Intercalated Intermetallic Compounds AlTi3 and Fe2Ti in Microrods and Microtubes Obtained by Invariant Reaction of Mechanically Milled System Al43Ti36Fe21
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
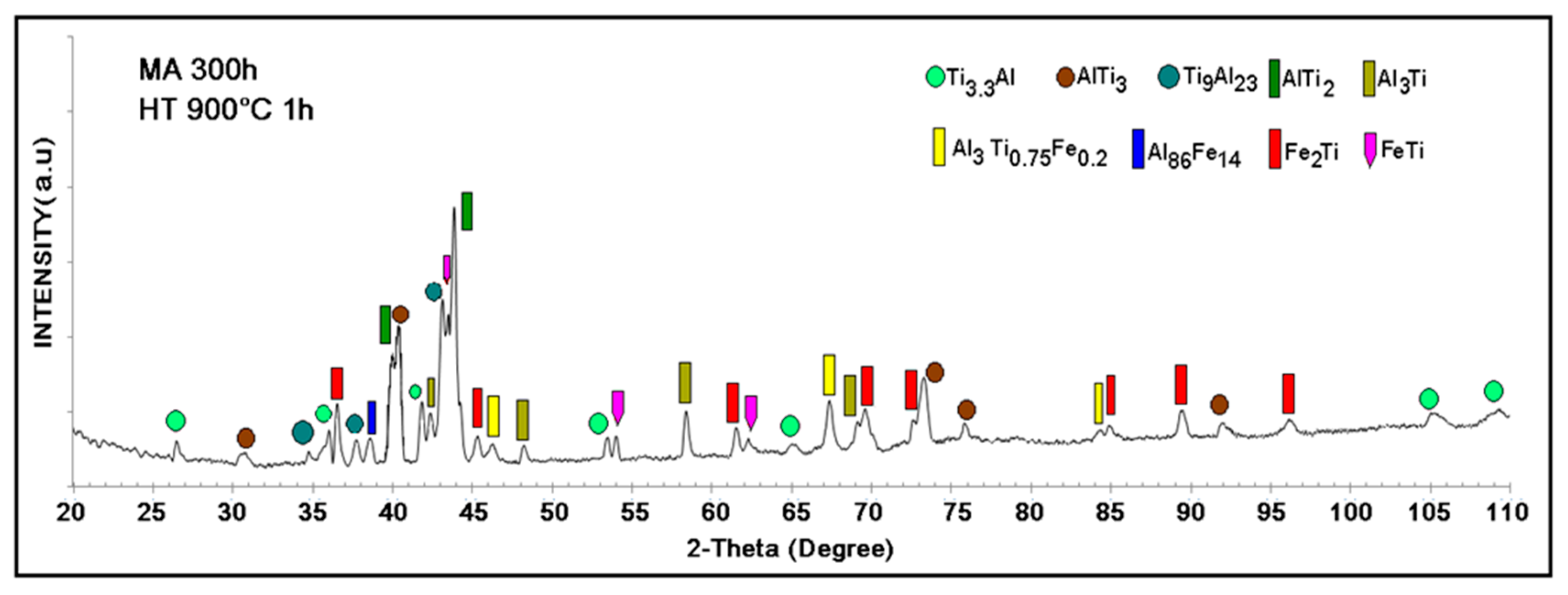
3.1. X-Ray Diffraction Microstructural Characterization
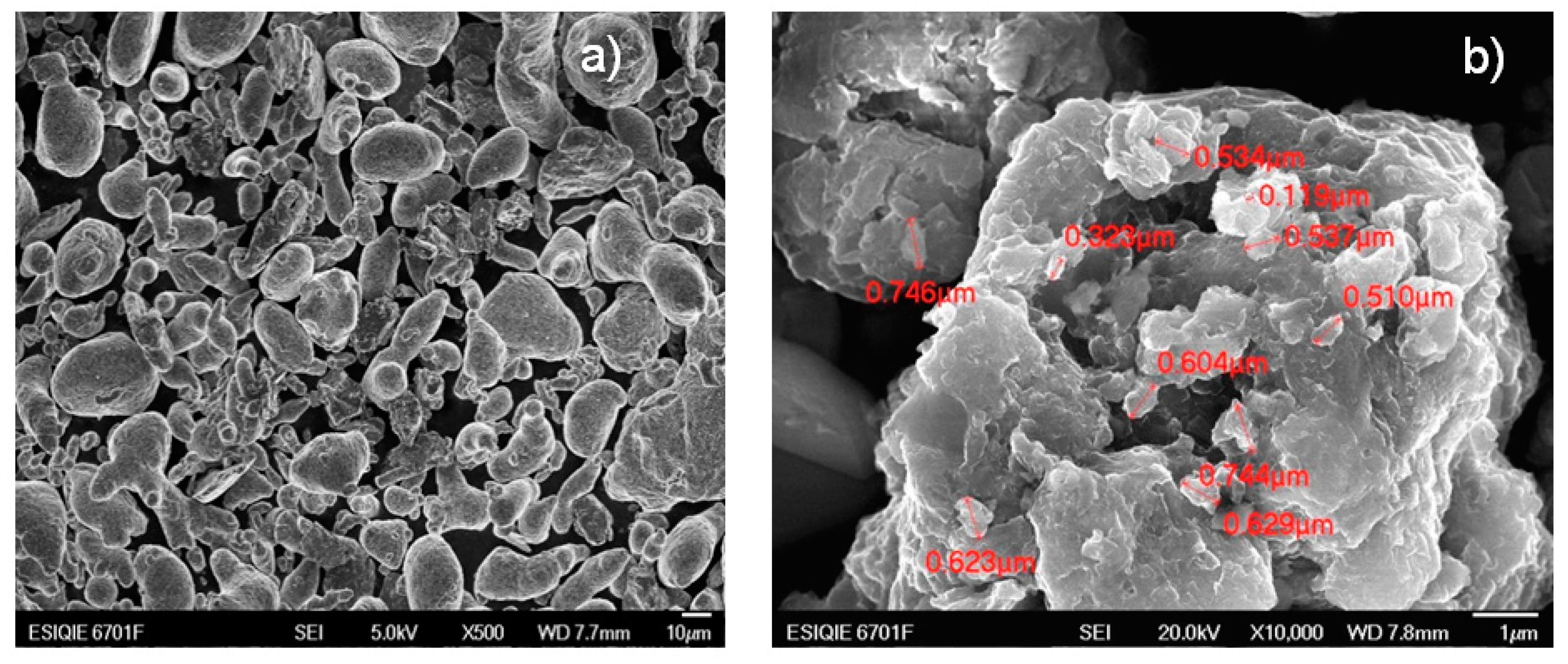
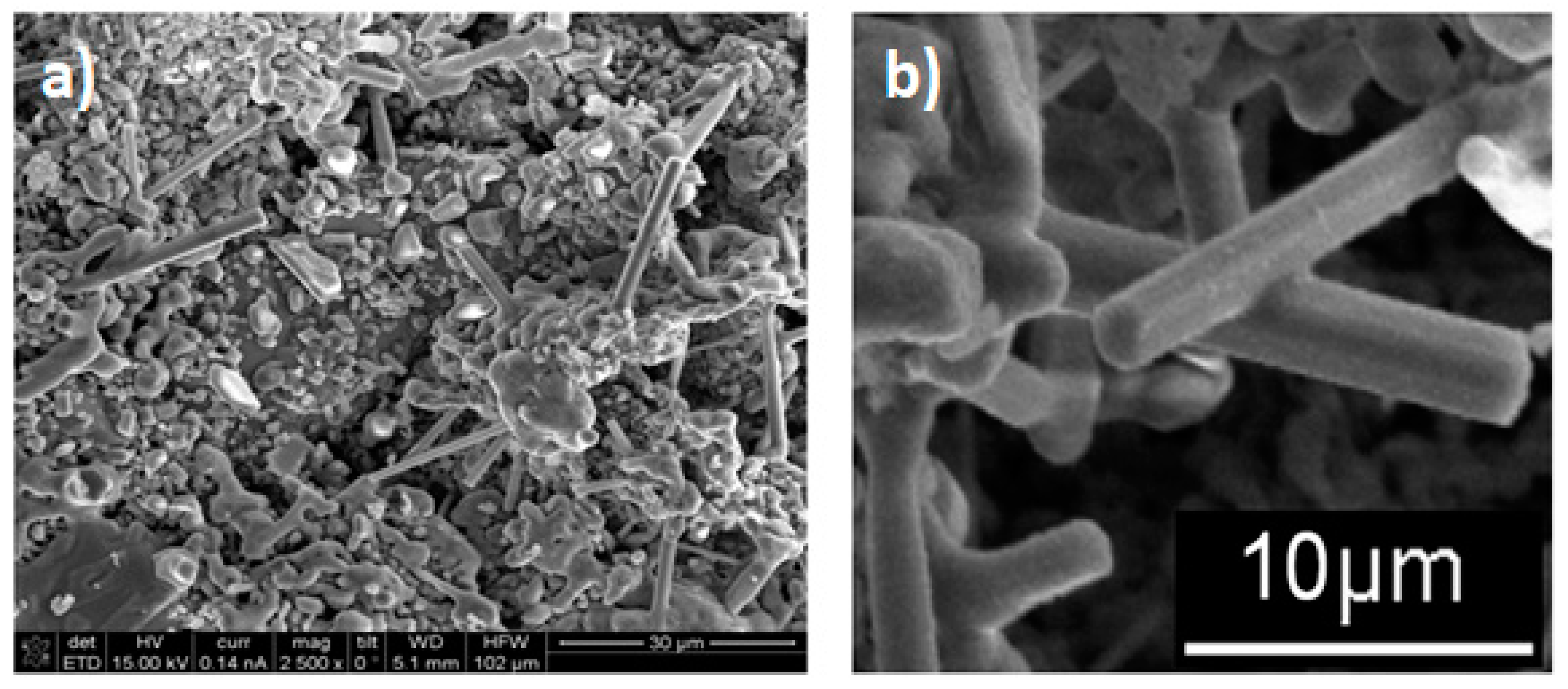
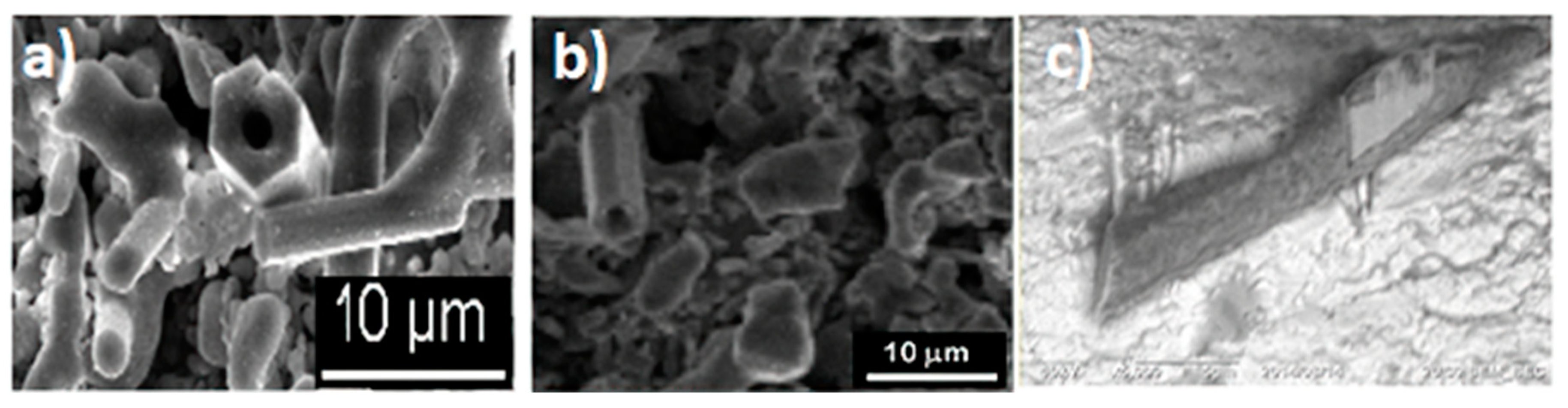
3.2. Scanning Electron Microscopy
3.3. High-Resolution Transmission Electron Microscopy (HRTEM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tavakoli, A.; Sohrabi, M.; Kargari, A. A Review of Methods for Synthesis of Nanostructured Metals with Emphasis on Iron Compounds, Institute of Chemistry, Slovac Academy of Sciences. Chem. Pap. Spinger Link 2007, 61, 151–170. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nat. Protoc. 2006, 1, 1711. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zheng, X.; Kempa, T.J.; Fang, Y.; Yu, N.; Yu, G.; Huang, J.; Lieber, C.M. Coaxial silicon nanowires as solar cells and nanoelectronic power sources. Nature 2007, 449, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wu, Y.S.; Li, W.; Xiu, Y. Preparation of La(OH)3 and La2O3 with Rod Morphology by Simple Hydration of La2O3. Ceram. Int. 2018, 44, 19301–19306. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Matin, S.M.; Sönke, F.; Milena, E.; Wei, J.; Wehmann, H.H.; Andreas, W.; Martin, M.; Werner, B. Dependence of N-polar GaN rod morphology on growth parameters during selective area growth by MOVPE. J. Cryst. Growth 2013, 364, 149–154. [Google Scholar] [CrossRef]
- Gromykoa, I.; Dedovaa, T.; Polivtsevaa, S.; Koisa, J.; Puust, L.; Sildos, I.; Merea, A.; Krunks, M. Electrodeposited ZnO morphology transformations under the influence of SeO2 additive: Rods, disks, nanosheets network. Thin Solid Film. 2018, 652, 10–15. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, C.; Zhang, H.; Lu, G. Effects of bath temperature on the morphology of ZnO nano-rods and its optical properties. Mater. Lett. 2015, 148, 1–4. [Google Scholar] [CrossRef]
- Quitoriano, N.J.; Evoy, S.; Belov, M.; Theodore, I.; Kamins, T.I. Single-Crystal Si Nanotubes, and Their Mechanical Resonant Properties. Nano Lett. 2009, 9, 1511–1516. [Google Scholar] [CrossRef]
- Mamat, M.H.; Khusaimi, Z.; Zahidi, M.M.; Bakar, S.A.; Siran, Y.; Mohd, R.; Syahril, A.; Asis, A.J.; Tahirudin, S.; Abdullah, S.; et al. Controllable Growth of Vertically Aligned Aluminum-Doped Zinc Oxide Nanorod Arrays by Sonicated Sol—Gel Immersion Method depending on Precursor Solution Volumes. Jpn. J. Appl. Phys. 2011, 50, 1–5. [Google Scholar] [CrossRef]
- Kumar, V.; Wang, X.; Lee, P.S. Synthesis of pyramidal and prismatic hexagonal MoO3 nanorods using thiourea. CrystEngComm 2013, 15, 7663–7669. [Google Scholar] [CrossRef]
- Xudong, W. Large-Scale Hexagonal-Patterned Growth of Aligned ZnO Nanorods for Nano-optoelectronics and Nano sensor Arrays. Nano Lett. 2004, 4, 423–426. [Google Scholar]
- Yazdanpanah, M.M.; Harfenist, S.A.; Safir, A.; Cohn, R.W. Selective self-assembly at room temperature of individual freestanding Ag2Ga alloy nanoneedles. J. Appl. Phys. 2005, 98, 073510. [Google Scholar] [CrossRef]
- Jalilian, R.; Rivera, J.; Askari, D.; Arva, S.; Rathfon, J.M.; Cohn, R.W.; Yazdanpanah, M.M. Toward wafer-scale patterning of freestanding intermetallic nanowires. Nanotechnology 2011, 22, 295601–295607. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.S.; Girolami, G.; Amano, J.; Islam, M.S.; Sharma, S.; Kamins, T.I.; Kimukin, I. InP nanobridges epitaxially formed between two vertical Si surfaces by metal-catalyzed chemical vapor deposition. Appl. Phys. Lett. 2006, 89, 133121. [Google Scholar] [CrossRef]
- Xu, C.K.; Shin, P.H.; Cao, L.; Wu, J.; Gao, D. Ordered TiO2 Nanotube Arrays on Transparent Conductive Oxide for Dye-Sensitized Solar Cells. Chem. Mater. 2010, 22, 143. [Google Scholar] [CrossRef]
- Lind, H. Modulations in Intermetallic Families of Compounds. Ph.D. Thesis, Department of Inorganic Chemistry, Stockholm University, Stockholm, Sweden, 2004; pp. 1–20. Available online: https://www.diva-portal.org/smash/get/diva2:192339/FULLTEXT01.pdf (accessed on 12 November 2019).
- Gutiérrez, K.; Rodríguez, J.; Aray, Y.; Luiggi, N. Topological study of Charge Density in AlTi, AlTi3 and Al3Ti intermetallics. J. Comput. Methods Sci. Eng. 2012, 12, 361–370. [Google Scholar] [CrossRef]
- Kostov, A.; Friedrich, B.; Živković, D. Thermodynamic calculations in alloys Ti-Al, Ti-Fe, Al-Fe AND Ti-Al-Fe. J. Min. Metall. 2008, 44, 49–61. [Google Scholar] [CrossRef]
- Suyanarayana, C. Progress in Materials Science; Elsevier: Amsterdam, The Netherlands, 2011; Volume 46, pp. 1–184. [Google Scholar]
- Chien, C.L.; Liou, S.H. Crystalline and amorphous FeTi and Fe2Ti. Phys. Rev. 1985, 31, 8238. [Google Scholar] [CrossRef]
- Davis, S.H. Theory of Solidification, 1st ed.; Cambridge University Press: Cambridge, UK, 2001; pp. 255–273. [Google Scholar]
- Suryanarayana, C. Mechanical Alloying and Milling; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloiteilchen mittels Röntgenstrahlen. Nachrichten von der Gesellschaft der Wissenschaften zu Gottingen. Math. Phys. 1918, 1–2, 96–100. [Google Scholar]
- Tetsuji, S. Magnetic properties of Ti–Fe alloy powders prepared by mechanical grinding. J. Alloy. Compd. 2004, 364, 113–116. [Google Scholar] [CrossRef]
- Flemings, M.C. Solidification Processing; McGraw Hill: New York, NY, USA, 1974; Volume 1, pp. 94–133. [Google Scholar]
- Petr, Š.; Martin, F.; David, H.; Monika, V.; Mojmír, Š. Strength and Brittleness of Interfaces in Fe-Al Superalloy Nanocomposites under Multiaxial Loading: An ab initio and Atomistic Study. Nanomaterials 2018, 8, 873. [Google Scholar] [CrossRef]
- Callister, W.D., Jr.; David, G.R. Materials Science and Engineering, 9th ed.; Wiley: Hoboken, NJ, USA, 2015; ISBN 978-0-470-41997-7. [Google Scholar]
- Patricia, D. Nanoengineering, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780444627452. [Google Scholar]





| Metal | PDF Card Number | Phase | Lattice Parameters |
|---|---|---|---|
| Al | 65–2869 | Cubic F | a = 4.049 Å |
| Ti | 89–2762 | Hexagonal | a = 2.951 Å, c = 4.684 Å |
| Fe | 65–4899 | Cubic I | a = 2.867 Å |
| Milled for 300 h (a) Milled and Sintered at 900 °C (b) | Phase | Wt % | Structure Lattice Parameters (Å) |
|---|---|---|---|
| a,b | AlTi3 | 68.11 (a) 17.82 (b) | Hexagonal a = 5.79, c = 4.64 |
| a | Fe3Al | 31.89 (a) | Cubic F, a = 5.791 |
| b | AlTi3.3 | 17.29 | Hexagonal a = 11.52, c = 4.65 |
| b | Al23Ti9 | 6.96 | Tetragonal I, a = 3.89, c = 33.46 |
| b | AlTi2 | 9.84 | Hexagonal a = 7.85, c = 4.79 |
| b | Al3Ti | 10.05 | Tetragonal I, a = 3.84, c = 8.59 |
| b | Fe2Ti | 20.02 | Hexagonal a = 7.85, c = 4.29 |
| b | FeTi | 9.01 | Cubic P a = 2.97 |
| b | Al86Fe14 | 2.11 | None reported |
| b | Al3Ti0.75Fe0.2 | 6.74 | Cubic P a = 3.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz Barriga Arceo, L.G.; González Reyes, L.; Rivera Olvera, J.N.; Medina Ovando, A.; Garibay Febles, V. Intercalated Intermetallic Compounds AlTi3 and Fe2Ti in Microrods and Microtubes Obtained by Invariant Reaction of Mechanically Milled System Al43Ti36Fe21. Materials 2019, 12, 3806. https://doi.org/10.3390/ma12233806
Díaz Barriga Arceo LG, González Reyes L, Rivera Olvera JN, Medina Ovando A, Garibay Febles V. Intercalated Intermetallic Compounds AlTi3 and Fe2Ti in Microrods and Microtubes Obtained by Invariant Reaction of Mechanically Milled System Al43Ti36Fe21. Materials. 2019; 12(23):3806. https://doi.org/10.3390/ma12233806
Chicago/Turabian StyleDíaz Barriga Arceo, Lucía G., Leonardo González Reyes, Jesús Noé Rivera Olvera, Abraham Medina Ovando, and Vicente Garibay Febles. 2019. "Intercalated Intermetallic Compounds AlTi3 and Fe2Ti in Microrods and Microtubes Obtained by Invariant Reaction of Mechanically Milled System Al43Ti36Fe21" Materials 12, no. 23: 3806. https://doi.org/10.3390/ma12233806
APA StyleDíaz Barriga Arceo, L. G., González Reyes, L., Rivera Olvera, J. N., Medina Ovando, A., & Garibay Febles, V. (2019). Intercalated Intermetallic Compounds AlTi3 and Fe2Ti in Microrods and Microtubes Obtained by Invariant Reaction of Mechanically Milled System Al43Ti36Fe21. Materials, 12(23), 3806. https://doi.org/10.3390/ma12233806





