Effects of C Addition on the Microstructures of As-Cast Cu–Fe–P Alloys
Abstract
:1. Introduction
2. Experimental Section
3. Results
3.1. Macroscopic and Microscopic Structures
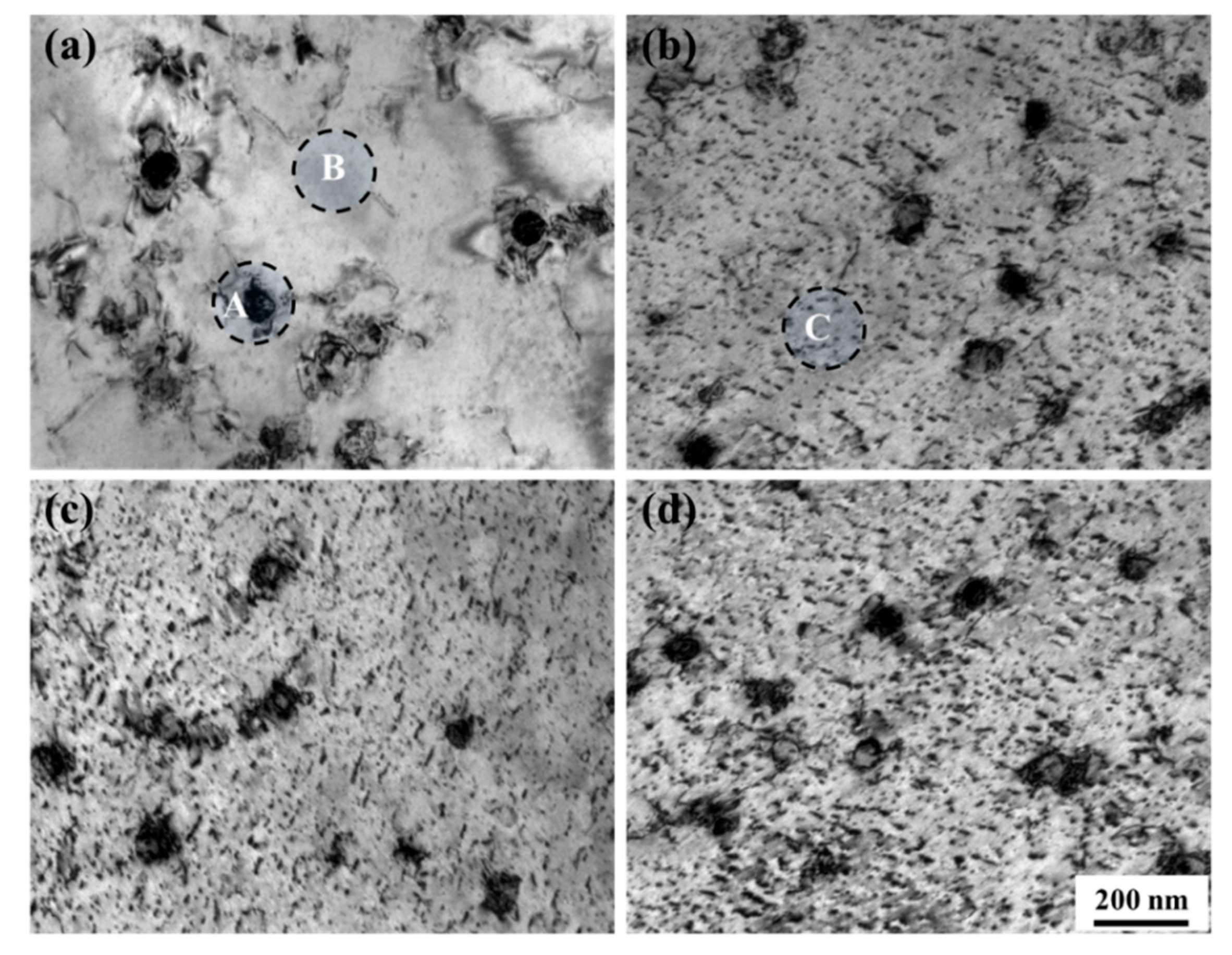
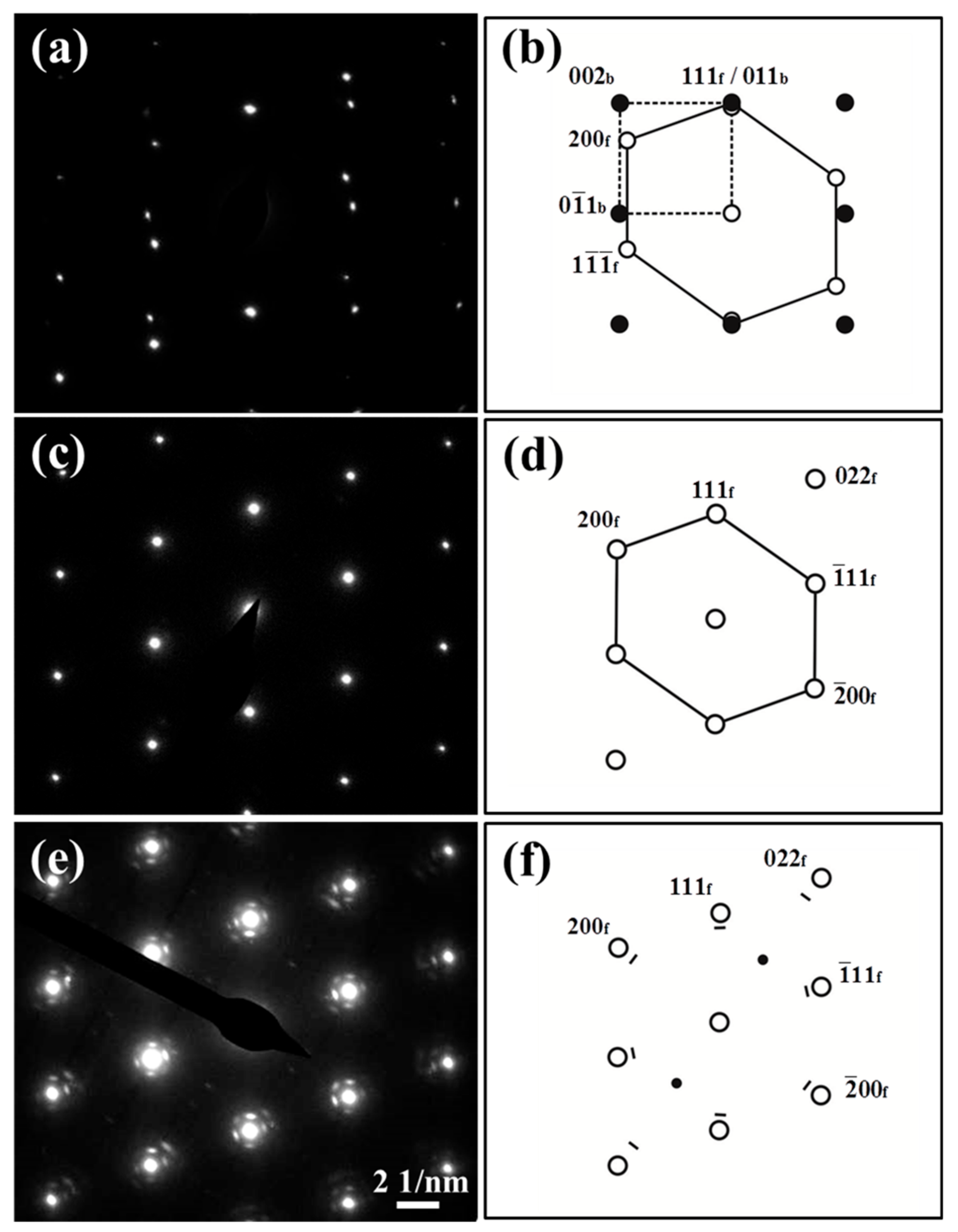
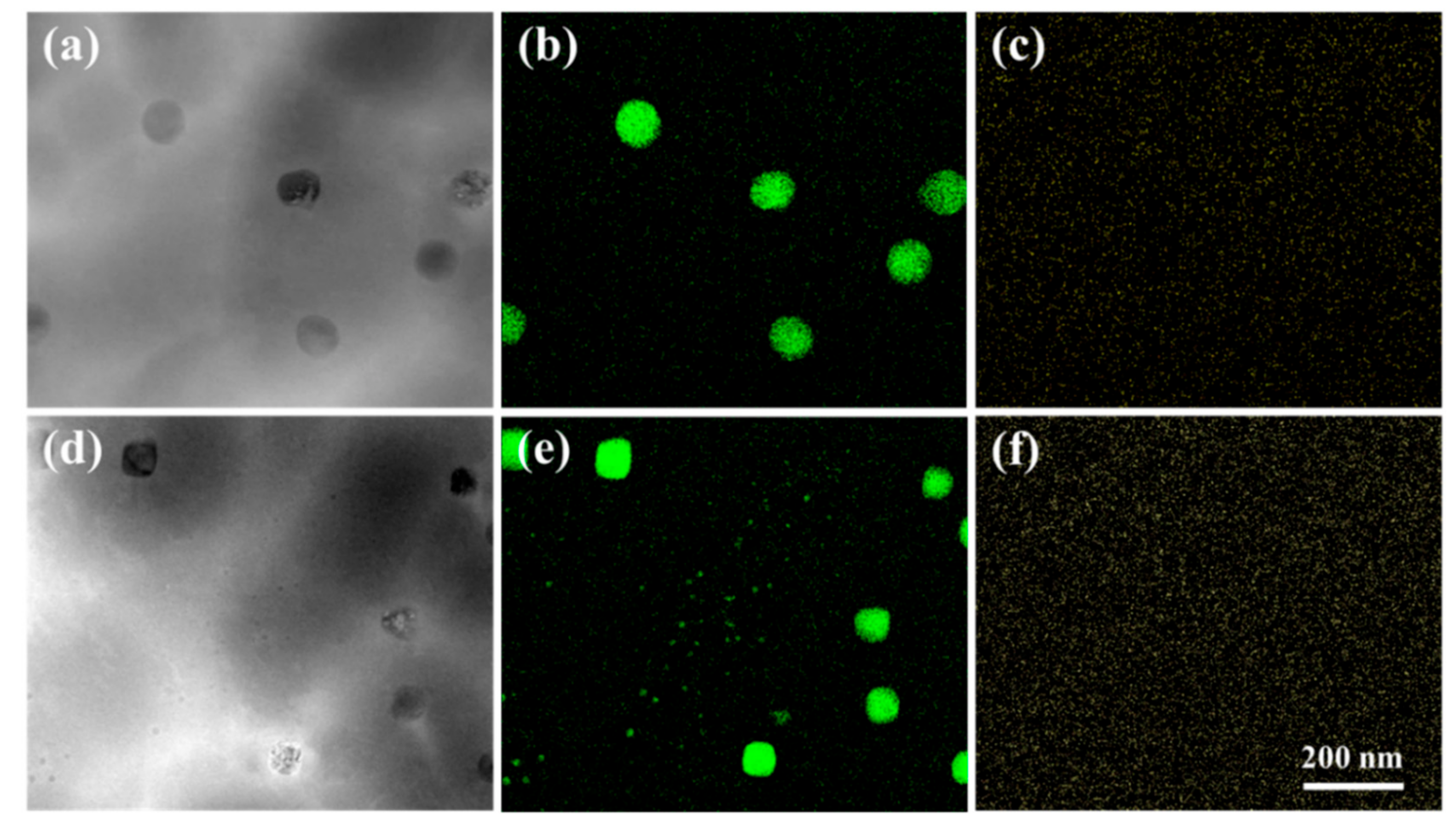
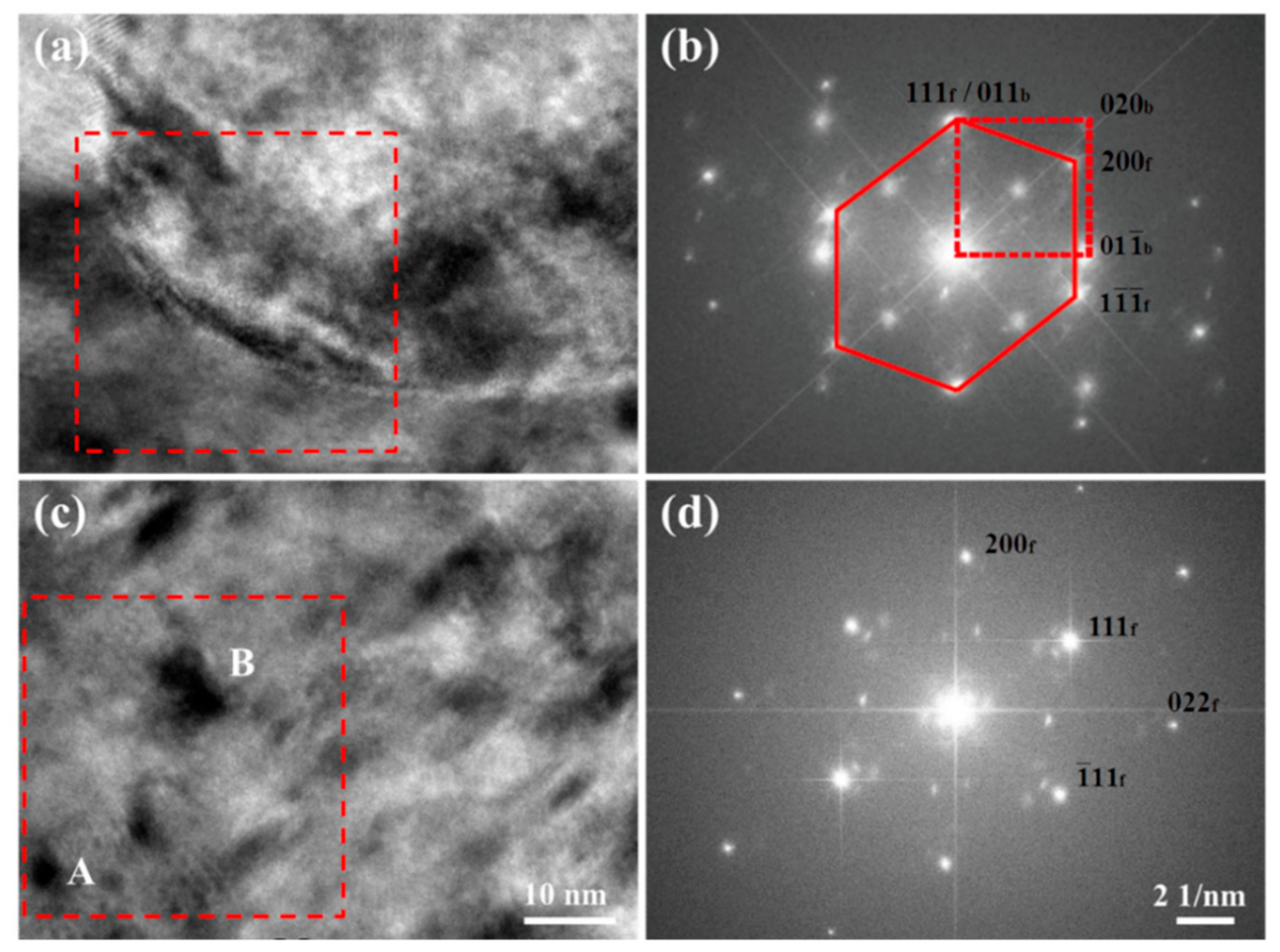
3.2. Fe Particles and Initial Decomposition Product
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Go, Y.S.; Spitzig, W.A. Strengthening in deformation-processed Cu–20% Fe composites. J. Mater. Sci. 1991, 26, 163–171. [Google Scholar] [CrossRef]
- Biselli, C.; Morris, D.G. Microstructure and strength of Cu-Fe in Situ composites after very high drawing strains. Acta Mater. 1996, 44, 493–504. [Google Scholar] [CrossRef]
- Verhoeven, J.D.; Chueh, S.C.; Gibson, E.D. Strength and conductivity of in situ Cu–Fe alloys. J. Mater. Sci. 1989, 24, 1748–1752. [Google Scholar] [CrossRef]
- Lu, D.P.; Wang, J.; Zeng, W.J.; Liu, Y.; Lu, L.; Sun, B.D. Study on high-strength and high-conductivity Cu–Fe–P alloys. Mater. Sci. Eng. A 2006, 421, 254–259. [Google Scholar] [CrossRef]
- Gao, H.Y.; Wang, J.; Shu, D.; Sun, B.D. Effect of Ag on the microstructure and properties of Cu–Fe in situ composites. Scr. Mater. 2005, 53, 1105–1109. [Google Scholar] [CrossRef]
- Song, J.S.; Hong, S.I.; Park, Y.G. Deformation processing and strength/conductivity properties of Cu–Fe–Ag microcomposites. J. Alloys Compd. 2005, 388, 69–74. [Google Scholar] [CrossRef]
- Cao, H.; Min, J.Y.; Wu, S.D.; Xian, A.P.; Shang, J.K. Pinning of grain boundaries by second phase particles in equal-channel angularly pressed Cu–Fe–P alloy. Mater. Sci. Eng. A 2006, 431, 86–91. [Google Scholar] [CrossRef]
- Liu, K.M.; Lu, D.P.; Zhou, H.T.; Atrens, A.; Zou, J.; Yang, Y.L.; Zeng, S.M. Effect of Ag micro-alloying on the microstructure and properties of Cu–14Fe in situ composite. Mater. Sci. Eng. A 2010, 527, 4953–4958. [Google Scholar] [CrossRef]
- Guo, F.A.; Xiang, C.J.; Yang, C.X.; Cao, X.M.; Mu, S.G.; Tang, Y.Q. Study of rare earth elements on the physical and mechanical properties of a Cu–Fe–P–Cr alloy. Mater. Sci. Eng. B 2008, 147, 1–6. [Google Scholar] [CrossRef]
- Zou, J.; Lu, D.P.; Liu, K.M.; Fu, Q.F. Effects of an Alternating Magnetic Field/Ag Multi-Alloying Combined Solidification Process on Cu–14Fe Alloy. Materials 2018, 11, 2501. [Google Scholar] [CrossRef]
- Papaefthymiou, S.; Bouzouni, M.; Gavalas, E. Theoretical Study of Particle Dissolution during Homogenization in Cu–Fe–P Alloy. Metals 2018, 8, 455. [Google Scholar] [CrossRef]
- López, G.A.; Mittemeijer, E.J. The solubility of C in solid Cu. Scr. Mater. 2004, 51, 1–5. [Google Scholar] [CrossRef]
- Jeong, E.; Han, S.; Goto, M.; Kim, S. Effects of thermo-mechanical processing and trace amount of carbon addition on tensile properties of Cu–2.5Fe–0.1P alloys. Mater. Sci. Eng. A 2009, 520, 66–74. [Google Scholar] [CrossRef]
- Kim, H.G.; Han, S.Z.; Euh, K.; Lim, S.H. Effects of C addition and thermo-mechanical treatments on microstructures and properties of Cu–Fe–P alloys. Mater. Sci. Eng. A 2011, 530, 652–658. [Google Scholar] [CrossRef]
- Guo, M.; Wang, F.; Yi, L. The microstructure controlling and deformation behaviors of Cu–Fe–C alloy prepared by rapid solidification. Mater. Sci. Eng. A 2016, 657, 197–209. [Google Scholar] [CrossRef]
- Guo, M.X.; Zhu, J.; Yi, L.; Wang, F.; Li, G.J.; Lei, R.S. Effects of precipitation and strain-induced martensitic transformation of Fe–C phases on the mechanical properties of Cu–Fe–C alloy. Mater. Sci. Eng. A 2017, 697, 119–125. [Google Scholar] [CrossRef]
- Wang, C.P.; Liu, X.J.; Kainuma, R.; Takaku, Y.; Ohnuma, I.; Ishida, K. Formation of core-type macroscopic morphologies in Cu–Fe base alloys with liquid miscibility gap. Metall. Mater. Trans. A 2004, 35, 1243–1253. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, W.; Guo, W.; Fu, Q.F.; Lu, D.P.; Yi, G.B.; Jing, Y.H.; Luo, L.; Liu, Y. Effect of C addition on the liquid phase separation in an as-cast Cu–14Fe alloy. Materialia 2019, 7, 100379. [Google Scholar] [CrossRef]
- Morris, M.A.; Leboeuf, M.; Morris, D.G. Recrystallization mechanisms in a Cu–Cr–Zr alloy with a bimodal distribution of particles. Mater. Sci. Eng. A 1994, 188, 255–265. [Google Scholar] [CrossRef]
- Dong, Q.Y.; Wang, M.P.; Shen, L.N.; Jia, Y.L.; Li, Z. Diffraction analysis of alpha-Fe precipitates in a polycrystalline Cu–Fe alloy. Mater. Charact. 2015, 105, 129–135. [Google Scholar] [CrossRef]
- Chbihi, A.; Sauvage, X.; Blavette, D. Atomic scale investigation of Cr precipitation in copper. Acta Mater. 2012, 60, 4575–4585. [Google Scholar] [CrossRef] [Green Version]
- Wada, M.; Yuchi, Y.; Uemori, R.; Tanino, M.; Mori, T. Fim study of F.C.C. iron particles in Cu. Acta Metall. 1988, 36, 333–339. [Google Scholar] [CrossRef]
- Heinrich, A.; Al-Kassab, T.A.; Kirchheim, R. Investigation of the early stages of decomposition of Cu–0.7at.% Fe with the tomographic atom probe. Mater. Sci. Eng. A 2003, 353, 92–98. [Google Scholar] [CrossRef]
- Batra, I.S.; Dey, G.K.; Kulkarni, U.D.; Banerjee, S. Precipitation in a Cu–Cr–Zr alloy. Mater. Sci. Eng. A 2002, 356, 32–36. [Google Scholar] [CrossRef]
- Baburaj, E.G.; Kulkarni, U.D.; Menon, E.S.K.; Krishnan, R. CuBe precipitation in Cu-Be alloys. Phase Transit. 1979, 1, 171–197. [Google Scholar] [CrossRef]
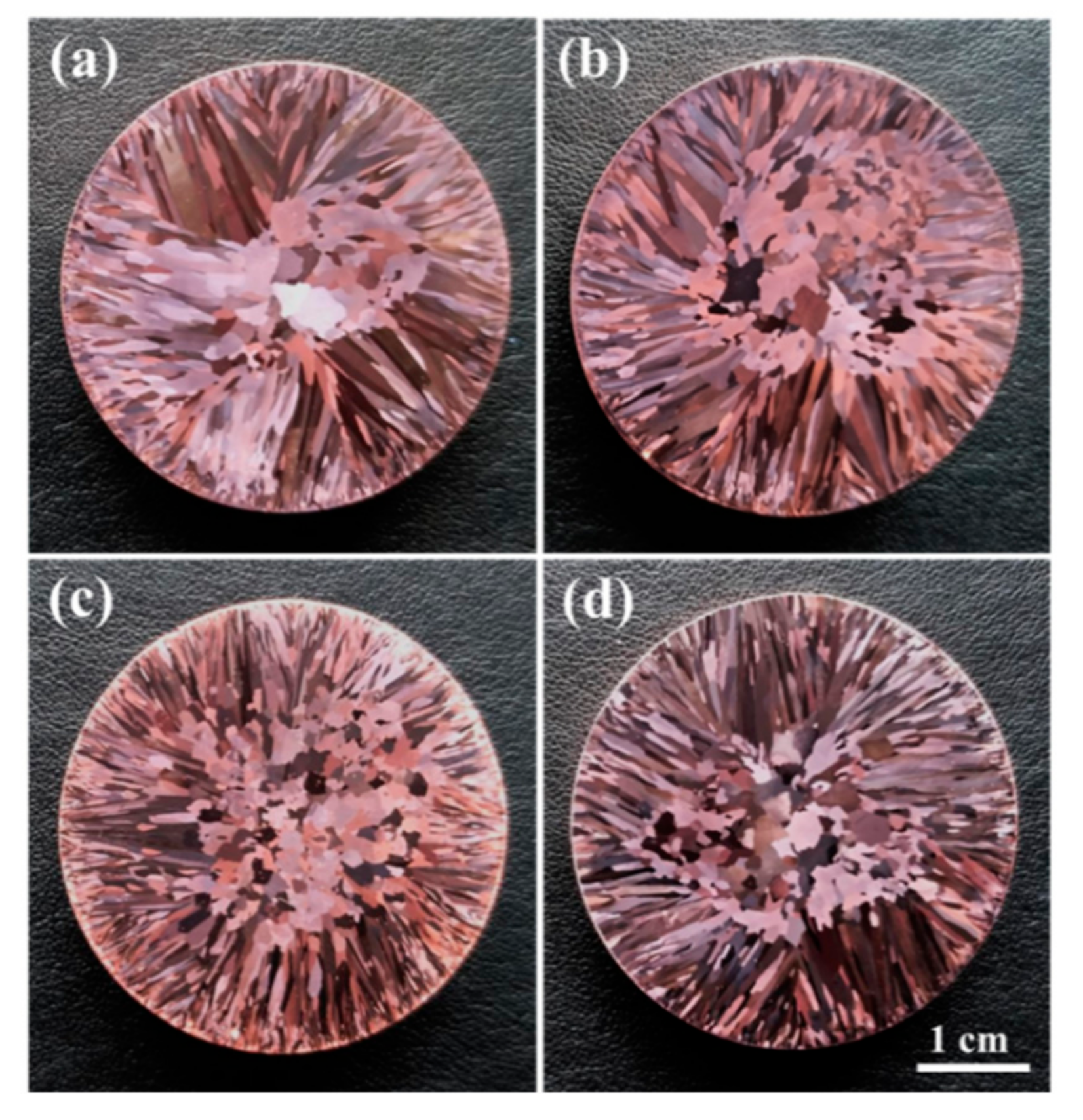
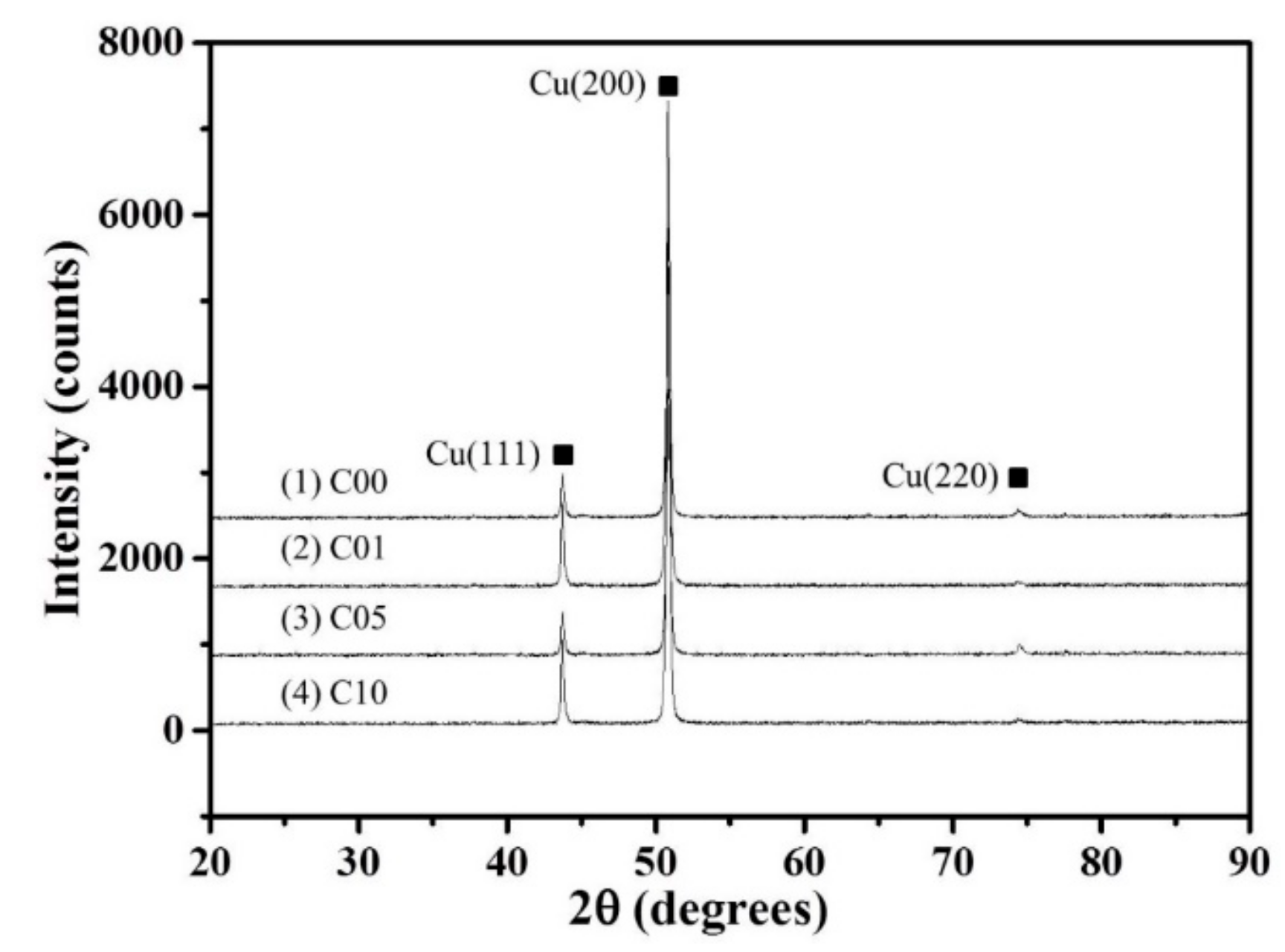
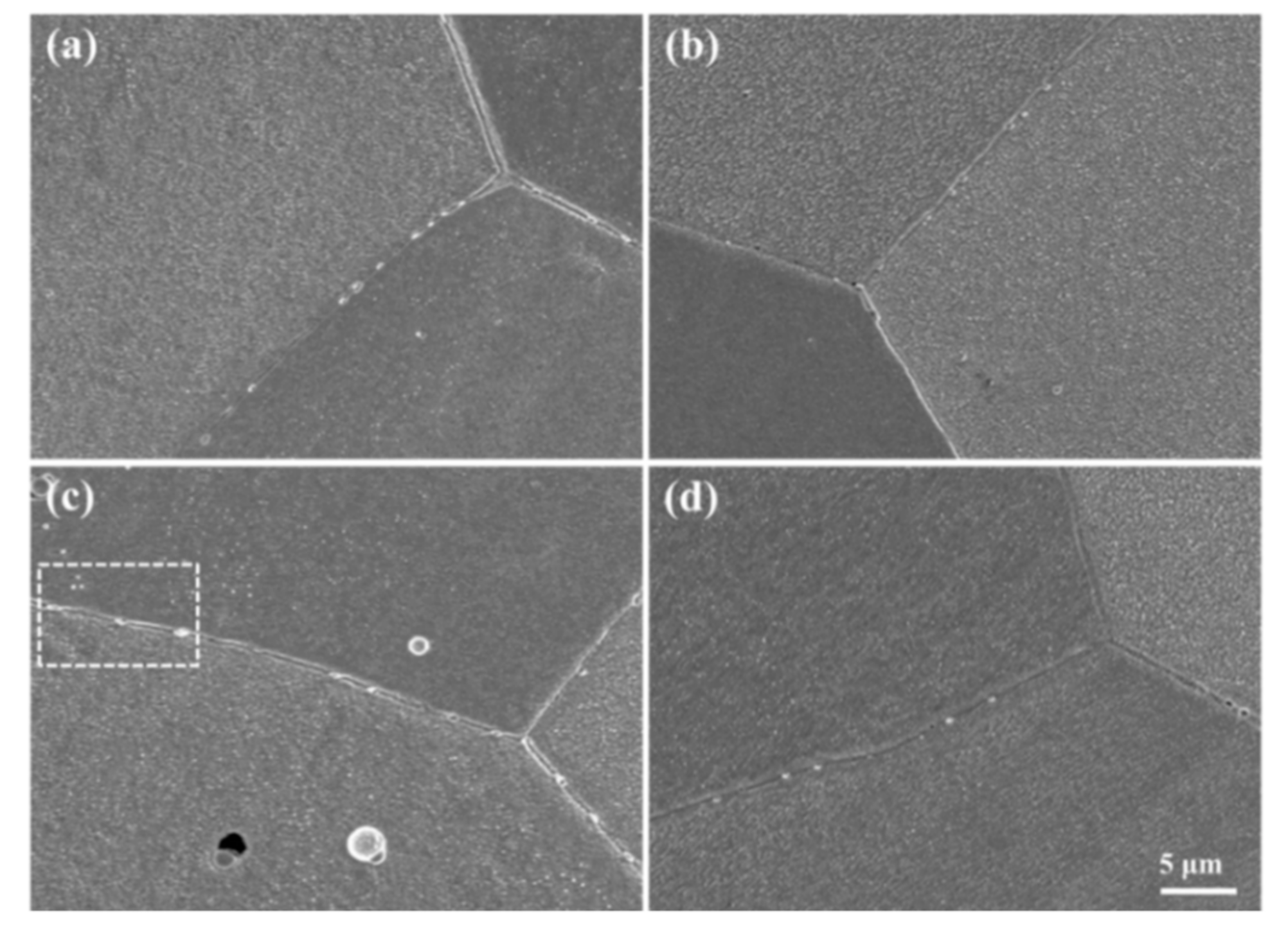

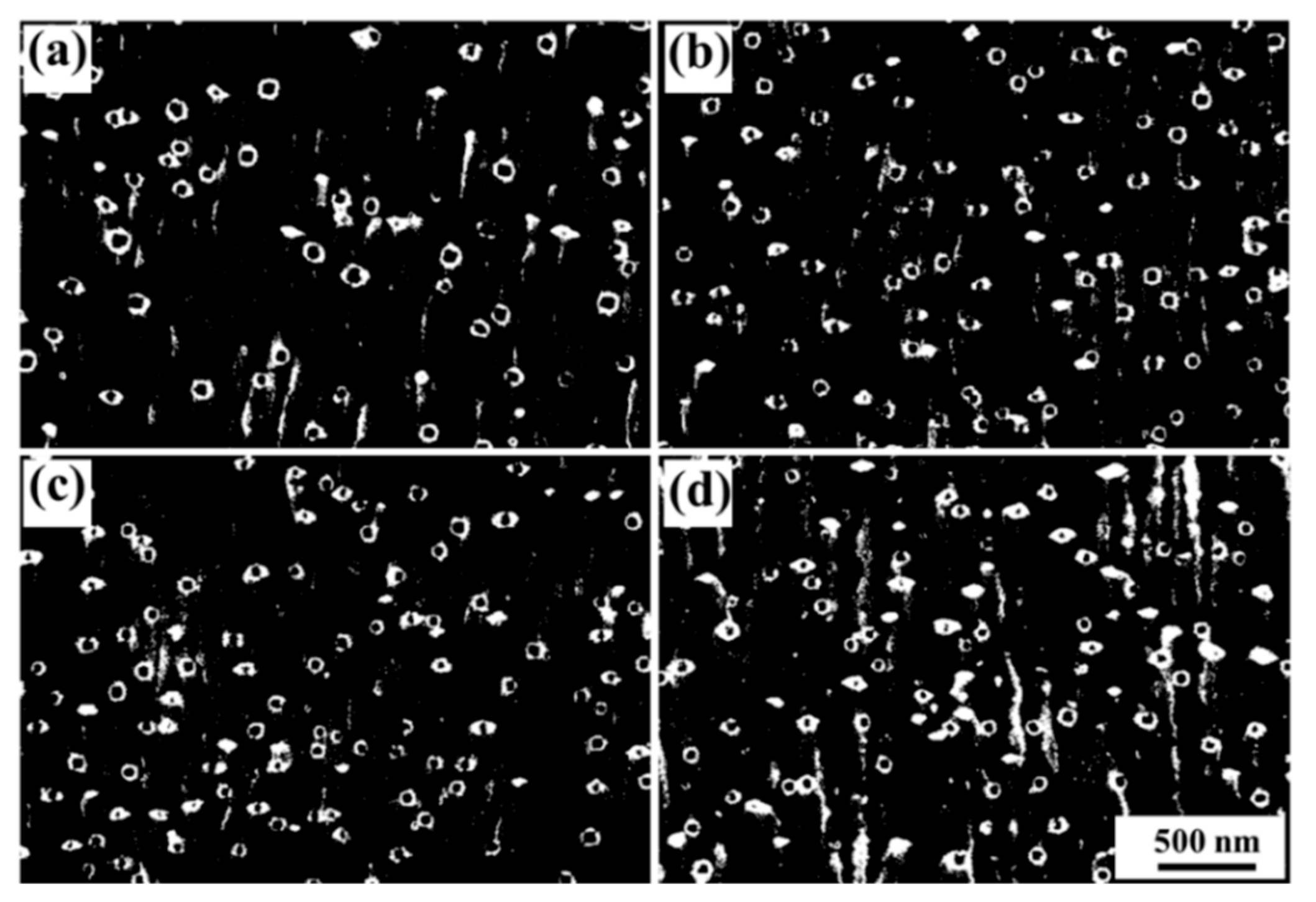
| Alloy | Fe | P | C | Cu |
|---|---|---|---|---|
| Cu–1.8Fe–0.02P (C00) | 1.79 | 0.025 | 0.001 | Balance |
| Cu–1.8Fe–0.02P–0.01C (C01) | 1.79 | 0.020 | 0.011 | Balance |
| Cu–1.8Fe–0.02P–0.05C (C05) | 1.78 | 0.056 | 0.013 | Balance |
| Cu–1.8Fe–0.02P–0.10C (C10) | 1.78 | 0.026 | 0.015 | Balance |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Hu, X.; Guo, W.; Zou, J.; Liu, K.; Lu, D.; Tan, D. Effects of C Addition on the Microstructures of As-Cast Cu–Fe–P Alloys. Materials 2019, 12, 2772. https://doi.org/10.3390/ma12172772
Chen W, Hu X, Guo W, Zou J, Liu K, Lu D, Tan D. Effects of C Addition on the Microstructures of As-Cast Cu–Fe–P Alloys. Materials. 2019; 12(17):2772. https://doi.org/10.3390/ma12172772
Chicago/Turabian StyleChen, Wei, Xiaona Hu, Wei Guo, Jin Zou, Keming Liu, Deping Lu, and Dunqiang Tan. 2019. "Effects of C Addition on the Microstructures of As-Cast Cu–Fe–P Alloys" Materials 12, no. 17: 2772. https://doi.org/10.3390/ma12172772
APA StyleChen, W., Hu, X., Guo, W., Zou, J., Liu, K., Lu, D., & Tan, D. (2019). Effects of C Addition on the Microstructures of As-Cast Cu–Fe–P Alloys. Materials, 12(17), 2772. https://doi.org/10.3390/ma12172772






