Pd-Ag Electrical Resistivity in Hydrogen and Deuterium: Temperature Effect
Abstract
1. Introduction
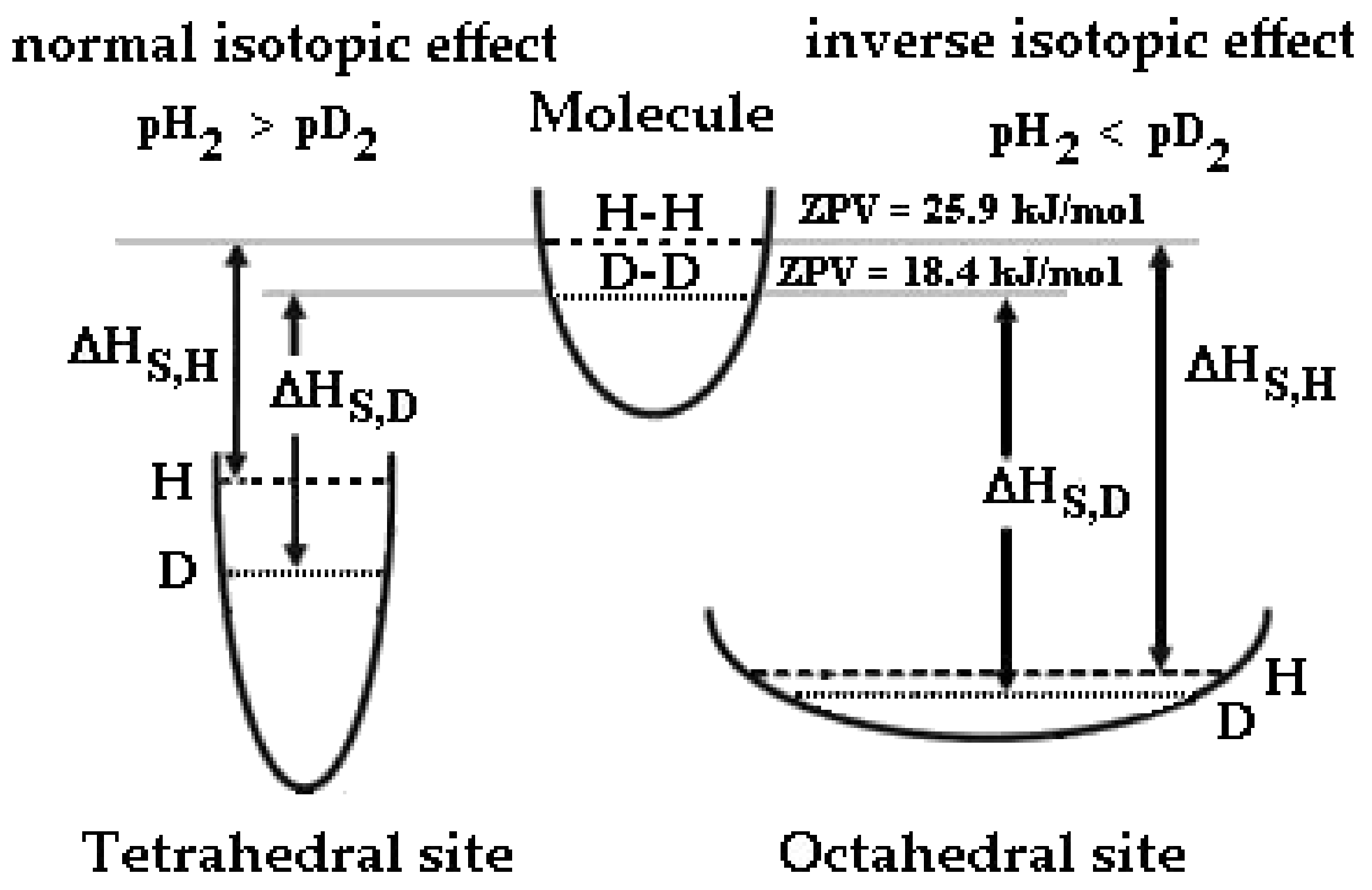
2. The Isotopic Effect on Pd-Ag Resistivity and Solubility
3. Experimental Methods
4. Results and Discussion
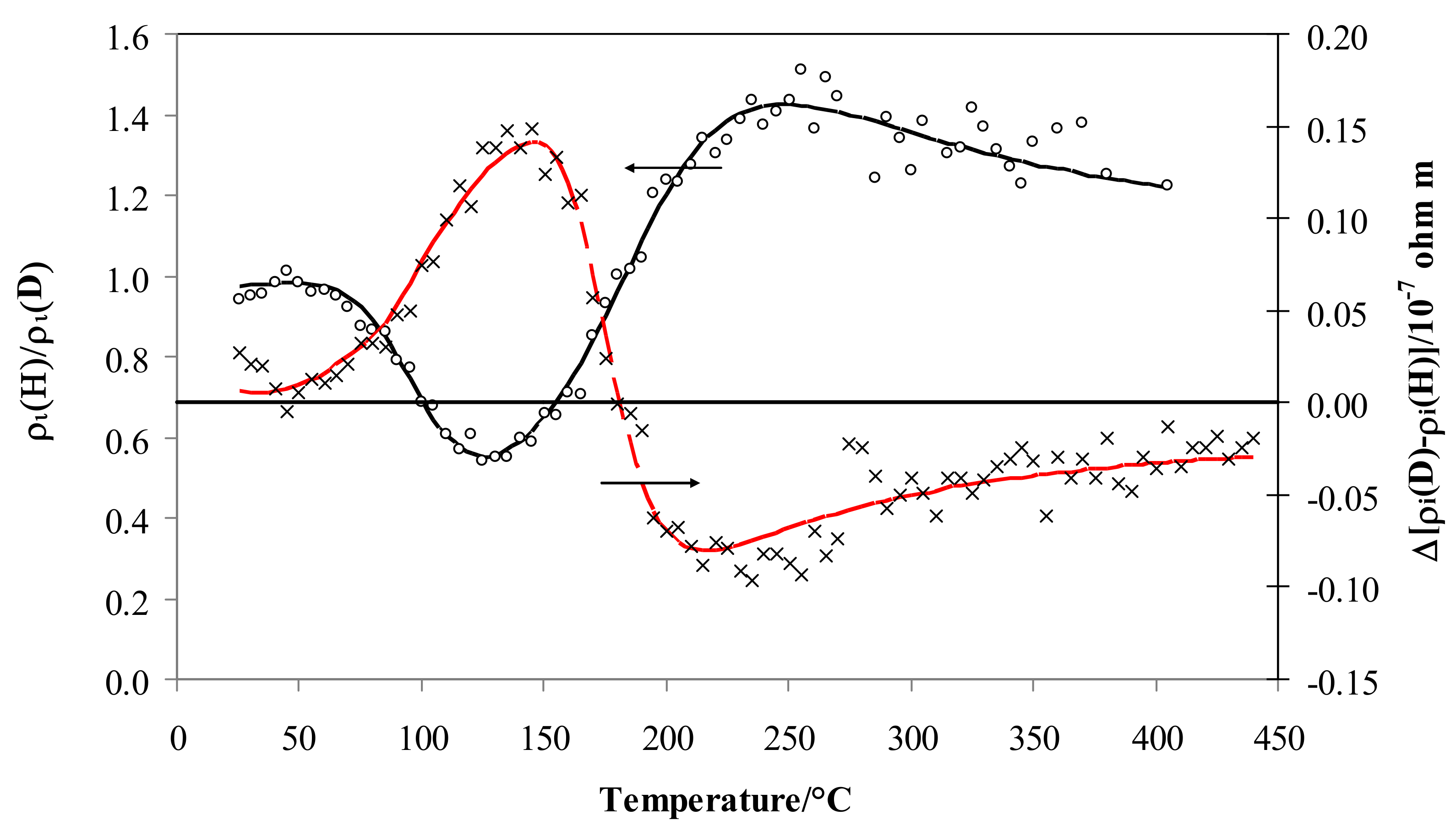
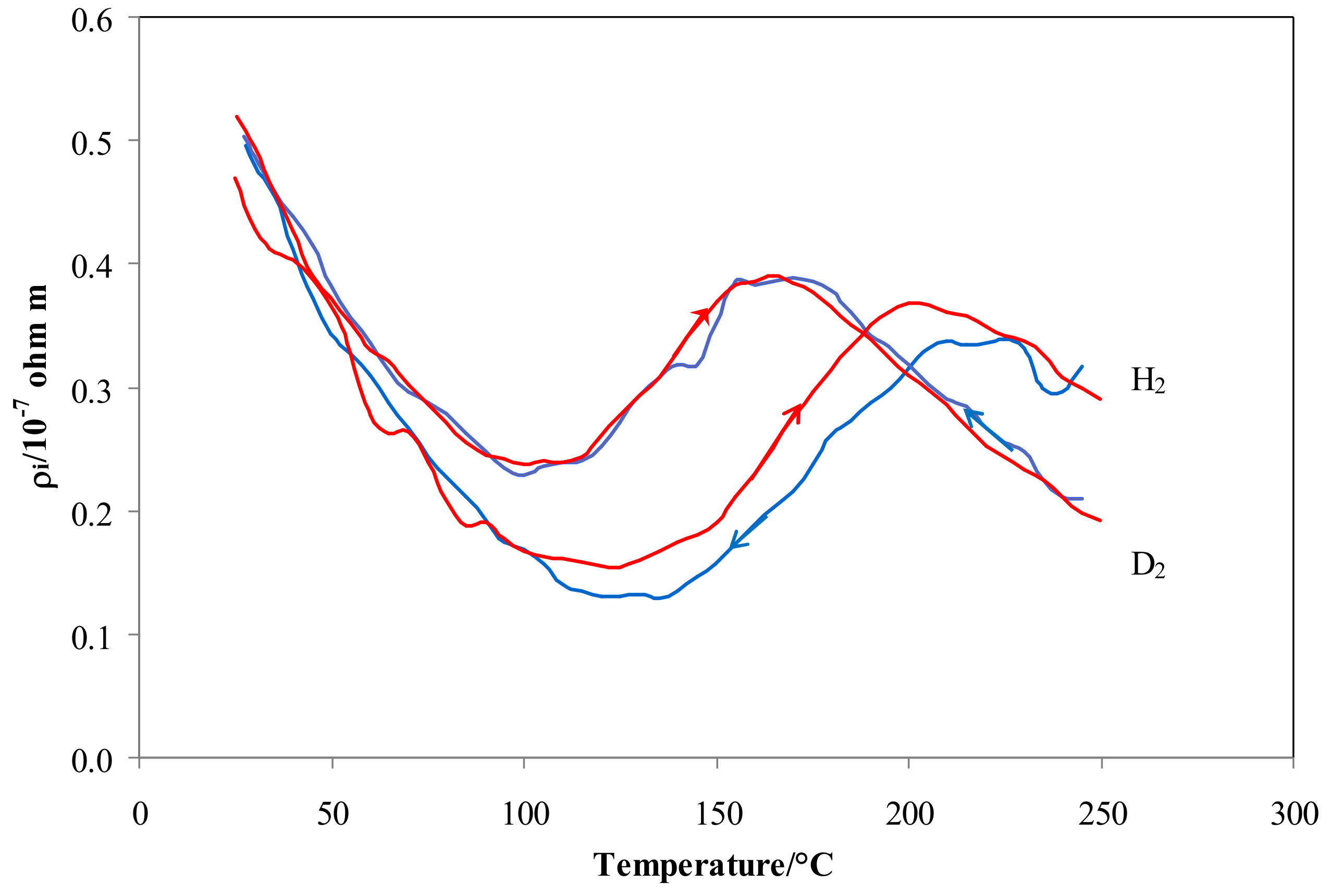
4.1. Electrical Resistivity vs. Temperature
4.2. Electrical Resistivity vs. Hydrogen Isotopic Content
5. Conclusions
- -
- The loading of hydrogen and deuterium atoms at low H/M ratios reduces the number of conduction electrons, thereby increasing resistivity,
- -
- Above a well-marked and similar H/M and D/M ratio (about 0.20), the further absorption of isotopes leads to an increase in the conduction electrons, thus reducing the resistivity,
- -
- Further increasing the H/M or D/M ratio beyond a certain value (about 0.34), the electron scattering against the large number of isotope atoms (operating as reticular defects) becomes prevalent, and the resistivity grows very rapidly.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darling, A.S. Thermal and Electrolytic Palladium Alloy Diffusion Cells. Platin. Met. Rev. 1963, 7, 126–129. [Google Scholar]
- Medrano, J.A.; Fernandez, E.; Melendez, J.; Parco, M.; Tanaka, D.A.P.; van Sint Annaland, M.; Gallucci, F. Pd-based metallic supported membranes: High-temperature stability and fluidized bed reactor testing. Int. J. Hydrog. Energy 2018, 41, 8706–8718. [Google Scholar] [CrossRef]
- Tosti, S.; Pozio, A. Membrane Processes for the Nuclear Fusion Fuel Cycle. Membranes 2018, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Kalyanam, K.; Sood, S. A Comparison of Process Characteristics for the Recovery of Tritium from Heavy Water and Light Water Systems. Fusion Technol. 1988, 14, 524–528. [Google Scholar] [CrossRef]
- Cristescu, I.R.; Tamm, U.; Caldwell-Nichols, C.; Glugla, M.; Murdoch, D.; Welte, S. Simultaneous tritium and deuterium transfer in a water detritiation CECE facility at TLK. Fusion Eng. Des. 2003, 69, 109–113. [Google Scholar] [CrossRef]
- Albrecht, H.; Hutter, E. Tritium recovery from an ITER ceramic test blanket module—Process options and critical R&D issues. Fusion Eng. Des. 2000, 49, 769–777. [Google Scholar]
- Kinjo, M.; Fukada, S.; Katayama, K.; Edao, Y.; Hayashi, T. Experiment on Recovery of Hydrogen Isotopes from Li 17 Pb 83 Blanket by Liquid-Gas Contact. Fusion Sci. Technol. 2017, 71, 520–526. [Google Scholar] [CrossRef]
- Fukada, S.; Nishikawa, T.; Kinjo, M.; Katayama, K. Study of hydrogen recovery from Li-Pb using packed tower. Fusion Eng. Des. 2018, 135, 74–80. [Google Scholar] [CrossRef]
- Alpy, N.; Terlain, A.; Lorentz, V. Hydrogen extraction from Pb–17Li: Results with a 800 mm high packed column. Fusion Eng. Des. 2000, 49, 775–780. [Google Scholar] [CrossRef]
- Tosti, S. Membranes and Membrane Reactors for tritium Separation. In Tritium in Fusion: Production, Uses and Environmental Impact; Tosti, S., Ghirelli, N., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 203–240. [Google Scholar]
- Shu, J.; Grandjean, B.P.A.; Van Neste, A.; Kaliaguine, S. Catalytic palladium-based membrane reactors: A review. Can. J. Chem. Eng. 1991, 69, 1036–1060. [Google Scholar] [CrossRef]
- Okazaki, J.; Tanaka, D.A.P.; Tanco, M.A.L.; Wakui, Y.; Mizukami, F.; Suzuki, T.M. Hydrogen permeability study of the thin Pd–Ag alloy membranes in the temperature range across the α–β phase transition. J. Membr. Sci. 2006, 282, 370–374. [Google Scholar] [CrossRef]
- Tosti, S.; Borgognoni, F.; Santucci, A. Electrical resistivity, strain and permeability of Pd–Ag membrane tubes. Int. J. Hydrog. Energy 2010, 35, 7796–7802. [Google Scholar] [CrossRef]
- Lewis, F. Hydrogen pressure-hydrogen content and electrical resistance-hydrogen content relationships of palladium and palladium alloy-hydrogen systems. Int. J. Hydrog. Energy 1995, 20, 369–372. [Google Scholar] [CrossRef]
- Tosti, S.; Rizzello, C.; Borgognoni, F.; Ghirelli, N.; Santucci, A.; Trabuc, P. Design of Pd-based membrane reactor for gas detritiation. Fusion Eng. Des. 2011, 86, 2180–2183. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kimura, S. Separation and concentration of hydrogen isotopes by palladium-alloy membrane. J. Nucl. Sci. Technol. Jpn. 1984, 26, 802–810. [Google Scholar] [CrossRef]
- Mitsuishi, N.; Yuki, T.; Ichihara, I. Characteristics of the permeation of hydrogen-inert gas mixtures through a palladium alloy tube wall. J. Less Common Met. 1983, 89, 415–422. [Google Scholar] [CrossRef]
- Luo, W.; Cowgill, D.F.; Stewart, K. Absorption isotherms for H2(D2)-Pd0.8Ag0.2 (198–323 K). J. Alloy. Compd. 2010, 489, 47–50. [Google Scholar] [CrossRef]
- Nishiwaka, M.; Shiraishi, T.; Kawamura, Y.; Takeishi, T. Permeation rate of hydrogen isotopes through palladium-silver alloy. J. Nucl. Sci. Technol. 1996, 33, 774–780. [Google Scholar] [CrossRef]
- Ackerman, F.J.; Koskinas, G.J. Permeation of Hydrogen and Deuterium through Palladium-Silver. J. Chem. Eng. Data 1972, 17, 51–55. [Google Scholar] [CrossRef]
- Lässer, R.; Powell, G. Solubility of protium, deuterium and tritium in palladium-silver alloys at low hydrogen concentrations. J. Less Common Met. 1987, 130, 387–394. [Google Scholar] [CrossRef]
- Paolone, A.; Tosti, S.; Santucci, A.; Palumbo, O.; Trequattrini, F. H2 and D2 Solubility in Commercial Pd-Ag Alloys for Hydrogen Purification. ChemEngineering 2017, 1, 14. [Google Scholar] [CrossRef]
- Anand, N.S.; Pati, S.; Jat, R.A.; Parida, S.C.; Mukerjee, S.K. Thermodynamics and kinetics of hydrogen/deuterium absorptione desorption in Pd0.77Ag0.23 alloy. Int. J. Hydrog. Energy 2015, 40, 444–450. [Google Scholar] [CrossRef]
- Hickman, R.G. Diffusion and permeation of deuterium in palladium-silver at high temperature and pressure. J. Less Common Met. 1969, 19, 369–383. [Google Scholar] [CrossRef]
- Bambakidis, G.; Smith, R.J.; Otterson, D.A. Electrical resisitivity versus Deuterium concentration in Palladium. Phys. Rev. 1969, 177, 1044–1048. [Google Scholar] [CrossRef]
- Smith, R.J. Anomalous Electrical Resitivity between 4.2 and 300 K of Palladium-Deuterium; NASA Technical Note D-2568; National Aeronautics and Space Administration: Washington, DC, USA, 1965. [Google Scholar]
- McNicholl, R.A.; Lewis, F.A. Hydrogen contents and electrical resistivity of palladium—Silver alloys. Int. J. Altern. Energy Ecol. 2004, 3, 32–33. [Google Scholar]
- Carson, A.W.; Lewis, A.; Schurter, H. Relationships between the Hydrogen Content and Electrical Resistance of Palladium + Silver Alloys. Trans. Faraday Sec. 1967, 43, 1447–1452. [Google Scholar] [CrossRef]
- Tóth, J.; Garaguly, J.; Peter, L.; Tompa, K. Resistivity changes during hydrogenation of Pd80Ag20 alloy in non-equilibrium circumstances. J. Alloy. Compd. 2000, 312, 117–120. [Google Scholar] [CrossRef]
- Pozio, A.; Jovanović, Z.; Presti, R.L.; De Francesco, M.; Tosti, S. Pd–Ag hydrogen content and electrical resistivity: Temperature and pressure effect. Int. J. Hydrog. Energy 2012, 37, 7925–7933. [Google Scholar] [CrossRef]
- Gallagher, P.T.; Oates, W.A. Vibrational entropies of hydrogen in palladium-silver-hydrogen alloys by the isotopic solubility ratio method. J. Phys. Chem. Solids 1971, 322, 2105–2110. [Google Scholar] [CrossRef]
- Bourgeois, N.; Crivello, J.-C.; Cenedese, P.; Paul-Boncour, V.; Joubert, J.-M. Vibration analysis of hydrogen, deuterium and tritium in metals: Consequences on the isotope effect. J. Phys. Condens. Matter 2018, 30, 335402. [Google Scholar] [CrossRef]
- Geerken, B.M.; Griessen, R.; Huisman, L.M.; Walker, E. Contribution of optical phonons to the elastic moduli of PdHx and PdDx. Phys. Rev. 1982, 26, 1637–1650. [Google Scholar] [CrossRef]
- Sonwane, C.G.; Wilcox, J.; Ma, Y.H. Achieving optimum hydrogen permeability in PdAg and PdAu alloys. J. Chem. Phys. 2006, 125, 184714. [Google Scholar] [CrossRef] [PubMed]
- Løvvik, O.M.; Olsen, R.A. Density functional calculations on hydrogen in palladium–silver alloys. J. Alloys Compd. 2002, 330, 332–337. [Google Scholar] [CrossRef]
- Sicking, G. Isotope effects in metal-hydrogen systems. J. Less Common Met. 1984, 101, 169–190. [Google Scholar] [CrossRef]
- Wiswall, R.H.; Reilly, J.J. Inverse Hydrogen Isotope Effects in Some Metal Hydride Systems. Inorg. Chem. 1972, 11, 1691–1696. [Google Scholar] [CrossRef]
- Bellini, S.; Sun, Y.; Gallucci, F.; Caravella, A. Thermodynamic Aspects in Non-Ideal Metal Membranes for Hydrogen Purification. Membrane 2018, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Pozio, A.; Jovanović, Z.; Tosti, S. Hydrogen absorption in Pd-Ag system: A TPD and electrical resistivity study. Materials 2019, 12, 3160. [Google Scholar] [CrossRef]
- Serra, E.; Kemali, M.; Perujo, A.; Ross, D.K. Hydrogen and Deuterium in Pd-25 Pct Ag Alloy: Permeation, Diffusion, Solubilization, and Surface Reaction. Metall. Mater. Trans. A 1998, 29, 1023–1028. [Google Scholar] [CrossRef]
- Nishiwaka, M.; Shiraishi, T.; Murakami, K. Solubility and Separation Factor of Protium-Deuterium Binary Component System in Palladium. J. Nucl. Sci. Technol. 1996, 33, 504–510. [Google Scholar] [CrossRef]
- Brodowsky, H.; Poeschel, E. Wasserstoff in Pd/Ag-Legierungen. Z. Phys. Chem. NF 1965, 44, 143–159. [Google Scholar] [CrossRef]
- Millet, P.; Lebouin, C.; Decaux, C.; Ngameni, R.; Ranjbari, A.; Guymont, M. Characterization of metal hydrides using pneumato-chemical impedance spectroscopy. Int. J. Hydrog. Energy 2009, 34, 4990–4996. [Google Scholar] [CrossRef]
- Jemaa, N.; Grandjean, B.P.A.; Kaliaguine, S. Diffusion coefficient of hydrogen in a Pd-Ag membrane: Effect of hydrogen solubility. Can. J. Chem. Eng. 1995, 73, 405–410. [Google Scholar] [CrossRef]
- Catti, M.; Fabelo, O.; Filabozzi, A.; Pietropaolo, A.; Tosti, S.; Pozio, A.; Santucci, A. Neutron diffraction study of the Pd0.772Ag0.228Dn membrane for hydrogen separation. Int. J. Hydrog. Energy 2017, 42, 6787–6792. [Google Scholar] [CrossRef]
- Oriani, R.A. The Physical and Metallurgical Aspects of Hydrogen in Metals. In Proceedings of the Fourth International Conference on Cold Fusion, Lahaina, Maui, HI, USA, 6–9 December 1993; p. 94304. [Google Scholar]
- Bickel, P.W.; Berlincourt, T.G. Optical Mode Scattering Contribution to Electrical Resistivity in Zirconium Hydride. Phys. Rev. B 1960, 119, 1603–1604. [Google Scholar] [CrossRef]
- Tsuchiya, B.; Teshigawara, M.; Konashi, K.; Nagata, S.; Shikama, T.; Yamawaki, M. Isotope Effects in Thermal Diffusivity and Electrical Resistivity of Zirconium Hydride and Deuteride. J. Nucl. Sci. Technol. 2002, 39, 402–406. [Google Scholar] [CrossRef]
- Bickel, P.W.; Berlincourt, T.G. Electrical properties of hydrides and deuterides of zirconium. Phys. Rev. B 1970, 2, 4807–4813. [Google Scholar] [CrossRef]
- Tripodi, P.; McKubre, M.; Tanzella, F.; Honnor, P.; Di Gioacchino, D.; Celani, F.; Violante, V. Temperature coefficient of resistivity at compositions approaching PdH. Phys. Lett. A 2000, 276, 122–126. [Google Scholar] [CrossRef]
- Rosenberg, H.M. The Solid State, 3th ed.; Oxford Science Publications: Oxford, UK, 1988. [Google Scholar]
- Mott, N.F. The Basis of the Electron Theory of Metals, with Special Reference to the Transition Metals. Proc. Phys. Soc. 1949, 62, 416–422. [Google Scholar] [CrossRef]
- Fort, D.; Harris, I.R. The physical properties of some palladium alloy hydrogen diffusion membrane material. J. Less Common Metal. 1975, 41, 313–327. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozio, A.; Tosti, S. Pd-Ag Electrical Resistivity in Hydrogen and Deuterium: Temperature Effect. Materials 2019, 12, 3551. https://doi.org/10.3390/ma12213551
Pozio A, Tosti S. Pd-Ag Electrical Resistivity in Hydrogen and Deuterium: Temperature Effect. Materials. 2019; 12(21):3551. https://doi.org/10.3390/ma12213551
Chicago/Turabian StylePozio, Alfonso, and Silvano Tosti. 2019. "Pd-Ag Electrical Resistivity in Hydrogen and Deuterium: Temperature Effect" Materials 12, no. 21: 3551. https://doi.org/10.3390/ma12213551
APA StylePozio, A., & Tosti, S. (2019). Pd-Ag Electrical Resistivity in Hydrogen and Deuterium: Temperature Effect. Materials, 12(21), 3551. https://doi.org/10.3390/ma12213551





