Ab Initio Study of Ternary W5Si3 Type TM5Sn2X Compounds (TM = Nb, Ti and X = Al, Si)
Abstract
:1. Introduction
2. Computational Details
2.1. Methodology
2.2. Finite Displacement (Supercell) Method
2.3. Elastic Properties
3. Results and Discussion
3.1. Elastic Properties
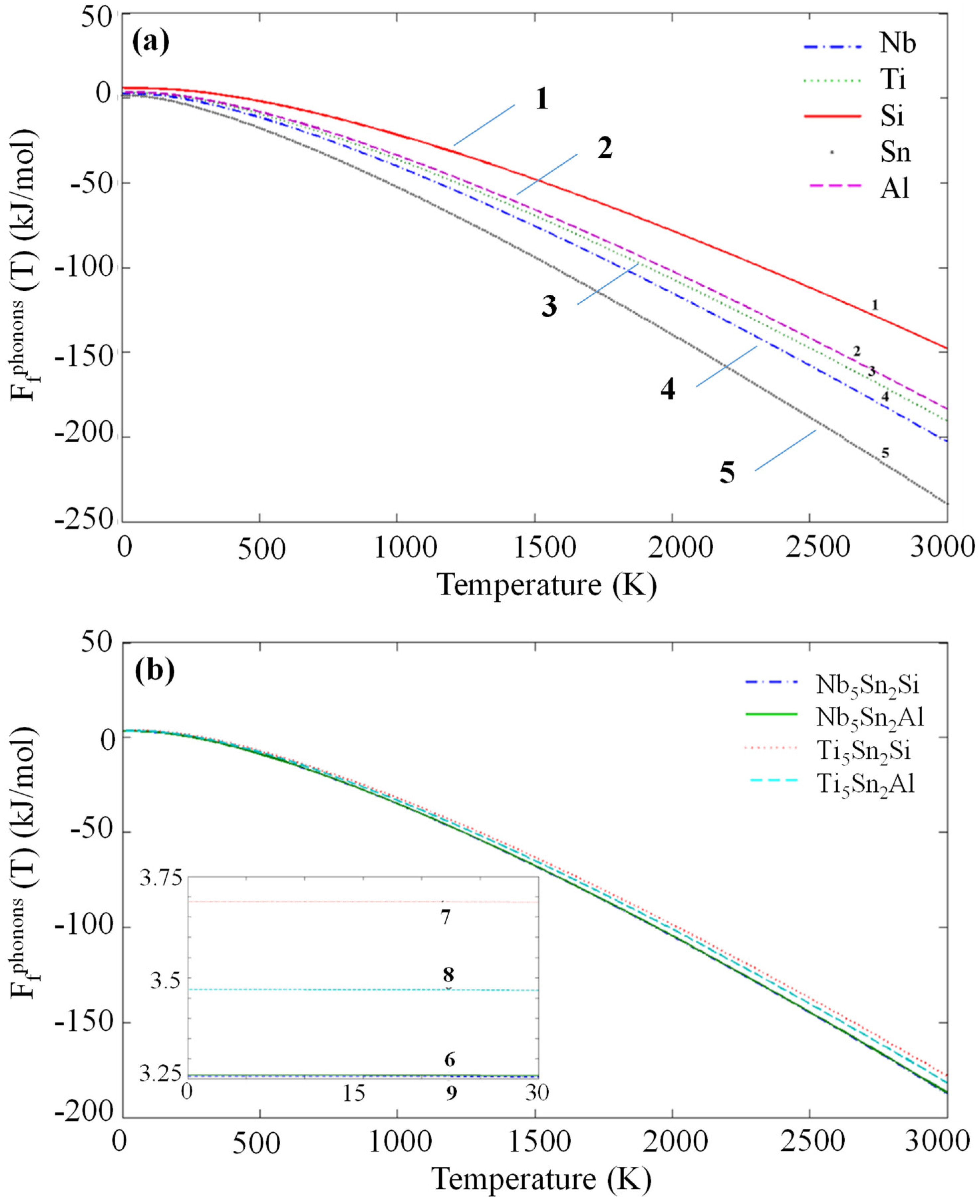
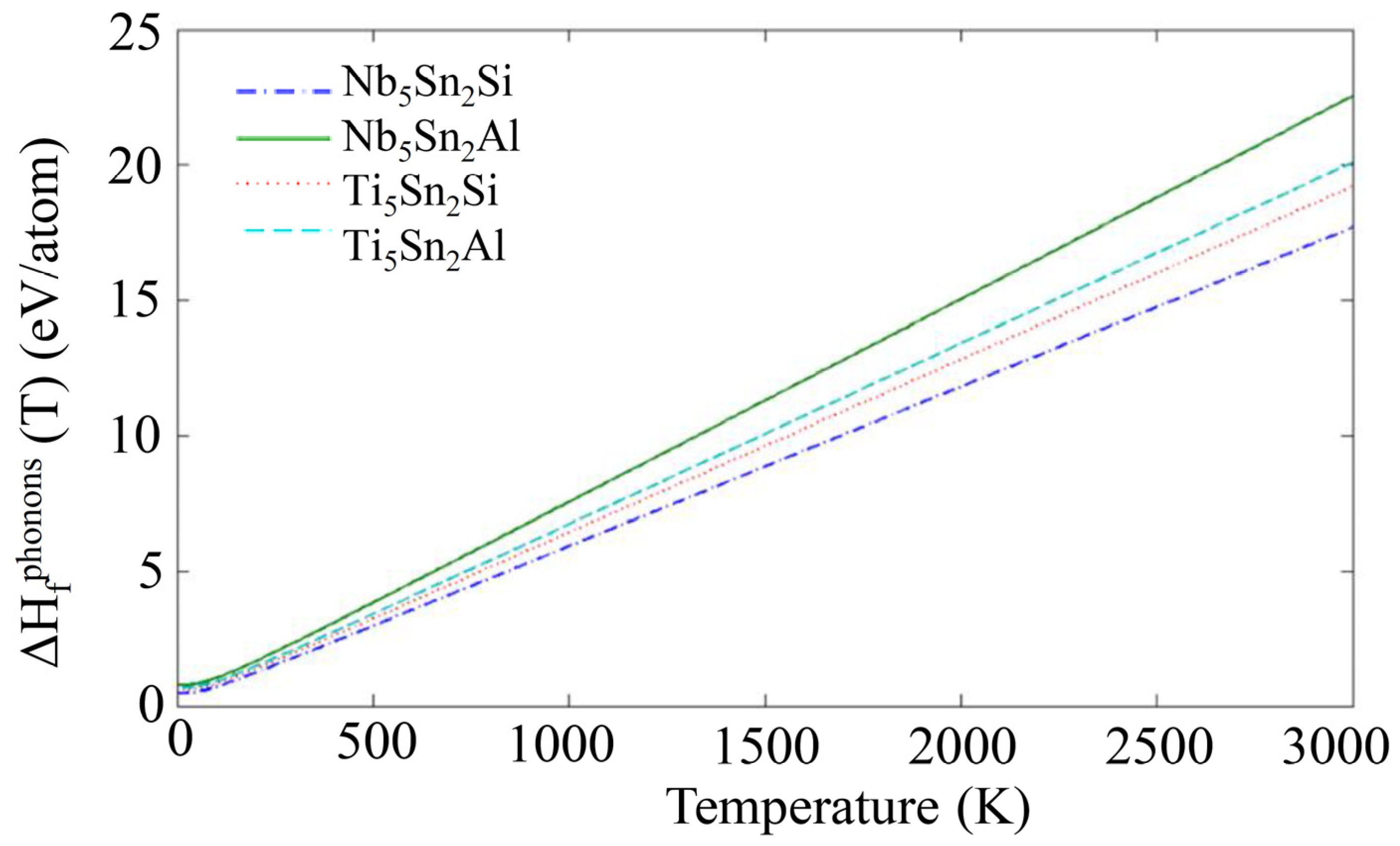
3.2. Enthalpies of Formation
3.3. Debye Temperatures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bewlay, B.P.; Jackson, M.R.; Gigliotti, M.F.X. Niobium silicide high temperature in situ composites. In Intermetallic Compounds: Principles and Practice; Fleischer, R.L., Westbrook, J.H., Eds.; John Wiley: New York, NY, USA, 2001; Volume 3, pp. 541–560. [Google Scholar]
- Tsakiropoulos, P. On Nb silicide based alloys: Alloy design and selection. Materials 2018, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Tsakiropoulos, P. On the macrosegregation of silicon in niobium silicide based alloys. Intermetallics 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Jackson, M.R.; Bewlay, B.P.; Zhao, J.C. Niobium-Silicide based Composites Resistant to High Temperature Oxidation. US Patent 6,913,655 B2, 5 July 2005. [Google Scholar]
- Xu, Z.; Utton, C.; Tsakiropoulos, P. A study of the effect of 2 at.% Sn on the microstructure and isothermal oxidation at 800 and 1200 °C of Nb-24Ti-18Si based alloys with Al and/or Cr additions. Materials 2018, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Al and Cr additions in the microstructure of Nb–Ti–Si in situ composites. Intermetallics 2005, 13, 1079–1095. [Google Scholar] [CrossRef]
- Vellios, N.; Tsakiropoulos, P. The role of Sn and Ti additions in the microstructure of Nb-18Si base alloys. Intermetallics 2007, 15, 1518–1528. [Google Scholar] [CrossRef]
- Zacharis, E.; Utto, C.; Tsakiropoulos, P. A study of the effects of Hf and Sn on the microstructure, hardness and oxidation of Nb-18Si silicide based alloys without Ti addition. Materials 2018, 11, 2447. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Tsakiropoulos, P.; Shao, G. A thermo-gravimetric and microstructural study of the oxidation of Nbss/Nb5Si3-based in situ composites with Sn addition. Intermetallics 2007, 15, 270–281. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. Alloying and properties of C14-NbCr2 and A15-Nb3X (X = Al, Ge, Si, Sn) in Nb-silicide based alloys. Materials 2018, 11, 395. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Scott, A.; Tsakiropoulos, P. Ab initio study of the intermetallics in Nb-Si binary system. Intermetallics 2014, 54, 125–132. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. Ab initio investigation of the intermetallics in the Nb-Sn binary system. Acta Mater. 2015, 86, 23–33. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Scott, A.; Tsakiropoulos, P. Ab initio study of binary and ternary Nb3(X,Y) A15 intermetallic phases (X,Y = Al, Ge, Si, Sn). Metall. Mater. Trans. A 2015, 46, 566–576. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, X.; Zhang, C. Thermodynamic modeling of the Nb-rich corner in the Nb-Si-Sn system. Calphad 2012, 36, 82–88. [Google Scholar] [CrossRef]
- Bulanova, M.; Tretyachenko, L.; Meleshevich, K.; Saltykov, V.; Vereshchaka, V.; Galadzhyj, O.; Kulak, L.; Firstov, S. Influence of tin on the structure and properties of as-cast Ti-rich Ti–Si alloys. J. Alloy. Compd. 2003, 350, 164–173. [Google Scholar] [CrossRef]
- Colinet, C.; Tedenac, J.-C. Structural stability of the - Ti5Sn2Si compound. Calphad 2011, 35, 643–647. [Google Scholar] [CrossRef]
- Horyn, R.; Lukaszewicz, K. The crystal structure of Nb5Sn2Si. Bull. Acad. Pol. Sci. Ser. Sci. Chim. 1970, 18, 59–64. [Google Scholar]
- Pietzka, M.A.; Schuster, J.C. New ternary aluminides T5M2Al having W5Si3-type structure. J. Alloy. Compd. 1995, 230, L10–L12. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. Phase equilibria in the Nb-rich region of Al-Nb-Sn at 900 and 1200 °C. Materials 2019, 12, 2759. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Krist. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef] [PubMed]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Cote, M.; Louie, S.G.; Cohen, M.L. Relaxation of crystals with the quasi-Newton method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Wang, Y.; Woodward, C.; Zhou, S.H.; Liu, Z.K.; Chen, L.Q. Structural stability of Ni-Mo compounds from first-principles calculations. Scr. Mater. 2005, 52, 17–20. [Google Scholar] [CrossRef]
- Montanari, B.; Harrison, N.M. Lattice dynamics of TiO2 rutile: Influence of gradient corrections in density functional calculations. Chem. Phys. Lett. 2002, 364, 528–534. [Google Scholar] [CrossRef]
- Baroni, S.; de Gironcoli, S.; Corso, A.D.; Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef] [Green Version]
- Born, M.; Huang, K. Dynamical Theory of Crystal Lattices; Clarendon Press Oxford: Oxford, UK, 1956. [Google Scholar]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. 1952, 65, 349–355. [Google Scholar] [CrossRef]
- Reuss, A. Account of the liquid limit of mixed crystals on the basis of the plasticity condition for single crystal. Z. Angew. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Anderson, O.L. A simplified method for calculating debye temperature from elastic constants. J. Phys. Chem. Solids 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Soderlind, P.; Eriksson, O.; Wills, J.M.; Boring, A.M. Theory of elastic-constants of cubic transition-metals and alloys. Phys. Rev. B 1993, 48, 5844–5851. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.; Wang, H. Single Crystal Elastic Constants and Calculated Aggregate Properties: A Handbook; MIT Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Rayne, J.A.; Chandrasekhar, B.S. Elastic Constants of β Tin from 4.2 K to 300 K. Phys. Rev. 1960, 120, 1658–1663. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. The impact of Ti and temperature on the stability of Nb5Si3 phases: A first principles study. Sci. Techn. Adv. Mater. 2017, 184, 67–469. [Google Scholar] [CrossRef] [PubMed]
- Tromans, D. Elastic anisotropy of HCP metal crystals and polycrystals. Int. J. Res. Rev. Appl. Sci. 2011, 6, 462–483. [Google Scholar]
- Pugh, S.F. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Pettifor, D.G. Theoretical predictions of structure and related properties of intermetallics. Mater. Sci. Technol. 1992, 8, 345–349. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 7th ed.; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Smithells, C.J. Metal References Book, 5th ed.; Butterworth: London, UK, 1976. [Google Scholar]
- Brantley, W.A. Calculated elastic-constants for stress problems associated with semiconductor devices. J. Appl. Phys. 1973, 44, 534–535. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, M.; Wang, R.; Mo, Z.; Tang, B.; Peng, L.; Ding, W. First-principles study of (Ti5−xMgx)Si3 phases with the hexagonal D88 structure: Elastic properties and electronic structure. Comput. Mater. Sci. 2012, 54, 287–292. [Google Scholar] [CrossRef]
- Chen, Y.; Hammerschmidt, T.; Pettifor, D.G.; Shang, J.-X.; Zhang, Y. Influence of vibrational entropy on structural stability of Nb–Si and Mo–Si systems at elevated temperatures. Acta Mater. 2009, 57, 2657–2664. [Google Scholar] [CrossRef]
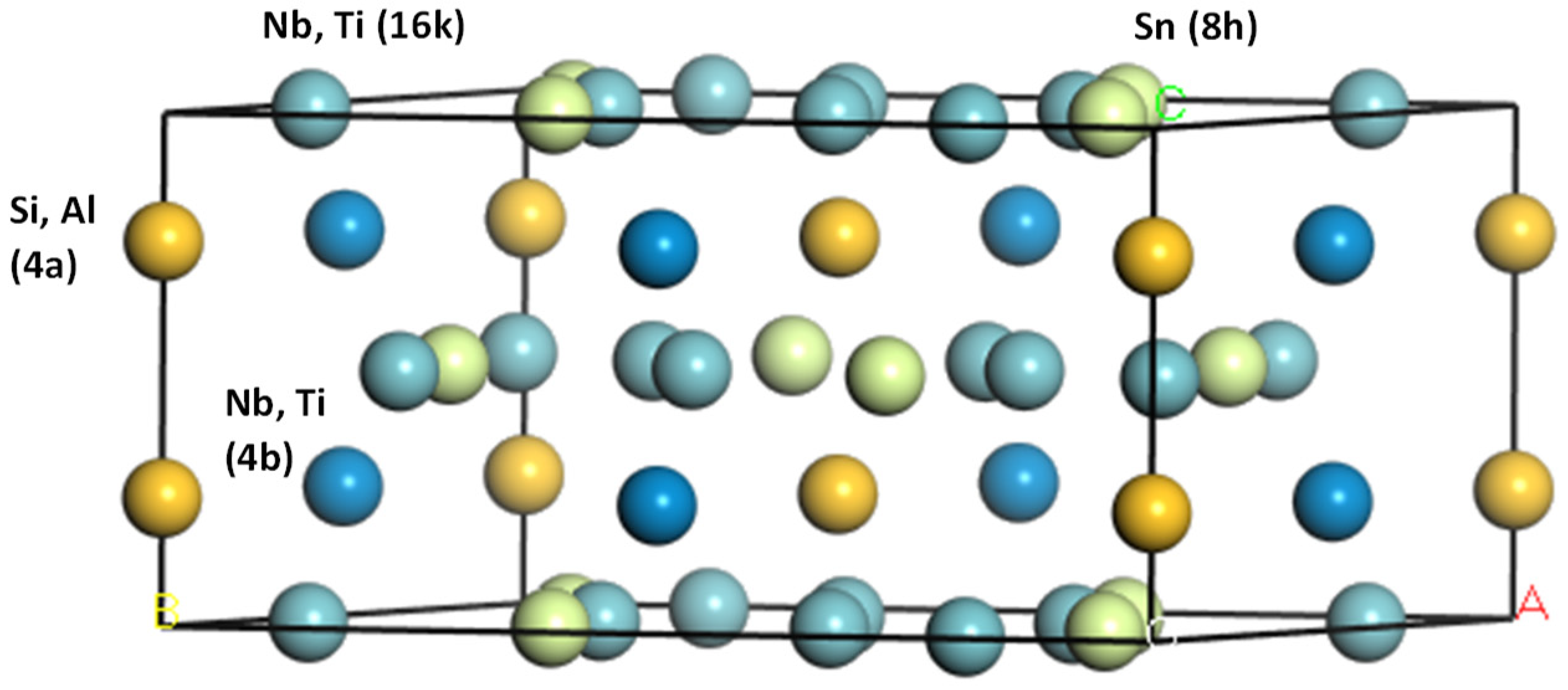


| Phase | Lattice Parameters | |
|---|---|---|
| a | c | |
| Nb5Sn2Si | 10.683 | 5.145 |
| - | 10.541 [17] | 5.138 [17] |
| Ti5Sn2Si | 10.582 | 5.05 |
| - | 10.558 [16] | 5.03 [16] |
| Nb5Sn2Al | 10.735 | 5.203 |
| - | 10.629 [18] | 5.216 [18] |
| Ti5Sn2Al | 10.612 | 5.184 |
| - | 10.549 [18] | 5.242 [18] |
| Element and Phase | VRH Approximation | B–M EOS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C11 | C12 | C13 | C33 | C44 | C66 | B | B | B’ | |
| Nb | 241 | 126.3 | - | - | 26.7 | - | 164.5 | 165.1 | 4.005 |
| - | 253 a | 133 a | - | - | 31 a | - | - | - | - |
| Si | 151.2 | 57.4 | - | - | 73.1 | - | 88.7 | 91.2 | 4.009 |
| - | 166 b | 64 b | - | - | 79.6 b | - | - | - | - |
| Al | 107.4 | 57.6 | - | - | 30.3 | - | 74.2 | 76.47 | 4.037 |
| - | 107 b | 61 b | - | - | 28 b | - | - | - | - |
| Sn | 74.2 | 58 | 22.2 | 81.2 | 23.4 | 9.9 | 51.8 | 52.01 | 3.703 |
| - | 72.3 c | 59.4 c | 35.8 c | 88.4 c | 22 c | 22.5 c | 54.9 c | - | - |
| Ti | 149.6 d | 97.5 d | 79.7 d | 186.1 d | 33 d | - | 110.9 d | 118.4 d | 4 d |
| - | 160 e | 90 e | 66 e | 181 e | 46.5 e | - | - | - | - |
| Nb5Sn2Si | 303.5 | 104.4 | 98.9 | 313.4 | 74.4 | 98.7 | 169.4 | 168.8 | 5 |
| Ti5Sn2Si | 214.8 | 73.6 | 71.1 | 189.6 | 51.6 | 75.3 | 116.6 | 119.7 | 5 |
| Nb5Sn2Al | 286.5 | 97 | 95.7 | 269.6 | 62.5 | 81.7 | 157.6 | 158.6 | 5 |
| Ti5Sn2Al | 211.5 | 75.1 | 63.3 | 178.6 | 47.3 | 69.8 | 111.1 | 118.9 | 5 |
| Element and Phase | G | E | - | - | - | - | - | θD (K) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| VRH | VRH | v | C12–C44 | C13–C44 | C12–C66 | G/B | Phonon DOS | Elastic Const. | Literature | |
| Nb | 36.5 | 101.9 | 0.396 | 99.6 | - | - | 0.228 | 277 | 268 | 275 a |
| - | 37.5 b | 104.9 b | 0.397 b | - | - | - | - | - | - | - |
| Si | 61.2 | 149.2 | 0.216 | −17.4 | - | - | 0.701 | 647 | 628 | 645 a |
| - | 64.1 c | 155.8 c | 0.215 c | - | - | - | - | - | - | - |
| Al | 28 | 74.7 | 0.334 | 27.3 | - | - | 0.377 | 394 | 420 | 428 a |
| - | 26.2 b | 70.6 b | 0.345 b | - | - | - | - | - | - | - |
| Sn | 16.3 | 44.3 | 0.357 | - | −1.2 | 48.1 | 0.315 | 254 | 217 | 230 a |
| - | 17.7 d | 48 d | 0.355 d | - | - | - | - | - | - | - |
| Ti | 32.7 e | 89.3 e | 0.366 e | - | 19.5 e | 0.295 e | 369 e | 346 e | 380 e | |
| Nb5Sn2Si | 89.7 | 228.7 | 0.275 | - | 24.5 | 5.7 | 0.53 | 311 | 327 | - |
| Ti5Sn2Si | 61.8 | 157.6 | 0.275 | - | 19.5 | −1.7 | 0.53 | 305 | 326 | - |
| Nb5Sn2Al | 77.1 | 198.9 | 0.29 | - | 33.2 | 15.3 | 0.489 | 298 | 305 | - |
| Ti5Sn2Al | 58.6 | 149.5 | 0.276 | - | 16 | 5.3 | 0.527 | 300 | 320 | - |
| Intermetallic | Enthalpy of Formation (kJ/mol) | |
|---|---|---|
| Current Study | Literature | |
| Nb5Sn2Si | −30.296 | - |
| Ti5Sn2Si | −50.655 | −50.751 [16] |
| Nb5Sn2Al | −21.516 | - |
| Ti5Sn2Al | −36.471 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. Ab Initio Study of Ternary W5Si3 Type TM5Sn2X Compounds (TM = Nb, Ti and X = Al, Si). Materials 2019, 12, 3217. https://doi.org/10.3390/ma12193217
Papadimitriou I, Utton C, Tsakiropoulos P. Ab Initio Study of Ternary W5Si3 Type TM5Sn2X Compounds (TM = Nb, Ti and X = Al, Si). Materials. 2019; 12(19):3217. https://doi.org/10.3390/ma12193217
Chicago/Turabian StylePapadimitriou, Ioannis, Claire Utton, and Panos Tsakiropoulos. 2019. "Ab Initio Study of Ternary W5Si3 Type TM5Sn2X Compounds (TM = Nb, Ti and X = Al, Si)" Materials 12, no. 19: 3217. https://doi.org/10.3390/ma12193217





