Feasibility of Using 3D Printed Polyvinyl Alcohol (PVA) for Creating Self-Healing Vascular Tunnels in Cement System
Abstract
:1. Introduction
2. Materials and Methods
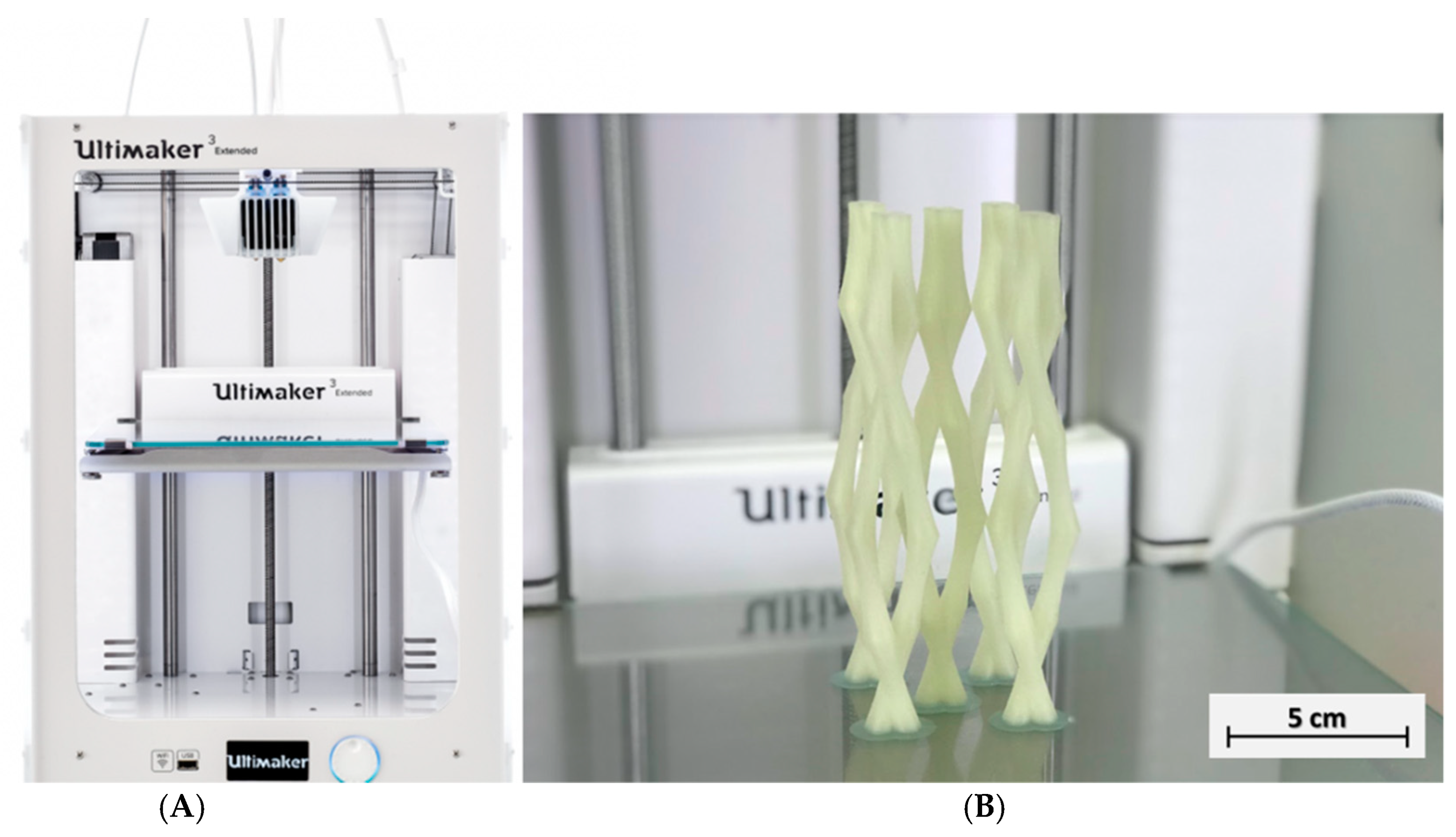
2.1. Fabrication of 3D Printed PVA Vascular Structures and Cement Samples
2.2. The Cementitious Matrix
2.3. Characterization Methods
3. Results and Discussion
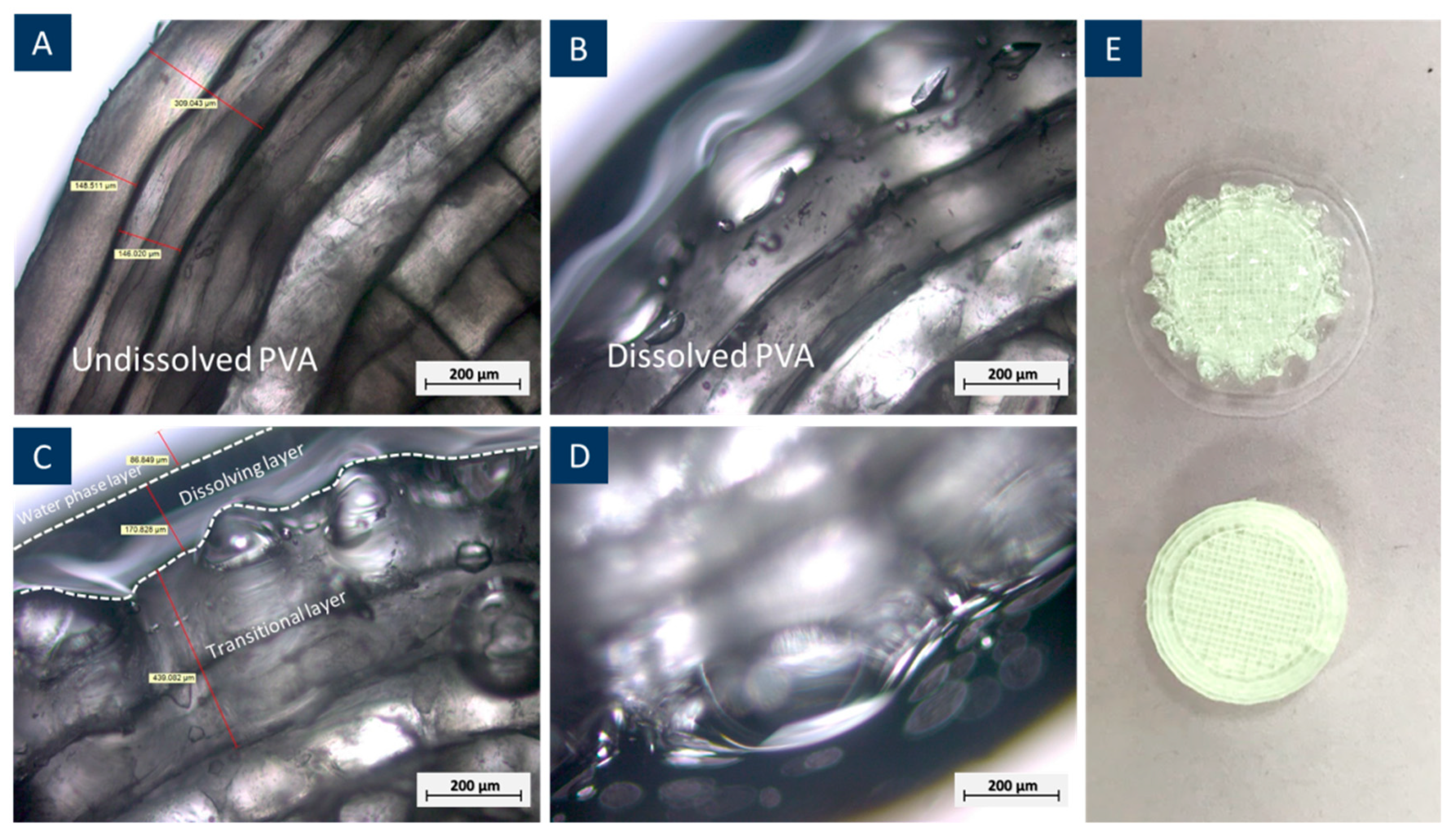
3.1. Chemical Property and Dissolution Behaviour of the 3D Printed PVA
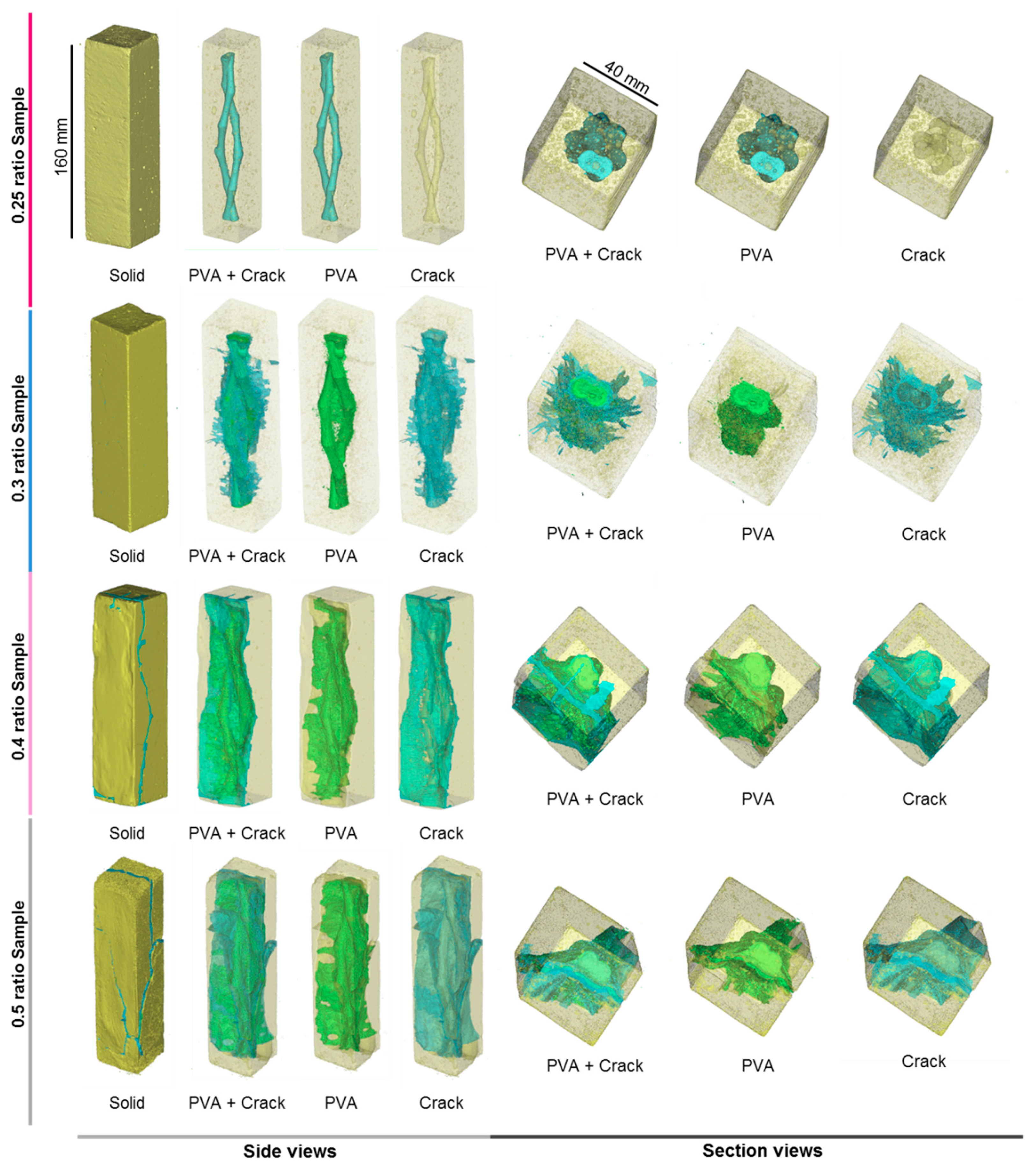
3.2. PVA Expansion in Cement Prisms
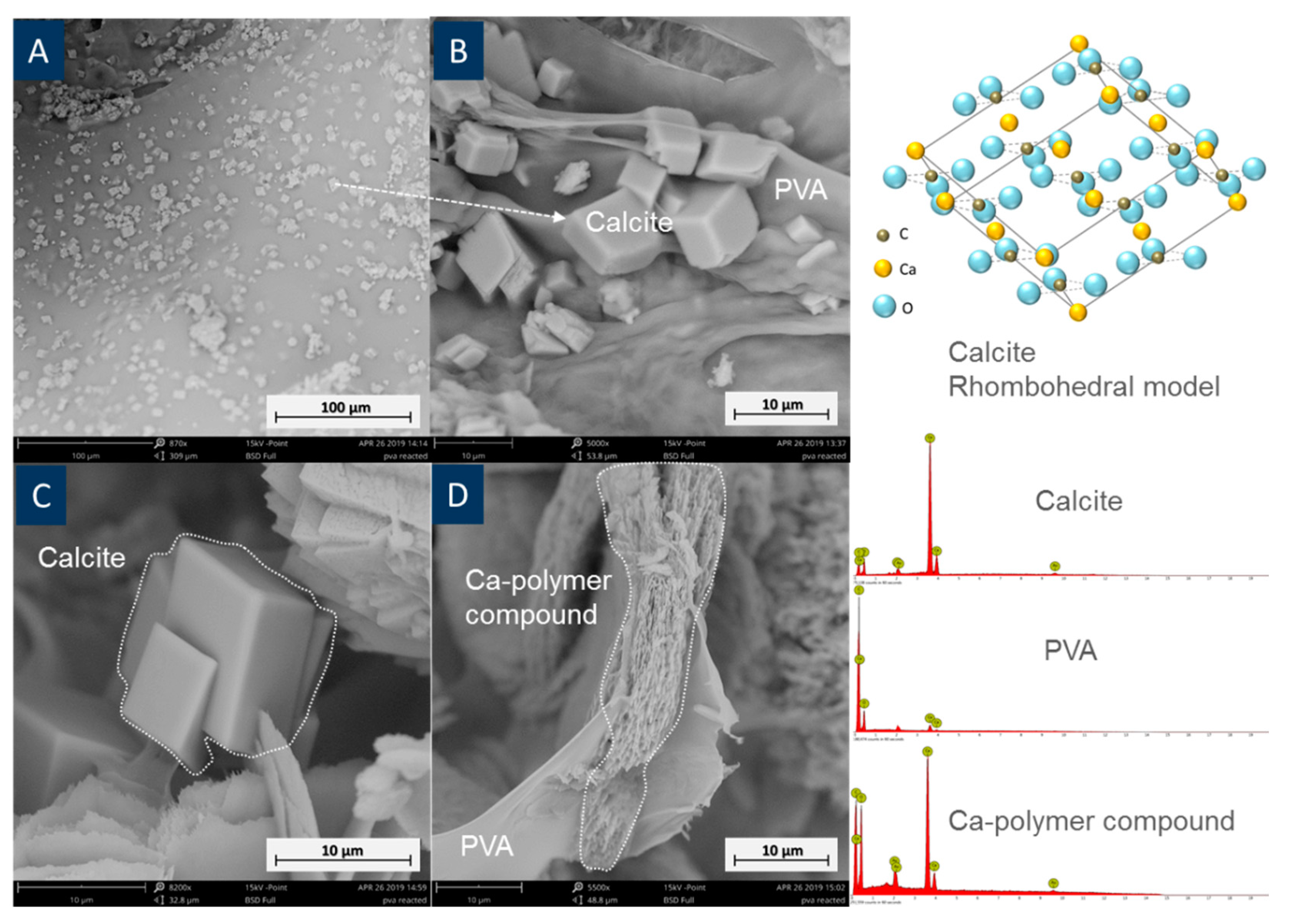
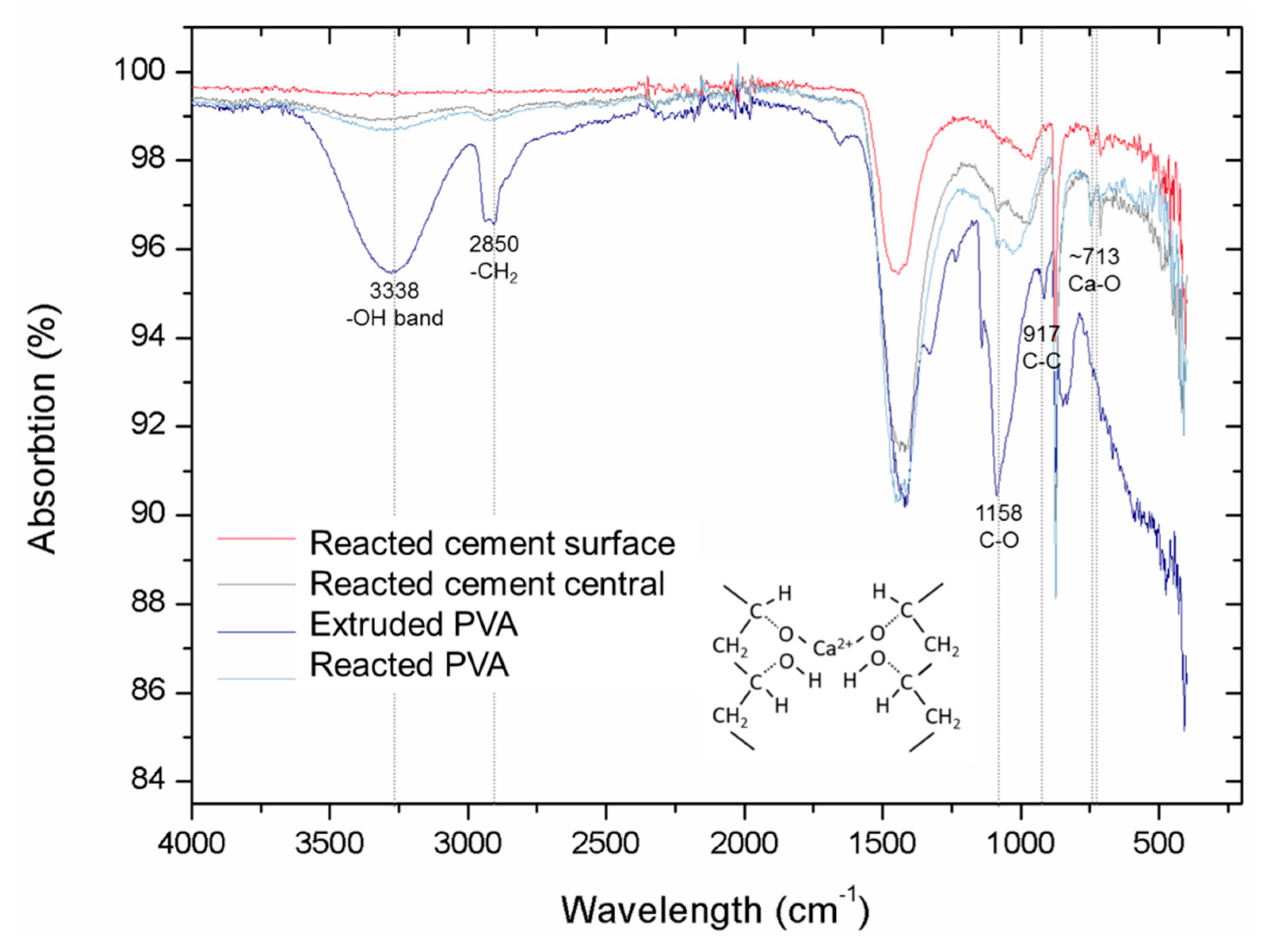
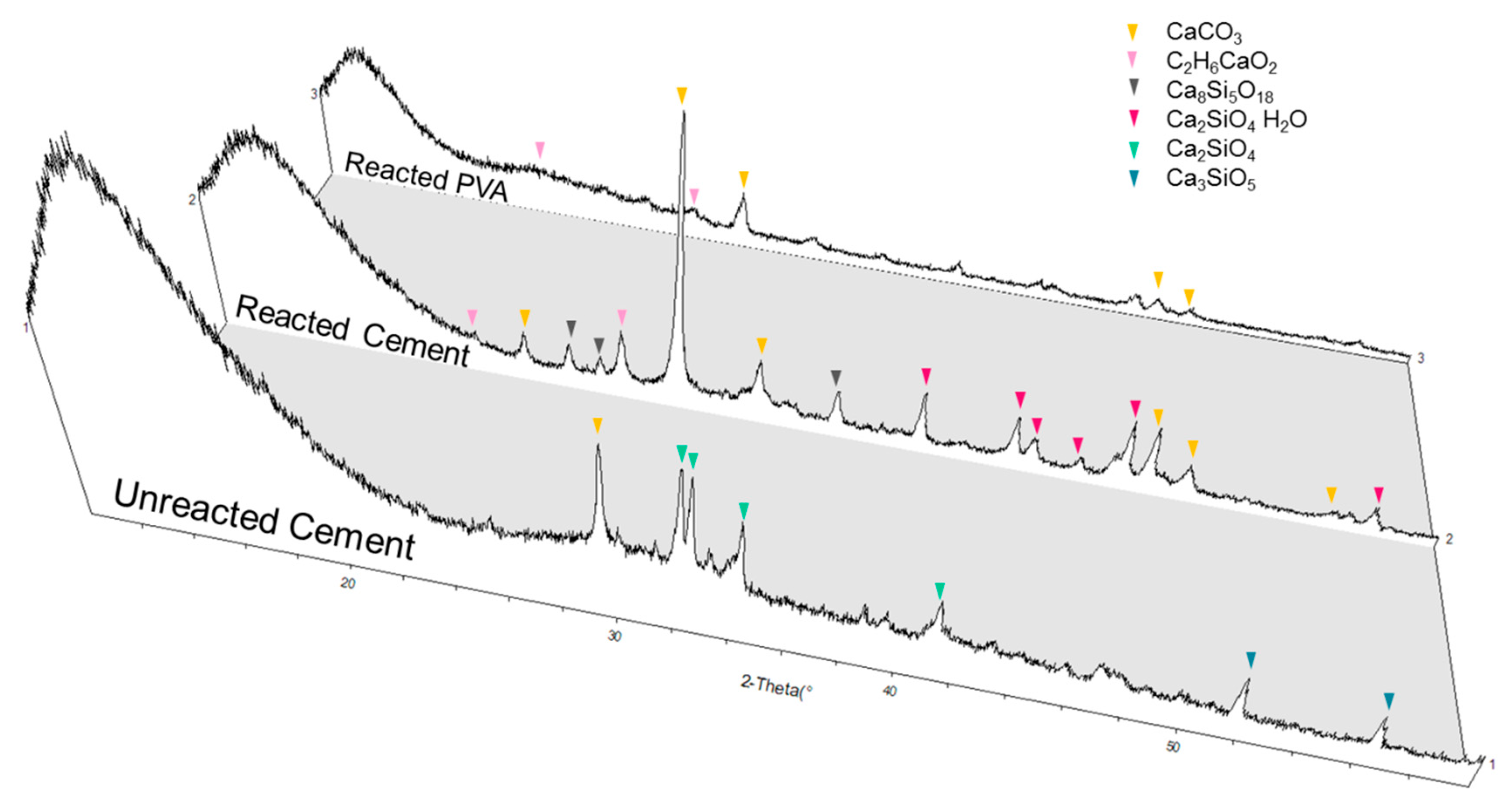
3.3. Interaction between PVA and Cement
3.4. Comparison with Existing Techniques and Cost Anaylsis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sangadji, S. Can self-healing mechanism helps concrete structures sustainable? Procedia Eng. 2017, 171, 238–249. [Google Scholar] [CrossRef]
- Concrete Society. Non-Structural Cracks in Concrete: A Concrete Society Report; Concrete Society: London, UK, 2010. [Google Scholar]
- Tilly, G.; Jacobs, J. Concrete Repairs: Performance in Service and Current Practice; Taylor and Francis: London, UK, 2007. [Google Scholar]
- De Rooij, M.; Van Tittelboom, K.; De Belie, N.; Schlangen, E. State-of-the-Art Report of RILEM Technical Committee 221-SHC: Self-Healing Phenomena in Cement-Based Materials; Springer: New York city, NY, USA, 2013; pp. 8–12. [Google Scholar]
- Aldea, C.M.; Song, W.J.; Popovics, J.S.; Shah, S.P. Extent of healing of cracked normal strength concrete. J. Mater. Civ. Eng. 2000, 12, 92–96. [Google Scholar] [CrossRef]
- Litina, C.; Kanellopoulos, A.; Al-Tabbaa, A. Alternative repair system for concrete using microencapsulated healing agents. Concr. Solut. 2014, 2014, 97–103. [Google Scholar]
- Alghamri, R.J.; Al-Tabbaa, A. Self-Healing Performance of Magnesia-Based Pellets in Concrete. In Proceedings of the 2nd International Conference on Concrete Sustainability (ICCS16), Madrid, Spain, 13–15 June 2016; International Centre for Numerical Methods in Engineering (CIMNE): Barcelona, Spain, 2016. [Google Scholar]
- Souza, L. Design and Synthesis of Microcapsules Using Microfluidics for Autonomic Self-Healing in Cementitious Materials. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2017. [Google Scholar]
- Li, V.C.; Lim, Y.M.; Chan, Y.W. Feasibility study of a passive smart self-healing cementitious composite. Compos. B Eng. 1998, 29, 819–827. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; de Belie, N.; van Loo, D.; Jacobs, P. Self-healing efficiency of cementitious materials containing tubular capsules filled with healing agent. Cem. Concr. Compos. 2011, 33, 497–505. [Google Scholar] [CrossRef]
- Minnebo, P.; Thierens, G.; de Valck, G.; van Tittelboom, K.; de Belie, N.; van Hemelrijck, D.; Tsangouri, E. A novel design of autonomously healed concrete: Towards a vascular healing network. Materials 2017, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Sisomphon, K.; Copuroglu, O.; Fraaij, A. Application of Encapsulated Lightweight Aggregate Impregnated with Sodium Monofluorophosphate as a Self-Healing Agent in Blast Furnace Slag Mortar. Heron 2011, 56, 17–36. [Google Scholar]
- Dry, C. Passive tuneable fibres and matrices. Int. J. Mod. Phys. B 1992, 6, 2763–2771. [Google Scholar] [CrossRef]
- Dry, C.M. Three designs for the internal release of sealants, adhesives, and waterproofing chemicals into concrete to reduce permeability. Cem. Concr. Res. 2000, 30, 1969–1977. [Google Scholar] [CrossRef]
- Joseph, C.; Jefferson, A.D.; Isaacs, B.; Lark, R.; Gardner, D. Experimental investigation of adhesive-based self-healing of cementitious materials. Mag. Concr. Res. 2010, 62, 831–843. [Google Scholar] [CrossRef]
- Davies, R.; Jefferson, A.; Lark, R.; Gardner, D. A novel 2D vascular network in cementitious materials. In Proceedings of the Concrete—Innovation and Design, Fib Symposium, Copenhagen, Denmark, 18–20 May 2015. [Google Scholar]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Formia, A.; Terranova, S.; Antonaci, P.; Pugno, N.; Tulliani, J. Setup of extruded cementitious hollow tubes as containing/releasing devices in self-healing systems. Materials 2015, 8, 1897–1923. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Wang, C.; Zhao, H. Fabrication of water-soluble poly (vinyl alcohol)-based composites with improved thermal behavior for potential three-dimensional printing application. J. Appl. Polym. 2017, 134. [Google Scholar] [CrossRef]
- Salentijn, G.I.; Oomen, P.E.; Grajewski, M.; Verpoorte, E. Fused deposition modeling 3D printing for (bio) analytical device fabrication: Procedures, materials, and applications. Anal. Chem. 2017, 89, 7053–7061. [Google Scholar] [CrossRef] [PubMed]
- Jamróza, W.; Kurek, M.; Łyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrekb, K.; Paluch, M.; Jachowicz, R. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm. 2017, 533, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Ore’fice, R.L.; Mansur, A.A.P. Characterization of poly (vinyl alcohol)/poly (ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Zheng, G.Q.; Li, X.D.; Wang, X.M.; Ma, J.F.; Gu, Z.W. Structural characteristics of poly (vinyl alcohol)–calcium carbonate composites prepared by sequential method. Adv. Appl. Ceram. 2008, 107, 46–51. [Google Scholar] [CrossRef]
- Danner, R.P.; High, M.S. Handbook of Polymer Solution Thermodynamics; American Institute of Chemical Engineers: New York, NY, USA, 1993. [Google Scholar]
- Park, S.J.; Lee, J.E.; Park, J.H.; Lee, N.K.; Lyu, M.; Park, K.; Koo, M.S.; Cho, S.H.; Son, Y.; Park, S. Enhanced Solubility of the Support in an FDM-Based 3D Printed Structure Using Hydrogen Peroxide under Ultrasonication. Adv. Mater. Sci. Eng. 2018, 2018, 3018761. [Google Scholar] [CrossRef]
- Bonapasta, A.A.; Buda, F.; Colombet, P.; Guerrini, G. Cross-linking of poly(vinyl alcohol) chains by Ca ions in macro-defect-free cements. Chem. Mater. 2002, 14, 1016–1022. [Google Scholar] [CrossRef]
- British Standards Institution, BS EN 00196-1-2005. Methods of Testing Cement; BSI: London, UK, 2005. [Google Scholar]
- Galván-Ruiz, M.; Hernández, J.; Baños, L.; Noriega-Montes, J.; Rodríguez-García, M.E. Characterization of calcium carbonate, calcium oxide, and calcium hydroxide as starting point to the improvement of lime for their use in construction. J. Mater. Civ. Eng. 2009, 21, 694–698. [Google Scholar] [CrossRef]
- MarketsandMarkets, Concrete Repair Mortars Market AD4535. Available online: https://www.marketsandmarkets.com/Market-Reports/concrete-repair-mortar-market-160103226.html (accessed on 5 August 2018).





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Souza, L.R.d.; Litina, C.; Markaki, A.E.; Al-Tabbaa, A. Feasibility of Using 3D Printed Polyvinyl Alcohol (PVA) for Creating Self-Healing Vascular Tunnels in Cement System. Materials 2019, 12, 3872. https://doi.org/10.3390/ma12233872
Li Z, Souza LRd, Litina C, Markaki AE, Al-Tabbaa A. Feasibility of Using 3D Printed Polyvinyl Alcohol (PVA) for Creating Self-Healing Vascular Tunnels in Cement System. Materials. 2019; 12(23):3872. https://doi.org/10.3390/ma12233872
Chicago/Turabian StyleLi, Zijing, Lívia Ribeiro de Souza, Chrysoula Litina, Athina E. Markaki, and Abir Al-Tabbaa. 2019. "Feasibility of Using 3D Printed Polyvinyl Alcohol (PVA) for Creating Self-Healing Vascular Tunnels in Cement System" Materials 12, no. 23: 3872. https://doi.org/10.3390/ma12233872




