Electrochemical Study of a Hybrid Polymethyl Methacrylate Coating using SiO2 Nanoparticles toward the Mitigation of the Corrosion in Marine Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Coatings
2.1.1. Synthesis of the (PMMA) Coating
2.1.2. Synthesis of the Hybrid PMMA Coating
2.2. Stainless Steel Sheet Preparation and Deposition of the Coatings
2.3. Physicochemical Characterization
2.4. Electrochemical Characterization
2.4.1. Electrochemical Impedance Spectroscopy
2.4.2. Polarization Curves
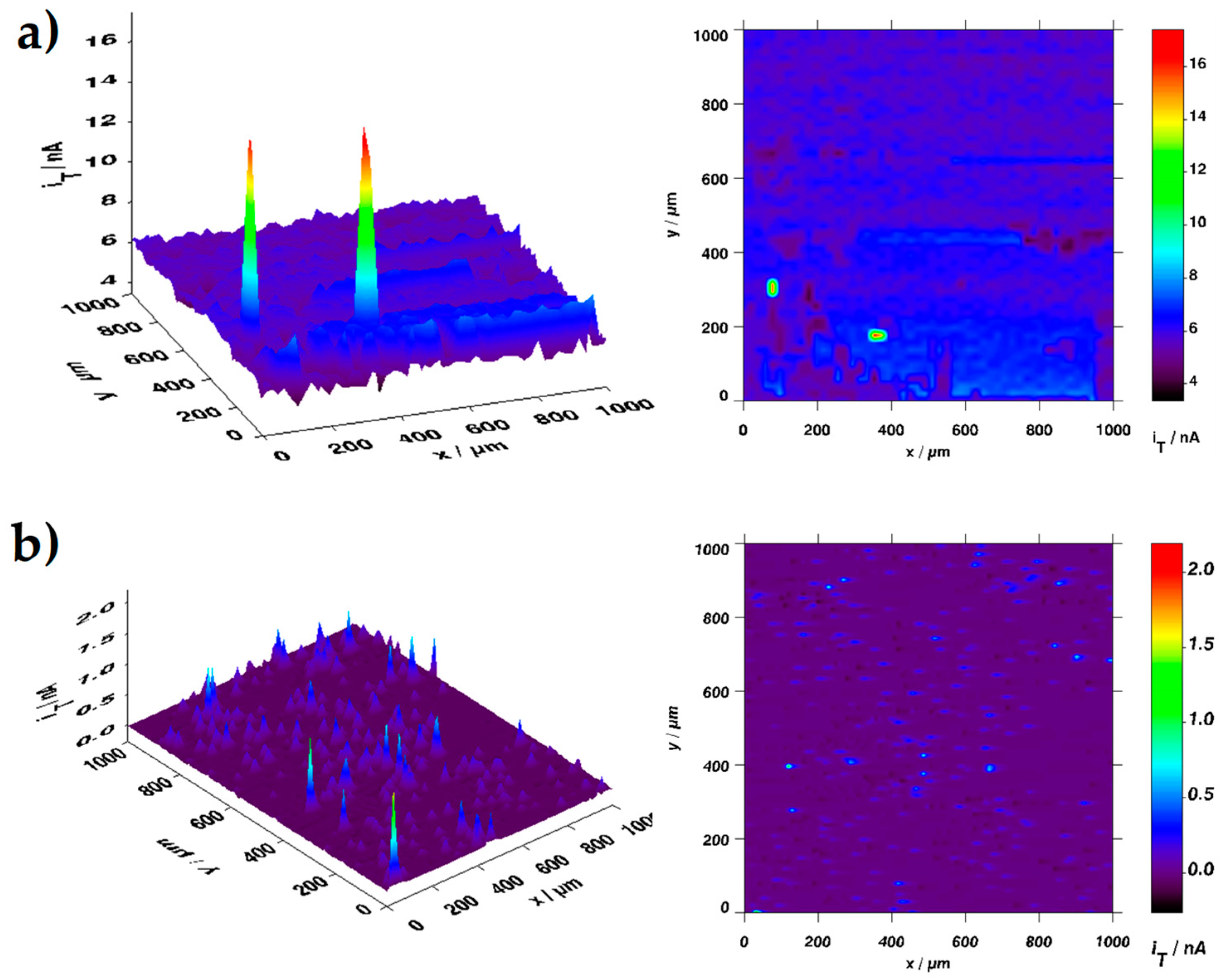
2.4.3. Coating Performance by Scanning Electrochemical Microscopy Evaluation
3. Results and Discussion
3.1. Microstructural Characterization of SiO2 Nanoparticles and PMMA + SiO2 Coating
3.2. Electrochemical Characterization of Hybrid PMMA + SiO2 Coating
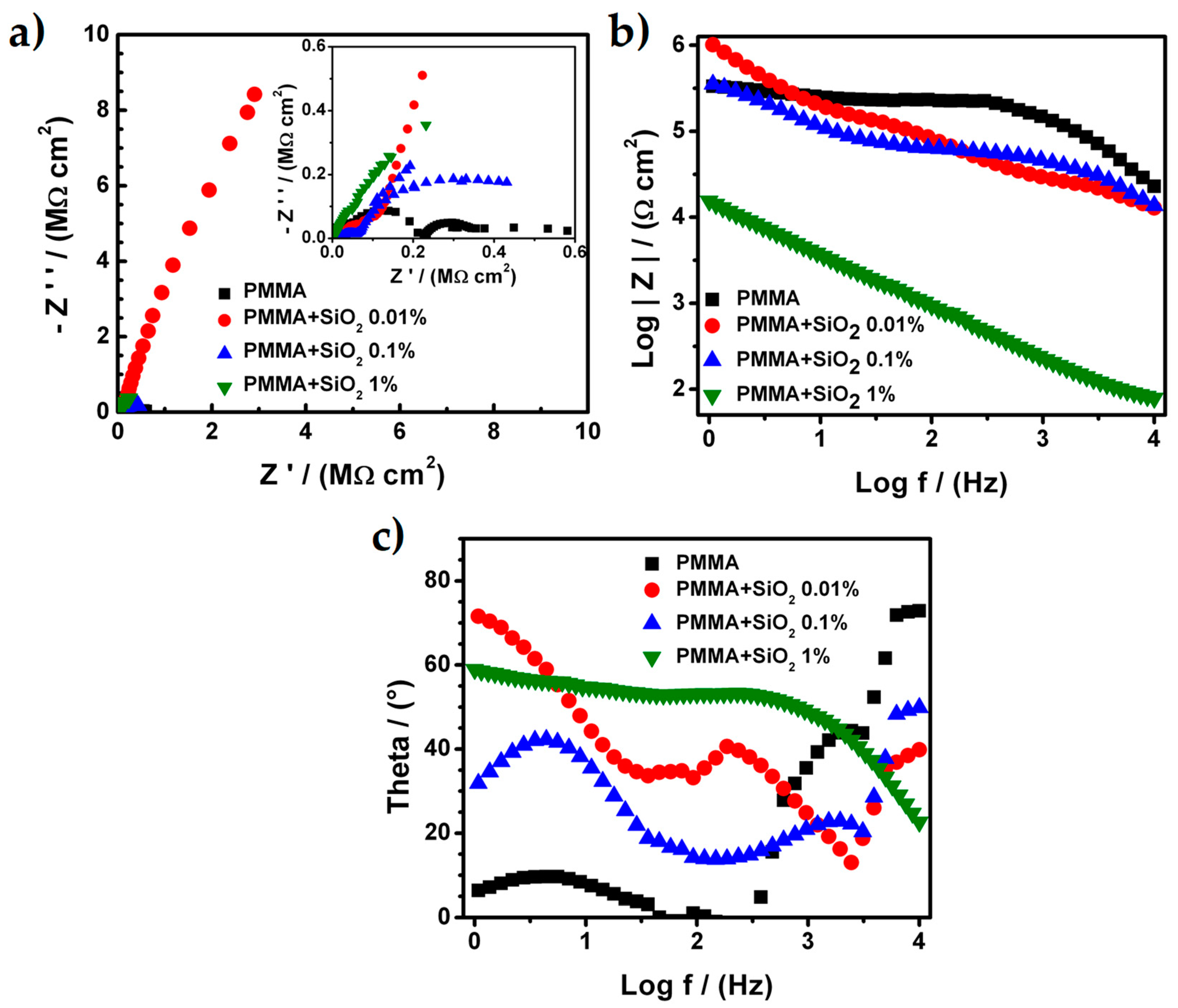
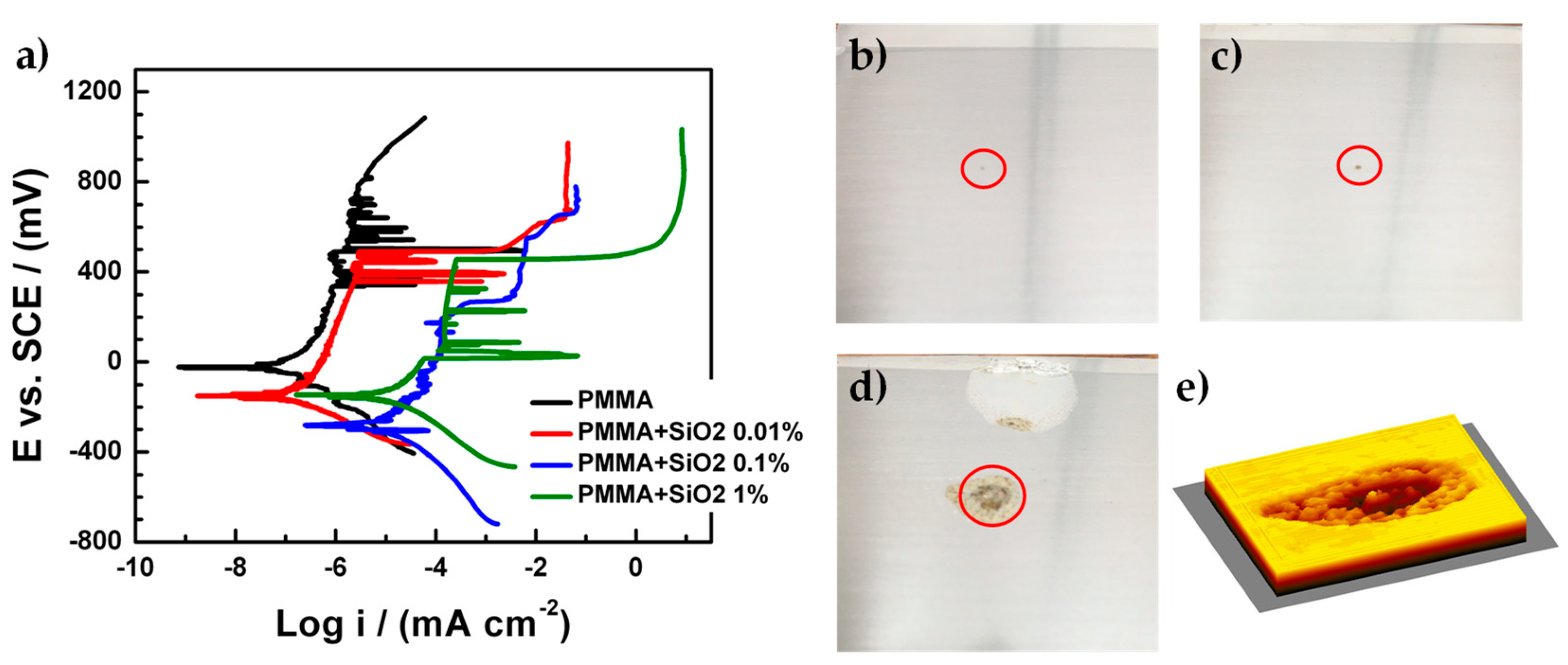
3.3. Electrochemical Characterization of PMMA and Hybrid PMMA + SiO2 Coating with an Artificial Defect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chandler, K.A. Marine and Offshore Corrosion; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Burstein, G.; Liu, C.; Souto, R.; Vines, S. Origins of pitting corrosion. Corros. Eng. Sci. Technol. 2004, 39, 25–30. [Google Scholar] [CrossRef]
- ASM. ASM handbook Volume 13A. Corrosion: Fundamentals, Testing and Protection; ASM International: Materials Park, OH, USA, 2003. [Google Scholar]
- Fontana, M.G.; Greene, N. Corrosion engineering, 2nd ed.; McGraw-Hill: New York, NY, USA, 1967. [Google Scholar]
- Chang, K.-C.; Ji, W.-F.; Lai, M.-C.; Hsiao, Y.-R.; Hsu, C.-H.; Chuang, T.-L.; Wei, Y.; Yeh, J.-M.; Liu, W.-R. Synergistic effects of hydrophobicity and gas barrier properties on the anticorrosion property of PMMA nanocomposite coatings embedded with graphene nanosheets. Polym. Chem. 2014, 5, 1049–1056. [Google Scholar] [CrossRef]
- Hammer, P.; dos Santos, F.C.; Cerrutti, B.M.; Pulcinelli, S.H.; Santilli, C.V. Carbon nanotube-reinforced siloxane-PMMA hybrid coatings with high corrosion resistance. Prog. Org. Coat. 2013, 76, 601–608. [Google Scholar] [CrossRef]
- Tallman, D.E.; Spinks, G.; Dominis, A.; Wallace, G.G. Electroactive conducting polymers for corrosion control. J. Solid State Electr. 2002, 6, 73–84. [Google Scholar] [CrossRef]
- Ates, M. A review on conducting polymer coatings for corrosion protection. J. Adhes. Sci. Technol. 2016, 30, 1510–1536. [Google Scholar] [CrossRef]
- Peabody, A.W. Control of Pipeline Corrosion, 2nd ed.; NACE International and The Corrosion Society: Houston, TX, USA, 2001. [Google Scholar]
- Knudsen, O.; Forsgren, A. Corrosion Control through Organic Coatings; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Coan, T.; Barroso, G.S.; Machado, R.A.F.; de Souza, F.S.; Spinelli, A.; Motz, G. A novel organic-inorganic PMMA/polysilazane hybrid polymer for corrosion protection. Prog. Org. Coat. 2015, 89, 220–230. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.; Yan, Y.; Huo, Z.; Hao, C.; Peng, J.; Zhang, G. Mechanical performance of indium zinc oxide films deposited on coating-treated PMMA substrates. Surf. Coat. Tech. 2019, 358, 604–610. [Google Scholar] [CrossRef]
- Biswas, S.; Roy, N.; Biswas, R.; Kuar, A.S. Experimental investigations into underwater laser transmission micro-channeling on PMMA. In Application of Lasers in Manufacturing. Lecture Notes on Multidisciplinary Industrial Engineering; Dixit, U., Joshi, S., Davim, J., Eds.; Springer: Singapore, 2019; pp. 209–229. [Google Scholar]
- Darwish, G.; Huang, S.; Knoernschild, K.; Sukotjo, C.; Campbell, S.; Bishal, A.K.; Barão, V.A.; Wu, C.D.; Taukodis, C.G.; Yang, B. Improving polymethyl methacrylate resin using a novel titanium dioxide coating. J. Prosthodont. 2019. [Google Scholar] [CrossRef]
- Kim, D.; Joo, S.-Y.; Lee, C.G.; Kim, B.-S.; Kim, W.-B. Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation. J. Photoch. Photobio. A 2019, 376, 206–211. [Google Scholar] [CrossRef]
- Yudoyono, G.; Puspitasari, N.; Prajitno, G.; Sunarno, H.; Rohedi, A.Y.; Pramono, Y.H.; Zainuri, M. Sol-gel deposition of TiO2/PMMA bilayer with the optical and antireflection properties. J. Phys. Conf. Ser. 2019, 1153, 012079. [Google Scholar] [CrossRef]
- Yanli, C. Enhanced interfacial adhesion between PMMA and carbon fiber by graphene oxide coating. Compos. Interface 2019, 26, 41–51. [Google Scholar] [CrossRef]
- Ling, J.Y.; Wang, L.; Bo, J.X.; He, L. Durable superhydrophobic/highly oleophobic coatings from multi-dome SiO2 nanoparticles and fluoroacrylate block copolymers on flat substrates. J. Mater. Chem. A 2015, 3, 20134–20144. [Google Scholar]
- Madhan Kumar, A.; Latthe, S.S.; Sudhagar, P.; Obot, I.B.; Gasem, Z.M. In-situ synthesis of hydrophobic SiO2-PMMA composite for surface protective coatings: Experimental and quantum chemical analysis. Polymer 2015, 77, 79–86. [Google Scholar] [CrossRef]
- Dos Santos, F.C.; Harb, S.V.; Menu, M.-J.; Turq, V.; Pulcinelli, S.H.; Santillia, C.V.; Hammer, P. On the structure of high performance anticorrosive PMMA-siloxane-silica hybrid coatings. RSC Adv. 2015, 5, 106754–106763. [Google Scholar] [CrossRef]
- Harb, S.V.; Cerrutti, B.M.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Siloxane-pmma hybrid anti-corrosion coatings reinforced by lignin. Surf. Coat. Technol. 2015, 275, 9–16. [Google Scholar] [CrossRef]
- Salazar-Bravo, P.; Del Ángel-López, D.; Torres-Huerta, A.; Domínguez-Crespo, M.; Palma-Ramírez, D.; López-Oyama, A. Corrosion investigation of new hybrid organic/inorganic coatings for carbon steel substrates: Electrochemical and surface characterizations. Prog. Org. Coat. 2019, 135, 51–64. [Google Scholar] [CrossRef]
- Augustinho, T.; Motz, G.; Ihlow, S.; Machado, R. Application of hybrid organic/inorganic polymers as coatings on metallic substrates. Mater. Res. Express 2016, 3, 095301. [Google Scholar] [CrossRef]
- Yeh, J.-M.; Weng, C.-J.; Liao, W.-J.; Mau, Y.-W. Anticorrosively enhanced PMMA-SiO2 hybrid coatings prepared from the sol-gel approach with msma as the coupling agent. Surf. Coat. Tech. 2006, 201, 1788–1795. [Google Scholar] [CrossRef]
- Ye, Z.N.; Zhu, Z.J.; Zhang, Q.H.; Liu, X.Y.; Zhang, J.Q.; Cao, F.H. In situ secm mapping of pitting corrosion in stainless steel using submicron Pt ultramicroelectrode and quantitative spatial resolution analysis. Corros. Sci. 2018, 143, 221–228. [Google Scholar] [CrossRef]
- Izquierdo, J.G.; S. Souto, R.M. Application of AC-SECM in corrosión science: Local visualization of heterogeneous chemical activity in AA2024 surfaces. Int. J. Electrochem. Sci. 2012, 7, 11377–11388. [Google Scholar]
- Deflorian, F.; Rossi, S. The role of ions diffusion in the cathodic delamination rate of polyester coated phosphatized steel. J. Adhes. Sci. Technol. 2003, 17, 291–306. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S. An EIS study of ion diffusion through organic coatings. Electrochim. Acta 2006, 51, 1736–1744. [Google Scholar] [CrossRef]
- Leidheiser Jr, H.; Wang, W.; Igetoft, L. The mechanism for the cathodic delamination of organic coatings from a metal surface. Prog. Org. Coat. 1983, 11, 19–40. [Google Scholar] [CrossRef]
- Sun, P.; Laforge, F.O.; Mirkin, M.V. Scanning electrochemical microscopy in the 21st century. Phys. Chem. Chem. Phys. 2007, 9, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Cornut, R.; Lefrou, C. New analytical approximation of feedback approach curves with a microdisk SECM tip and irreversible kinetic reaction at the substrate. J. Electroanal. Chem. 2008, 621, 178–184. [Google Scholar] [CrossRef]
- Lefrou, C.; Cornut, R. Analytical expressions for quantitative scanning electrochemical microscopy (SECM). ChemPhysChem 2010, 11, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.A.; Dam-Johansen, K.; Weinell, C.E.; Kiil, S. Cathodic delamination: Quantification of ionic transport rates along coating-steel interfaces. Prog. Org. Coat. 2010, 68, 70–78. [Google Scholar] [CrossRef]
- Collazo, A.; Izquierdo, M.; Nóvoa, X.; Pérez, C. Surface treatment of carbon steel substrates to prevent cathodic delamination. Electrochim. Acta 2007, 52, 7513–7518. [Google Scholar] [CrossRef]
- Maeztu, J.D.; Rivero, P.J.; Berlanga, C.; Bastidas, D.M.; Palacio, J.F.; Rodriguez, R. Effect of graphene oxide and fluorinated polymeric chains incorporated in a multilayered sol-gel nanocoating for the design of corrosion resistant and hydrophobic surfaces. Appl. Surf. Sci. 2017, 419, 138–149. [Google Scholar] [CrossRef]

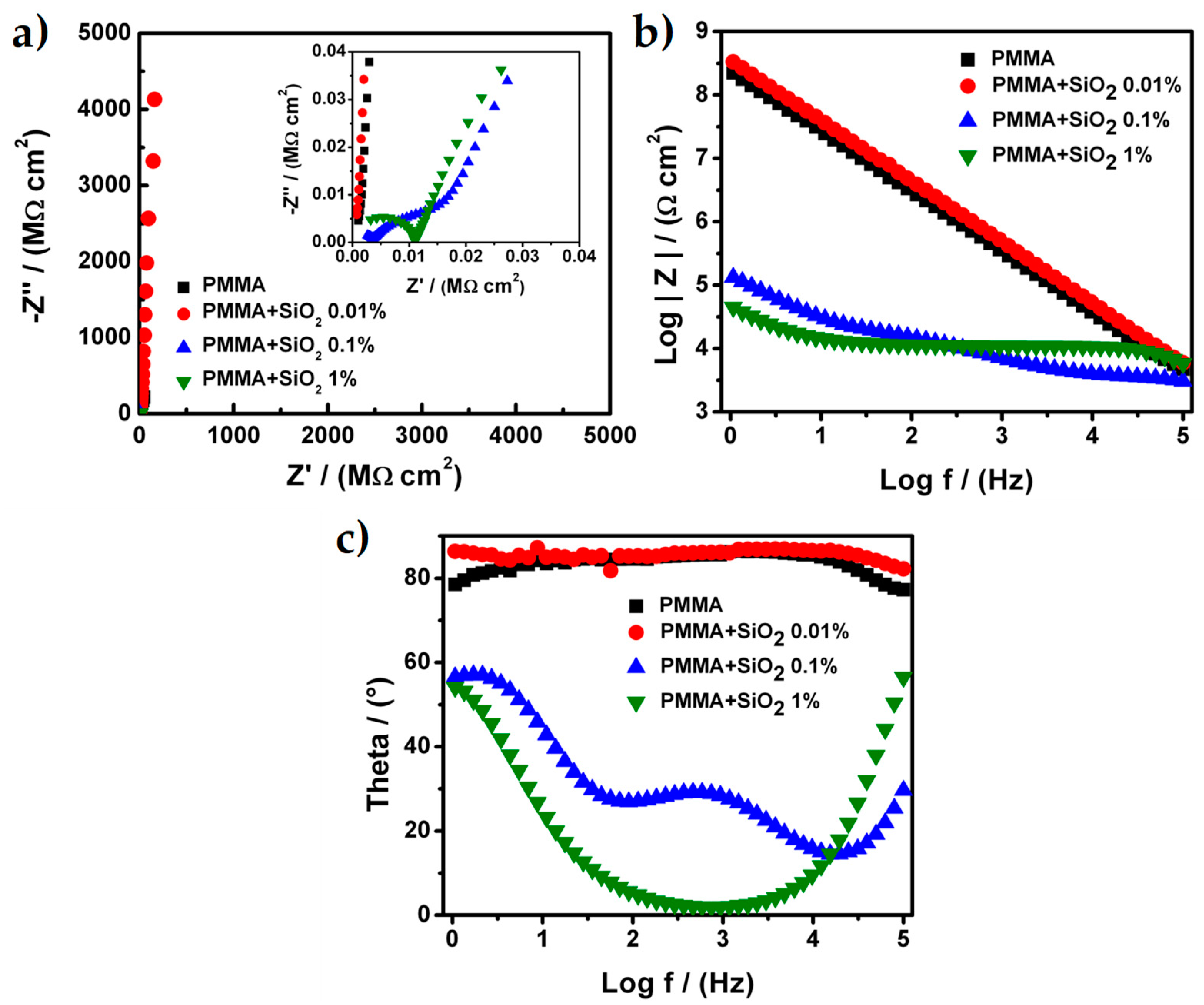
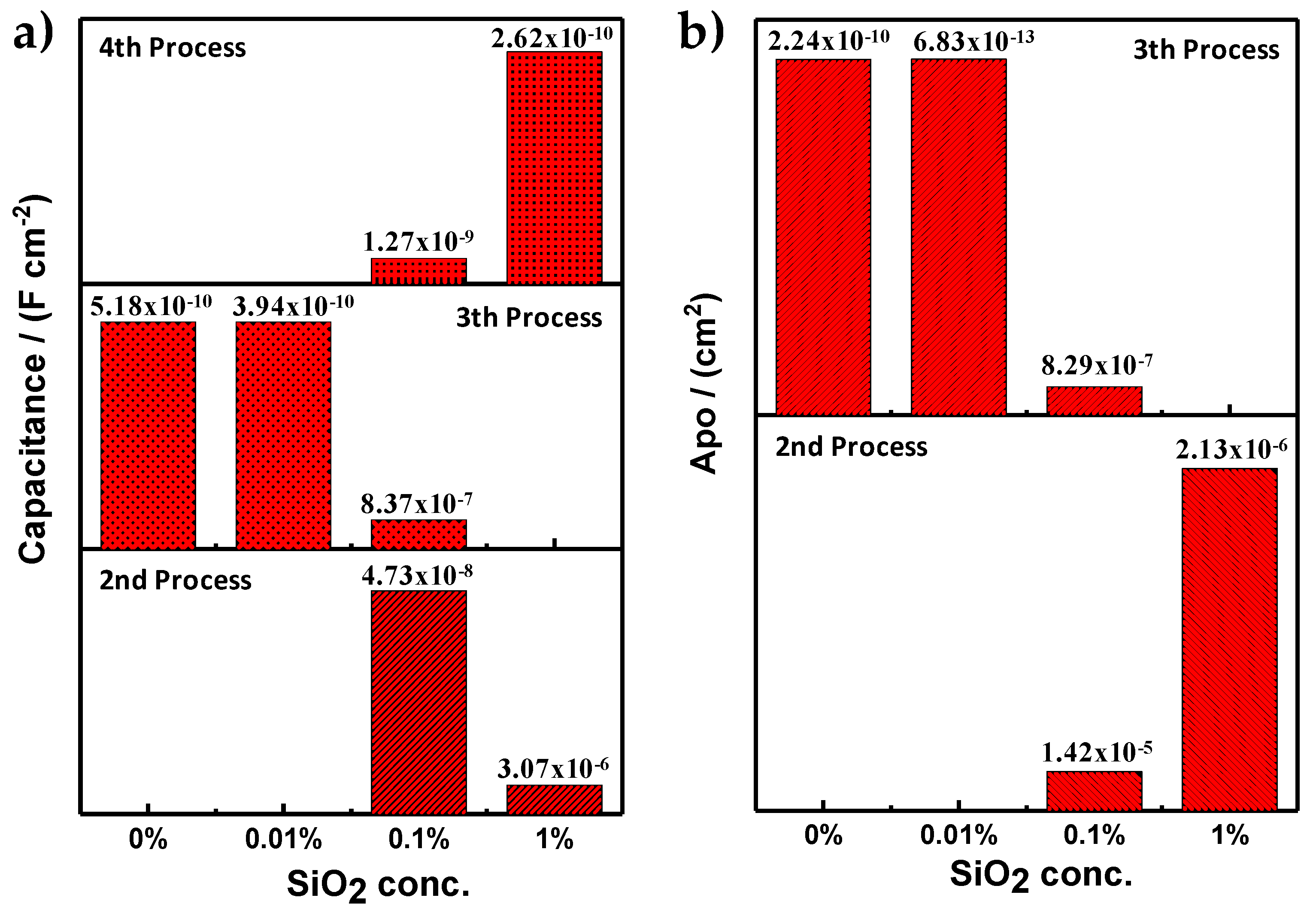
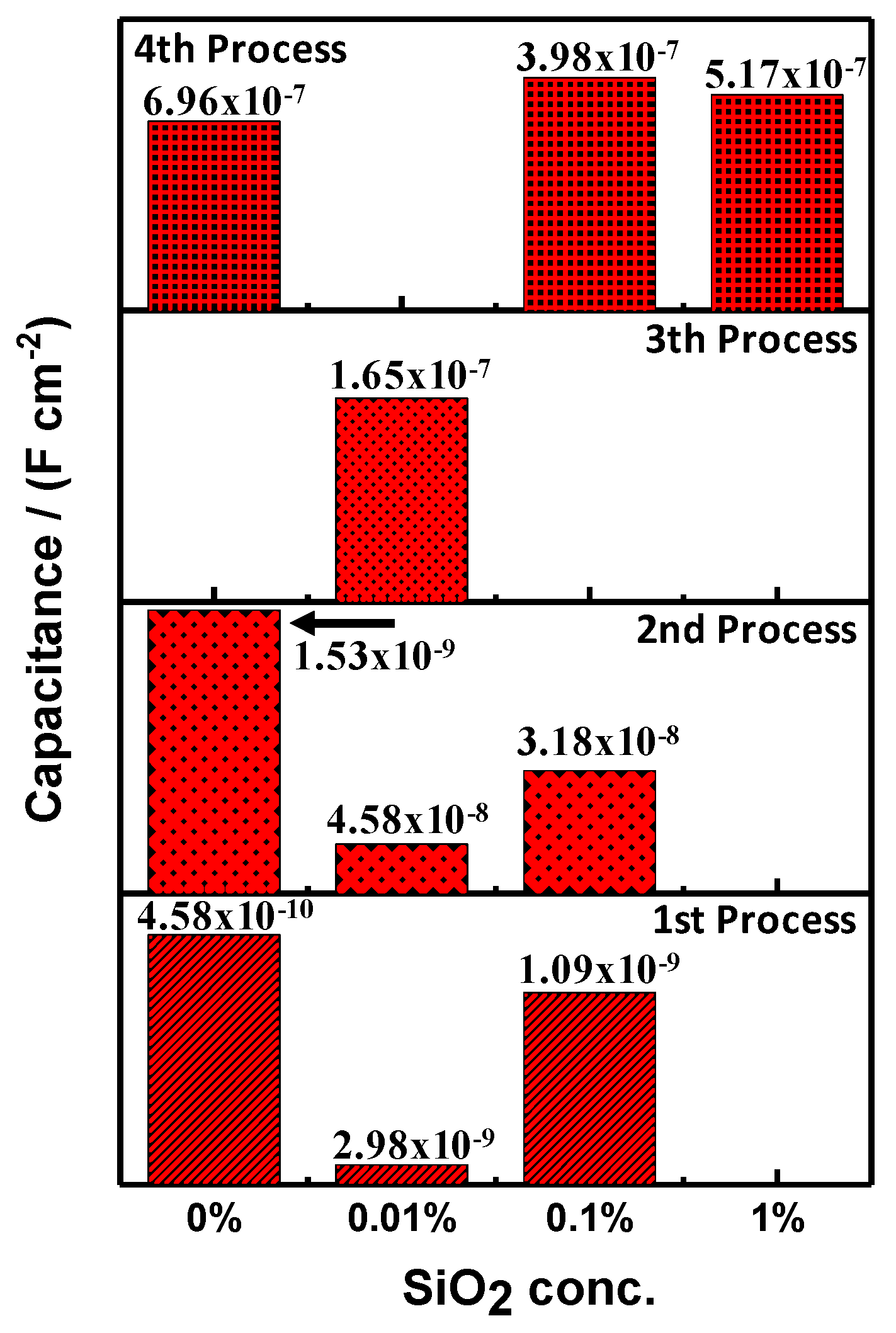
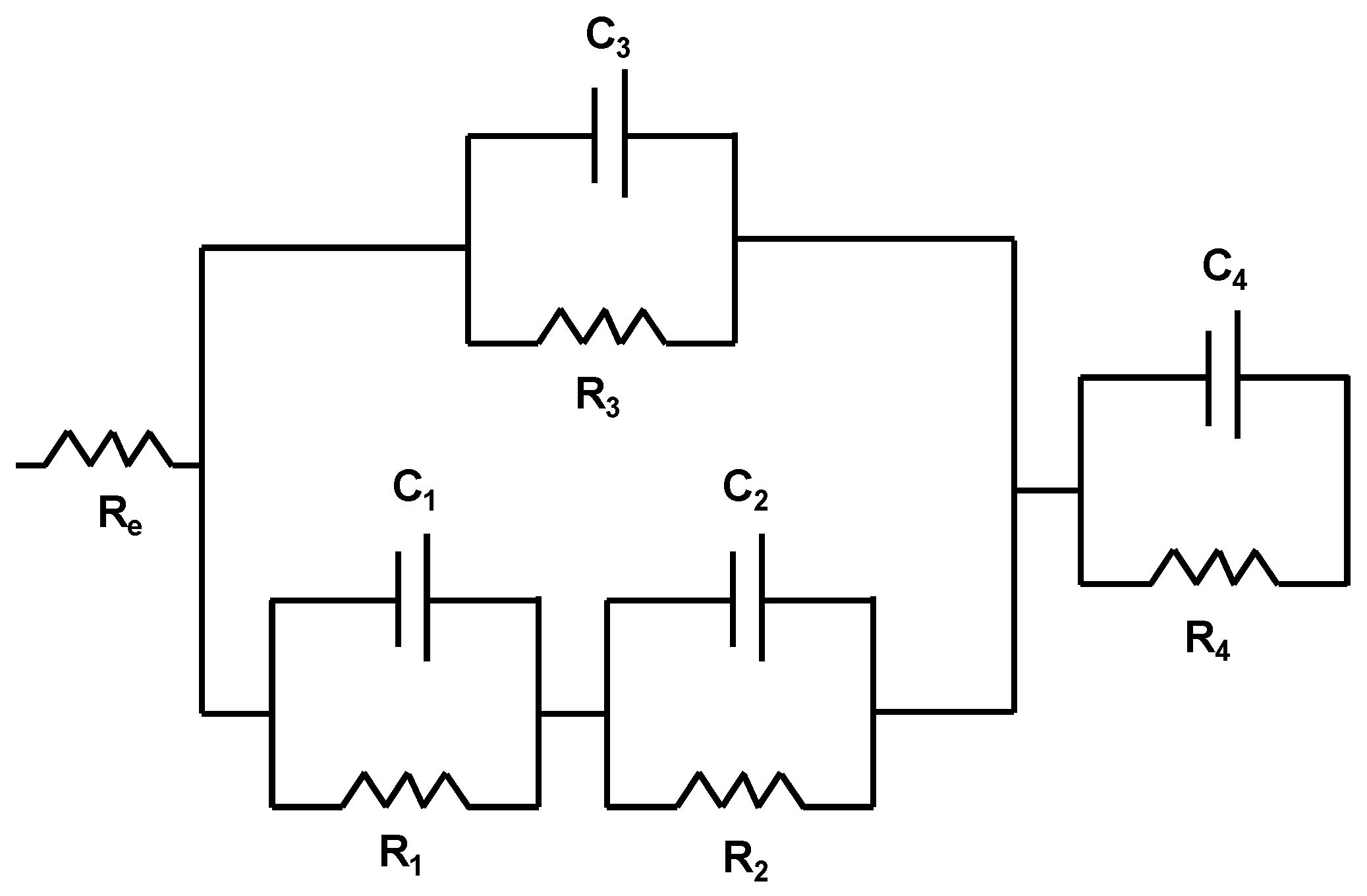

| Sample | Process | RE (Ω⋅cm2) | RPRO (Ω⋅cm2) | Capacitance (F⋅cm−2) | APO (cm2) |
|---|---|---|---|---|---|
| PMMA | 3th | 1372 | 7.55 × 108 | 5.18 × 10−10 | 2.24 × 10−10 |
| PMMA + SiO2 0.01% | 3th | 1060 | 2.48 × 1011 | 3.94 × 10−10 | 6.83 × 10−13 |
| PMMA + SiO2 0.1% | 2nd | 1920 | 11.88 × 103 | 4.73 × 10−8 | 1.42 × 10−5 |
| 3th | - | 204.167 × 103 | 8.37 × 10−7 | 8.29 × 10−7 | |
| 4th | - | 2.402 × 103 | 1.27 × 10−9 | - | |
| PMMA + SiO2 1% | 2nd | 584 | 79.514 × 103 | 3.07 × 10−6 | 2.13 × 10−6 |
| 4th | - | 10.727 × 103 | 2.62 × 10−10 | - |
| Sample | Process | RE (Ω⋅cm2) | RPRO (Ω⋅cm2) | Capacitance (F⋅cm−2) |
|---|---|---|---|---|
| PMMA | 1st | 1803 | 9.55 × 104 | 4.58 × 10−10 |
| 2nd | - | 1.91 × 105 | 1.53 × 10−9 | |
| 4th | - | 1.37 × 105 | 6.96 × 10−7 | |
| PMMA + SiO2 0.01% | 1st | 11.158 × 103 | 2.11 × 104 | 2.98 × 10−9 |
| 2nd | - | 1.46 × 105 | 4.58 × 10−8 | |
| 3th | - | 2.97 × 107 | 1.65 × 10−7 | |
| PMMA + SiO2 0.1% | 1st | 965 | 2.86 × 104 | 1.09 × 10−9 |
| 2nd | - | 4.82 × 104 | 3.18 × 10−8 | |
| 4th | - | 4.82 × 105 | 3.98 × 10−7 | |
| PMMA + SiO2 1% | 4th | 63 | 72.8 | 5.17 × 10−7 |
| Sample | Ecorr (mV) | icorr (mA·cm−2) | Corrosion Rate (mmpy) |
|---|---|---|---|
| PMMA | −24 | 3.258 × 10−7 | 3.783 × 10−6 |
| PMMA + SiO2 0.01% | −155 | 3.090 × 10−7 | 3.588 × 10−6 |
| PMMA + SiO2 0.1% | −228 | - | - |
| PMMA + SiO2 1% | −144 | 2.472 × 10−5 | 2.870 × 10−4 |
| Sample | Contact angle (°) |
|---|---|
| PMMA | 84.71 ± 0.82 |
| PMMA + SiO2 0.01% | 87.06 ± 0.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maya-Cornejo, J.; Rodríguez-Gómez, F.J.; Molina, G.A.; Galindo-de-la-Rosa, J.; Ledesma-García, J.; Hernández-Martínez, Á.R.; Esparza, R.; Pérez, R.; Estévez, M. Electrochemical Study of a Hybrid Polymethyl Methacrylate Coating using SiO2 Nanoparticles toward the Mitigation of the Corrosion in Marine Environments. Materials 2019, 12, 3216. https://doi.org/10.3390/ma12193216
Maya-Cornejo J, Rodríguez-Gómez FJ, Molina GA, Galindo-de-la-Rosa J, Ledesma-García J, Hernández-Martínez ÁR, Esparza R, Pérez R, Estévez M. Electrochemical Study of a Hybrid Polymethyl Methacrylate Coating using SiO2 Nanoparticles toward the Mitigation of the Corrosion in Marine Environments. Materials. 2019; 12(19):3216. https://doi.org/10.3390/ma12193216
Chicago/Turabian StyleMaya-Cornejo, José, Francisco J. Rodríguez-Gómez, Gustavo A. Molina, Juan Galindo-de-la-Rosa, Janet Ledesma-García, Ángel R. Hernández-Martínez, Rodrigo Esparza, Ramiro Pérez, and Miriam Estévez. 2019. "Electrochemical Study of a Hybrid Polymethyl Methacrylate Coating using SiO2 Nanoparticles toward the Mitigation of the Corrosion in Marine Environments" Materials 12, no. 19: 3216. https://doi.org/10.3390/ma12193216
APA StyleMaya-Cornejo, J., Rodríguez-Gómez, F. J., Molina, G. A., Galindo-de-la-Rosa, J., Ledesma-García, J., Hernández-Martínez, Á. R., Esparza, R., Pérez, R., & Estévez, M. (2019). Electrochemical Study of a Hybrid Polymethyl Methacrylate Coating using SiO2 Nanoparticles toward the Mitigation of the Corrosion in Marine Environments. Materials, 12(19), 3216. https://doi.org/10.3390/ma12193216






