Research on the Oxidation Mechanism of Vermicular Graphite Cast Iron
Abstract
:1. Introduction
2. Experimental
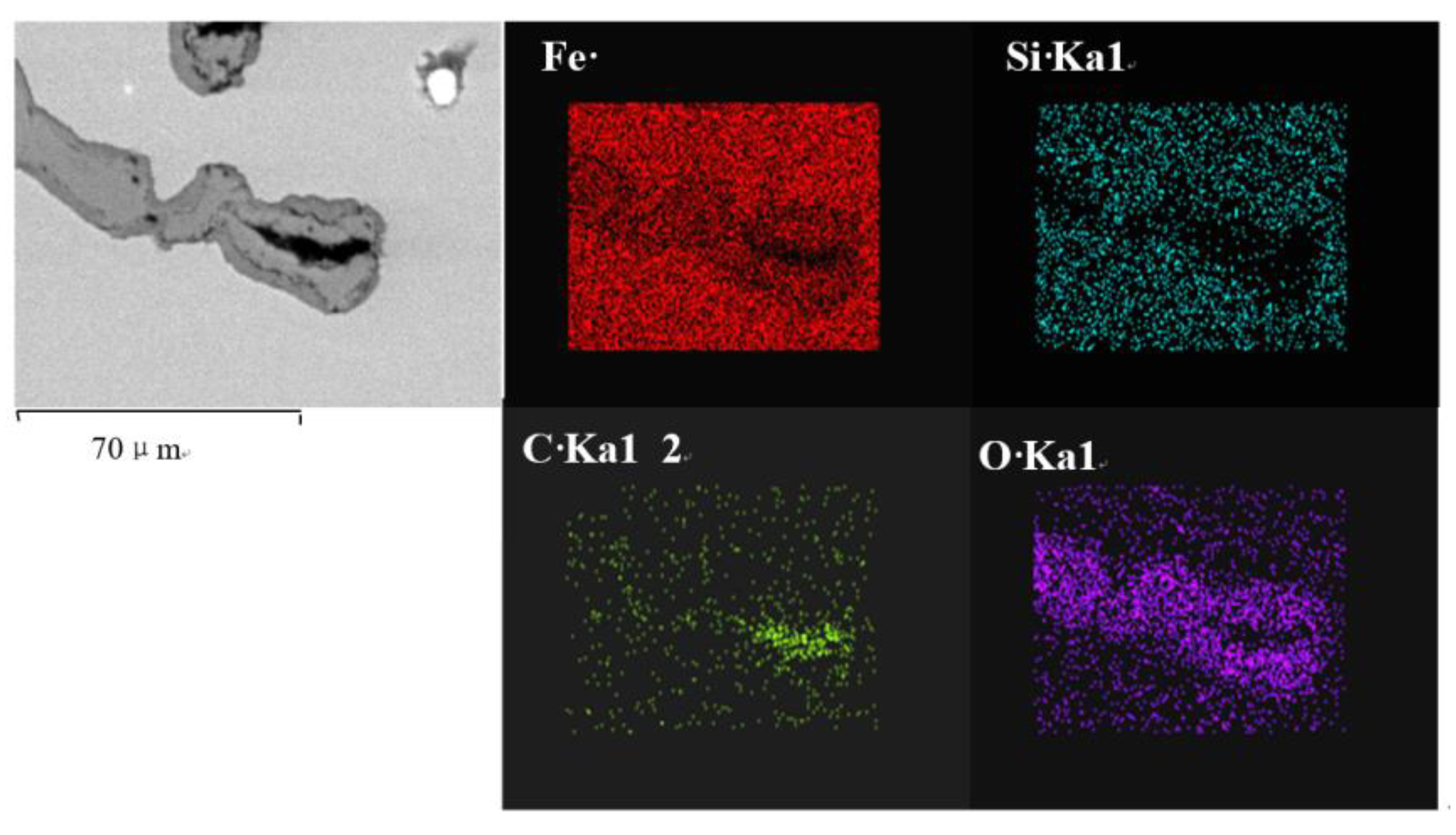
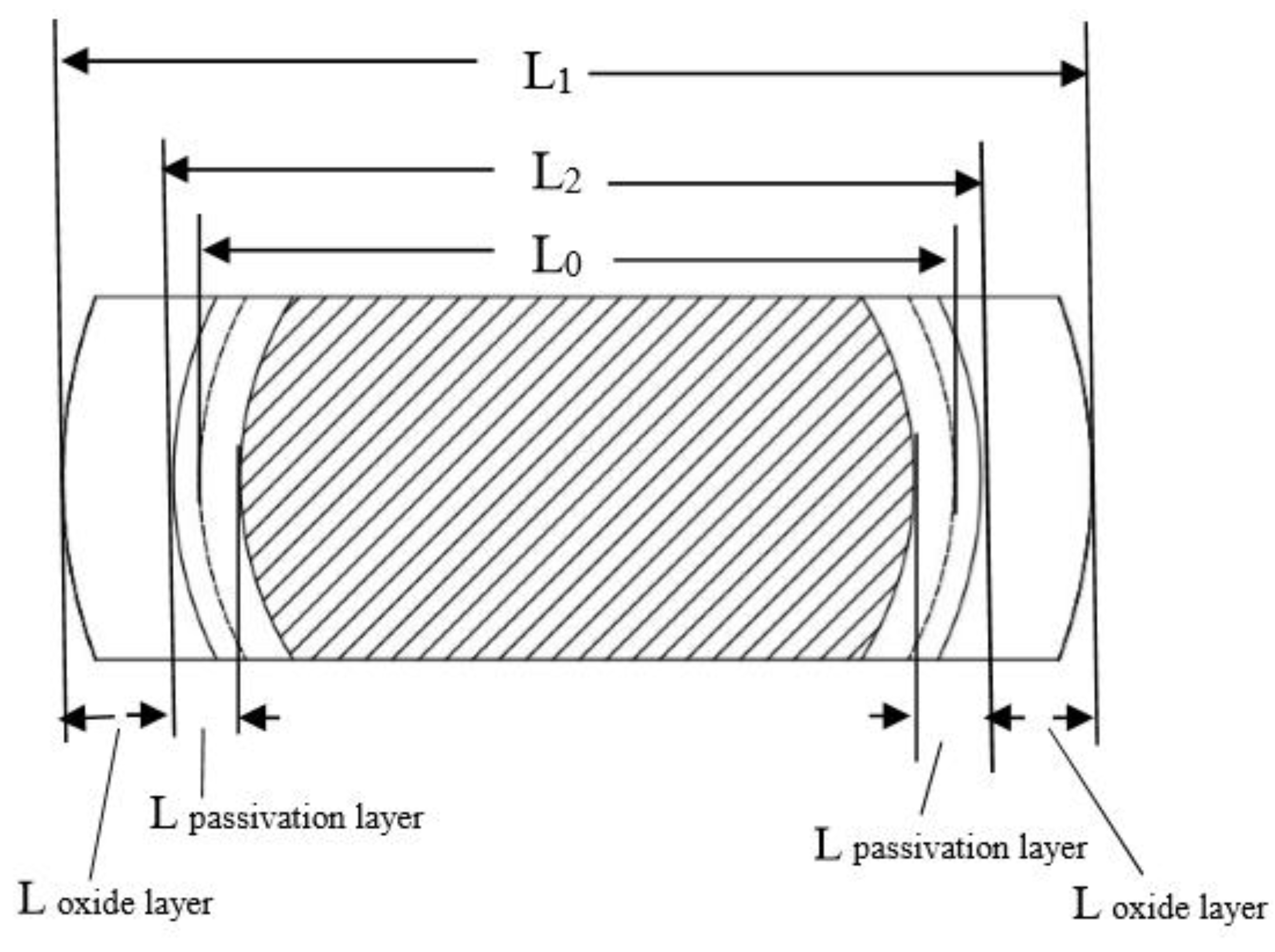
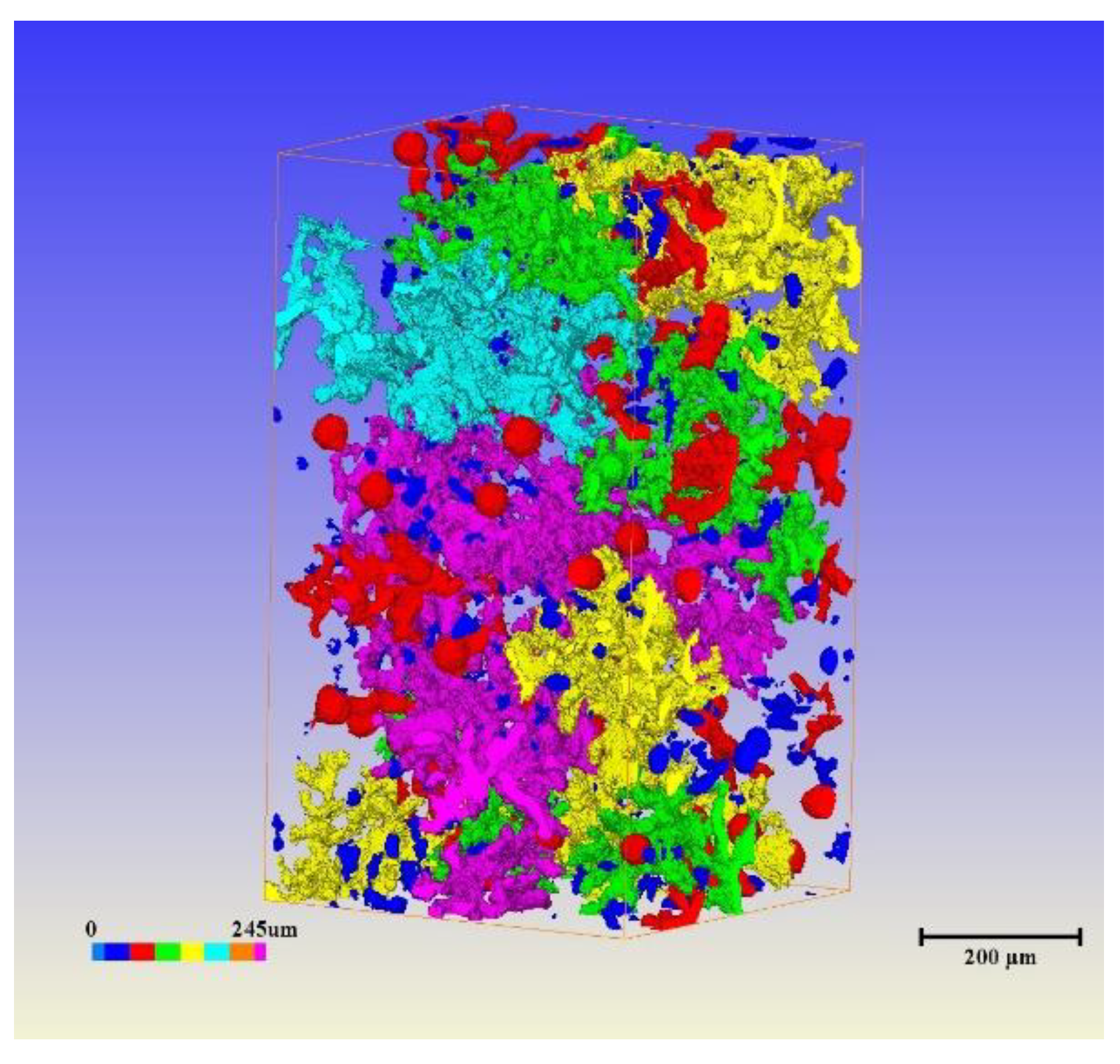
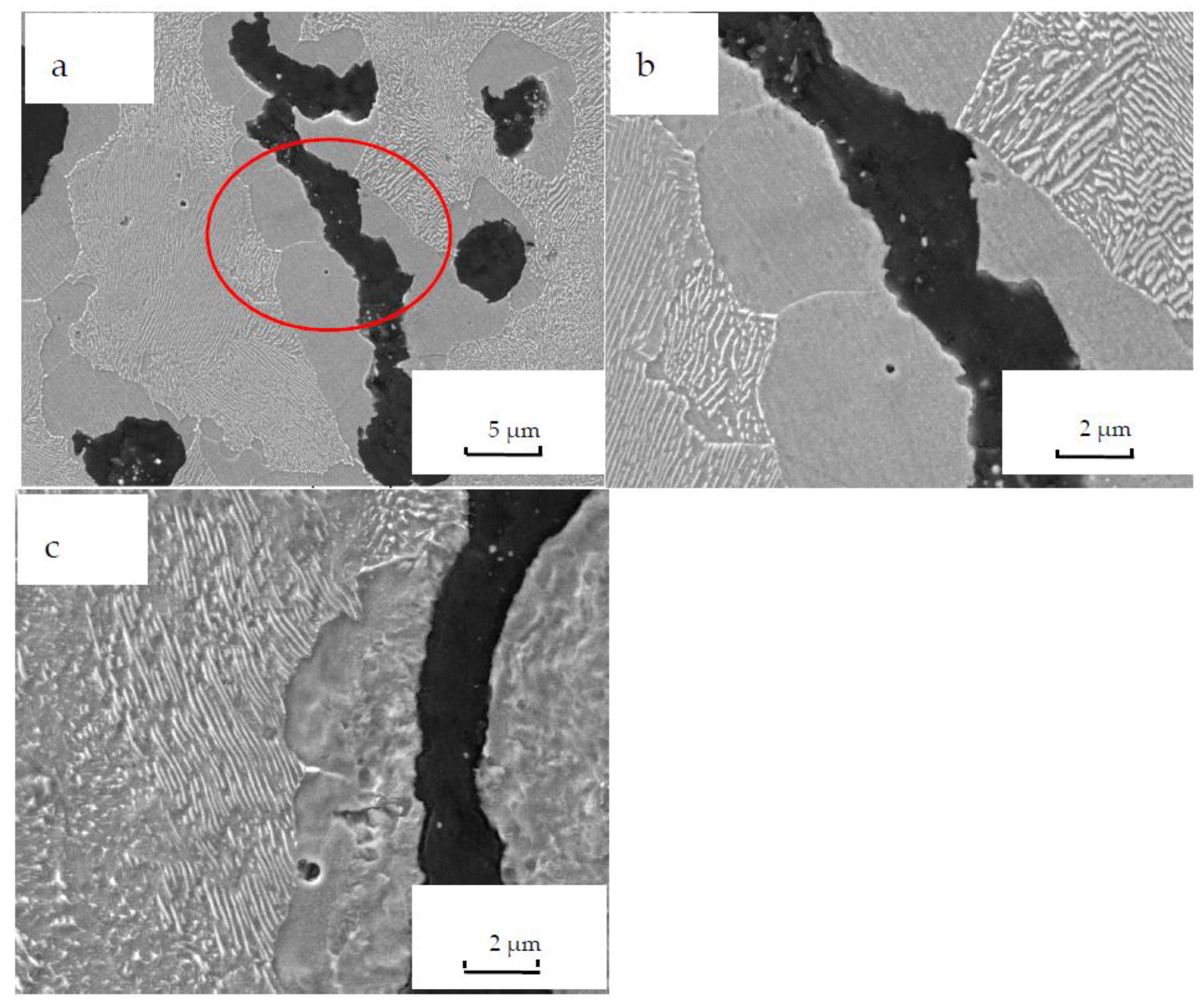
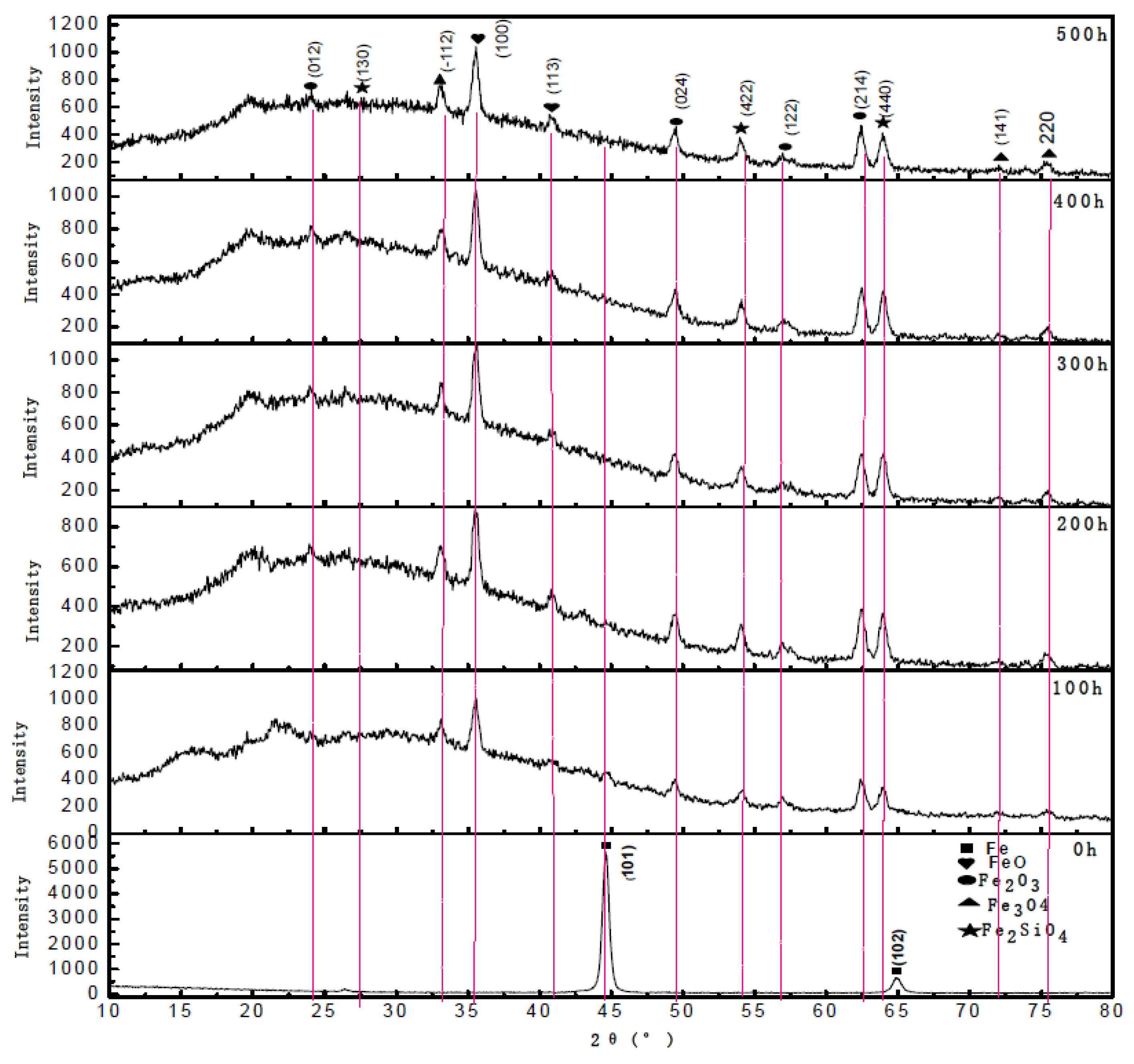
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, J.P.; Kim, H.G.; Park, J.Y. A study of the oxidation of FeCrAl alloy in pressurized water and high-temperature steam environment. Corros. Sci. 2015, 94, 459–465. [Google Scholar]
- Samira, R.; Behzad, N. Effect of microstructure and surface features on wetting angle of a Fe-3.2wt% C.E. cast iron with water. Appl. Surf. Sci. 2018, 440, 341–350. [Google Scholar]
- Zhang, M.X.; Pang, J.C.; Qiu, Y. Thermo-mechanical fatigue property and life prediction of vermicular graphite iron. Mater. Sci. Eng. A 2017, 698, 63–72. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Hussain, M.S.; Bakhsheshi-Rad, H.R. Formation of a dense and continuous Al2O3 layer in nano thermal barrier coating systems for the suppression of spinel growth on the Al2O3 oxide scale during oxidation. J. Alloys Compd. 2013, 571, 205–220. [Google Scholar] [CrossRef]
- Sohi, M.H.; Ghasemi, H.M.; Shahripour, A. Microstructural study of surface melted and chromium surface alloyed ductile iron. Appl. Surf. Sci. 2012, 258, 7348–7353. [Google Scholar] [CrossRef]
- Kováčiková, P.; Bezdedová, R.; Vavro, J. Comparison of Numerical Analysis of Stress-Strain States of Cast Iron with Vermicular Graphite Shape and Globular Graphite Shape. Procedia Eng. 2016, 136, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.Q.; Yang, Z.; Tao, D.; Gao, P.H. Effects of vermicular graphite rate on the oxidation resistance and mechanical properties of vermicular graphite iron. J. Alloys Compd. 2018, 765, 213–220. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, P.J. Multi-layer compound coating on cast iron piston ring by multi-arc and magnetron sputtering ion compound plating technique. Surf. Coat. Technol. 2000, 131, 422–427. [Google Scholar]
- Abdoos, M.; Yamamoto, K.; Bose, B.; Fox-Rabinovich, G.; Veldhuis, S. Effect of coating thickness on the tool wear performance of low stress TiAlN PVD coating during turning of compacted graphite iron (CGI). Wear 2019, 422–423, 128–136. [Google Scholar] [CrossRef]
- Gao, P.H.; Cao, S.T.; Li, J.P. High temperature oxidation resistance of M42C stainless steel coatings deposited on the surface of cast iron through atmospheric plasma spraying. J. Alloys Compd. 2016, 684, 188–194. [Google Scholar] [CrossRef]
- Chen, P.G.; Liu, Z.L.; Li, R.Q.; Li, X.Q. The effect of manganese additions on the high temperature oxidation behaviour of the high-vanadium cast iron. J. Alloys Compd. 2018, 767, 181–187. [Google Scholar] [CrossRef]
- Sah, I.; Kim, D.; Lee, H.J.; Jang, C. Development and oxidation resistance evaluation of Al-rich surface layer on Alloy 617. Surf. Coat. Technol. 2013, 236, 400–404. [Google Scholar] [CrossRef]
- Albuquerque, A.D.; Sartori, J.R. Nucleation and growth of graphite particles in ductile cast iron. J. Alloys Compd. 2019, 775, 1230–1234. [Google Scholar]
- Wu, Y.; Li, J.P.; Yang, Z. Creep behavior accompanying oxidation of compacted graphite cast iron. Mater. Sci. Eng. A 2018, 723, 174–181. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Ma, Y.Q. Effect of aluminum on the oxidation resistance of heat resistant cast iron. Mech. Eng. Mater. 2011, 138, 32–34. [Google Scholar]
- Han, Y.J.; Ye, X.F.; Lu, X.G.; Liu, C.; Hao, J.L. Residual stress evolution of thermally grown oxide in thermal barrier coatings deposited onto nickel-base superalloy and iron-base alloy with thermal exposure ageing. J. Alloys Compd. 2014, 584, 19–27. [Google Scholar] [CrossRef]
- Moonesan, M.; Madah, F.; Zadeh, A.H. Effect of alloying elements on thermal shock resistance of gray cast iron. J. Alloys Compd. 2012, 520, 226–231. [Google Scholar] [CrossRef]
- Dawson, S. Compacted graphite iron—A material solution for modern diesel engine cylinder lock and heads. China Foundry 2009, 6, 241–246. [Google Scholar]
- Zhang, X.; Zhang, G.; Xu, H. Homogeneity research on microstructure and properties of vermicular graphite cast iron. Hot Work. Technol. 2014, 43, 27–30. [Google Scholar]
- Yue, X.; Song, C.; Yan, Z.; Shen, X.; Ke, W.; Ji, Z.; Zhu, G.; Yuan, A.; Zhu, J.; Li, B. Reduced graphene oxide supported nitrogen-doped porous carbon-coated NiFe alloy composite with excellent electrocatalytic activity for oxygen evolution reaction. Appl. Surf. Sci. 2019, 493, 963–974. [Google Scholar] [CrossRef]
- Li, M. Technical route of new material for engine iron castings. Intern. Combust. Engine Powerpl. 2010, 124, 49–58. [Google Scholar]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, D.; Dioszegi, A. Effects of nodularity on thermal conductivity of cast iron. Int. J. Cast Met. Res. 2013, 20, 30–41. [Google Scholar] [CrossRef]
- Daniel, H.; Rikard, K. Influences of the graphite growth direction on the thermal conductivity of cast iron. Metall. Mater. Trans. 2007, 38, 268–275. [Google Scholar]
- Sitek, R.; Bolek, T.; Dobosz, R.; Plocinski, T.; Mizera, J. Microstructure and oxidation resistance of aluminide layer produced on Inconel 100 nickel alloy by CVD method. Surf. Coat. Technol. 2016, 304, 584–591. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Guo, Y.C.; Zhong, Y. Study on microstructure and tribological properties of AlSn films deposited via magnetron sputtering ion plating. Appl. Surf. Sci. 2019, 483, 123–132. [Google Scholar] [CrossRef]
- Benedetti, M.; Fontanari, V.; Lusuardi, D. Effect of graphite morphology on the fatigue and fracture resistance of ferritic ductile cast iron. Eng. Fract. Mech. 2019, 206, 427–441. [Google Scholar] [CrossRef]
- Ramírez-Ramírez, J.H.; Colas, R.; Garza-Montes-De-Oca, N.F. High Temperature Oxidation of a Work Roll Grade High-Chromium White Cast Iron. J. Iron Steel Res. Int. 2013, 20, 122–129. [Google Scholar] [CrossRef]
- Kim, S.; Cockcroft, S. Mechanical, wear and heat exposure properties of compacted graphite cast iron at elevated temperatures. J. Alloys Compd. 2009, 31, 253–257. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhou, J.R.; Su, W. Casting of cylinder block and cylinder head of engine. J. Netshape Form. Eng. 2010, 2, 79–86. [Google Scholar]
- Hecht, R.L.; Dinw, R.B.; Wang, H. The effect of graphite flake morphology on the thermal diffusivity of gray cast irons used for automotive brake discs. J. Mater. Sci. 2013, 34, 4775–4781. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Yang, Z.; Guo, D.; Tao, D.; Guo, Y.; Li, J.; Bai, Y. Research on the Oxidation Mechanism of Vermicular Graphite Cast Iron. Materials 2019, 12, 3130. https://doi.org/10.3390/ma12193130
Guo Q, Yang Z, Guo D, Tao D, Guo Y, Li J, Bai Y. Research on the Oxidation Mechanism of Vermicular Graphite Cast Iron. Materials. 2019; 12(19):3130. https://doi.org/10.3390/ma12193130
Chicago/Turabian StyleGuo, Qiaoqin, Zhong Yang, Ding Guo, Dong Tao, Yongchun Guo, Jianping Li, and Yaping Bai. 2019. "Research on the Oxidation Mechanism of Vermicular Graphite Cast Iron" Materials 12, no. 19: 3130. https://doi.org/10.3390/ma12193130
APA StyleGuo, Q., Yang, Z., Guo, D., Tao, D., Guo, Y., Li, J., & Bai, Y. (2019). Research on the Oxidation Mechanism of Vermicular Graphite Cast Iron. Materials, 12(19), 3130. https://doi.org/10.3390/ma12193130






