Abstract
Y2BaCuO5 often occurs as an accompanying phase of the well-known high-temperature superconductor YBa2Cu3O7 (also known as YBCO). Y2BaCuO5, easily identifiable due to its characteristic green coloration, is often referred to as ‘green phase’ or ‘Y-211’. In this contribution, Y2BaCuO5 phase was studied in detail with a focus on its thermal and thermodynamic properties. Energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), and scanning electron microscopy (SEM) were employed in the study of sample’s morphology and chemical composition. XRD data were further analyzed and lattice parameters refined by Rietveld analysis. Simultaneous thermal analysis was employed to study thermal stability. Particle size distribution was analyzed by laser diffraction. Finally, thermodynamic properties, namely heat capacity and relative enthalpy, were measured by drop calorimetry, differential scanning calorimetry (DSC), and physical properties measurement system (PPMS). Enthalpy of formation was assessed from ab-initio DFT calculations.
1. Introduction
Transition metal (TM) oxides constitute a large family of oxides, which possess a variety of interesting properties [1,2,3,4]. Cobalt mixed oxides with misfit structure are thermoelectric materials for the recovery of waste heat, and misfit cobaltites also show unique catalytic properties [5,6,7]. Mixed oxides containing lithium found a crucial application in accumulator industry [8]. Nano-sized oxides are widely used in catalysis, electrochemistry, and solar cells [9,10,11,12,13]. Among TM oxides, the high-temperature superconductors (HTS) revealing critical temperature close to the temperature of liquid nitrogen have been attracting permanent attention since their discovery in 1986 [14]. Although enhanced critical temperatures and intriguing superconducting behavior have been later reported in several systems, for example in layered ternary and quaternary arsenide systems [15,16], the HTS cuprates became the most wide-spread group of high-temperature superconductors, still being at the forefront of both theoretical and applied research in the field of superconductivity.
Their discovery in the Ba–La–Cu–O system [14] was immediately followed by an intensive search for novel HTS phases in congeners systems such as Y–Ba–Cu–O, Bi–Sr–Ca–Cu–O, Tl–Ba–Ca–Cu–O, and Hg–Ba–Ca–Cu–O [17,18,19,20,21,22,23,24,25]. Superconducting phases in these systems are usually derived from perovskites by inserting other structural motifs such as rock-salt type blocks, and thus forming layered structures. In general, perovskites and similar layered oxides exhibit a wide variety of interesting properties, such as high electrochemical stability, catalytic activity, low electric resistivity, and low thermal conductivity [26,27,28,29]. For the properties listed above, layered inorganic oxides are still studied extensively, even to this day. One of the most significant systems relevant to HTS is the quasi-ternary Y–Ba–Cu–O system [30], with YBa2Cu3O7-δ (YBCO) being the superconductive phase [31]. This phase is usually accompanied by Y2BaCuO5 [32,33,34,35], which is often formed during the solid-state reaction [36], as a result of surplus of yttrium. This can be caused by partial melting during the processing yielding a Ba- and Cu-rich melt, which subsequently crystallizes into Ba3Cu5O8 phase during cooling if the equilibrium is not achieved. As a result, the rest of the system is shifted towards Y-rich compositions, inducing a formation of Y2BaCuO5. However, this effect is not necessarily detrimental for the superconductivity of the material, since Y2BaCuO5, if properly nanostructured, can act as a source of pinning centers for vortices. The goal of this paper is to study Y2BaCuO5 phase that is present in single-domain YBCO superconductors in a form of pinning centers. The main focus is put on the thermal properties, which are decisive for tailoring the material properties by high temperature processing.
Similarly to YBa2Cu3O7-δ, copper is five-fold coordinated by oxygen atoms; however, the tetragonal pyramids are not interconnected, yielding an insulating ground state. Moreover, at temperatures below 30 K, an antiferromagnetic arrangement of spins ½ localized on Cu(+II) sites is established [37]. These physical characteristics have to be considered when interpreting the heat capacity at low temperatures.
In our contribution, we prepared pure polycrystalline phase Y2BaCuO5. Phase purity, morphology, and thermal and thermodynamic properties were studied in detail. The precise knowledge of thermodynamic properties of phases involved in the Y–Ba–Cu–O system is essential for modeling phase equilibria in YBCO-based superconducting materials (including systems with additional components) and tailoring their micro/nanostructural characteristics to enhance their performance.
2. Materials and Methods
We used the following chemicals for the synthesis of Y2BaCuO5: Barium carbonate (Sigma-Aldrich, Prague, Czech Republic; 99 + %), yttrium (III) oxide (Chemapol, Prague, Czech Republic; 99.99%), and copper (II) oxide (Sigma-Aldrich; 99.99%). Powders were homogenized in the ratio corresponding to Y2BaCuOx stoichiometry and repeatedly calcined in corundum crucible at 1123 K for 12 h, then at temperatures of 1138 K, 1153 K, and 1163 K for 24 h with a homogenization between each step. Finally, the sample was pressed into pellets and calcined for additional 24 h at 1173 K. The sample was slowly cooled in order to obtain a thermodynamically stable phase fully saturated with oxygen. The sample with the stoichiometry Y2BaCuO5 is termed Y-211 hereinafter.
X-ray powder diffraction (XRD) was measured at 298 K on Bruker D8 Discoverer powder diffractometer (Bruker, Karlsruhe, Germany) in a standard parafocusing Bragg–Brentano geometry using CuKα radiation (λ = 1.5418 Å, U = 40 kV, I = 40 mA). Rietveld refinement fit was performed using Topas software v. 5 (Bruker, Karlsruhe, Germany).
The microstructure was analyzed using scanning electron microscopy (SEM). The elemental composition was studied using energy-dispersive spectroscopy (EDS) analyzer X-MaxN with 20 mm2 silicon drift detector from Oxford instruments (Abingdon, UK) and software package Aztec Energy v. 3 (Asylum Research-Nanoanalysis, Wycombe, UK) using a Tescan Lyra dual beam microscope (Tescan, Brno, Czech Republic) with a field emission gun electron source.
The thermal stability Y2BaCuO5 was analyzed by simultaneous thermal analysis (STA) using Setaram Setsys Evolution (Setaram, Lyon, France). The heating rate during the measurement performed in dynamic air atmosphere (50 cm3 min−1) was 10 K min−1.
The particle size distribution was analyzed by the laser diffraction method; Malvern Panalytical Mastersizer 3000 device (Malvern, UK) with 4 mW He–Ne 632.8 nm Red light source and 10 mW LED 470 nm Blue light source was used. The range of measurement was set from 0.1 to 1000 μm. The measurement was done using dispersion (1 g/100 mL) of the material in isopropyl alcohol. Y-211 sample was measured 5 times (5 scans) and distribution was created as an average value.
The heat capacity in the low-temperature region was measured using the physical properties measurement system (PPMS) equipment, Evercool-II 9 T-type (Quantum Design, San Diego, CA, USA). The heat capacity was obtained by the relaxation method under high vacuum.
A Tian–Calvet-type calorimeter μDSC IIIa (Setaram, Lyon, France) was used for the measurement of heat capacities in the temperature range 262 to 358 K, employing NIST Standard reference material No. 720 as a reference material. Performance of the calorimeter is regularly checked by measurement of compounds with well-established Cpm data (naphthalene, benzophenone, benzoic acid). Based on these checking experiments, the combined expanded uncertainty of the heat capacity measurements is estimated to be Uc(Cpm) = 0.01 Cpm. More details can be found in paper [38]. Data obtained from this calorimeter are referred as DSC data.
Multi HTC 96 (Setaram, Lyon, France) high-temperature calorimeter (operated in a static air atmosphere) was used to measure enthalpy increments. The measurement was performed by dropping of the reference material (synthetic sapphire, NIST No. 720) and the sample in the sequence standard–sample–standard–sample–standard. The delays between two subsequent drops were 25 min in order to stabilize the heat flow. The estimated combined expanded uncertainty of the heat capacity measurements is estimated to be Uc(Cpm) = 0.03 Cpm.
Enthalpy of formation was assessed from ab-initio density functional theory (DFT)-based calculations. The electronic structure and total energies of Y2BaCuO5 and the constituent oxides, Y2O3, BaO, and CuO, were calculated by means MedeA-VASP [39] program using projector augmented plane waves basis set and generalized gradient approximation (GGA-PBE0 [40]) to exchange-correlation functional combined with additional local orbital specific coulomb potential U = 3 eV acting on Cu-3d states [41]. An antiferromagnetic ground state was considered for both CuO [42] and Y2BaCuO5 [37]. The basis set with a cut-off energy of 400 eV was applied. The k-point mesh was constructed inside the first Brillouin zone with k-point spacing smaller than 0.25 Å−1. A tetrahedron integration scheme was applied for electron density of states calculation.
3. Results and Discussion
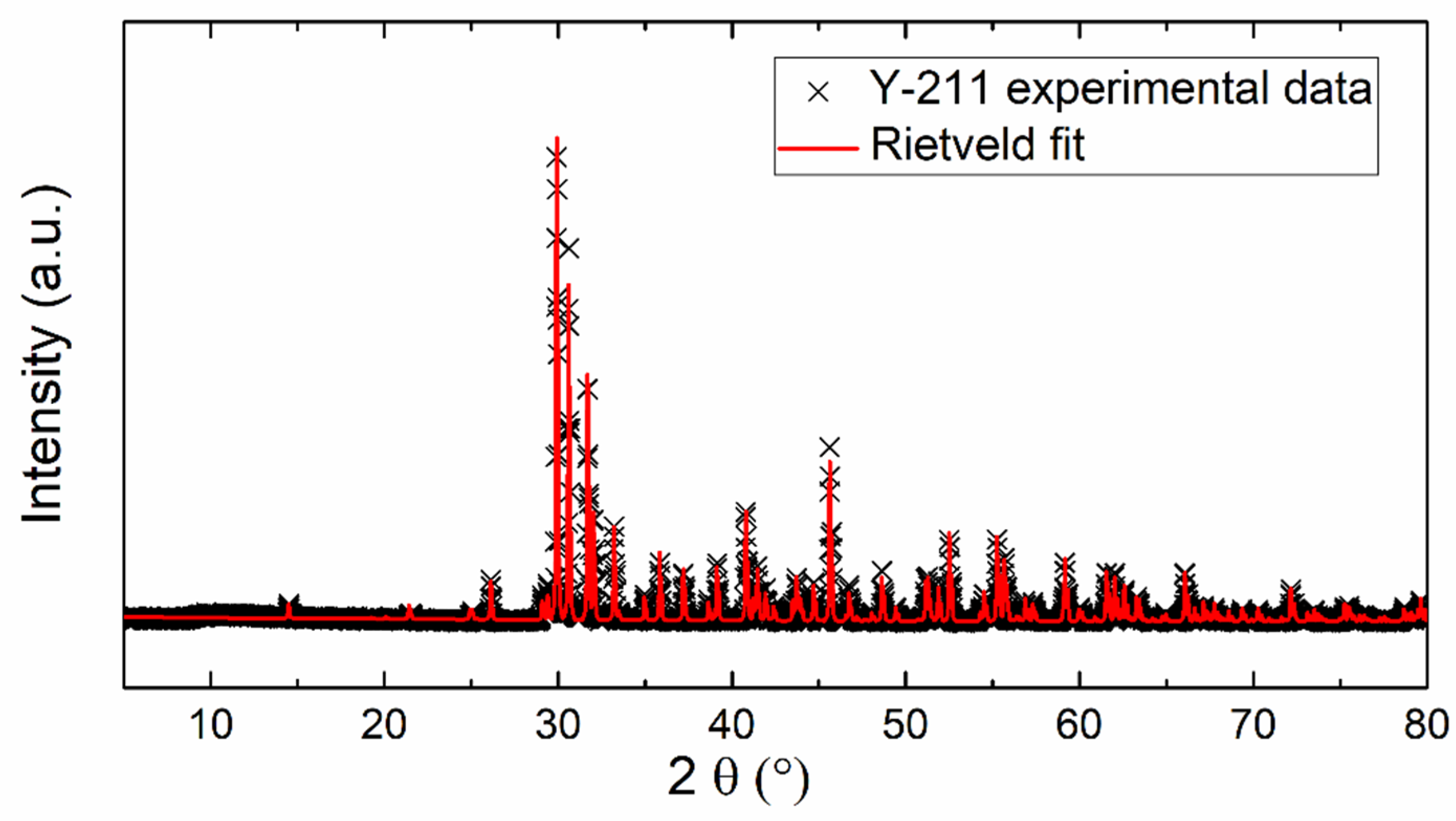
Figure 1 shows the XRD pattern of the prepared Y2BaCuO5 sample. No visible impurities were detected by X-ray diffraction, only the presence of the Y-211 phase (Y2BaCuO5). The Rietveld fit (based on the orthorhombic space group Pnma) was applied to refine the lattice parameters. The following lattice parameters were obtained: a = 12.134 Å, b = 5.6614 Å, and c = 7.1347 Å (α = β = γ = 90° being fixed). The obtained data are in good agreement with the already published results a = 12.1630 Å, b = 5.6490 Å, and c = 7.1230 Å (JCPDS 01-078-2214).
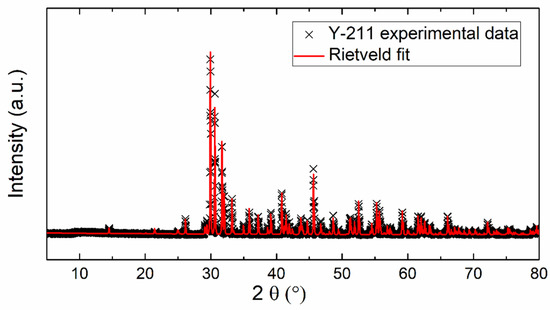
Figure 1.
XRD powder diffractogram and Rietveld fit of experimental data.
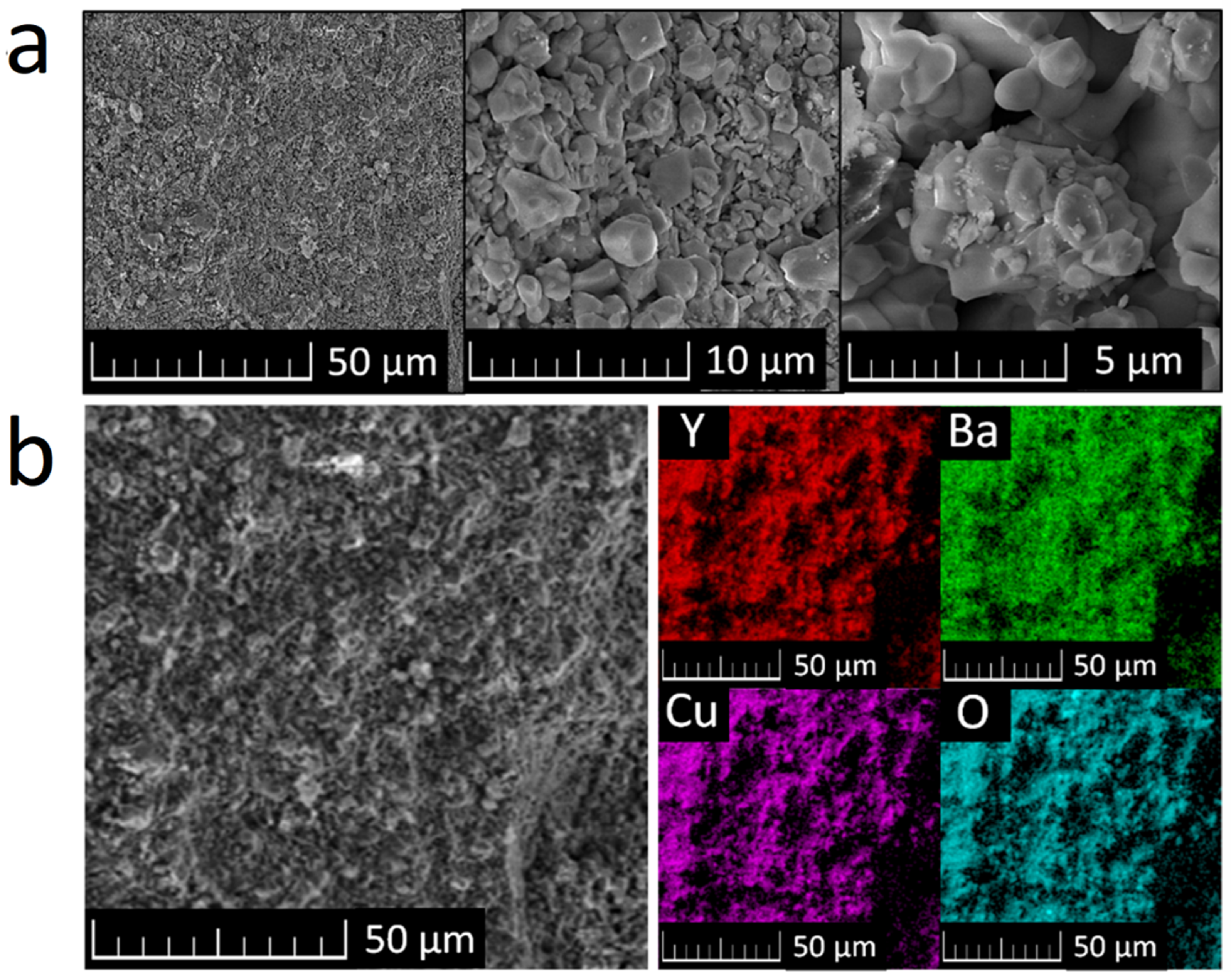
SEM micrographs of the sample show that the Y2BaCuO5 particle size lies mostly between 1 and 5 μm; the particle size remained very homogeneous across the sample, as can be seen from Figure 2a. This is in good agreement with the microstructures typically observed in polycrystalline cuprates. Elemental distribution maps were obtained by EDS and can be seen in Figure 2b. The sample appears to be homogenous and pure from a chemical point of view. Only Y, Ba, Cu, and O were detected. These results correspond with the results obtained by X-ray diffraction and confirm purity and phase composition of the prepared sample.
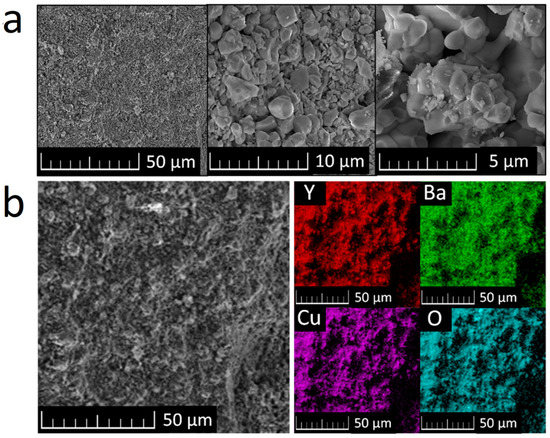
Figure 2.
Y2BaCuO5 SEM micrographs (a) and elemental distribution maps of obtained by EDS (b).
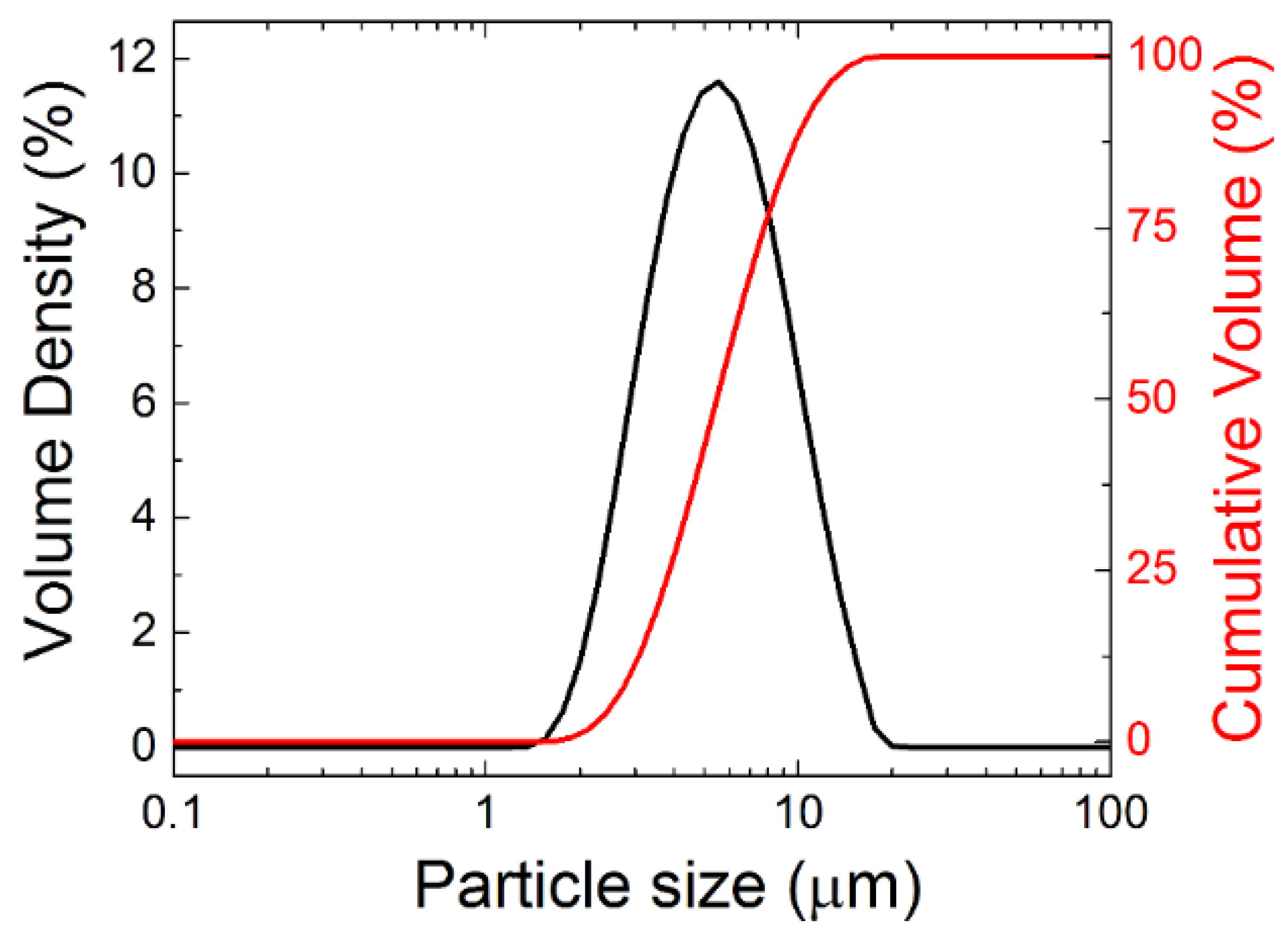
In order to confirm the sizes of grains obtained from SEM, the powder was also analyzed by laser diffraction. Particle size distribution is shown in Figure 3, revealing that the grains are mainly of the size between 1 and 10 μm, dx(50) = 5.2 μm, dx(10) = 2.8 μm and dx(90) = 10.3 μm. These data are in good agreement with SEM.
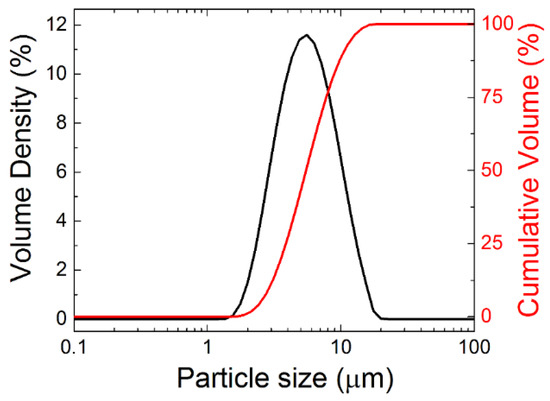
Figure 3.
Particle size distribution of Y2BaCuO5 powder using laser diffraction.
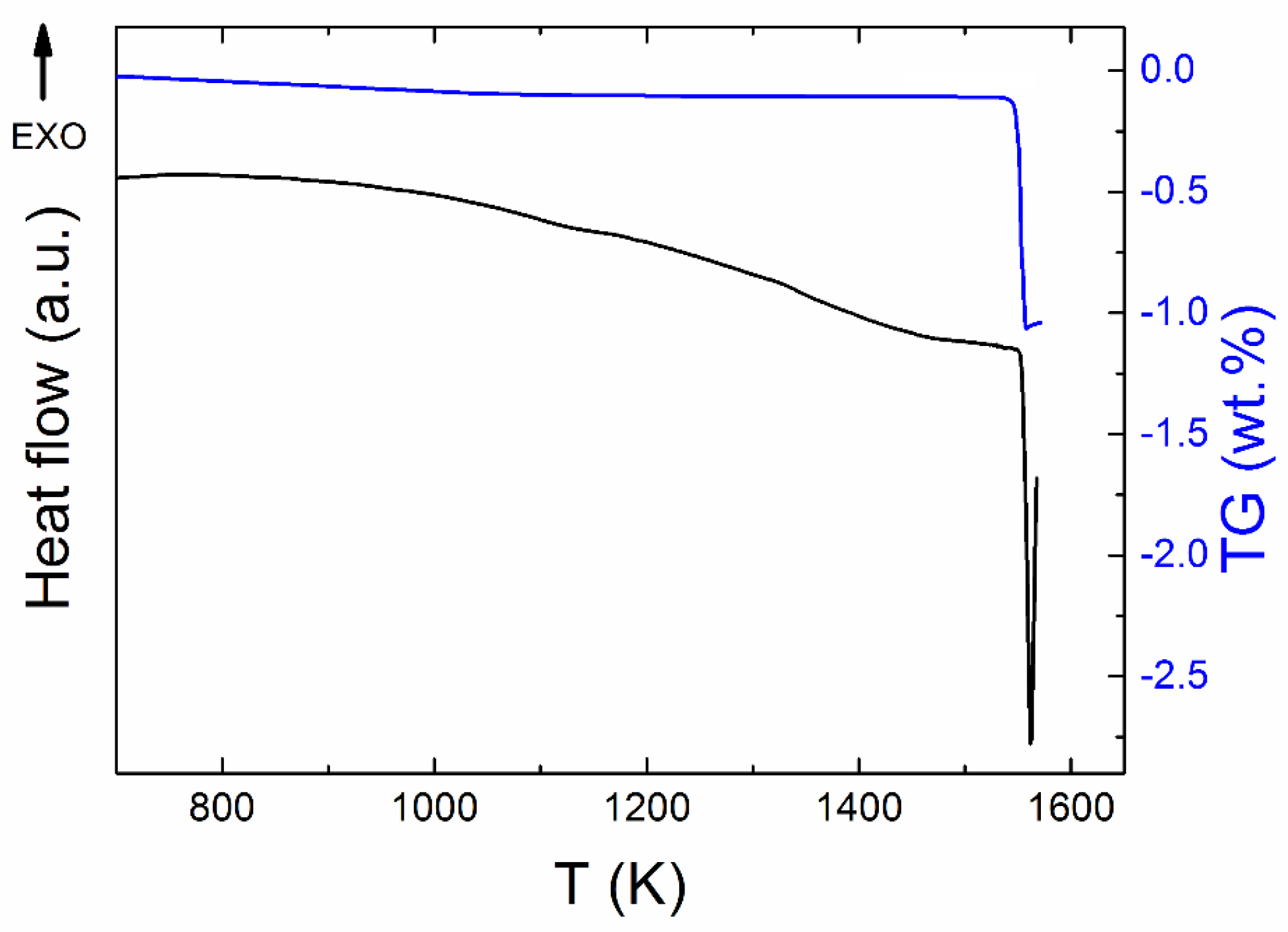
Simultaneous thermal analysis (STA) was employed to study the thermal behavior of Y2BaCuO5 (Figure 4). The phase Y-211 decomposed in air atmosphere at 1551 K. Throughout the measurement, the mass was almost constant, i.e., the phase was stable up to temperatures of ~1550 K and we did not detect any release of oxygen, suggesting the presence of the stoichiometric phase. The weight decrease related to the original mass of Y2BaCuO5 was approximately 1 wt % suggesting formation of Y2O3 (s), BaO (s), and Cu–O (l).
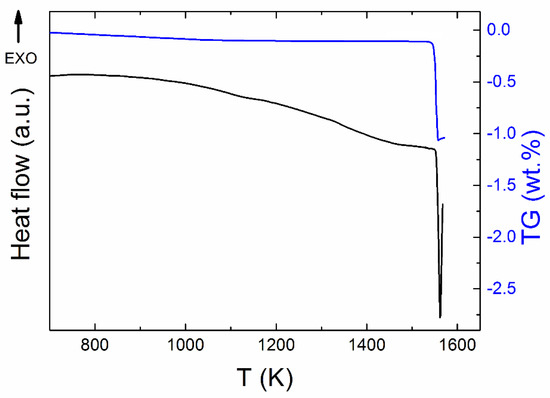
Figure 4.
STA measurement of Y2BaCuO5 in dynamic air atmosphere.
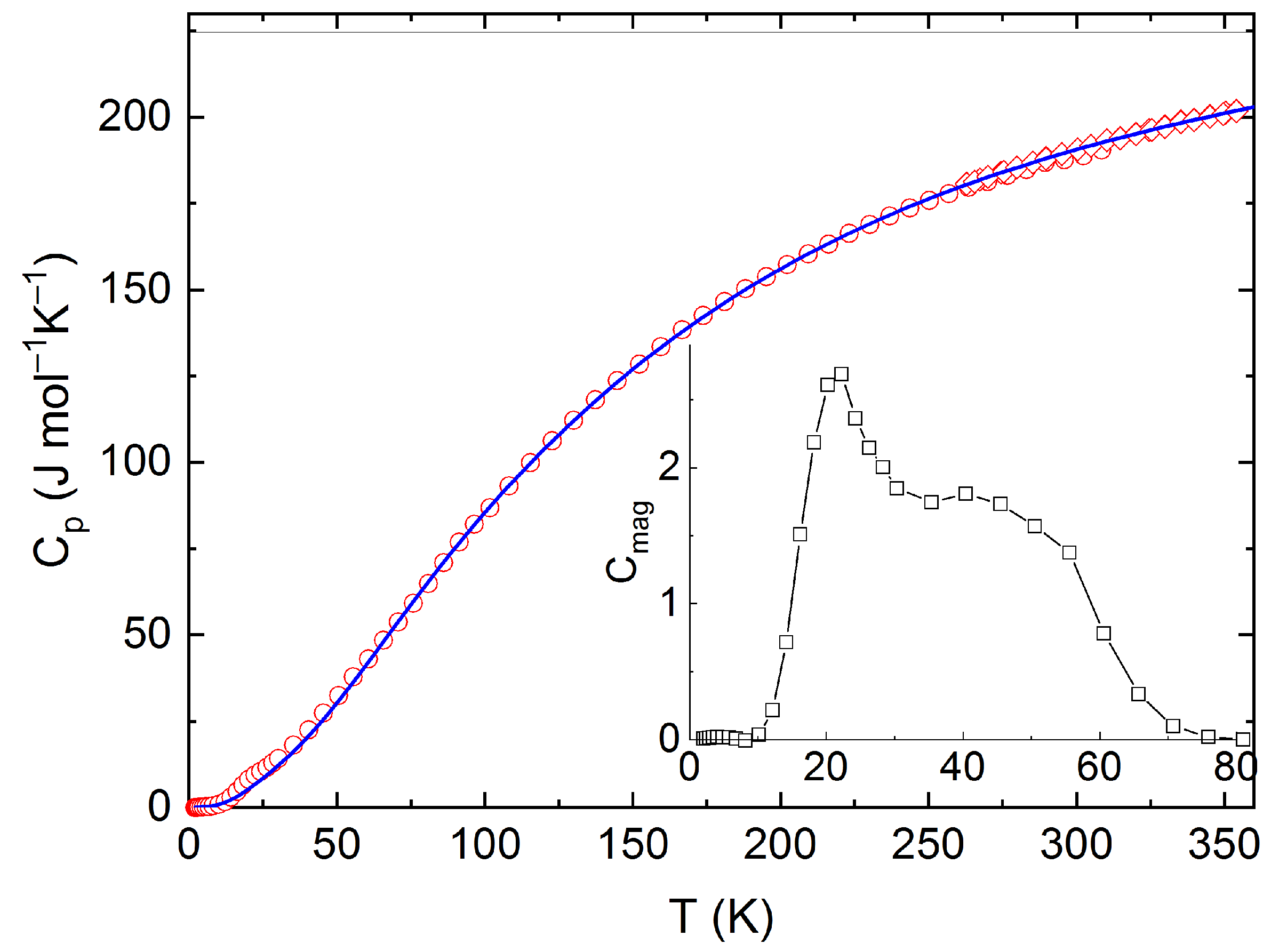
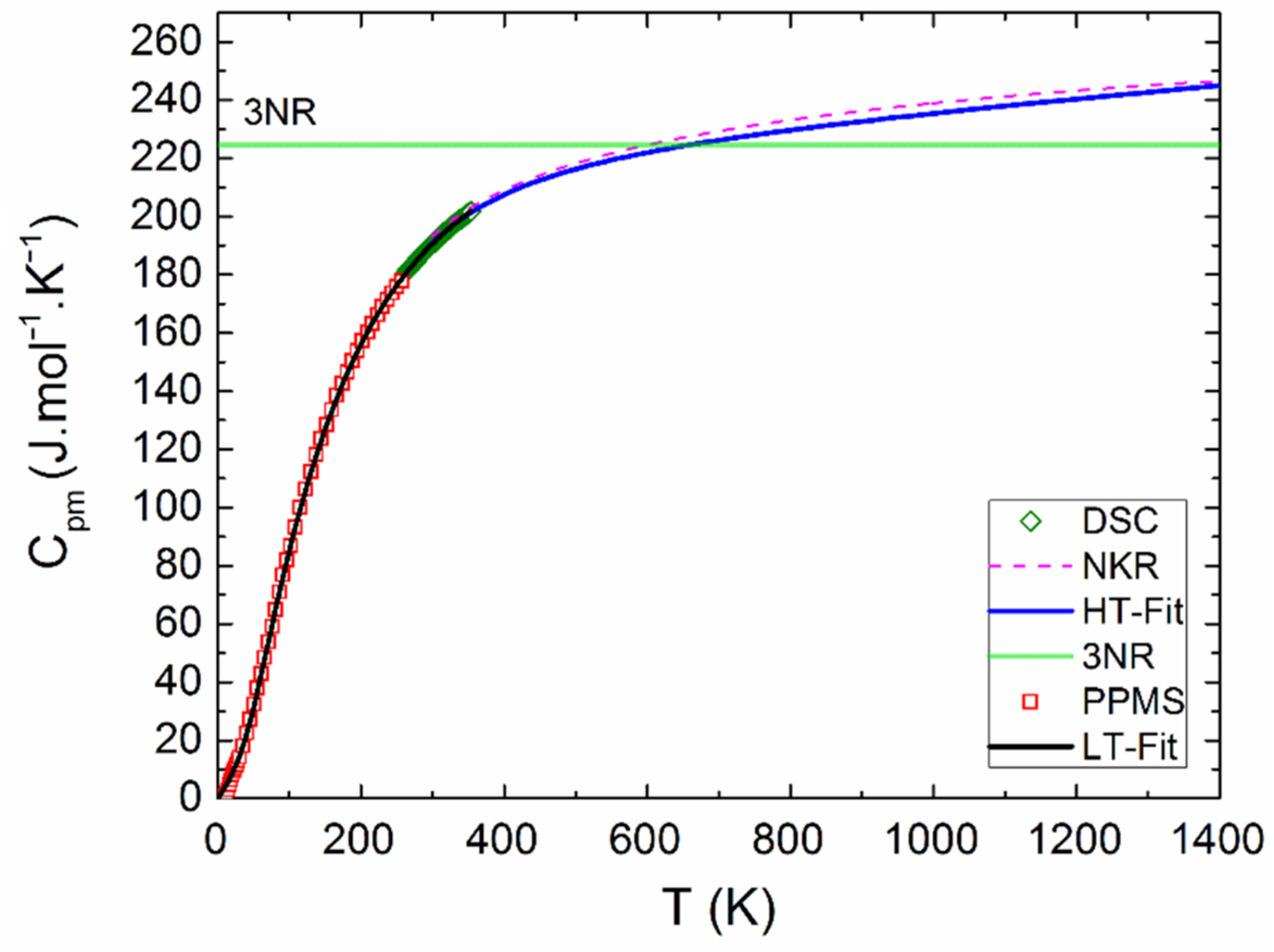
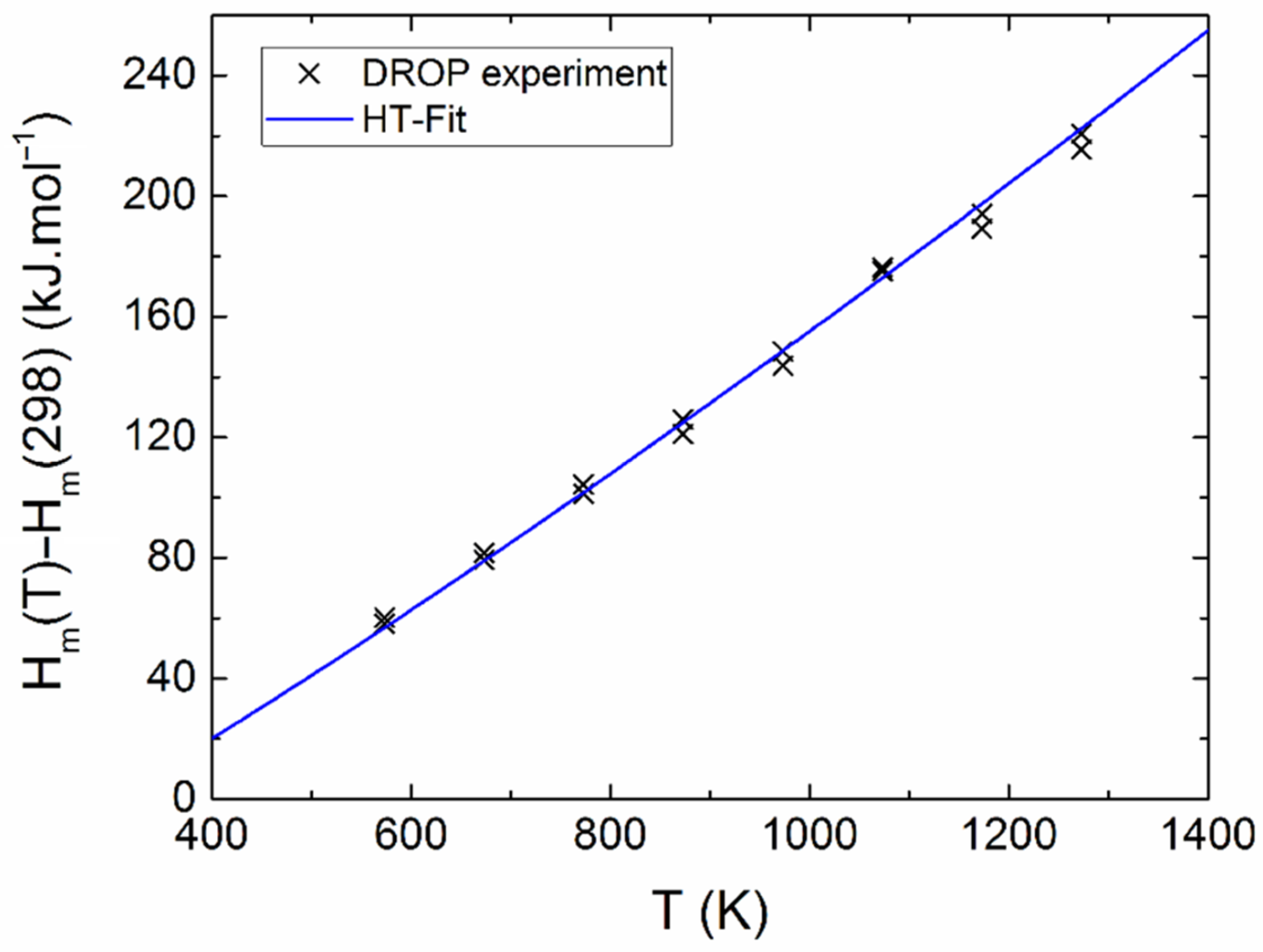
The measured calorimetric data used for further analysis involved 37 Cpm values from relaxation time method (30–257 K), 40 Cpm values from DSC (263–354 K) (see Figure 5 and Figure 6), and 16 values of the enthalpy increments from the drop measurements from the range 573–1273 K (see Figure 7).
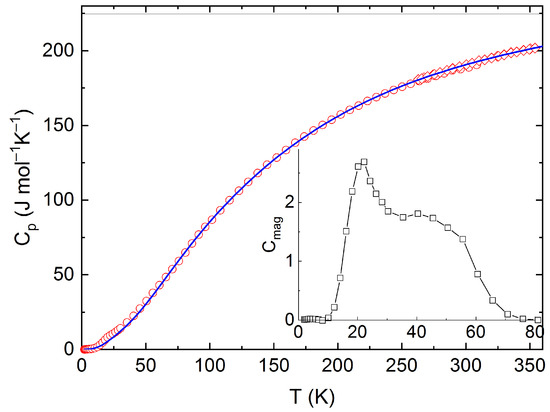
Figure 5.
Low temperature heat capacity of Y2BaCuO5 obtained from PPMS and DSC, including the Debye–Einstein fit and the excess magnetic term in the inset.
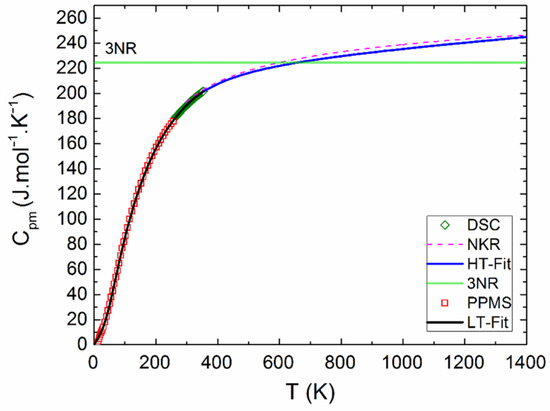
Figure 6.
Heat capacity of Y2BaCuO5 obtained from PPMS and HT-fit.
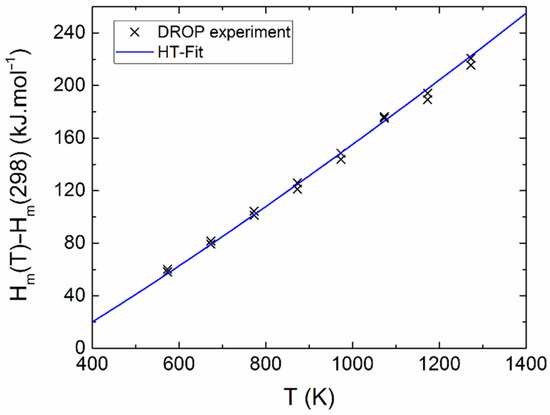
Figure 7.
Relative enthalpy of Y2BaCuO5 obtained from drop experiments.
For further analysis of low temperature heat capacity data, two sets of the Cpm data (relaxation time + DSC) were considered. Since the studied material is a magnetic insulator, only the lattice part of heat capacity is relevant for temperatures well above the Néel temperature, T = 16.2 K. For this reason, only the data above 30 K were considered for the analysis of phonon heat capacity, which was performed using the combination of the Debye and Einstein models according to the following Equation (1):
Here R is the gas constant, θD and θEi are the Debye and Einstein characteristic temperatures, and xD = θD/T, xEi = θEi/T, and wi refer to a degeneracy of the corresponding Einstein mode.
It is a well-known fact that the phonon spectrum of a mixed oxide contains 3n–3 optical branches and three acoustic branches (n is number of atoms per formula unit). In Y2BaCuO5, there are 9 atoms per formula unit representing 24 optical branches which were grouped into three 8-fold degenerate Einstein modes.
The Debye–Einstein model is based on a harmonic crystal approximation describing the heat capacity at constant volume. As can be seen Figure 6, the experimentally obtained heat capacity exceeded the Dulong–Petit limit valid for CV at ~670 K. It was previously described [43] that a multiplication factor 1/(1−αT) can be introduced to any vibration mode (in order to take into account the anharmonic effects) and to describe the Cp–CV difference using a semi-empirical approach. Hence, in our analysis, we used such an anharmonicity parameter that was combined only with the Debye mode in Equation (1). The analysis of the phonon heat capacity is shown in Table 1. The fitted curve based on PPMS and DSC data corresponding to LT-fit in Figure 5 describes the low temperature data satisfactorily above 60 K. Below this temperature, a substantial excess term due to magnetic ordering is clearly visible (see the inset in Figure 5).

Table 1.
Evaluated parameters of Debye–Einstein model based on PPMS and DSC data.
The absolute entropy at the reference temperature (T = 298.15 K) was obtained by integrating the experimental data of the heat capacity divided by the thermodynamic temperature from 2 K up to ambient temperature as Sm(298.15) = 209.47 J mol−1 K−1. This experimental value can be decomposed into a lattice vibrations contribution, Slat(298.15) = 206.9 J mol−1 K−1, and an excess entropy due to magnetic ordering disruption Slat(298.15) = 2.8 J mol−1 K−1, which is about two times lower than the theoretical value R ln2 corresponding to spin ½ of Cu(+II) and also lower than the experimental value reported by Knafo et al. [43] who, however, derived the phonon contribution by scaling the Cp data of YBa2Cu3O7.
Above room temperature, dependence of the heat capacity was also determined. The linear least-squares method was used for treatment of the heat capacity data from DSC and the enthalpy increment data from drop calorimetry. The temperature dependence of Cpm was obtained in Equation (2):
Let us note that the related temperature function of enthalpy, ΔHm(T) = Hm(T) − Hm(T0), is given by the following Equation (3):
The sum of squares was applied on both functions, Equations (2) and (3), and both sets of data were simultaneously minimized. Different weights wi were assigned to the individual points (calculated as wi = 1/δi, where δi is the absolute error bar). The obtained temperature dependence of heat capacity can be expressed as (valid between 298 and 1400 K) Equation (4):
We compared obtained data with the curve calculated using the Neumann–Kopp rule (NKR), where the heat capacity of Y2BaCuO5 was evaluated by the following Equation (5):
As can be seen from Figure 6, our data are in good agreement with the calculated NKR curve, suggesting that the lattice dynamics—including the anharmonic effects—are comparable in Y2BaCuO5 and the constituent binary oxides.
We took advantage of this similarity in chemical bonding and used the binary oxides as a reference for evaluating the enthalpy of formation from GGA+U ab-initio calculations. Hence, the ground state enthalpy of formation from oxides, ΔoxH = −32.2 kJ mol−1, is the primary value derived from DFT while the enthalpy of formation from elements, ΔfH = −2640.9 kJ mol−1, was obtained from ΔoxH and the tabulated ΔfH values of the binary oxides. The enthalpy of formation is underestimated compared to dissolution calorimetry results ΔfH = −2656 kJ mol−1 [44] and −2660 kJ mol−1 [45]. A part of this discrepancy can by accounted for by the negative integral of ΔoxCp used to recalculate the ab-initio value from 0 to 298 K, for which the experimental data are reported.
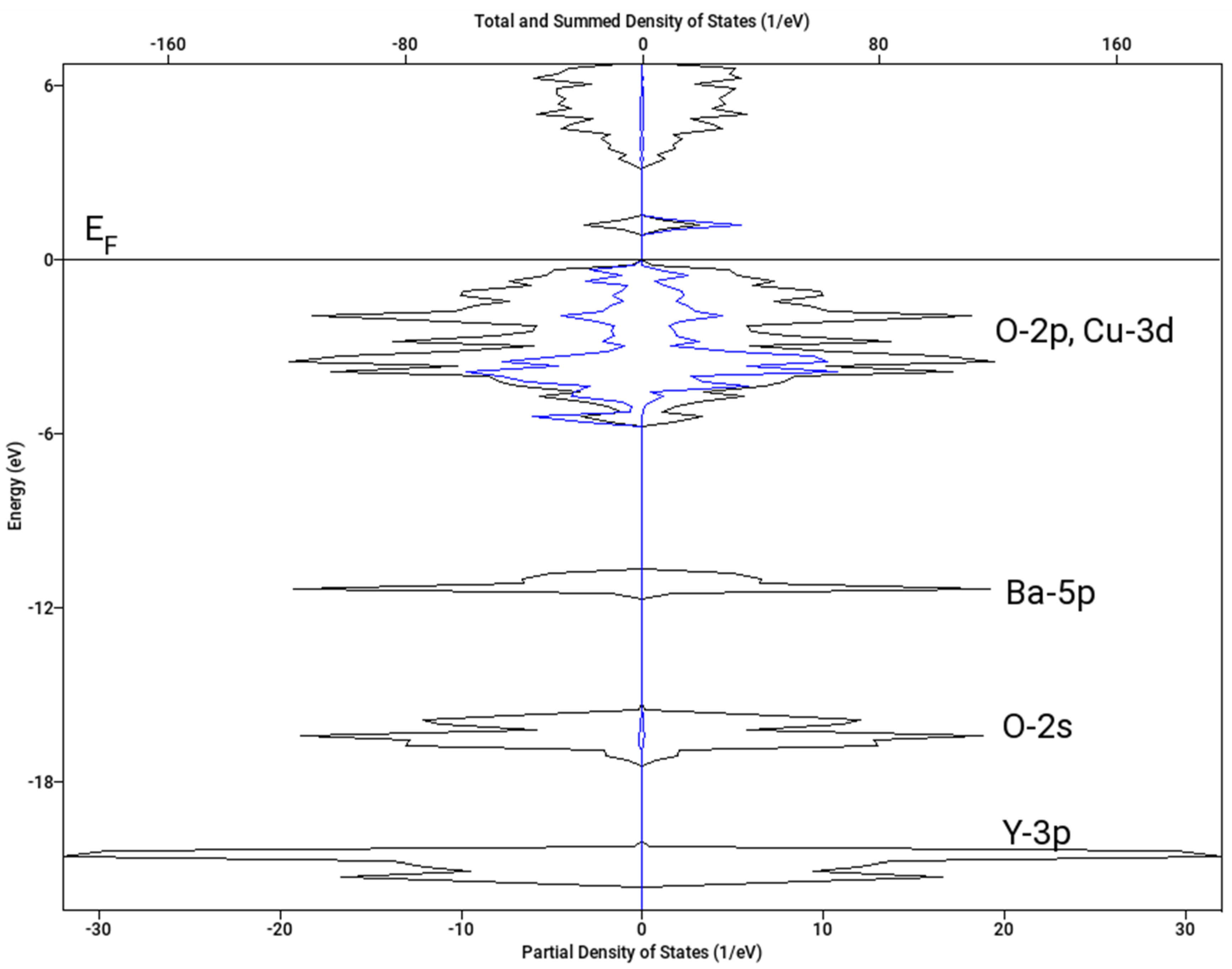
Let us note that the magnetic structure has a substantial effect on the resulting value of ΔoxH. Here, we present the results for A-type spin arrangement (two spins +½ and two spins −½ within the chemical unit cell) and no magnetic superstructure. The resulting density of states for both spin channels is shown in Figure 8. The spin polarization on copper sites clearly visible on Cu(1)-3d projection in Figure 8 amounts to 0.59 Bohr magnetons, which is in nice agreement with the neutron diffraction data (0.55 μB) [37]. If we considered the F type arrangement (all four spins aligned parallel) in the chemical unit cell and a magnetic superstructure with the wave vector k = [0, ½, ½], which has been reported as an alternative ordering, the calculation converged to a non-spin polarized state and ΔoxH = −0.5 kJ mol−1. Interestingly, a similar result (zero spin and low stabilization) was obtained for A-type ordering and k = [0, ½, ½] superstructure. Hence, the magnetic superstructure reported in [37] was not confirmed.
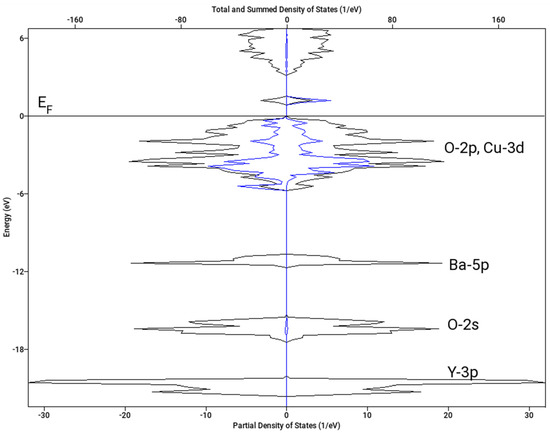
Figure 8.
Calculated density of states (DOS) of Y2BaCuO5 (A-type antiferromagnetic arrangement). Spin-down projections is shown as negative. Total DOS—black line; Cu-3d projection for Cu(1) atom with down majority spin—blue line. Fermi level (EF) is set to 0 eV.
4. Conclusions
In our contribution, we prepared a polycrystalline cuprate Y2BaCuO5 by conventional solid-state reaction. The sample was thoroughly analyzed in order to ensure its purity. Morphology and thermal properties were further studied in detail. Using X-ray diffraction, we confirmed the presence of pure Y2BaCuO5 without any detectable impurities. Very high thermal stability (up to ~1550 K) of Y2BaCuO5 (compared to phase YBa2Cu3O7) is important for its inert behavior during the manufacturing of YBCO bulk ceramics. Moreover, the obtained calorimetry data are important for thermodynamic modeling of high temperature material processing, which can underpin future development of high-temperature superconductors and their possible applications in superconducting bearings, transport, or superconducting transmission lines.
Author Contributions
Conceptualization, O.J. and D.S.; investigation, F.A., M.L., A.J., K.R. and T.H.; writing—original draft preparation, F.A.; writing—review and editing, O.J. and D.S.
Funding
This research was funded by the Czech Science Foundation, grant number 17-13161S. This research was also funded by TACR, program THETA, grant number TK01030200.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Xiong, D.; Li, D.; Lawes, S.; Sun, X. Recent developments and understanding of novel mixed transition-metal oxides as anodes in lithium ion batteries. Adv. Energy Mater. 2016, 6, 1502175. [Google Scholar] [CrossRef]
- Jankovský, O.; Sedmidubský, D.; Šimek, P.; Sofer, Z.; Ulbrich, P.; Bartůněk, V. Synthesis of MnO, Mn2O3 and Mn3O4 nanocrystal clusters by thermal decomposition of manganese glycerolate. Ceram. Int. 2015, 41, 595–601. [Google Scholar] [CrossRef]
- Bartůněk, V.; Huber, Š.; Sedmidubský, D.; Sofer, Z.; Šimek, P.; Jankovský, O. CoO and Co3O4 nanoparticles with a tunable particle size. Ceram. Int. 2014, 40, 12591–12595. [Google Scholar] [CrossRef]
- Kang, M.-G.; Cho, K.-H.; Kim, J.-S.; Nahm, S.; Yoon, S.-J.; Kang, C.-Y. Post-calcination, a novel method to synthesize cobalt oxide-based thermoelectric materials. Acta Mater. 2014, 73, 251–258. [Google Scholar] [CrossRef]
- Prasad, K.R.; Miura, N. Electrochemically synthesized MnO2-based mixed oxides for high performance redox supercapacitors. Electrochem. Commun. 2004, 6, 1004–1008. [Google Scholar] [CrossRef]
- Rao, K.; Smakula, A. Dielectric properties of cobalt oxide, nickel oxide, and their mixed crystals. J. Appl. Phys. 1965, 36, 2031–2038. [Google Scholar] [CrossRef]
- Van Schalkwijk, W.; Scrosati, B. Advances in lithium ion batteries introduction. In Advances in Lithium-Ion Batteries; Kluwer Academic/Plenum Publisher: London, UK, 2002; pp. 1–5. [Google Scholar]
- Andersen, N.I.; Serov, A.; Atanassov, P. Metal oxides/CNT nano-composite catalysts for oxygen reduction/oxygen evolution in alkaline media. Appl. Catal. B Environ. 2015, 163, 623–627. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Rao, M.M.; Venugopal, N.; Kim, K.-B. Hydrothermal synthesis of SnO2–V2O5 mixed oxide and electrochemical screening of carbon nano-tubes (CNT), V2O5, V2O5–CNT, and SnO2–V2O5–CNT electrodes for supercapacitor applications. J. Power Sour. 2007, 166, 578–583. [Google Scholar] [CrossRef]
- Ito, S.; Makari, Y.; Kitamura, T.; Wada, Y.; Yanagida, S. Fabrication and characterization of mesoporous SnO2/ZnO-composite electrodes for efficient dye solar cells. J. Mater. Chem. 2004, 14, 385–390. [Google Scholar] [CrossRef]
- Rusevova, K.; Kopinke, F.-D.; Georgi, A. Nano-sized magnetic iron oxides as catalysts for heterogeneous Fenton-like reactions—Influence of Fe (II)/Fe (III) ratio on catalytic performance. J. Hazard. Mater. 2012, 241, 433–440. [Google Scholar] [CrossRef]
- Leitner, J.; Bartůněk, V.; Sedmidubský, D.; Jankovský, O. Thermodynamic properties of nanostructured ZnO. Appl. Mater. Today 2018, 10, 1–11. [Google Scholar] [CrossRef]
- Bednorz, J.G.; Müller, K.A. Possible highT c superconductivity in the Ba−La−Cu−O system. Z. Phys. B Condens. Matter 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Rotter, M.; Tegel, M.; Johrendt, D. Superconductivity at 38 K in the iron arsenide (Ba1−xKx)Fe2As2. Phys. Rev. Lett. 2008, 101, 107006. [Google Scholar] [CrossRef]
- Tallapally, V.; Damma, D.; Darmakkolla, S.R. Facile synthesis of size-tunable tin arsenide nanocrystals. Chem. Commun. 2019, 55, 1560–1563. [Google Scholar] [CrossRef]
- Sleight, A.W.; Gillson, J.; Bierstedt, P. High-temperature superconductivity in the BaPb1−xBixO3 system. Solid State Commun. 1993, 88, 841–842. [Google Scholar] [CrossRef]
- Paglione, J.; Greene, R.L. High-temperature superconductivity in iron-based materials. Nat. Phys. 2010, 6, 645. [Google Scholar] [CrossRef]
- Jankovský, O.; Antončík, F.; Hlásek, T.; Plecháček, V.; Sedmidubský, D.; Huber, Š.; Lojka, M.; Bartůněk, V. Synthesis and properties of YBa2Cu3O7-δ–Y2Ba4CuWO10. 8 superconducting composites. J. Eur. Ceram. Soc. 2018, 38, 2541–2546. [Google Scholar] [CrossRef]
- Drozdov, A.; Eremets, M.; Troyan, I.; Ksenofontov, V.; Shylin, S. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature 2015, 525, 73. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Lv, Y.; Gao, W.; Yang, L.; Yu, R.; Li, F.; Jin, C. The superconductivity at 18 K in LiFeAs system. Solid State Commun. 2008, 148, 538–540. [Google Scholar] [CrossRef]
- Lim, C.S.; Wang, L.; Chua, C.K.; Sofer, Z.; Jankovský, O.; Pumera, M. High temperature superconducting materials as bi-functional catalysts for hydrogen evolution and oxygen reduction. J. Mater. Chem. A 2015, 3, 8346–8352. [Google Scholar] [CrossRef]
- Goldschmidt, D.; Reisner, G.; Direktovitch, Y.; Knizhnik, A.; Gartstein, E.; Kimmel, G.; Eckstein, Y. Tetragonal superconducting family (CaxLa1−x)(Ba1.75−xLa0.25+x)Cu3Oy: The effect of cosubstitution on the transition temperature. Phys. Rev. B 1993, 48, 532. [Google Scholar] [CrossRef]
- Mirmelshtein, A.; Podlesnyak, A.; Bobrovskii, V.; Davydov, S.; Karkin, A.; Kozhevnikov, V.; Goshchitskii, B.; Cheshnitskii, S. Electrical crystal field effects in high-temperature superconductor HoBa2Cu3O7. Phys. C Supercond. 1988, 153, 176–177. [Google Scholar] [CrossRef]
- Maple, M.; Dalichaouch, Y.; Ferreira, J.; Hake, R.; Lee, B.; Neumeier, J.; Torikachvili, M.; Yang, K.; Zhou, H.; Guertin, R. RBa2Cu3O7–δ (R = Rare earth) High-Tc magnetic superconductors. In Proceedings of the Yamada Conference XVIII on Superconductivity in Highly Correlated Fermion Systems, Sendai, Japan, 31 August–3 September 1987; Elsevier: Amsterdam, The Netherlands, 1987; pp. 155–162. [Google Scholar]
- Jankovský, O.; Sedmidubský, D.; Rubešová, K.; Sofer, Z.; Leitner, J.; Ružička, K.; Svoboda, P. Structure, non-stoichiometry and thermodynamic properties of Bi1. 85Sr2Co1.85O7. 7−δ ceramics. Thermochim. Acta 2014, 582, 40–45. [Google Scholar] [CrossRef]
- Jankovský, O.; Sedmidubský, D.; Sofer, Z.; Šimek, P.; Hejtmánek, J. Thermodynamic behavior of Ca3Co3. 93O9+δ ceramics. Ceram. Silik. 2012, 56, 139–144. [Google Scholar]
- Jankovský, O.; Sedmidubský, D.; Sofer, Z. Phase diagram of the pseudobinary system Bi–Co–O. J. Eur. Ceram. Soc. 2013, 33, 2699–2704. [Google Scholar] [CrossRef]
- Kamihara, Y.; Hiramatsu, H.; Hirano, M.; Kawamura, R.; Yanagi, H.; Kamiya, T.; Hosono, H. Iron-based layered superconductor: LaOFeP. J. Am. Chem. Soc. 2006, 128, 10012–10013. [Google Scholar] [CrossRef]
- Wu, M.-K.; Ashburn, J.R.; Torng, C.; Hor, P.H.; Meng, R.L.; Gao, L.; Huang, Z.J.; Wang, Y.; Chu, A. Superconductivity at 93 K in a new mixed-phase Y-Ba-Cu-O compound system at ambient pressure. Phys. Rev. Lett. 1987, 58, 908. [Google Scholar] [CrossRef]
- Schneemeyer, L.; Waszczak, J.; Siegrist, T.; Van Dover, R.; Rupp, L.; Batlogg, B.; Cava, R.J.; Murphy, D. Superconductivity in YBa2Cu3O7 single crystals. Nature 1987, 328, 601. [Google Scholar] [CrossRef]
- Watkins, S.; Fronczek, F.; Wheelock, K.; Goodrich, R.; Hamilton, W.; Johnson, W. Structure of Y2BaCuO5. Acta Crystallogr. Sect. C 1988, 44, 3–6. [Google Scholar]
- Lee, D.; Selvamanickam, V.; Salama, K. Influences of Y2BaCuO5 particle size and content on the transport critical current density of YBa2Cu3Ox superconductor. Phys. C Supercond. 1992, 202, 83–96. [Google Scholar] [CrossRef]
- McGinn, P.; Chen, W.; Zhu, N.; Tan, L.; Varanasi, C.; Sengupta, S. Microstructure and critical current density of zone melt textured YBa2Cu3O6+x/Y2BaCuO5 with BaSnO3 additions. Appl. Phys. Lett. 1991, 59, 120–122. [Google Scholar] [CrossRef]
- Jin, S.; Tiefel, T.; Kammlott, G. Effect of Y2BaCuO5 inclusions on flux pinning in YBa2Cu3O7−δ. Appl. Phys. Lett. 1991, 59, 540–542. [Google Scholar] [CrossRef]
- Hlásek, T.; Shi, Y.; Durrell, J.H.; Dennis, A.R.; Namburi, D.K.; Plecháček, V.; Rubešová, K.; Cardwell, D.A.; Jankovský, O. Cost-effective isothermal top-seeded melt-growth of single-domain YBCO superconducting ceramics. Solid State Sci. 2019, 88, 74–80. [Google Scholar] [CrossRef]
- Golosovsky, I.; Böni, P.; Fischer, P. Magnetic structure of Y2BaCuO5. Solid State Commun. 1993, 87, 1035–1037. [Google Scholar] [CrossRef]
- Štejfa, V.; Fulem, M.; Růžička, K.; Červinka, C. Thermodynamic study of selected monoterpenes III. J. Chem. Thermodyn. 2014, 79, 280–289. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Dudarev, S.; Botton, G.; Savrasov, S.; Humphreys, C.; Sutton, A. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA + U study. Phys. Rev. B 1998, 57, 1505. [Google Scholar] [CrossRef]
- Forsyth, J.; Brown, P.; Wanklyn, B. Magnetism in cupric oxide. J. Phys. C 1988, 21, 2917. [Google Scholar] [CrossRef]
- Martin, C. Simple treatment of anharmonic effects on the specific heat. J. Phys. Condens. Matter 1991, 3, 5967. [Google Scholar] [CrossRef]
- Hengzhong, Z.; Zheng, F.; Pingmin, Z.; Xinmin, C. Enthalpies of formation of some phases present in the Y−Ba−Cu−O system by solution calorimetry. J. Solut. Chem. 1995, 24, 565–578. [Google Scholar] [CrossRef]
- Zhou, Z.; Navrotsky, A. Thermochemistry of the Y2O3–BaO–Cu–O system. J. Mater. Res. 1992, 7, 2920–2935. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).