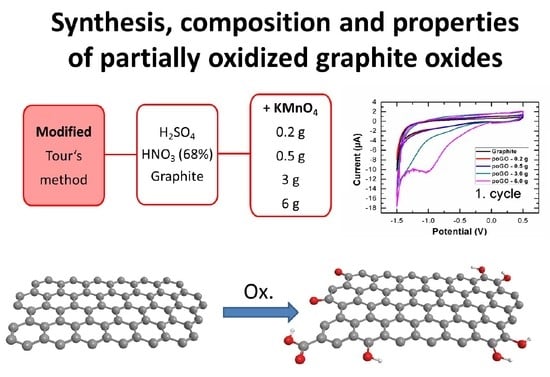
Synthesis, Composition, and Properties of Partially Oxidized Graphite Oxides
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Magnuson, C.W.; Venugopal, A.; An, J.H.; Suk, J.W.; Han, B.Y.; Borysiak, M.; Cai, W.W.; Velamakanni, A.; Zhu, Y.W.; et al. Graphene films with large domain size by a two-step chemical vapor deposition process. Nano Lett. 2010, 10, 4328–4334. [Google Scholar] [CrossRef] [PubMed]
- Sturala, J.; Luxa, J.; Pumera, M.; Sofer, Z. Chemistry of graphene derivatives: Synthesis, applications, and perspectives. Chem. Eur. J. 2018, 24, 5992–6006. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chopra, N. Progress in large-scale production of graphene. Part 1: Chemical methods. JOM 2015, 67, 34–43. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Jankovsky, O.; Kuckova, S.H.; Pumera, M.; Simek, P.; Sedmidubsky, D.; Sofer, Z. Carbon fragments are ripped off from graphite oxide sheets during their thermal reduction. N. J. Chem. 2014, 38, 5700–5705. [Google Scholar] [CrossRef]
- Li, Y.; Chopra, N. Progress in large-scale production of graphene. Part 2: Vapor methods. JOM 2015, 67, 44–52. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Zhong, Z.H. Wafer scale homogeneous bilayer graphene films by chemical vapor deposition. Nano Lett. 2010, 10, 4702–4707. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.T.; Ho, J.; Nezich, D.; Son, H.B.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Dimiev, A.; Kosynkin, D.V.; Sinitskii, A.; Slesarev, A.; Sun, Z.Z.; Tour, J.M. Layer-by-layer removal of graphene for device patterning. Science 2011, 331, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Ruess, G.; Vogt, F. Hochstlamellarer kohlenstoff aus graphitoxyhydroxyd-uber den ort der aktiven eigenschaften am kohlenstoffkristall. Mon. Chem. 1948, 78, 222–242. [Google Scholar] [CrossRef]
- Clauss, A.; Plass, R.; Boehm, H.P.; Hofmann, U. Untersuchungen zur struktur des graphitoxyds. Z. Anorg. Allg. Chem. 1957, 291, 205–220. [Google Scholar] [CrossRef]
- Mermoux, M.; Chabre, Y.; Rousseau, A. Ftir and c-13 nmr-study of graphite oxide. Carbon 1991, 29, 469–474. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.Y.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Nakajima, T.; Mabuchi, A.; Hagiwara, R. A new structure model of graphite oxide. Carbon 1988, 26, 357–361. [Google Scholar] [CrossRef]
- Szabo, T.; Berkesi, O.; Forgo, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dekany, I. Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Jankovsky, O.; Marvan, P.; Novacek, M.; Luxa, J.; Mazanek, V.; Klimova, K.; Sedmidubsky, D.; Sofer, Z. Synthesis procedure and type of graphite oxide strongly influence resulting graphene properties. Appl. Mater. Today 2016, 4, 45–53. [Google Scholar] [CrossRef]
- Bannov, A.G.; Manakhov, A.; Shibaev, A.A.; Ukhina, A.V.; Polčák, J.; Maksimovskii, E.A. Synthesis dynamics of graphite oxide. Thermochim. Acta 2018, 663, 165–175. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Sofer, Z.; Simek, P.; Jankovsky, O.; Sedmidubsky, D.; Beran, P.; Pumera, M. Neutron diffraction as a precise and reliable method for obtaining structural properties of bulk quantities of graphene. Nanoscale 2014, 6, 13082–13089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimiev, A.; Kosynkin, D.V.; Alemany, L.B.; Chaguine, P.; Tour, J.M. Pristine graphite oxide. J. Am. Chem. Soc. 2012, 134, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Matsuo, Y. Formation process and structure of graphite oxide. Carbon 1994, 32, 469–475. [Google Scholar] [CrossRef]
- Gao, W. The chemistry of graphene oxide. In Graphene Oxide; Springer: Berlin, Germany, 2015; pp. 61–95. [Google Scholar]
- Talyzin, A.V.; Mercier, G.; Klechikov, A.; Hedenstrom, M.; Johnels, D.; Wei, D.; Cotton, D.; Opitz, A.; Moons, E. Brodie vs. hummers graphite oxides for preparation of multi-layered materials. Carbon 2017, 115, 430–440. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, T.; Choi, J.; Park, J.; Kim, Y.S.; Chang, M.S.; Jung, H.; Park, K.T.; Yang, S.J.; Park, C.R. Hidden second oxidation step of hummers method. Chem. Mater. 2016, 28, 756–764. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Staudenmeier, L. Verfahren zur darstellung der graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1499. [Google Scholar] [CrossRef]
- Ulrich Hofmann, E.K. Untersuchungen über graphitoxyd. Z. Anorg. Allg. Chem. 1937, 234, 311–336. [Google Scholar] [CrossRef]
- Simek, P.; Klimova, K.; Sedmidubsky, D.; Jankovsky, O.; Pumera, M.; Sofer, Z. Towards graphene iodide: Iodination of graphite oxide. Nanoscale 2015, 7, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. Acs Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Jankovsky, O.; Jirickova, A.; Luxa, J.; Sedmidubsky, D.; Pumera, M.; Sofer, Z. Fast synthesis of highly oxidized graphene oxide. ChemistrySelect 2017, 2, 9000–9006. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Wei, Y.Y.; Sun, H.Y.; Li, Z.; Zhao, X.L.; Gao, C. An iron-based green approach to 1-h production of single-layer graphene oxide. Nat. Commun. 2015, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Sofer, Z.; Luxa, J.; Jankovsky, O.; Sedmidubsky, D.; Bystron, T.; Pumera, M. Synthesis of graphene oxide by oxidation of graphite with ferrate(vi) compounds: Myth or reality? Angew. Chem. Int. Edit. 2016, 55, 11965–11969. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Alwarappan, S.; Yoshimura, M.; Sahajwalla, V.; Nishina, Y. Graphene oxide: The new membrane material. Appl. Mater. Today 2015, 1, 1–12. [Google Scholar] [CrossRef]
- Jankovsky, O.; Storti, E.; Schmidt, G.; Dudczig, S.; Sofer, Z.; Aneziris, C.G. Unique wettability phenomenon of carbon-bonded alumina with advanced nanocoating. Appl. Mater. Today 2018, 13, 24–31. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Jankovsky, O.; Lojka, M.; Jan, L.X.; Sedmidubsky, D.; Tomanec, O.; Zboril, R.; Pumera, M.; Sofer, Z. Selective bromination of graphene oxide by the hunsdiecker reaction. Chem. Eur. J. 2017, 23, 10473–10479. [Google Scholar] [CrossRef]
- Jankovsky, O.; Novacek, M.; Luxa, J.; Sedmidubsky, D.; Fila, V.; Pumera, M.; Sofer, Z. A new member of the graphene family: Graphene acid. Chem. Eur. J. 2016, 22, 17416–17424. [Google Scholar] [CrossRef]
- Novacek, M.; Jankovsky, O.; Luxa, J.; Sedmidubsky, D.; Pumera, M.; Fila, V.; Lhotka, M.; Klimova, K.; Matejkova, S.; Sofer, Z. Tuning of graphene oxide composition by multiple oxidations for carbon dioxide storage and capture of toxic metals. J. Mater. Chem. A 2017, 5, 2739–2748. [Google Scholar] [CrossRef]
- Klimova, K.; Pumera, M.; Luxa, J.; Jankovsky, O.; Sedmidubsky, D.; Matejkova, S.; Sofer, Z. Graphene oxide sorption capacity toward elements over the whole periodic table: A comparative study. J. Phys. Chem. C 2016, 120, 24203–24212. [Google Scholar] [CrossRef]
- Jankovsky, O.; Simek, P.; Klimova, K.; Sedmidubsky, D.; Pumera, M.; Sofer, Z. Highly selective removal of ga3+ ions from al3+/ga3+ mixtures using graphite oxide. Carbon 2015, 89, 121–129. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 13. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman-spectroscopy of carbon materials-structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Arrais, A.; Diana, E.; Boccaleri, E. A study on the carbon soot derived from the wood combustion and on the relative alkali-extractable fraction. J. Mater. Sci. 2006, 41, 6035–6045. [Google Scholar] [CrossRef]
- Sofer, Z.; Jankovsky, O.; Simek, P.; Sedmidubsky, D.; Sturala, J.; Kosina, J.; Miksova, R.; Mackova, A.; Mikulics, M.; Pumera, M. Insight into the mechanism of the thermal reduction of graphite oxide: Deuterium-labeled graphite oxide is the key. ACS Nano 2015, 9, 5478–5485. [Google Scholar] [CrossRef]
- Jankovsky, O.; Lojka, M.; Novacek, M.; Luxa, J.; Sedmidubsky, D.; Pumera, M.; Kosina, J.; Sofer, Z. Reducing emission of carcinogenic by-products in the production of thermally reduced graphene oxide. Green Chem. 2016, 18, 6618–6629. [Google Scholar] [CrossRef] [Green Version]
- Eng, A.Y.S.; Ambrosi, A.; Chua, C.K.; Sanek, F.; Sofer, Z.; Pumera, M. Unusual inherent electrochemistry of graphene oxides prepared using permanganate oxidants. Chem. Eur. J. 2013, 19, 12673–12683. [Google Scholar] [CrossRef] [PubMed]





| Sample | C/O (EA) | C/O (EDS) | C/O (XPS) |
|---|---|---|---|
| PoGO-0.2 g | 12.0 | 12.5 | 7.3 |
| poGO-0.5 g | 8.7 | 9.1 | 5.5 |
| poGO-3.0 g | 3.7 | 3.3 | 3.9 |
| poGO-6.0 g | 3.1 | 2.5 | 3.8 |
| Sample | C=C | C–C | C–O | C=O | C(O)–O |
|---|---|---|---|---|---|
| poGO-0.2 g | 87.3 | 6.1 | 5.1 | 0.9 | 0.6 |
| poGO-0.5 g | 77.9 | 9.8 | 8.6 | 2.4 | 1.3 |
| poGO-3.0 g | 61.8 | 15.4 | 14.9 | 5.0 | 2.9 |
| poGO-6.0 g | 41.5 | 23.2 | 26.7 | 3.5 | 5.1 |
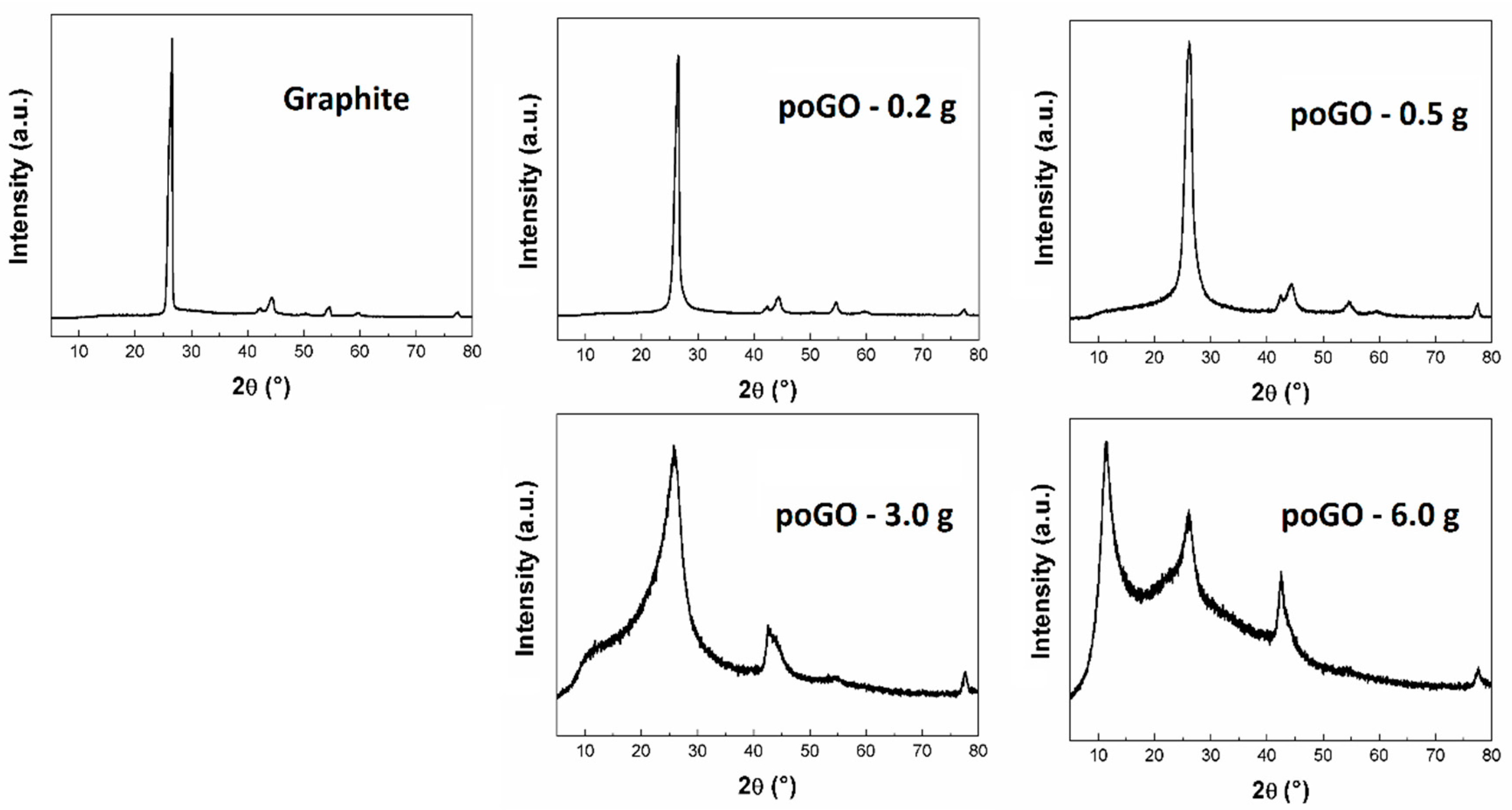
| Sample | Diffraction Angle (°) | Interlayer Distance (Å) | Average Particle Size (Å) |
|---|---|---|---|
| poGO-0.2 g | 26.48 | 3.36 | 94.61 |
| poGO-0.5 g | 26.14 | 3.41 | 43.59 |
| poGO-3.0 g | 25.76 | 3.46 | 12.43 |
| poGO-6.0 g | 11.35 | 7.79 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lojka, M.; Lochman, B.; Jankovský, O.; Jiříčková, A.; Sofer, Z.; Sedmidubský, D. Synthesis, Composition, and Properties of Partially Oxidized Graphite Oxides. Materials 2019, 12, 2367. https://doi.org/10.3390/ma12152367
Lojka M, Lochman B, Jankovský O, Jiříčková A, Sofer Z, Sedmidubský D. Synthesis, Composition, and Properties of Partially Oxidized Graphite Oxides. Materials. 2019; 12(15):2367. https://doi.org/10.3390/ma12152367
Chicago/Turabian StyleLojka, Michal, Boris Lochman, Ondřej Jankovský, Adéla Jiříčková, Zdeněk Sofer, and David Sedmidubský. 2019. "Synthesis, Composition, and Properties of Partially Oxidized Graphite Oxides" Materials 12, no. 15: 2367. https://doi.org/10.3390/ma12152367
APA StyleLojka, M., Lochman, B., Jankovský, O., Jiříčková, A., Sofer, Z., & Sedmidubský, D. (2019). Synthesis, Composition, and Properties of Partially Oxidized Graphite Oxides. Materials, 12(15), 2367. https://doi.org/10.3390/ma12152367