Photo-Induced Super-hydrophilic Thin Films on Quartz Glass by UV Irradiation of Precursor Films Involving a Ti(IV) Complex at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Precursor Solution Involving Ti(IV) Complex
2.3. Fabrication of Precursor Film F0 and Ultraviolet (UV)-Irradiated Thin Films Fx
2.4. Characterization of Precursor Solution S, Precursor Film F0, and UV-Irradiated Films Fx
2.4.1. Optical Characterization of S, F0, and Fx
2.4.2. X-Ray Diffraction (XRD) Patterns of F0 and Fx
2.4.3. Chemical Characterization of F0 and Fx
2.4.4. Surface Morphology and Film Thickness of Fx
2.4.5. Pencil Scratching Test for Adhesion Strength of Fx
2.4.6. Photo-Induced Hydrophilicity of Fx
3. Results
3.1. Absorption Spectrum of S
3.2. Optical Properties of Precursor Film F0 and UV-Irradiated Films Fx
3.3. XRD Patterns of F0 and Fx
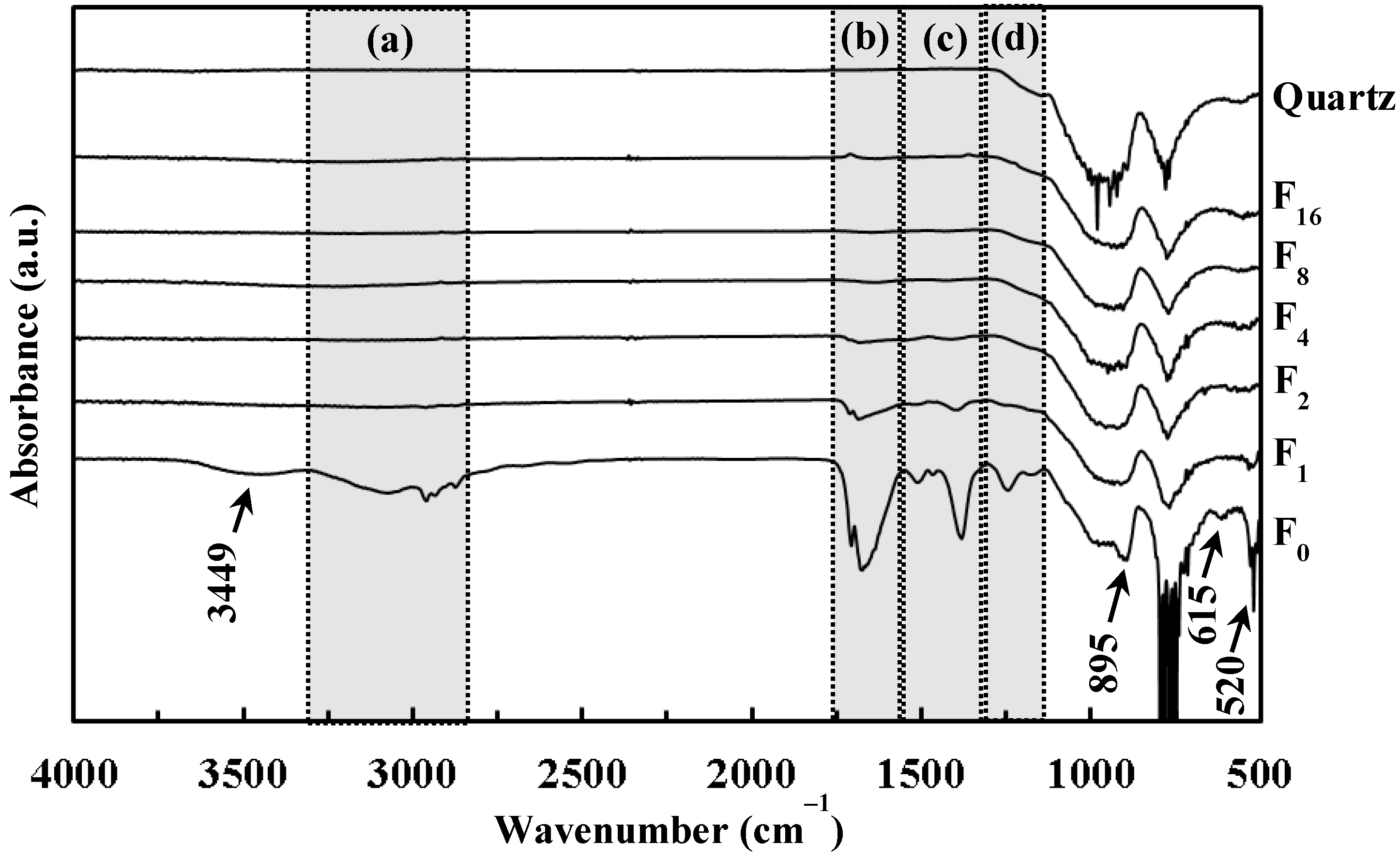
3.4. Fourier Transform-Infrared (FT-IR) Spectra of F0 and UV-Irradiated Films Fx, and Quartz Glass Substrate
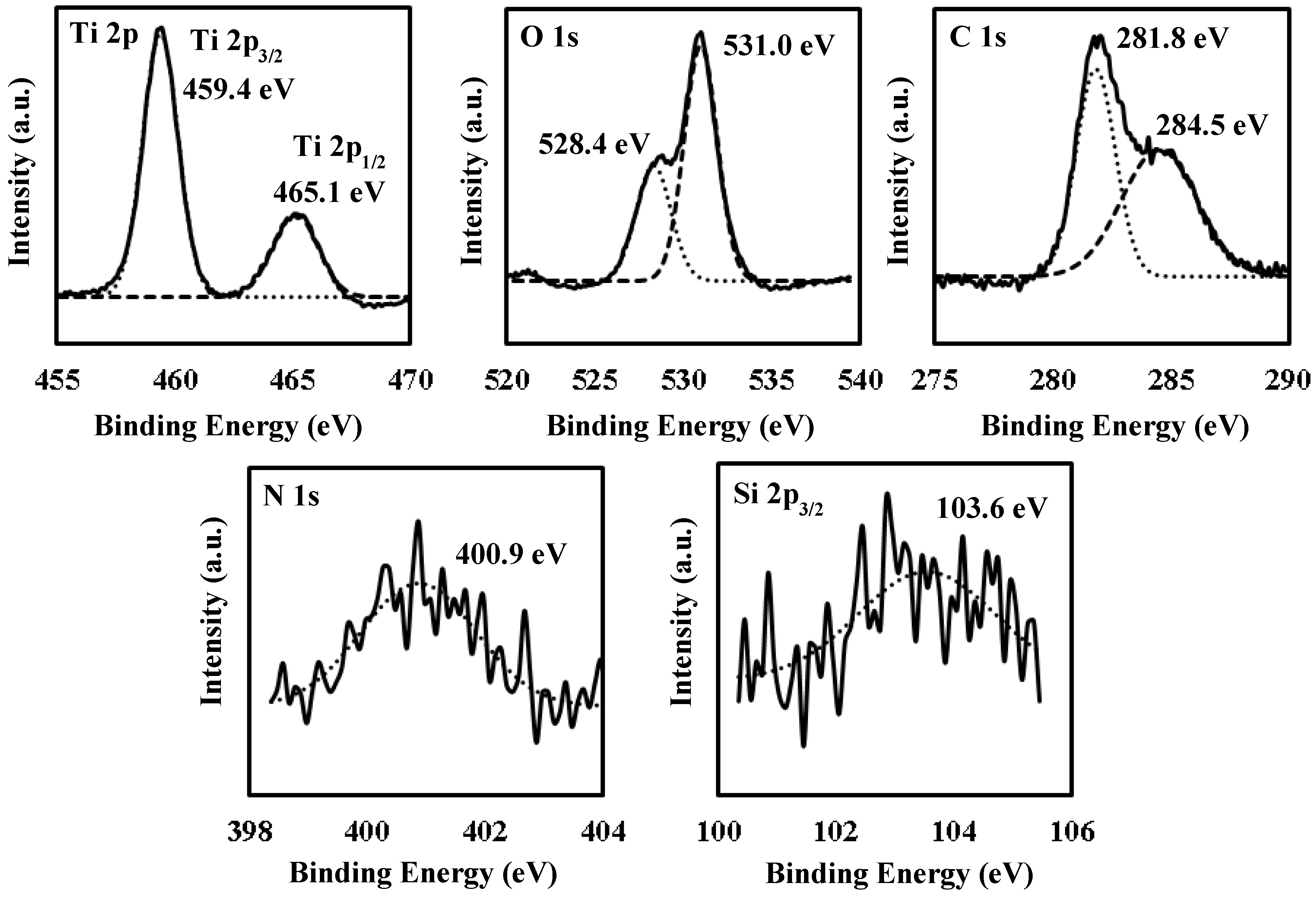
3.5. X-Ray Photoelectron Spectroscopy (XPS) Spectra of UV-Irradiated Film F4
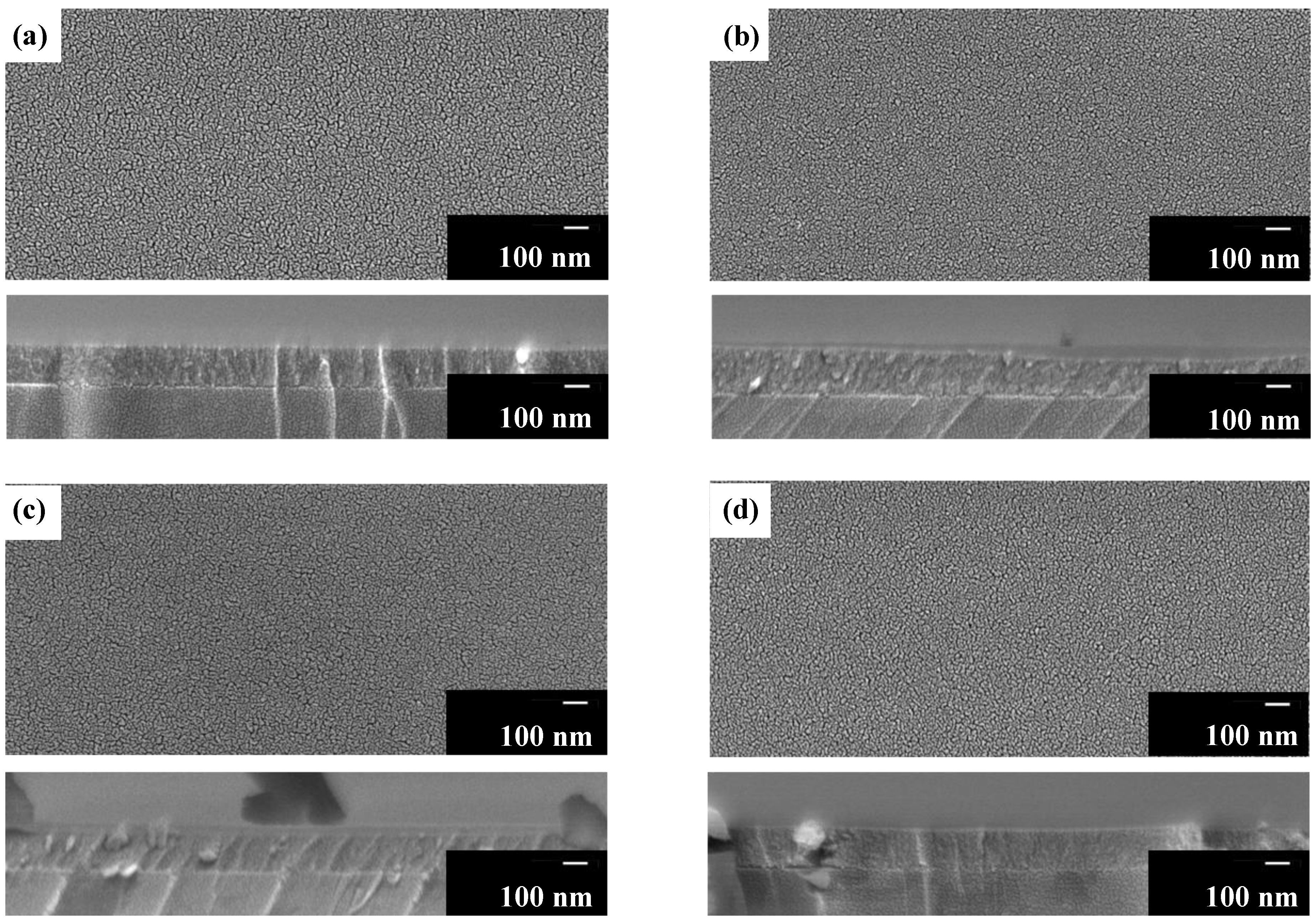
3.6. Surface Morphology, Film Thickness, and Adhesion Strength of UV-Irradiated Films Fx
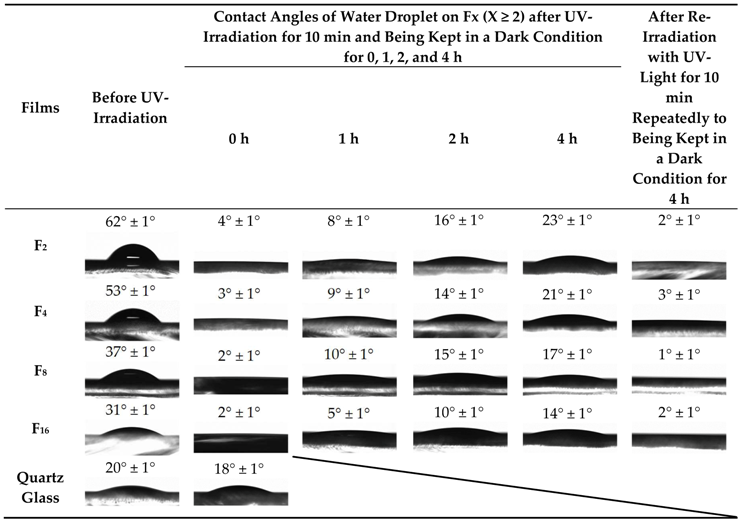
3.7. Contact Angles of Water Droplet on UV-Irradiated Films Fx
4. Discussion
4.1. Precursor Film Involving Ti(IV) Complex of Oxalato and Peroxo Ligands with Butylamine
4.2. Conversion of Precursor Film Involving Ti(IV) Complex Salt to Amorphous Thin Films by Irradiating with Weak UV Light
4.3. Optical and Mechanical Properties of the Amorphous Thin Films
4.4. Photo-Induced Super-Hydrophilicity of the Amorphous Thin Films
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fateh, R.; Dillert, R.; Bahnemann, D. Self-cleaning properties, mechanical stability, and adhesion strength of transparent photocatalytic TiO2-ZnO coatings on polycarbonate. ACS Appl. Mater. Interfaces 2014, 6, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Latthe, S.; Liu, S.; Terashima, C.; Nakata, K.; Fujishima, A. Transparent, Adherent, and Photocatalytic SiO2-TiO2 Coatings on Polycarbonate for Self-Cleaning Applications. Coatings 2014, 4, 497–507. [Google Scholar] [CrossRef]
- Adachi, T.; Latthe, S.S.; Gosavi, S.W.; Roy, N.; Suzuki, N.; Ikari, H.; Kato, K.; Katsumata, K.I.; Nakata, K.; Furudate, M.; et al. Photocatalytic, superhydrophilic, self-cleaning TiO2 coating on cheap, light-weight, flexible polycarbonate substrates. Appl. Surf. Sci. 2018, 458, 917–923. [Google Scholar] [CrossRef]
- Perkas, N.; Gedanken, A. Coatings of polymers with TiO2 nanoparticles by sonochemical method. In Proceedings of the 2011 NSTI Nanotechnology Conference and Expo, NSTI-Nanotech 2011, Boston, MA, USA, 13–16 June 2011; Volume 1, pp. 391–394. [Google Scholar]
- Miyauchi, M. Photocatalysis and photoinduced hydrophilicity of WO3 thin films with underlying Pt nanoparticles. Phys. Chem. Chem. Phys. 2008, 10, 6258–6265. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Bashir, M.; Casadevall, X.; Rees, J.M.; Zimmerman, W.B. Hydrophilic Surface Modification of PDMS Microchannel for O/W and W/O/W Emulsions. Micromachines 2015, 6, 1445–1458. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Chaiyakun, S.; Buranawong, A.; Deelert, T.; Witit-anun, N. The Influence of Total and Oxygen Partial Pressures on Structure and Hydrophilic Property of TiO2 Thin Films Deposited by Reactive DC Magnetron Sputtering. Adv. Mater. Res. 2008, 55–57, 465–468. [Google Scholar] [CrossRef]
- Nagai, H.; Aoyama, S.; Hara, H.; Mochizuki, C.; Takano, I.; Baba, N.; Sato, M. Rutile thin film responsive to visible light and with high UV light sensitivity. J. Mater. Sci. 2009, 44, 861–868. [Google Scholar] [CrossRef]
- Nagai, H.; Sato, M. Heat Treatment in Molecular Precursor Method for Fabricating Metal Oxide Thin Films. In Heat Treatment—Conventional and Novel Applications; Czerwinski, F., Ed.; InTech: Rijeka, Croatia, 2012; pp. 297–322. [Google Scholar]
- Nagai, H.; Sato, M. Molecular Precursor Method for Fabricating p-Type Cu2O and Metallic Cu Thin Films. In Modern Technologies for Creating the Thin-Film Systems and Coatings; Nikitenkov, N., Ed.; InTech: Rijeka, Croatia, 2017; pp. 3–20. [Google Scholar]
- Hishimone, P.; Nagai, H.; Morita, M.; Sakamoto, T.; Sato, M. Highly-Conductive and Well-Adhered Cu Thin Film Fabricated on Quartz Glass by Heat Treatment of a Precursor Film Obtained Via Spray-Coating of an Aqueous Solution Involving Cu(II) Complexes. Coatings 2018, 8, 352. [Google Scholar] [CrossRef]
- Wu, H.J.; Nagai, H.; Sato, M. Fabrication of a p-type Cu2O thin film via UV-irradiation of a patternable molecular-precursor film containing Cu(II) complexes. J. Cryst. Growth 2019, 509, 112–117. [Google Scholar] [CrossRef]
- Nagai, H.; Mochizuki, C.; Hara, H.; Takano, I.; Sato, M. Enhanced UV-sensitivity of vis-responsive anatase thin films fabricated by using precursor solutions involving Ti complexes. Sol. Energy Mater. Sol. Cells 2008, 92, 1136–1144. [Google Scholar] [CrossRef]
- Guo, X.; Navrotsky, A. Hydration dynamics in zeolite A—An X-ray diffraction and infrared spectroscopic study. Microporous Mesoporous Mater. 2018, 268, 197–201. [Google Scholar] [CrossRef]
- Sajan, D.; Joe, H.; Jayakumar, V.S.; Zaleski, J. Structural and electronic contributions to hyperpolarizability in methyl p-hydroxy benzoate. J. Mol. Struct. 2006, 785, 43–53. [Google Scholar] [CrossRef]
- Pandiarajan, S.; Umadevi, M.; Rajaram, R.K.; Ramakrishnan, V. Infrared and Raman spectroscopic studies of L-valine L-valinium perchlorate monohydrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 630–636. [Google Scholar] [CrossRef]
- Suryanarayanan, C.; Prasannan, A.; Hong, P.D.; Sambathkumar, B.; Somanathan, N. Variable temperature studies on mesogenic polythiophenes using 2D-IR and WAXS. Mater. Chem. Phys. 2014, 143, 1352–1363. [Google Scholar] [CrossRef]
- Weisz, A.D.; Garc, L.; Regazzoni, A.E.; Blesa, M.A. FTIR study of the adsorption of single pollutants and mixtures of pollutants onto titanium dioxide in water: Oxalic and salicylic acids. Catal. Today 2002, 76, 103–112. [Google Scholar] [CrossRef]
- Kurar, S.; Rathore, D.S.; Garg, G.; Khatri, K.; Saxena, R.; Sahu, S.K. Synthesis and Evaluation of Some Benzothiazole Derivatives As Antidiabetic Agents. Int. J. Pharm. Pharm. Sci. 2017, 9, 60–68. [Google Scholar]
- Xu, C.H.; Sun, S.Q.; Guo, C.Q.; Zhou, Q.; Tao, J.X.; Noda, I. Multi-steps Infrared Macro-fingerprint Analysis for thermal processing of Fructus viticis. Vib. Spectrosc. 2006, 41, 118–125. [Google Scholar] [CrossRef]
- Vogel, E.; Gbureck, A.; Kiefer, W. Vibrational spectroscopic studies on the dyes cresyl violet and coumarin 152. J. Mol. Struct. 2000, 550–551, 177–190. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J. Colloid Interface Sci. 2012, 371, 101–106. [Google Scholar] [CrossRef]
- Ohno, T.; Masaki, Y.; Hirayama, S.; Matsumura, M. TiO2-photocatalyzed epoxidation of 1-Decene by H2O2 under visible light. J. Catal. 2001, 204, 163–168. [Google Scholar] [CrossRef]
- Dakanali, M.; Kefalas, E.T.; Raptopoulou, C.P.; Terzis, A.; Voyiatzis, G.; Kyrikou, I.; Mavromoustakos, T.; Salifoglou, A. A New Dinuclear Ti(IV)-Peroxo-Citrate Complex from Aqueous Solutions. Synthetic, Structural, and Spectroscopic Studies in Relevance to Aqueous Titanium(IV)-Peroxo-Citrate Speciation. Inorg. Chem. 2003, 42, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Sofetis, A.; Fotopoulou, F.; Raptopoulou, C.P.; Zafiropoulos, T.F.; Perlepes, S.P.; Klouras, N. Reactions of titanocene dihalides with N,N′,N″-chelates: Preparation, X-ray structure and characterization of bis(chloro){2,6-bis[(3,5-dimethyl)pyrazol-1-yl]pyridine}(η2-peroxo)titanium(IV). Polyhedron 2009, 28, 3356–3360. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, J.; Chen, F. New approaches to prepare nitrogen-doped TiO2 photocatalysts and study on their photocatalytic activities in visible light. Appl. Catal. B Environ. 2009, 89, 563–569. [Google Scholar] [CrossRef]
- Jedsukontorn, T.; Saito, N. Photoinduced Glycerol Oxidation over Plasmonic Au and AuM (M = Pt, Pd and Bi) Nanoparticle-Decorated TiO2 Photocatalysts. Nanomaterials 2018, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Cui, X.; Shen, J.I.E.; Zhang, Q.U.N.; Yang, X.; Zhang, Z.; Ming, L.U. Study on the Superhydrophilicity of the SiO2-TiO2 Thin Films Prepared by Sol-Gel Method at Room Temperature. J. Sol-Gel Sci. Technol. 2004, 29, 131–136. [Google Scholar] [CrossRef]
- Baghriche, O.; Rtimi, S.; Pulgarin, C.; Roussel, C.; Kiwi, J. RF-plasma pretreatment of surfaces leading to TiO2 coatings with improved optical absorption and OH-radical production. Appl. Catal. B Environ. 2013, 130–131, 65–72. [Google Scholar] [CrossRef]
- Chen, X.; Glans, P.; Qiu, X.; Dayal, S.; Jennings, W.D.; Smith, K.E.; Burda, C.; Guo, J. X-ray spectroscopic study of the electronic structure of visible-light. J. Electron Spectrosc. Relat. Phenom. 2008, 162, 67–73. [Google Scholar] [CrossRef]
- Yang, J.; Bai, H.; Tan, X.; Lian, J. IR and XPS investigation of visible-light photocatalysis-Nitrogen-carbon-doped TiO2 film. Appl. Surf. Sci. 2006, 253, 1988–1994. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Y.; Zhao, X. Low-temperature preparation and visible-light-induced catalytic activity of anatase F-N-codoped TiO2. J. Mol. Catal. A Chem. 2007, 277, 119–126. [Google Scholar] [CrossRef]
- Zakaznova-Herzog, V.P.; Nesbitt, H.W.; Bancroft, G.M.; Tse, J.S.; Gao, X.; Skinner, W. High-resolution valence-band XPS spectra of the nonconductors quartz and olivine. Phys. Rev. B 2005, 72, 205113. [Google Scholar] [CrossRef]
- Mehra, M.C.; Easter, A. Colorimetric Determination of Titanium as Peroxomethylenediaminetetracarboxylate. Asian J. Chem. 1997, 9, 819–823. [Google Scholar]
- Hattori, A.; Tokihisa, Y.; Tada, H.; Tohge, N.; Ito, S.; Hongo, K.; Shiratsuchi, R.; Nogami, G. Patterning Effect of a Sol-Gel TiO2 Overlayer on the Photocatalytic Activity of a TiO2/SnO2 Bilayer-Type Photocatalyst. J. Sol-Gel Sci. Technol. 2001, 22, 53–61. [Google Scholar] [CrossRef]
- Li, C.; Colella, N.S.; Watkins, J.J. Low-Temperature Fabrication of Mesoporous Titanium Dioxide Thin Films with Tunable Refractive Indices for One-Dimensional Photonic Crystals and Sensors on Rigid and Flexible Substrates. ACS Appl. Mater. Interfaces 2015, 7, 13180–13188. [Google Scholar] [CrossRef]
- Zou, L.; Hu, W.; Xie, W.; Chen, R.; Qin, N.; Li, B.; Bao, D. Applied Surface Science Excellent resistive switching property and physical mechanism of amorphous TiO2 thin films fabricated by a low-temperature photochemical solution deposition method. Appl. Surf. Sci. 2014, 311, 697–702. [Google Scholar] [CrossRef]
- Miyauchi, M.; Tokudome, H. Low-reflective and super-hydrophilic properties of titanate or titania nanotube thin films via layer-by-layer assembly. Thin Solid Film 2006, 515, 2091–2096. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Eshaghi, A.; Eshaghi, A. Investigation of superhydrophilic mechanism of titania nano layer thin film-Silica and indium oxide dopant effect. Bull. Mater. Sci. 2012, 35, 137–142. [Google Scholar] [CrossRef]
- Gao, Y.; Masuda, Y.; Koumoto, K. Light-Excited Superhydrophilicity of Amorphous TiO2 Thin Films Deposited in an Aqueous Peroxotitanate Solution. Longmuir 2004, 20, 3188–3194. [Google Scholar] [CrossRef]
- Vrakatseli, V.E.; Pagonis, E.; Amanatides, E.; Mataras, D. Photoinduced superhydrophilicity of amorphous TiOx-like thin films by a simple room temperature sol-gel deposition and atmospheric plasma jet treatment. J. Phys. Conf. Ser. 2014, 550, 012034. [Google Scholar] [CrossRef]



| Sample | F1 | F2 | F4 | F8 | F16 |
|---|---|---|---|---|---|
| Refractive index | 1.70 ± 0.01 | 1.79 ± 0.01 | 1.78 ± 0.01 | 1.79 ± 0.01 | 1.79 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-J.; Tanabe, K.; Nagai, H.; Sato, M. Photo-Induced Super-hydrophilic Thin Films on Quartz Glass by UV Irradiation of Precursor Films Involving a Ti(IV) Complex at Room Temperature. Materials 2019, 12, 348. https://doi.org/10.3390/ma12030348
Wu H-J, Tanabe K, Nagai H, Sato M. Photo-Induced Super-hydrophilic Thin Films on Quartz Glass by UV Irradiation of Precursor Films Involving a Ti(IV) Complex at Room Temperature. Materials. 2019; 12(3):348. https://doi.org/10.3390/ma12030348
Chicago/Turabian StyleWu, Hsiang-Jung, Kota Tanabe, Hiroki Nagai, and Mitsunobu Sato. 2019. "Photo-Induced Super-hydrophilic Thin Films on Quartz Glass by UV Irradiation of Precursor Films Involving a Ti(IV) Complex at Room Temperature" Materials 12, no. 3: 348. https://doi.org/10.3390/ma12030348








