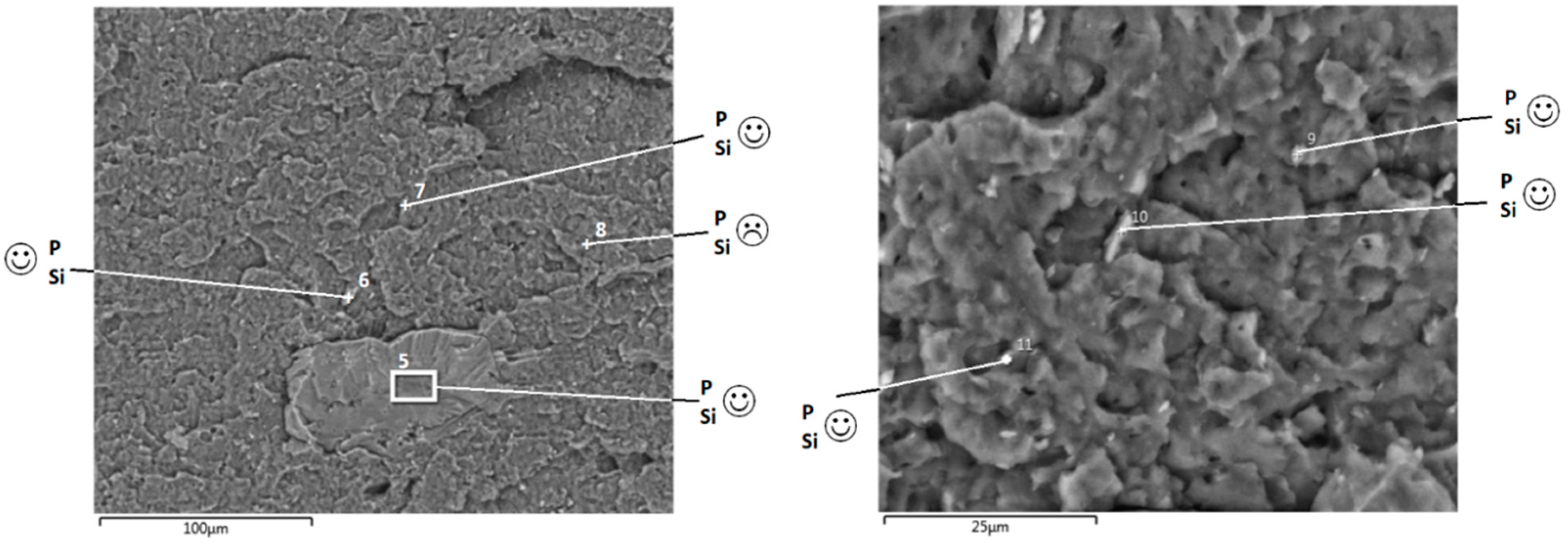
3.2.2. Effect of Phosphorylated Lignin
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Sonnier, R.; Taguet, A.; Ferry, L.; Lopez-Cuesta, J.M. Towards Bio-Based Flame Retardant Polymers; Springer Briefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Sakharov, A.M.; Sakharov, P.A.; Lomakin, S.M.; Zaikov, G.E. Chapter 7—Novel Class of Eco-Flame Retardants Based on the Renewable Raw Materials. In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 255–266. [Google Scholar]
- Hu, S.; Song, L.; Pan, H.; Hu, Y.; Gong, X. Thermal properties and combustion behaviors of flame retarded epoxy acrylate with a chitosan based flame retardant containing phosphorus and acrylate structure. J. Anal. Appl. Pyrolysis 2012, 97, 109–115. [Google Scholar] [CrossRef]
- Carosio, F.; di Blasio, A.; Cuttica, F.; Alongi, J.; Malucelli, G. Flame retardancy of polyester and polyester–cotton blends treated with caseins. Ind. Eng. Chem. Res. 2014, 53, 3917–3923. [Google Scholar] [CrossRef]
- Howell, B.; Oberdorfer, K.L.; Ostrander, E.A. Phosphorus Flame Retardants for Polymeric Materials from Gallic Acid and Other Naturally Occurring Multihydroxybenzoic Acids. Int. J. Polym. Sci. 2018, 3, 1–12. [Google Scholar] [CrossRef]
- Dehne, L.; Vila Babarro, C.; Saake, B.; Schwarz, K.U. Influence of lignin source and esterification on properties of lignin-polyethylene blends. Ind. Crops Prod. 2016, 86, 320–328. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Naseem, A.; Tabasum, S.; Zia, K.M.; Zuber, M.; Ali, M.; Noreen, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef]
- De Chirico, A.; Armanini, M.; Chini, P.; Cioccolo, G.; Provasoli, F.; Audisio, G. Flame retardants for polypropylene based on lignin. Polym. Degrad. Stab. 2003, 79, 139–145. [Google Scholar] [CrossRef]
- Song, P.; Cao, Z.; Fu, S.; Fang, Z.; Wu, Q.; Ye, J. Thermal degradation and flame retardancy properties of ABS/lignin: Effects of lignin content and reactive compatibilization. Thermochim. Acta 2011, 518, 59–65. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Aguedo, M.; Richel, A.; Brohez, S.; Delvosalle, C.; Dubois, P. Phosphorus and nitrogen derivatization as efficient route for improvement of lignin flame retardant action in PLA. Eur. Polym. J. 2016, 84, 652–667. [Google Scholar] [CrossRef]
- Laachachi, A.; Leroy, E.; Cochez, M.; Ferriol, M.; Lopez Cuesta, J.M. Use of oxide nanoparticles and organoclays to improve thermal stability and fire retardancy of poly(methyl methacrylate). Polym. Degrad. Stab. 2005, 89, 344–352. [Google Scholar] [CrossRef]
- Zanetti, M.; Kashiwagi, T.; Falqui, L.; Camino, G. Cone calorimeter combustion and gasification studies of polymer layered silicate nanocomposites. Chem. Mater. 2002, 14, 881–887. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Khelifa, F.; Rose, G.; Brohez, S.; Delvosalle, C.; Dubois, P. Cellulose/phosphorus combinations for sustainable fire retarded polylactide. Eur. Polym. J. 2016, 74, 218–228. [Google Scholar] [CrossRef]
- Gilca, I.A.; Popa, V.I.; Crestini, C. Obtaining lignin nanoparticles by sonication. Ultrason. Sonochem. 2015, 23, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gilca, I.A.; Ghitescu, R.E.; Puitel, A.C.; Popa, V.I. Preparation of lignin nanoparticles by chemical modification. Iran. Polym. J. 2014, 23, 355–363. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of environmentally biodegradable lignin nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef]
- Yang, W.; Kenny, J.M.; Puglia, D. Structure and properties of biodegradable wheat gluten bionanocomposites containing lignin nanoparticles. Ind. Crops Prod. 2015, 74, 348–356. [Google Scholar] [CrossRef]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J.M. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Prieur, B.; Meub, M.; Wittemann, M.; Klein, R.; Bellayer, S.; Fontaine, G.; Bourbigot, S. Phosphorylation of lignin to flame retard acrylonitrile butadiene styrene (ABS). Polym. Degrad. Stab. 2016, 127, 32–43. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Delvosalle, C.; Dubois, P. Phytic acid-lignin combination: A simple and efficient route for enhancing thermal and flame retardant properties of polylactide. Eur. Polym. J. 2017, 94, 270–285. [Google Scholar] [CrossRef]
- Ray, S.; Cooney, R.P. Thermal degradation of polymer and polymer composites. In Handbook of Environmental Degradation of Materials; Elsevier: Amsterdam, The Netherlands, 2012; pp. 213–242. [Google Scholar]

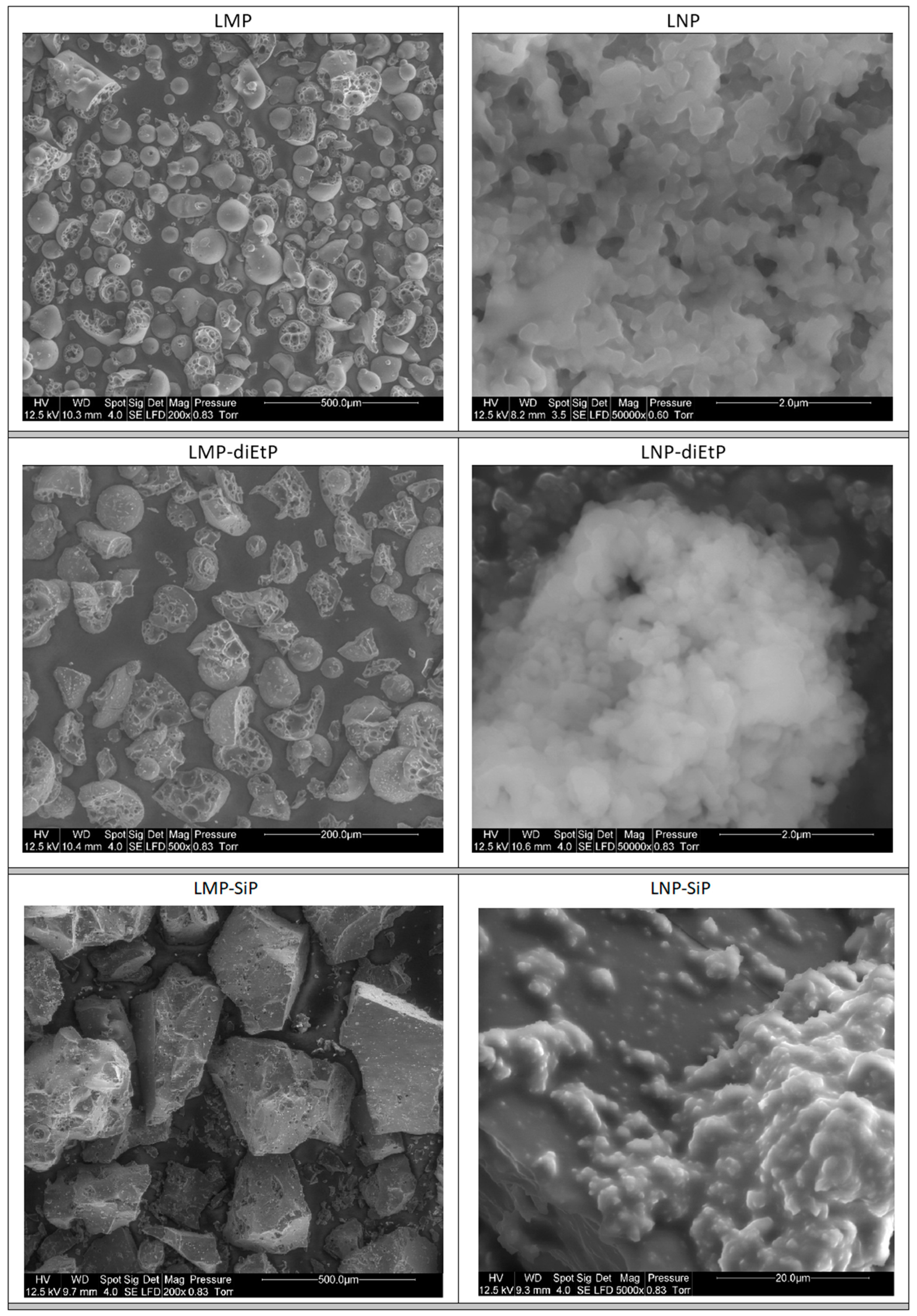
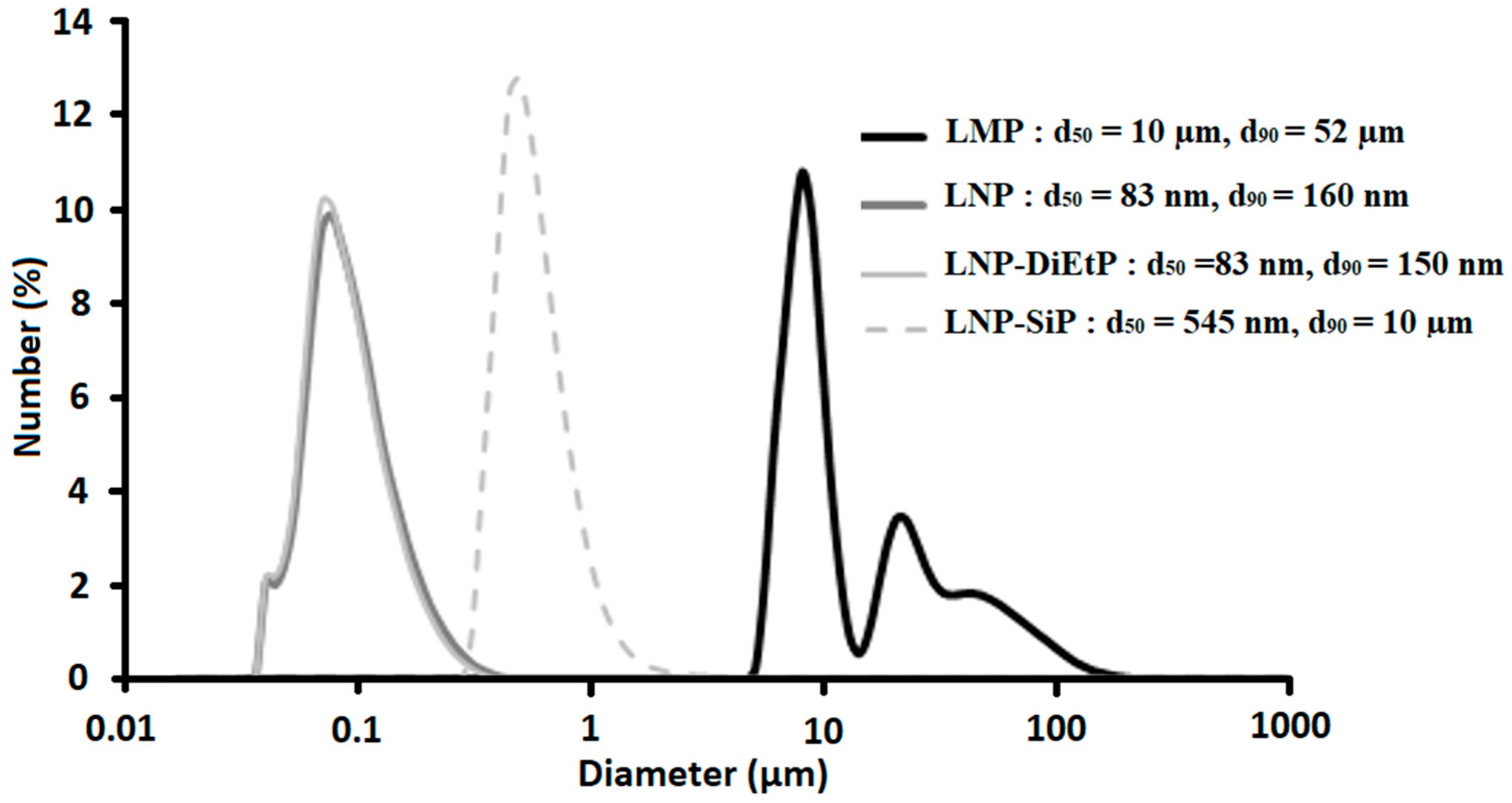
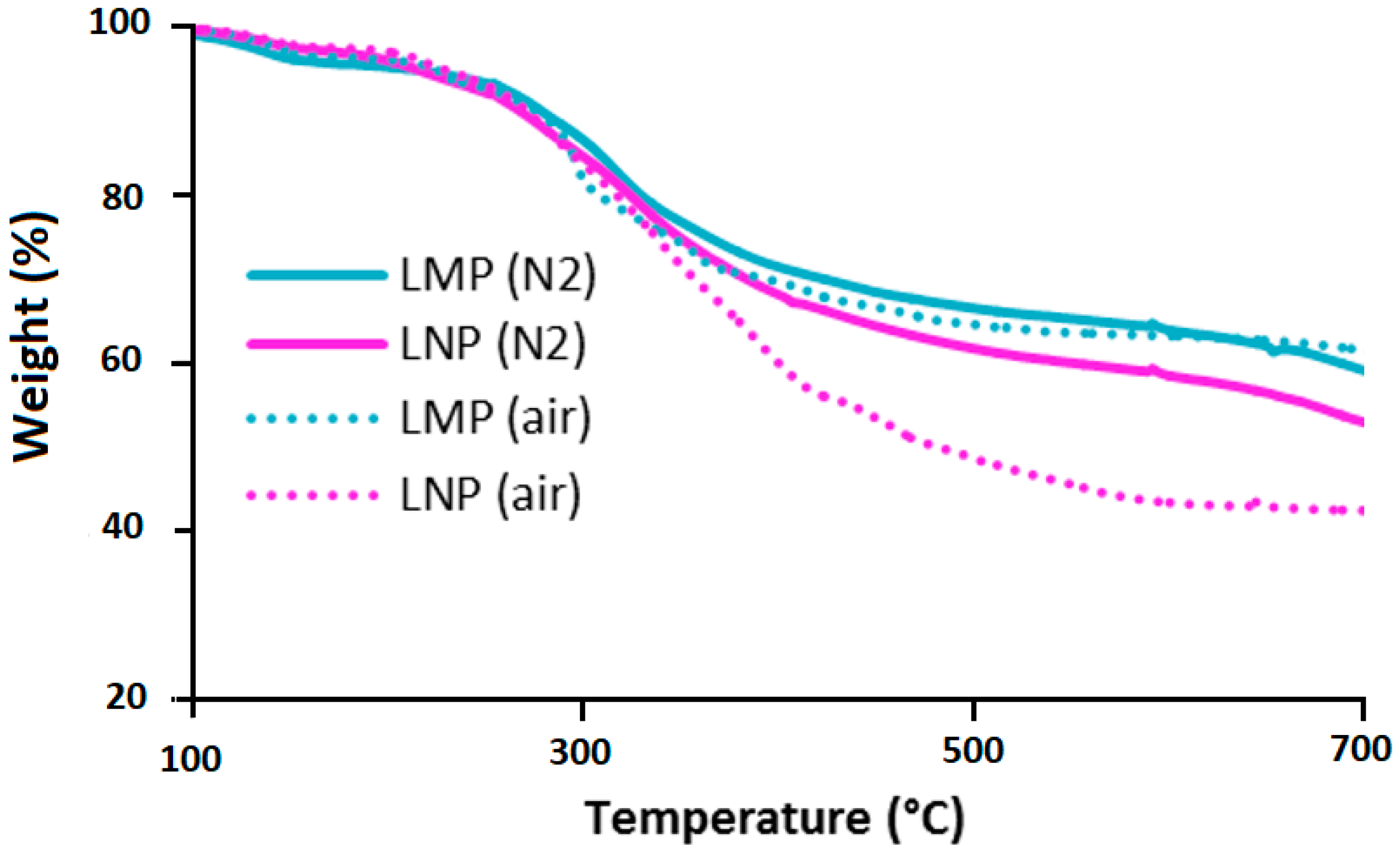

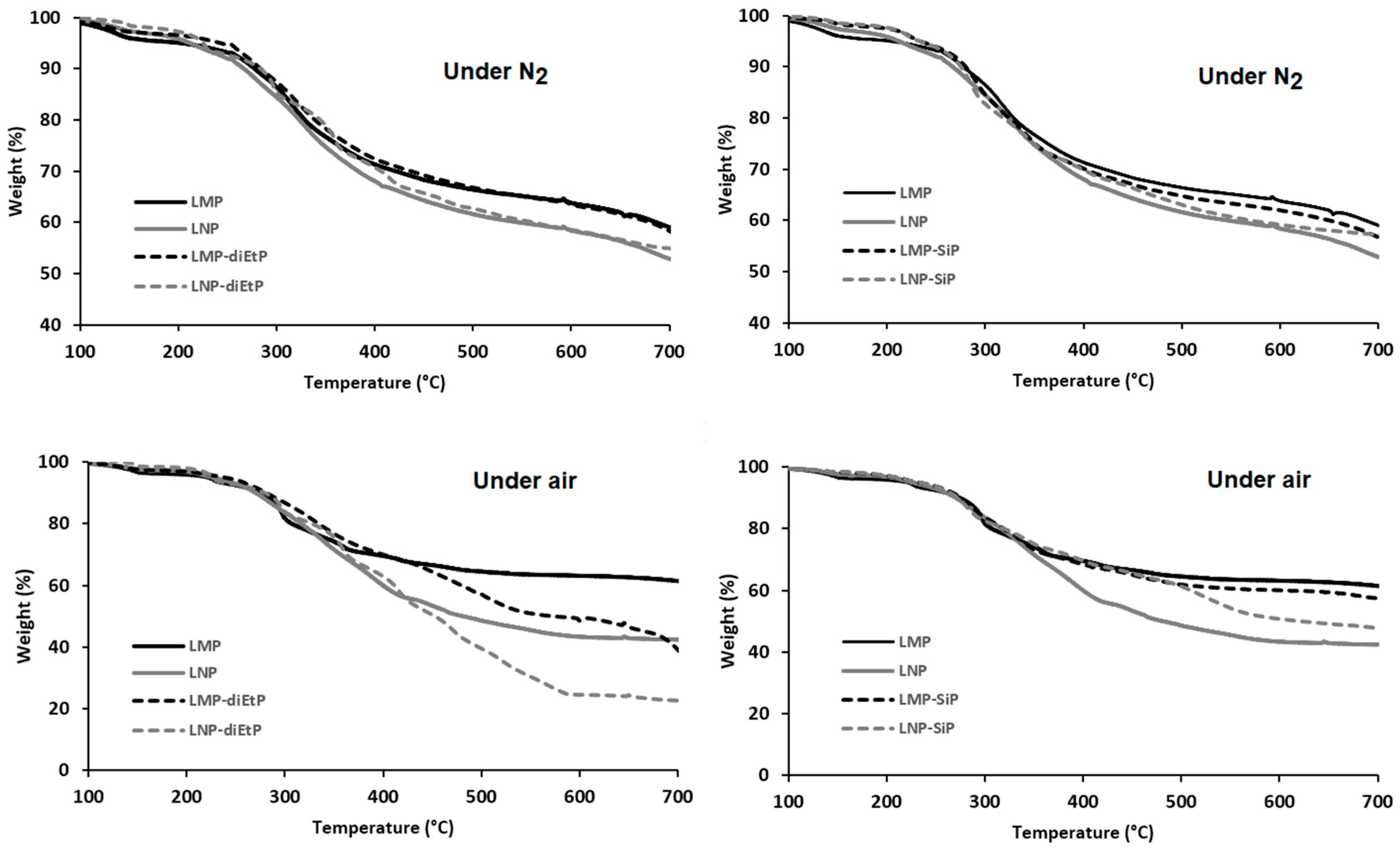



| Sample | Phosphorus Content (wt%) |
|---|---|
| LMP-diEtP | 0.35 ± 0.02 |
| LNP-diEtP | 0.67 ± 0.02 |
| LMP-SiP | 0.1 ± 0.02 |
| LNP-SiP | 3.2 ± 0.02 |
| Mn (g/mol) | |
|---|---|
| PLA | 80,000 |
| 10 wt% LMP | 36,000 |
| 10 wt% LNP | 39,000 |
| 10 wt% LMP-diEtP | 33,000 |
| 10 wt% LNP-diEtP | 55,000 |
| 10 wt% LMP-SiP | 30,000 |
| 10 wt% LNP-SiP | 40,000 |
| T5% (°C) | T10% (°C) | T50% (°C) | Residue at 650 °C (%) | |
|---|---|---|---|---|
| PLA | 333 | 340 | 360 | 0.9 |
| 10% LMP | 278 | 285 | 308 | 10.8 |
| 10% LNP | 277 | 285 | 313 | 8.5 |
| 10% LMP-diEtP | 263 | 278 | 294 | 9.7 |
| 10% LNP-diEtP | 297 | 300 | 325 | 8.3 |
| 10% LMP-SiP | 273 | 282 | 300 | 8.8 |
| 10% LNP-SiP | 315 | 330 | 350 | 5.7 |
| TTI (s) | pHRR Variation (%) | Char (%) | |
|---|---|---|---|
| PLA | 68 | / | 0.1 |
| 10% LMP | 65 ± 4 | +12 ± 2 | 11.5 ± 0.4 |
| 10% LNP | 34 ± 2 | +5 ± 2 | 2 ± 0.1 |
| 10% LMP-diEtP | 67 ± 2 | +20 ± 8 | 8 ± 1.2 |
| 10% LNP-diEtP | 54 ± 2 | −6 ± 1 | 4.2 ± 1.3 |
| 10% LMP SiP | 57 ± 4 | +20 ± 5 | 7.3 ± 0.3 |
| 10% LNP SiP | 86 ± 7 | −18 ± 1 | 2 ± 4 |
| 5% LMP-SiP | 57 ± 3 | + 18 ± 2 | 4.5 ± 0.2 |
| 5% LNP-SiP | 84 ± 4 | −11 ± 2 | 1 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chollet, B.; Lopez-Cuesta, J.-M.; Laoutid, F.; Ferry, L. Lignin Nanoparticles as A Promising Way for Enhancing Lignin Flame Retardant Effect in Polylactide. Materials 2019, 12, 2132. https://doi.org/10.3390/ma12132132
Chollet B, Lopez-Cuesta J-M, Laoutid F, Ferry L. Lignin Nanoparticles as A Promising Way for Enhancing Lignin Flame Retardant Effect in Polylactide. Materials. 2019; 12(13):2132. https://doi.org/10.3390/ma12132132
Chicago/Turabian StyleChollet, Benjamin, José-Marie Lopez-Cuesta, Fouad Laoutid, and Laurent Ferry. 2019. "Lignin Nanoparticles as A Promising Way for Enhancing Lignin Flame Retardant Effect in Polylactide" Materials 12, no. 13: 2132. https://doi.org/10.3390/ma12132132
APA StyleChollet, B., Lopez-Cuesta, J.-M., Laoutid, F., & Ferry, L. (2019). Lignin Nanoparticles as A Promising Way for Enhancing Lignin Flame Retardant Effect in Polylactide. Materials, 12(13), 2132. https://doi.org/10.3390/ma12132132








