Hydration Resistance of CaO Material Prepared by Ca(OH)2 Calcination with Chelating Compound
Abstract
:1. Introduction
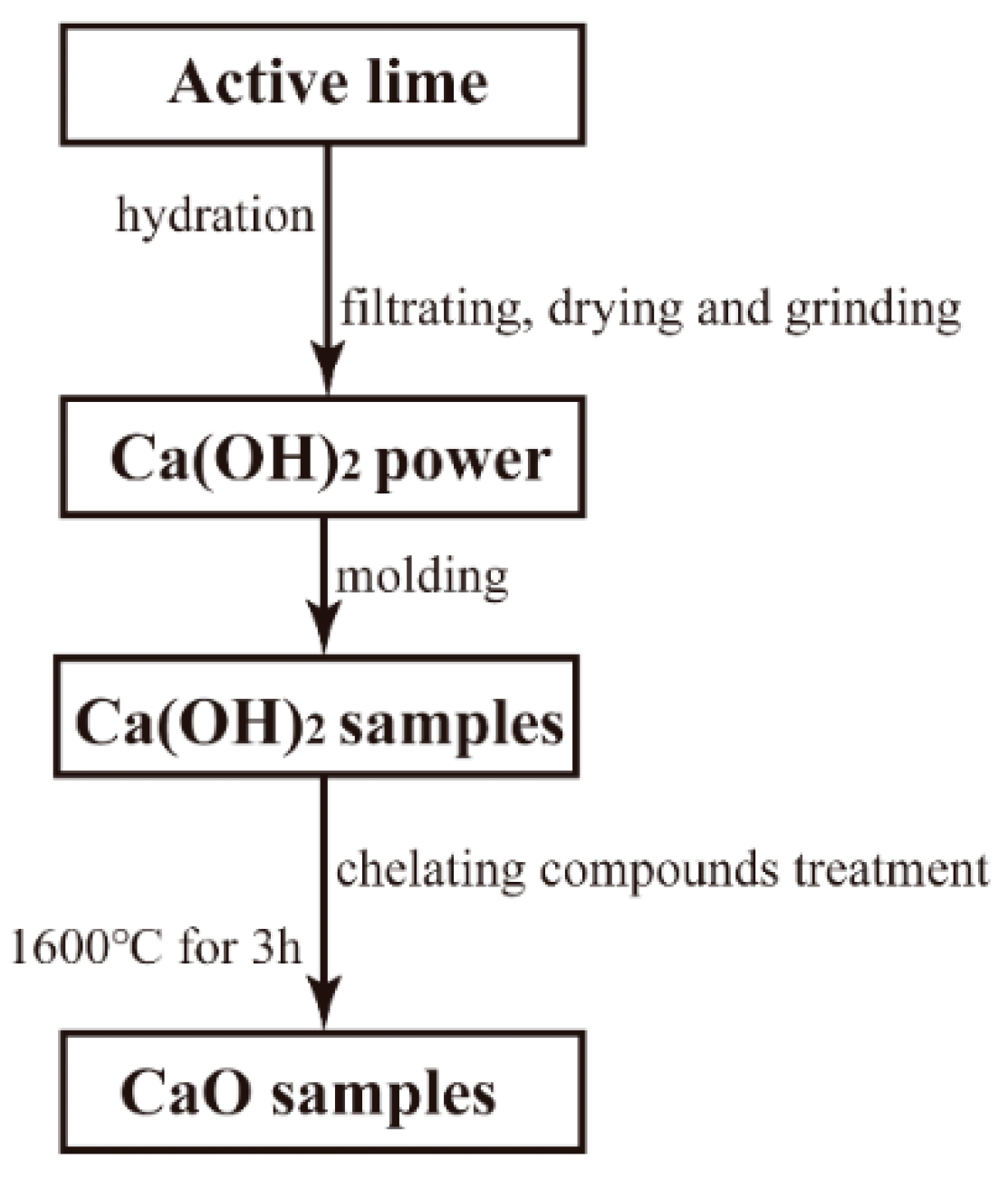
2. Experimental Procedure
3. Results and Discussion
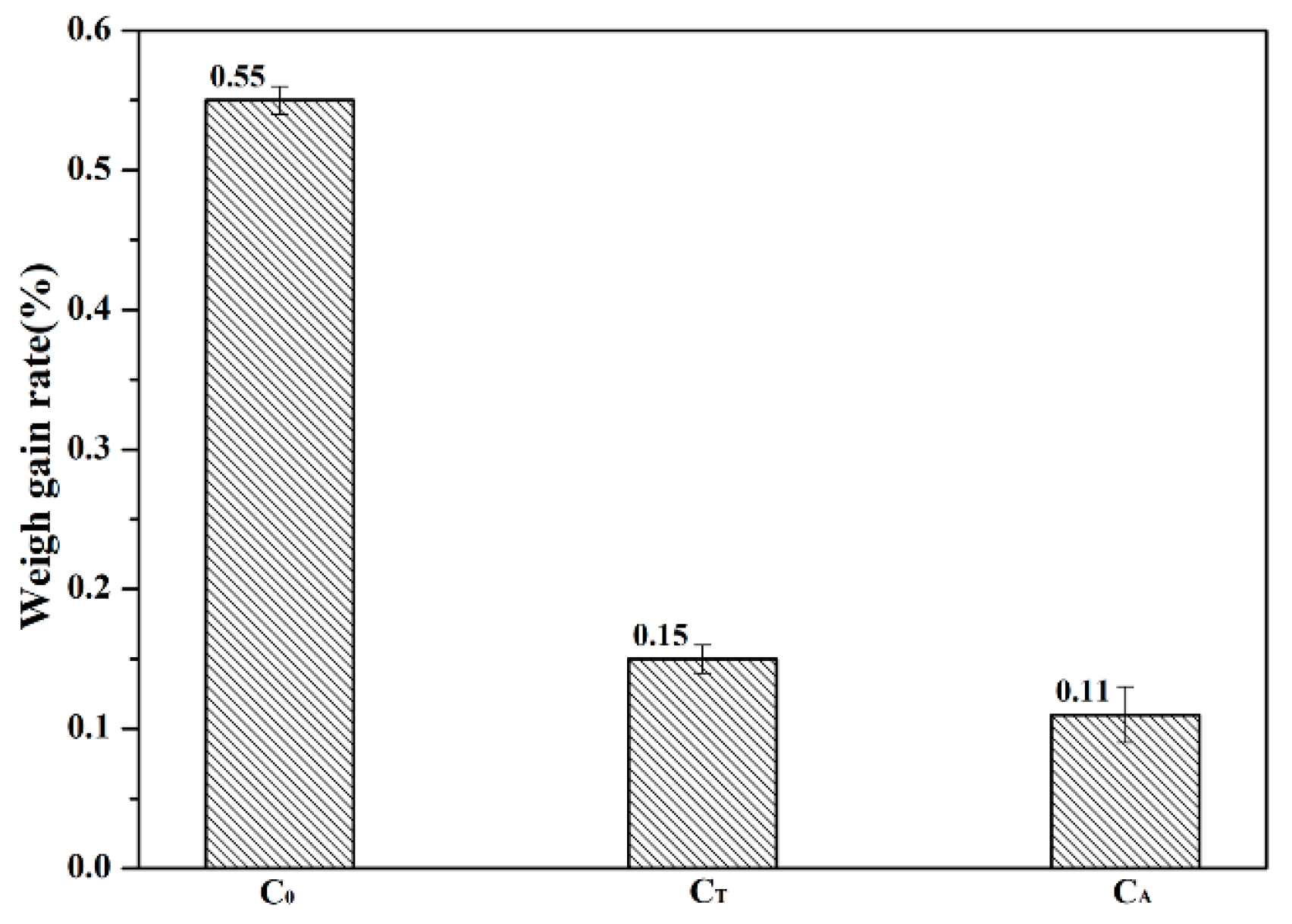
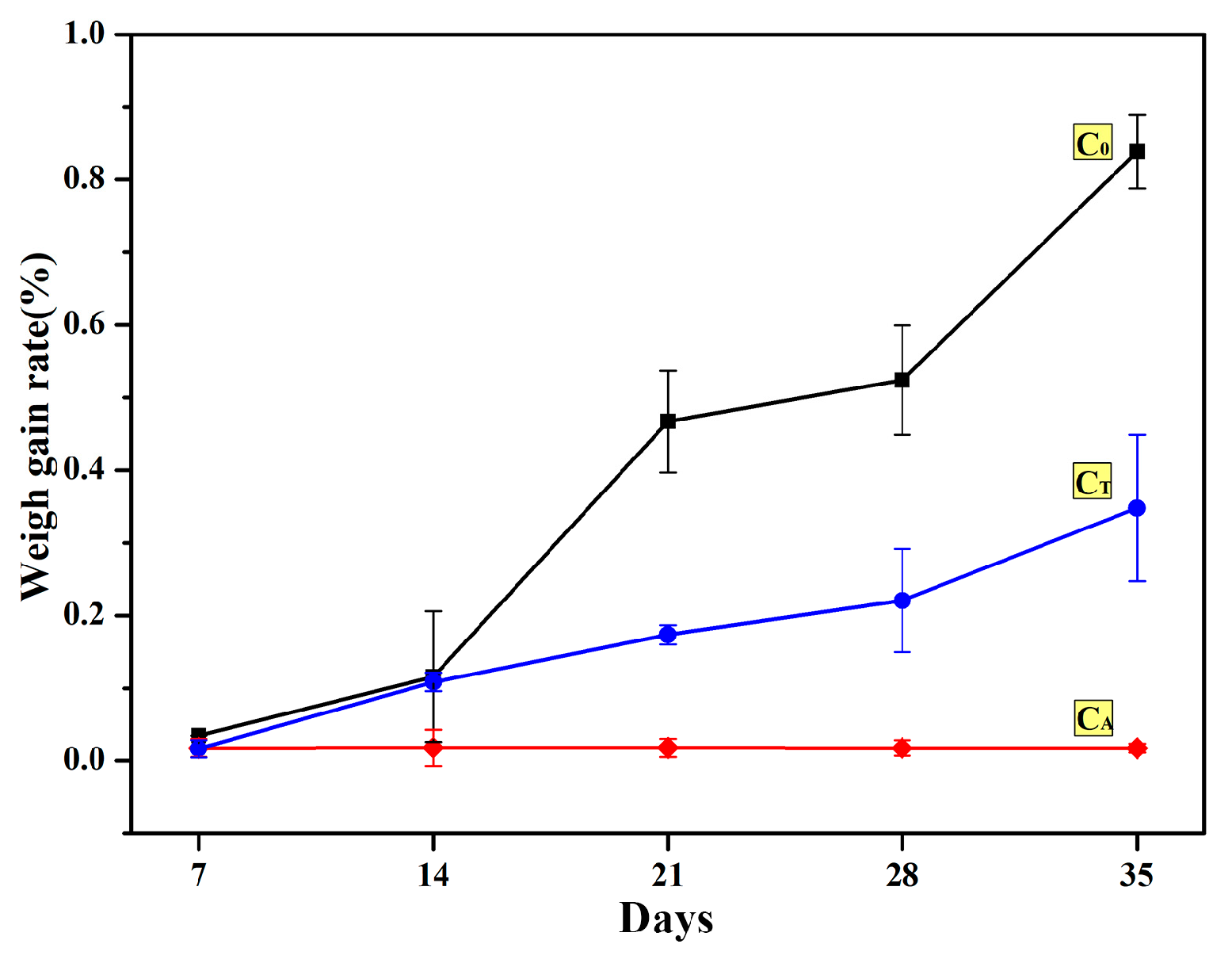
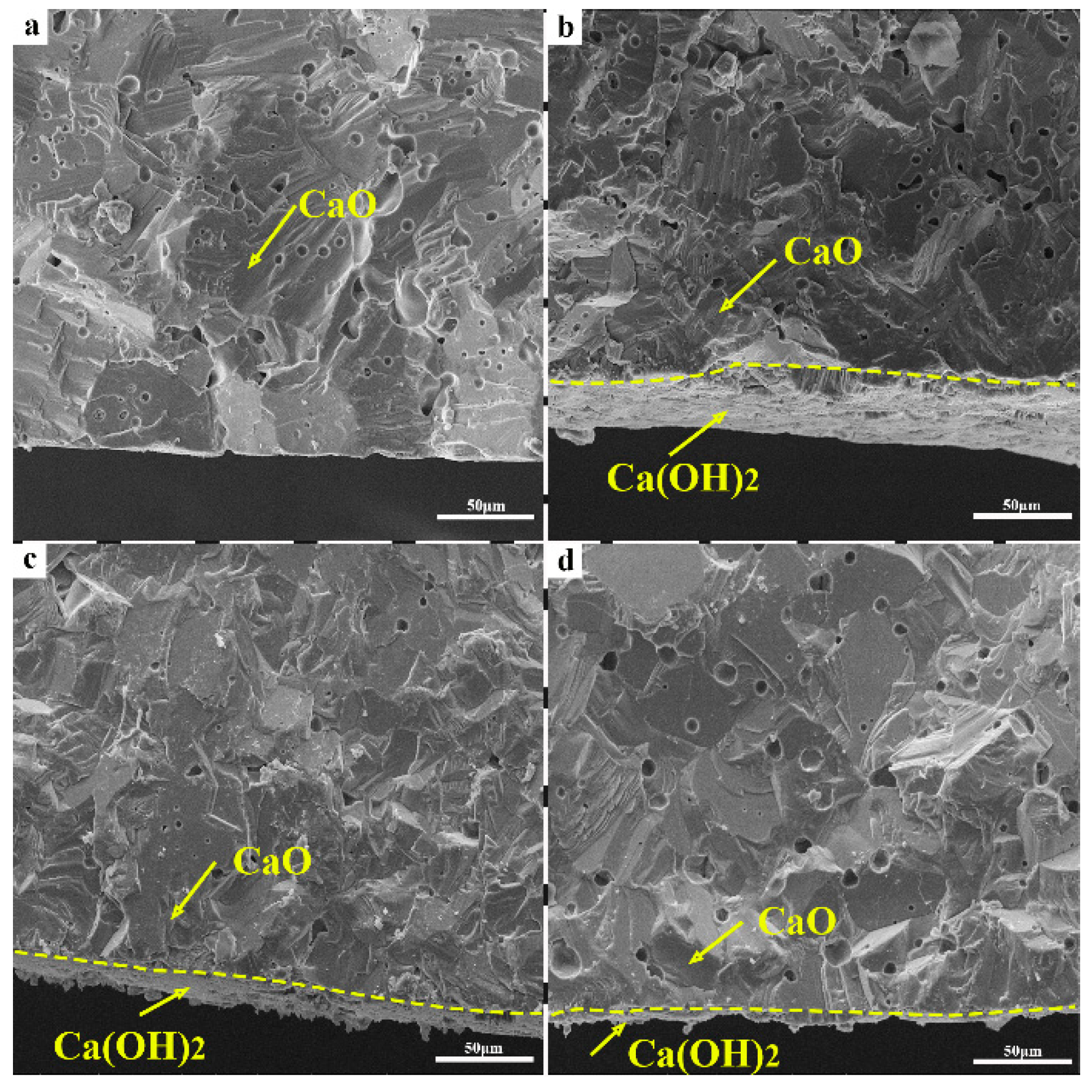
3.1. Hydration Resistance of CaO Samples
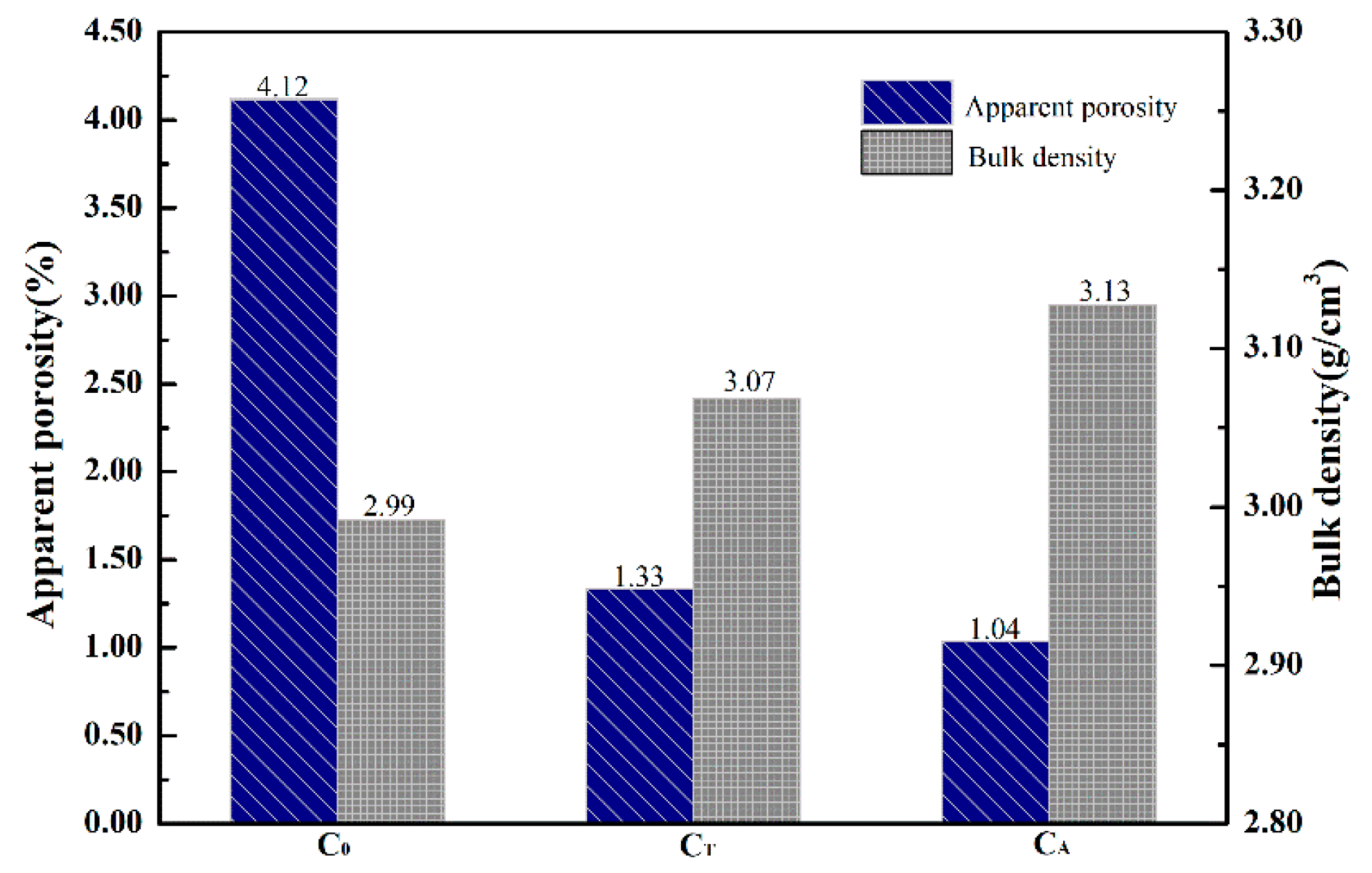
3.2. Densification
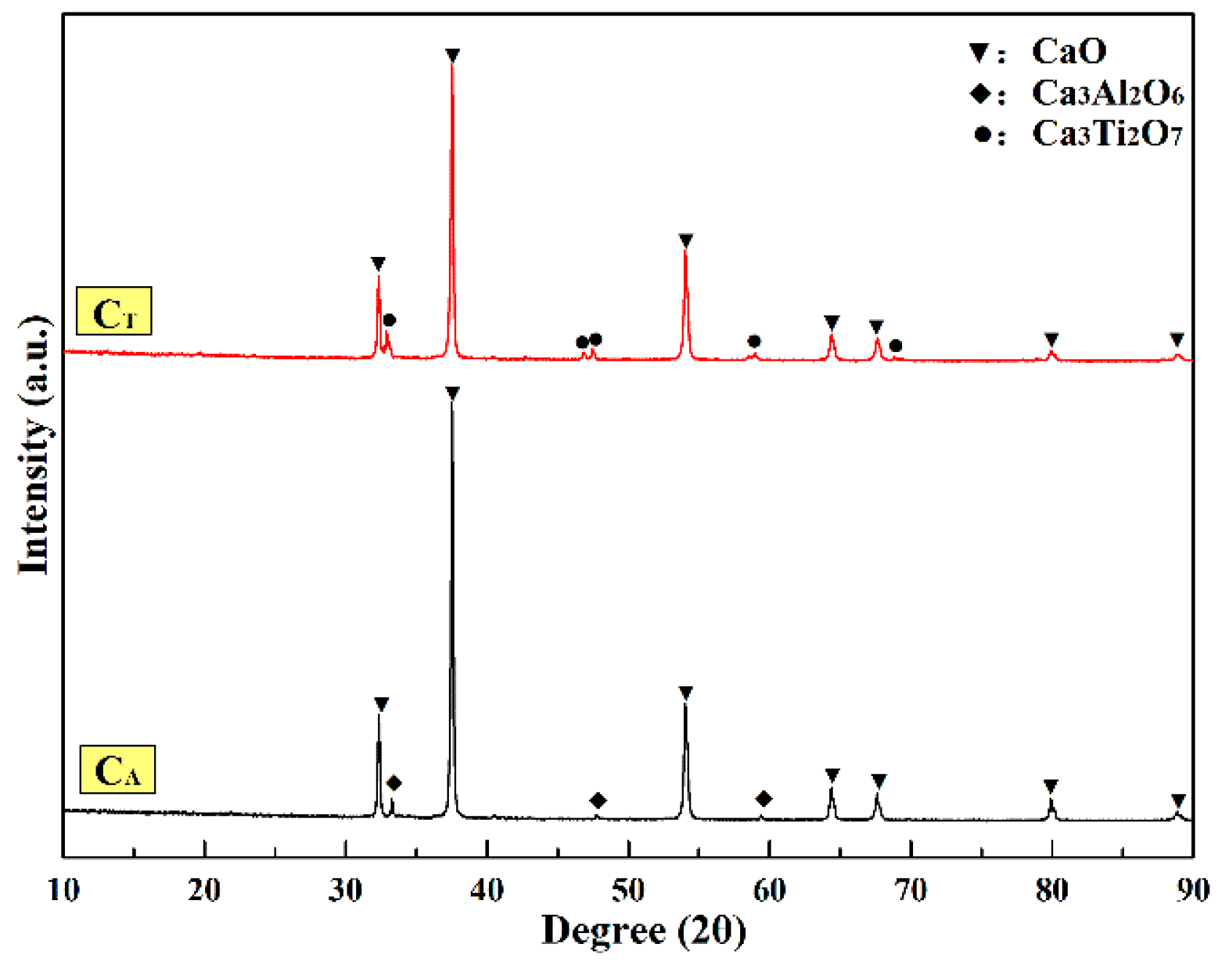
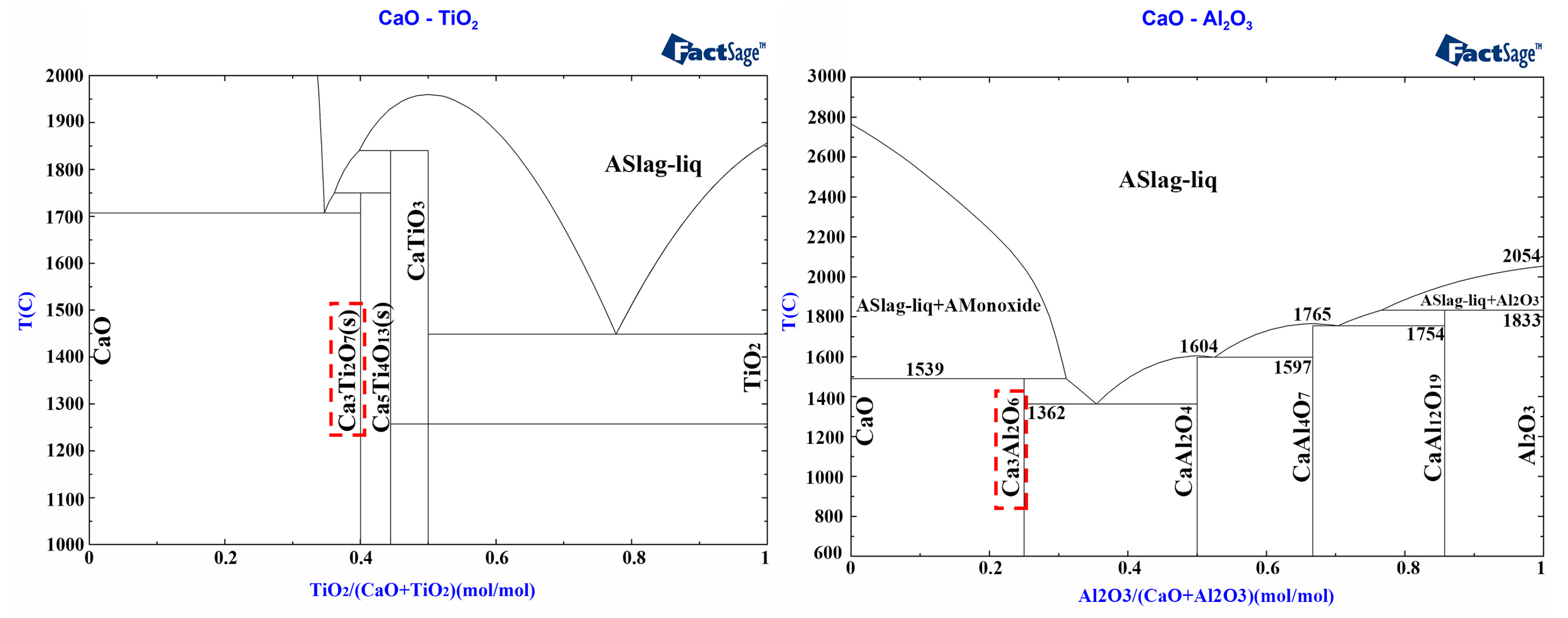
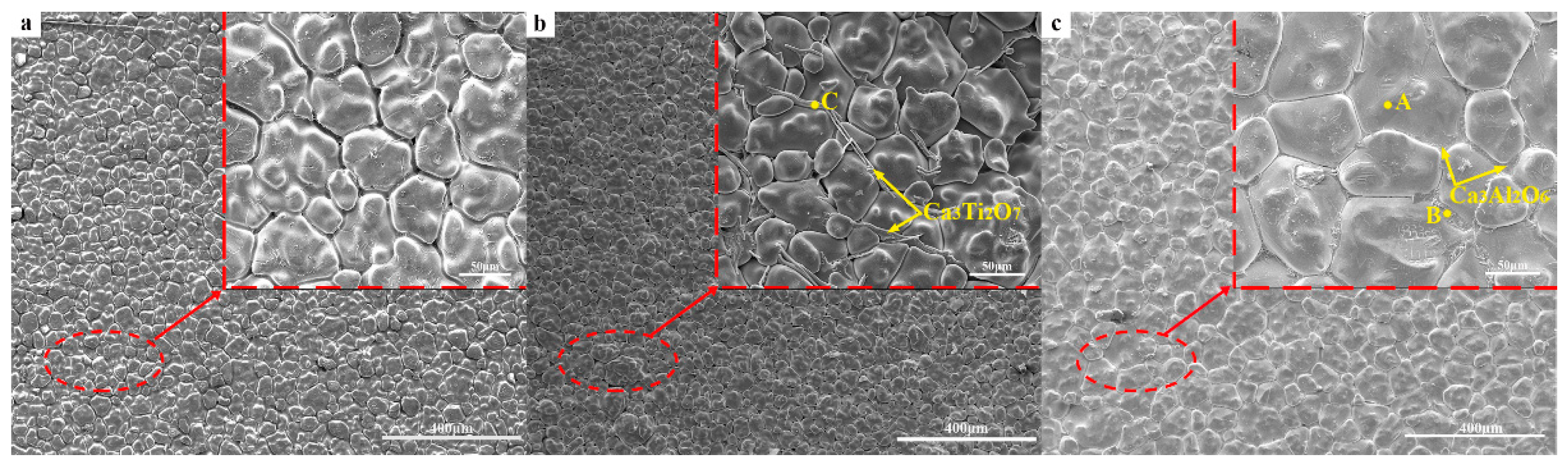
3.3. Phase Analysis of CaO Samples
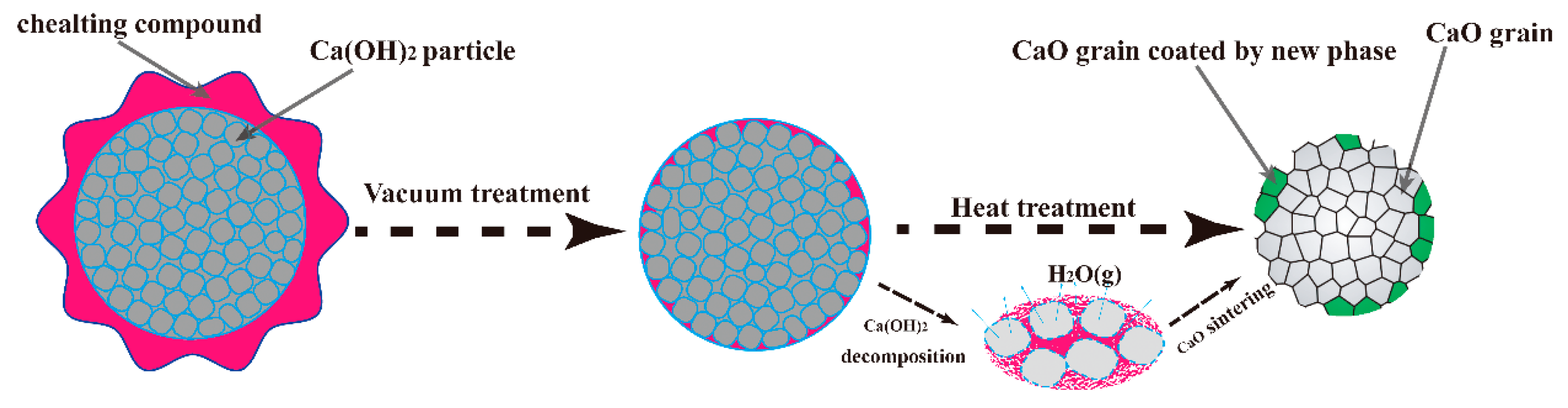
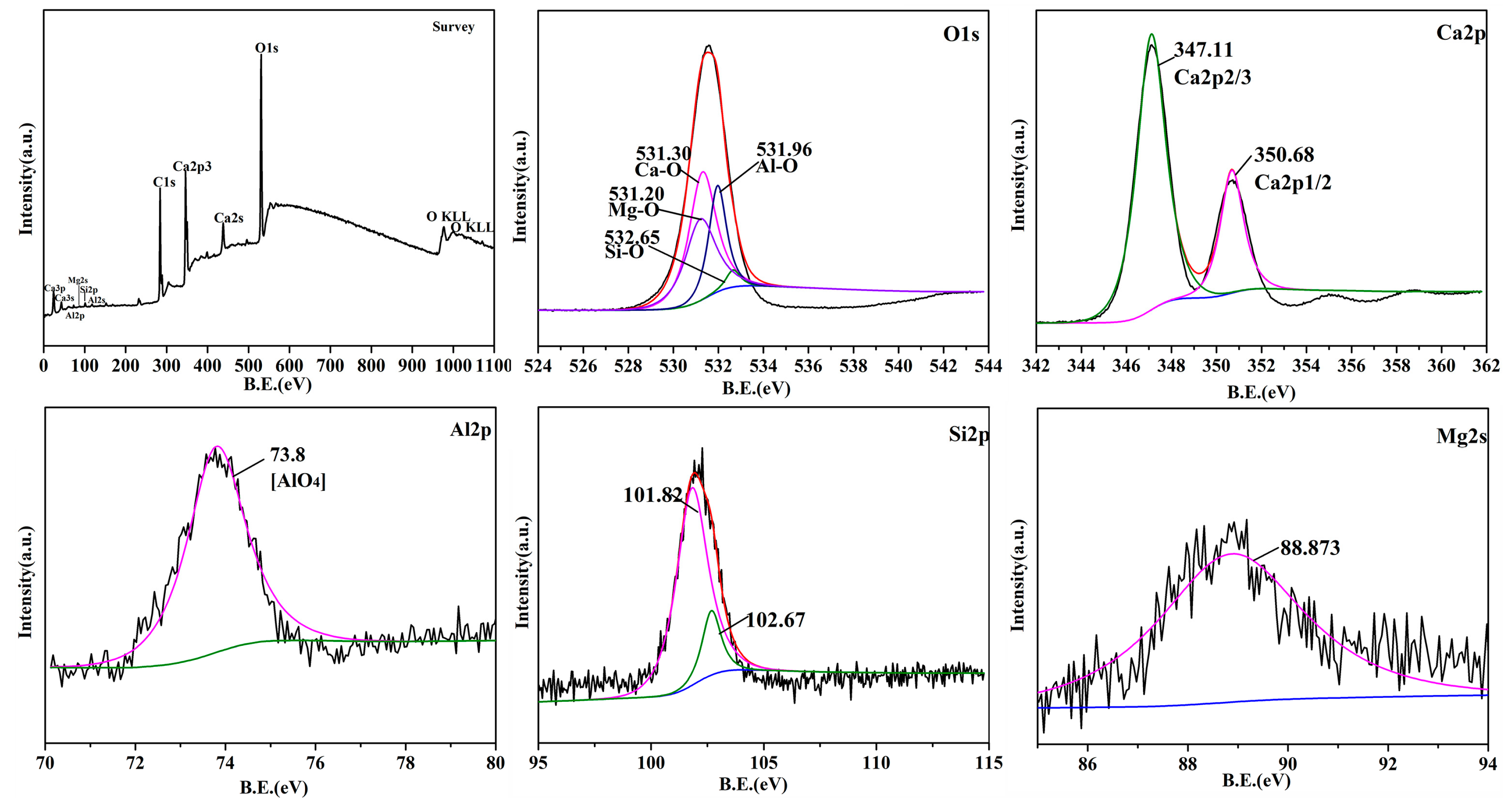
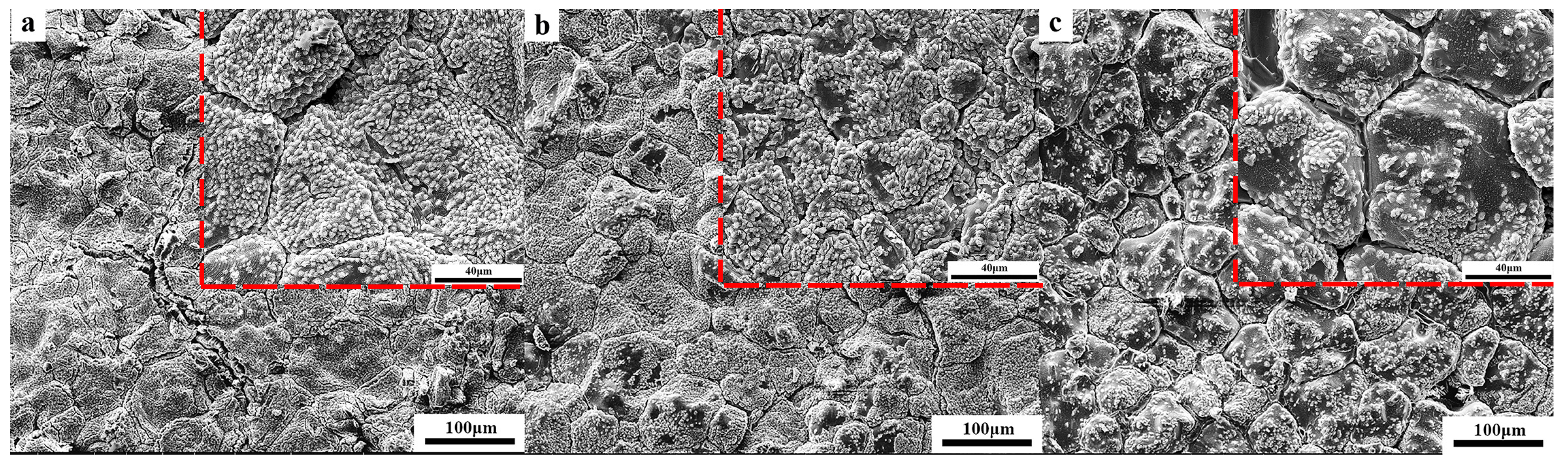
3.4. Microstructural Analysis of CaO Material
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Otham, A.G.M.; Abuel, M.A.; Serry, M.A. Hydration-resistant lime refractories from egyptian lime stone and ilmenite raw materials. Ceram. Int. 2001, 27, 801–807. [Google Scholar]
- Liu, X.; Zhou, Y.; Shang, B. Effect of oxides on the sintering process and the hydration resistance of CaO clinkers. Ceram. Int. 1996, 45, 78–80. [Google Scholar]
- Kahrizsangi, S.G.; Nemati, A.; Shahraki, A.; Farooghi, M. Effect of nano-sized Fe2O3 on microstructure and hydration resistance of MgO-CaO refractories. Int. J. Nanosci. Nanotechnol. 2016, 12, 19–26. [Google Scholar]
- Zhang, H.; Zhao, H.Z.; Zhen, J.; Li, J.; Yu, J.; Nie, J. Defect study of MgO-CaO material doped with CeO2. J. Adv. Mater. Sci. Eng. 2013, 20, 1–5. [Google Scholar] [CrossRef]
- Khlebnikova, Y.; Zhukovskaya, A.E.; Seliovanova, A.N. Methods for determining hydration resistance of refractories. Refract. Ind. Ceram. 2007, 48, 2–6. [Google Scholar] [CrossRef]
- Chen, S.; Chen, G.; Cheng, J. Effect of additive on the hydration resistance of material synthesized from the magnesia-calcia system. J. Am. Ceram. Soc. 2000, 83, 1810–1812. [Google Scholar] [CrossRef]
- Peng, C.; Li, N.; Han, B. Effect of zircon on sintering, composition and microstructure of magnesia powders. Sci. Sinter. 2009, 41, 11–17. [Google Scholar] [CrossRef]
- Kahrizsangi, S.G.; Barati, M.; Gheisari, H.; Shahraki, A.; Farooghi, M. Densification and properties of ZrO2 nanoparticles added magnesia-doloma refractories. Ceram. Int. 2016, 42, 15658–15663. [Google Scholar] [CrossRef]
- Chen, M.; Lu, C.; Yu, J. Improvement in performance of MgO-CaO refractories by addition of nano-sized ZrO2. J. Eur. Ceram. Soc. 2007, 27, 4633–4638. [Google Scholar] [CrossRef]
- Kahrizsangi, S.G.; Shahraki, A.; Farooghi, M. Effect of nano-TiO2 additions on the densification and properties of magnesite-dolomite ceramic composite. Iran. J. Sci. Technol. Trans. A 2018, 42, 567–575. [Google Scholar] [CrossRef]
- Ghonemi, N.M.; Mandourand, M.A.; Serry, M.A. Phase composition, microstructure and properties of sintered La2O3-doped lime and dolomite grains. Ceram. Int. 1990, 16, 215–223. [Google Scholar] [CrossRef]
- Ghoneim, N.M.; Mandour, M.A.; Serry, M.A. Sintering of lime doped with La2O3 and CeO2. Ceram. Int. 1989, 15, 357–362. [Google Scholar] [CrossRef]
- Yu, H.; Han, B.; Zhang, T. The effect of Al2O3 addition on the sintering properties of CaO clinker. J. Ceram. 2017, 38, 700–705. [Google Scholar]
- Kahrizsangi, S.G.; Nemati, A.; Shahraki, A.; Farooghi, M. Densification and Properties of Fe2O3 nanoparticles added CaO refractories. Ceram. Int. 2016, 42, 12270–12275. [Google Scholar] [CrossRef]
- Wei, Y.W.; Li, N. Effect of Fe2O3 addition in MgO-CaO refractory on desulfurization of liquid iron. J. Iron Steel Res. Int. 2003, 4, 4–7. [Google Scholar]
- Ghosh, A.; Bhattacharay, T.K.; Mukherjee, B.; Das, S.K. The effect of CuO addition on the sintering of lime. Ceram. Int. 2001, 27, 201–204. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhattacharay, T.K.; Mukherjee, B.; Maiti, S.; Tripathi, H.S.; Das, S.K. Densification and properties of lime with V2O5 additions. Ceram. Int. 2004, 30, 2117–2120. [Google Scholar] [CrossRef]
- Kahrizsangi, S.G.; Dehsheikh, H.G. MgO-CaO-Cr2O3 composition as a novel refractory brick: use of Cr2O3 nanoparticles. Bol. Soc. Esp. Ceram. Vidr. 2017, 56, 83–89. [Google Scholar] [CrossRef]
- Wei, Y.W.; Zhang, T.; Zhang, Q.; Han, B.; Li, N. Improvement in hydration resistance of CaO granules by addition of Zr(OH)4 and Al(OH)3. J. Am. Ceram. Soc. 2019, 102, 1414–1424. [Google Scholar] [CrossRef]
- Sanchez-Coronado, J.; Chung, D.D.L.; Martinez-Escandell, M.; Narciso, J.; Rodriguez-Reinoso, F. Effect of boron carbide particle addition on the thermomechanical behavior of carbon matrix silicon carbide particle composites. Carbon 2003, 41, 1096–1099. [Google Scholar] [CrossRef]
- Song, H.S.; Kim, C.H. The effect of surface carbonation on the hydration of CaO. Cement Concrete Res. 1990, 20, 815–823. [Google Scholar] [CrossRef]
- Gu, H.Z.; Wang, H.Z.; Sun, J.L.; Zhang, W.J.; Hou, D.Z.; Deng, C.J.; Hong, Y.R. Hydration kinetics of CaO-MgO clinker after surface treatment through H3PO4 or Al(H2PO4)3 Solution. Key Eng. Mater. 2002, 6, 224–226. [Google Scholar]
- Li, Z.; Zhang, S.; Lee, W.E. Improving the hydration resistance of lime-based refractory materials. Int. Mater. Rev. 2008, 53, 1–20. [Google Scholar] [CrossRef]
- Chen, M.; Wang, N.; Yu, J.; Yamaguchi, A. Effect of porosity on carbonation and hydration resistance of CaO materials. J. Eur. Ceram. Soc. 2007, 27, 1953–1959. [Google Scholar] [CrossRef]
- Caccia, M.; Camarano, A.; Sergi, D.; Ortona, A.; Narciso, J. Wetting and Navier-Stokes Equation—The Manufacture of Composite Materials. In Wetting and Wettability; IntechOpen: London, UK, 2015; pp. 105–135. [Google Scholar]
- Shahraki, A.; Ghasemi-kahrizsangi, S.; Nemati, A. Performance improvement of MgO-CaO refractories by the addition of nano-sized Al2O3. Mater. Chem. Phys. 2017, 198, 354–359. [Google Scholar] [CrossRef]
- Lucion, T.; Duvigneaud, P.H.; Laudet, A.; Stenger, J.F.; Gueguen, E. Effect of TiO2 additions on the densification of MgO and MgO-CaO mixtures. Key Eng. Mater. 2004, 264, 209–212. [Google Scholar] [CrossRef]
- German, R.M. Sintering Theory and Practice; Wiley-VCH: Weinheim, Germany, 1996. [Google Scholar]
- Cheng-li, W.; Bei-bei, W.; Ran, T.; Liu-wei, F.; Han-xu, L. Study of mineral structure transformation of coal ash with high ash melting temperature by XPS. Pctrosc Spect Anal. 2018, 38, 2296–2301. [Google Scholar]
- Kahrizsangi, S.G.; Dehsheikh, H.G.; Karamian, E.; Boroujerdnia, M.; Payandeh, K. Effect of MgAl2O4 nanoparticles addition on the densification and properties of MgO-CaO refractories. Ceram. Int. 2017, 43, 5014–5019. [Google Scholar] [CrossRef]

| Raw Material | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | Na2O | TiO2 | L.O.I. |
|---|---|---|---|---|---|---|---|---|---|
| Active lime | 96.80 | 0.44 | 0.47 | 0.30 | 1.59 | 0.007 | 0.007 | 0.005 | 0.23 |
| Item | Molecular Formula | Ti (Al) Content (%) | Relative Molecular Mass | Density (g/cm3) |
|---|---|---|---|---|
| Ti chelating compound | C15H26TiO6 | 9.8 | 274.29 | 1.040 |
| Al chelating compound | C12H23AlO5 | 8.9 | 350.25 | 0.975 |
| Element | Point A (wt.%) | Point B (wt.%) | Point C (wt.%) |
|---|---|---|---|
| O | 45.07 | 54.06 | 66.79 |
| Ca | 54.81 | 28.54 | 21.09 |
| Al | 0.12 | 17.40 | - |
| Ti | - | - | 12.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wei, Y.; Li, N.; Chen, J. Hydration Resistance of CaO Material Prepared by Ca(OH)2 Calcination with Chelating Compound. Materials 2019, 12, 2325. https://doi.org/10.3390/ma12142325
Wang J, Wei Y, Li N, Chen J. Hydration Resistance of CaO Material Prepared by Ca(OH)2 Calcination with Chelating Compound. Materials. 2019; 12(14):2325. https://doi.org/10.3390/ma12142325
Chicago/Turabian StyleWang, Jinhu, Yaowu Wei, Nan Li, and Junfeng Chen. 2019. "Hydration Resistance of CaO Material Prepared by Ca(OH)2 Calcination with Chelating Compound" Materials 12, no. 14: 2325. https://doi.org/10.3390/ma12142325
APA StyleWang, J., Wei, Y., Li, N., & Chen, J. (2019). Hydration Resistance of CaO Material Prepared by Ca(OH)2 Calcination with Chelating Compound. Materials, 12(14), 2325. https://doi.org/10.3390/ma12142325




