Multi-Functional Laccase Immobilized Hydrogel Microparticles for Efficient Removal of Bisphenol A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
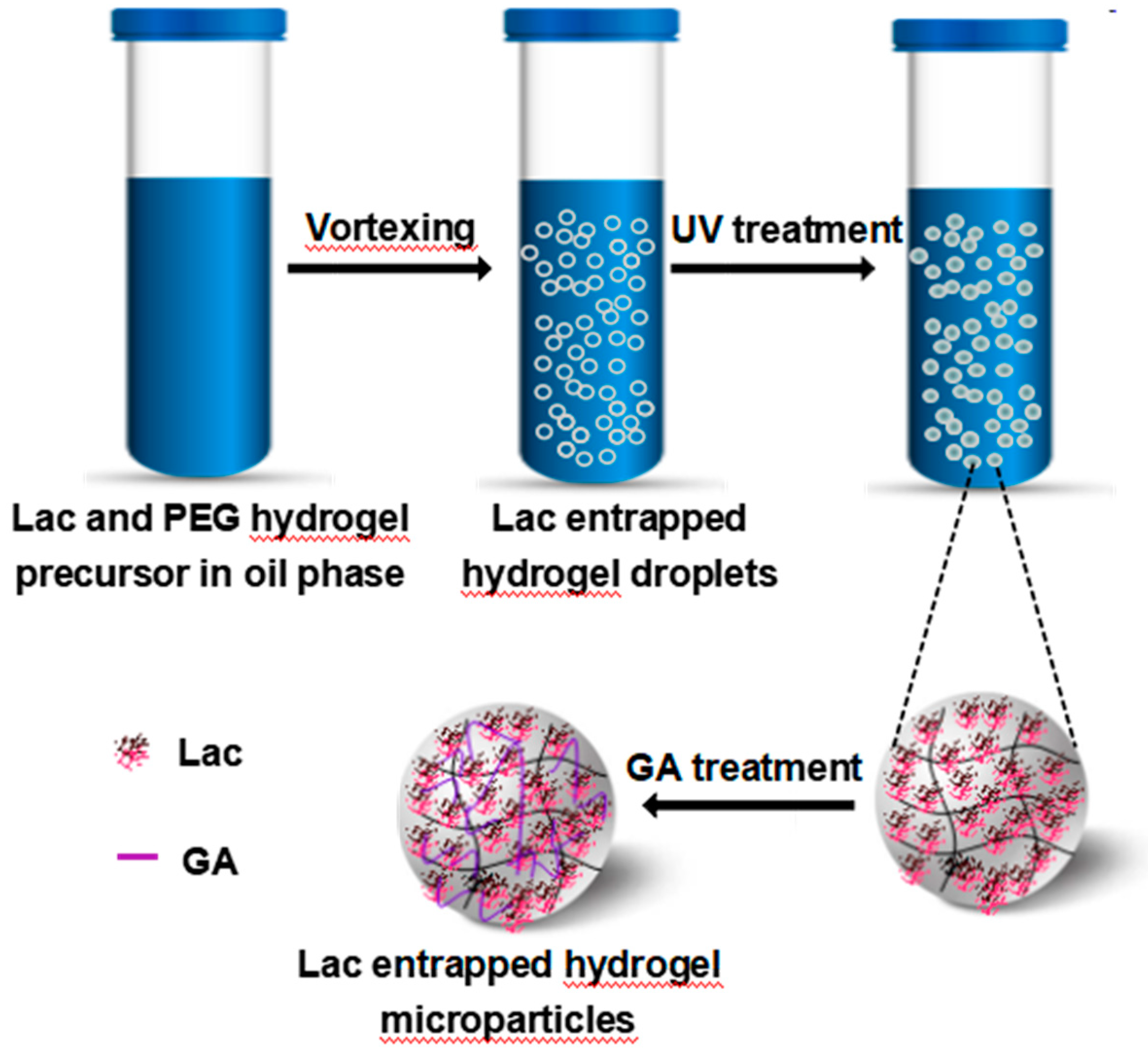
2.2. Preparation of Lac/Particles
2.3. Labeling of FITC to Lac
2.4. Activity Assay
2.5. Determination of Enzyme Kinetics
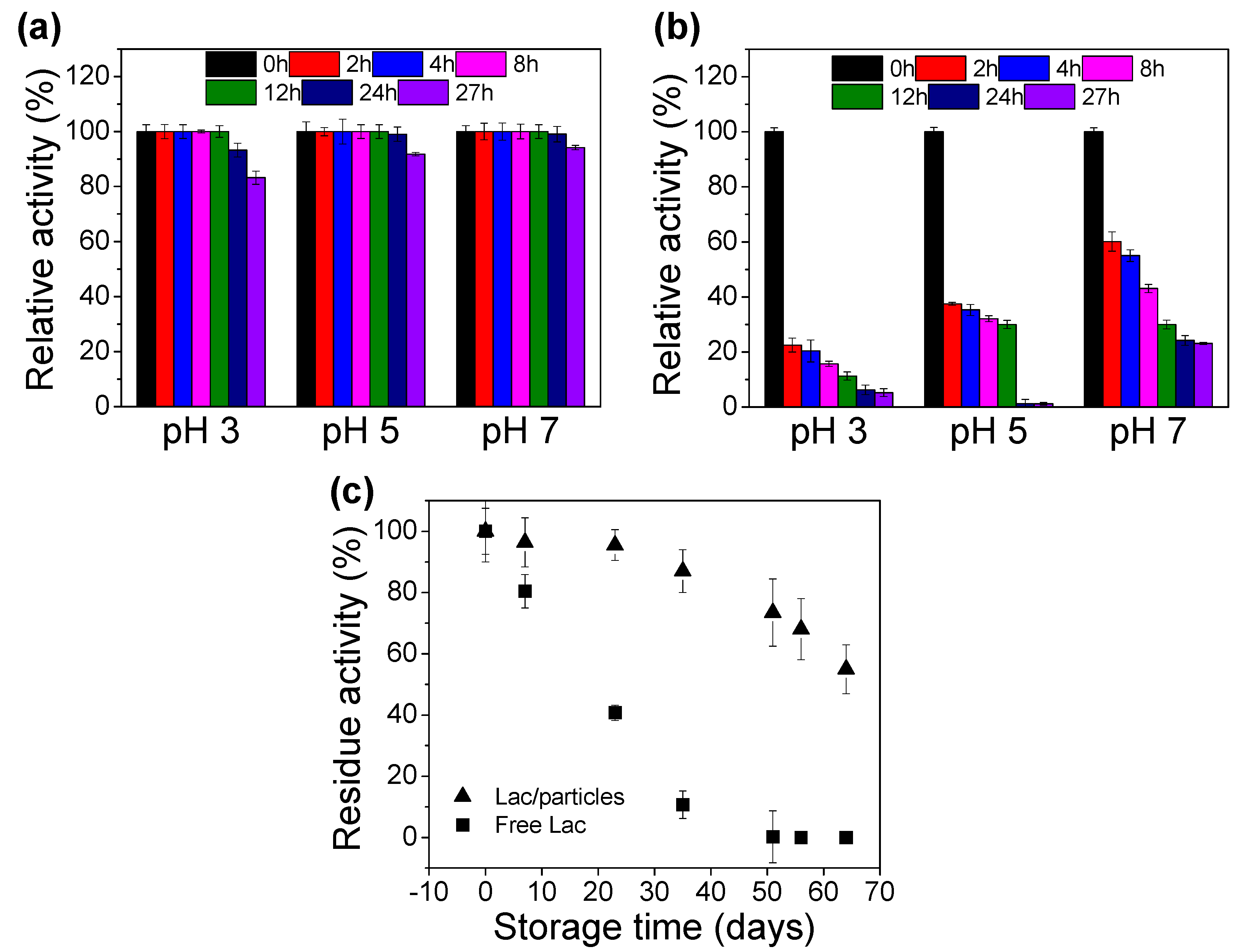
2.6. Stability of Free and Lac/Particles
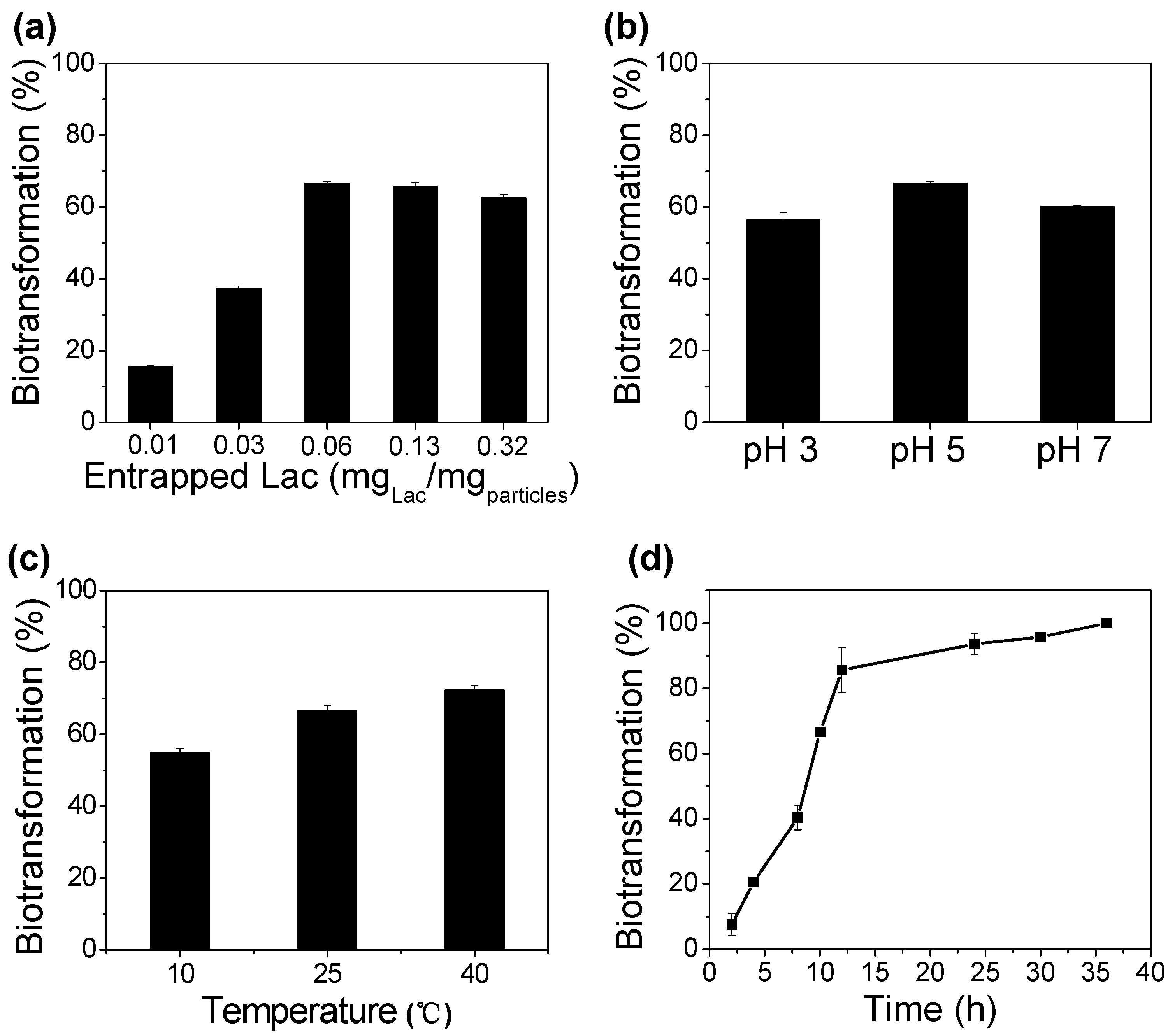
2.7. Biotransformation of BPA
2.8. Reusability of Lac/particles
3. Results and Discussion
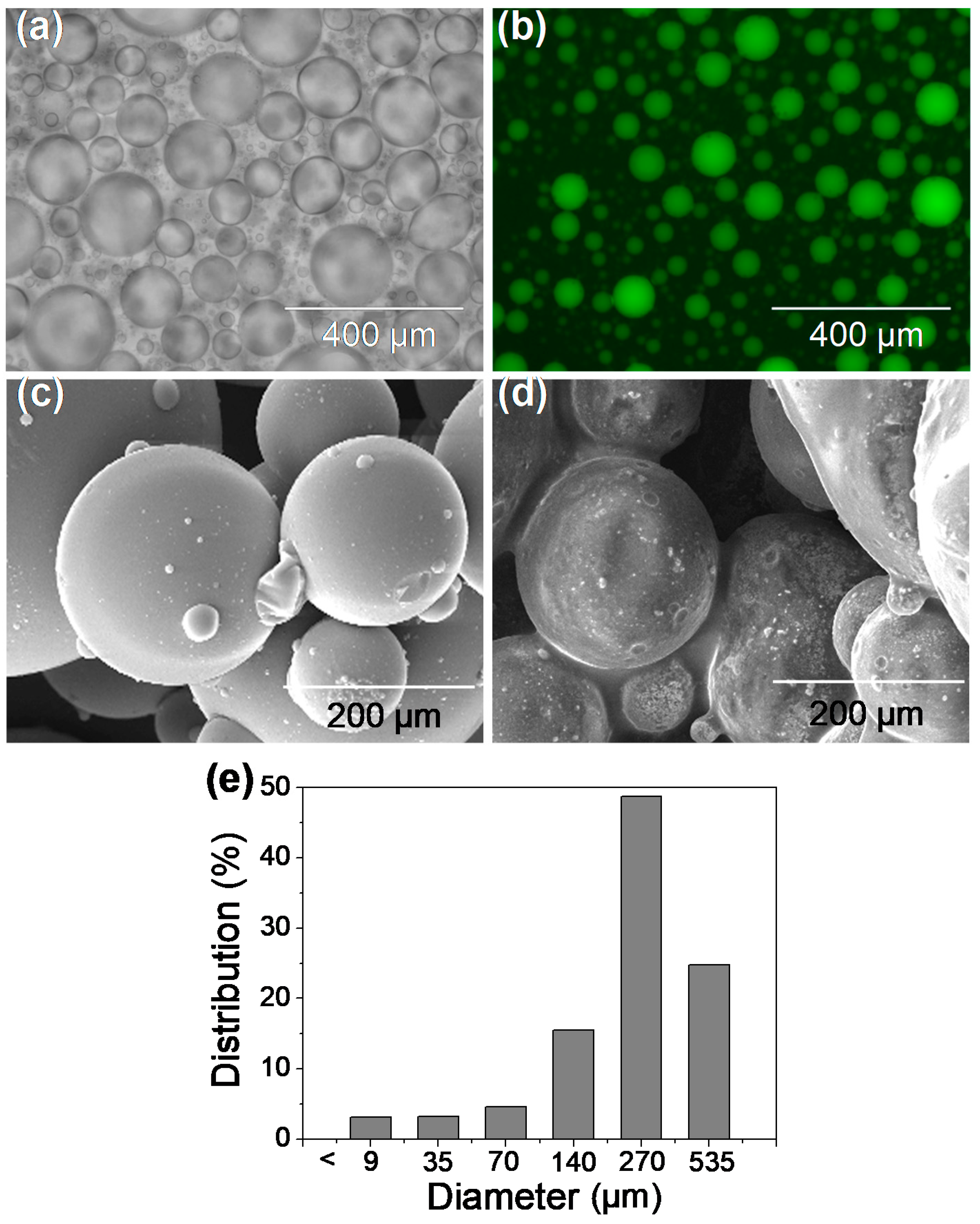
3.1. Properties of Lac/particles
3.2. Kinetics and Stability of Lac/Particles
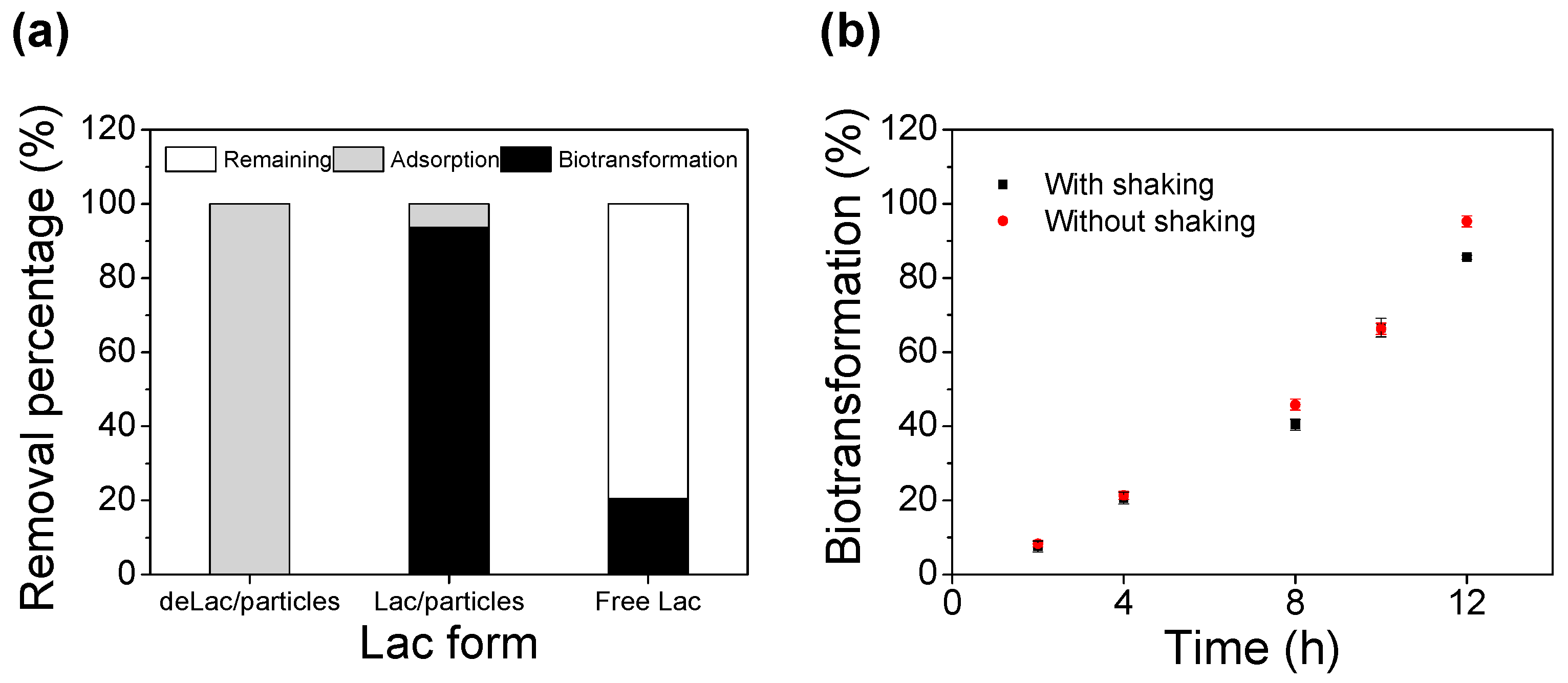
3.3. Enzymatic Biotransformation of BPA
3.4. Synergistic Removal of BPA
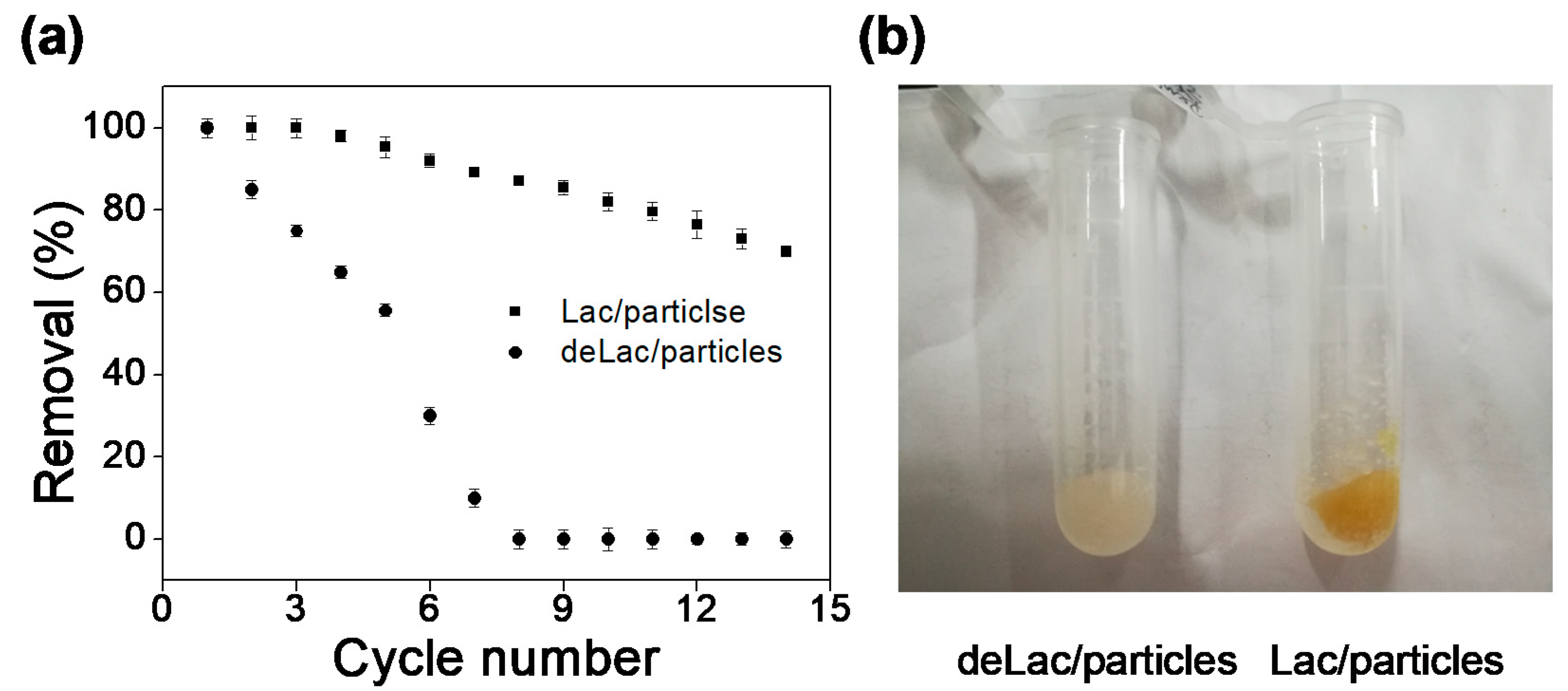
3.5. Reusability of Lac/Particles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Y.Q.; Wong, C.K.C.; Zheng, J.S.; Bouwman, H.; Barra, R.; Wahlström, B.; Neretin, L.; Wong, M.H. Bisphenol a (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef] [PubMed]
- vom Saal, F.S.; Hughes, C. An extensive new literature concerning low-dose effects of bisphenol a shows the need for a new risk assessment. Environ. Health Perspect. 2005, 113, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Javed, H.; Zhang, D.; Kim, J.H.; Westerhoff, P.; Li, Q.; Alvarez, P.J. Porous electrospun fibers embedding TiO2 for adsorption and photocatalytic degradation of water pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, E.; Niinipuu, M.; Fick, J.; Jansson, S. Using carbonized low-cost materials for removal of chemicals of environmental concern from water. Environ. Sci. Pollut. Res. 2018, 25, 15793–15801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, B.; Li, Y.; Tan, W.; Wang, Z.; Yu, Z.; Xing, S.; Lin, H.; Zhang, H. Degradation of bisphenol A by electro-enhanced heterogeneous activation of peroxydisulfate using Mn-Zn ferrite from spent alkaline Zn-Mn batteries. Chemosphere 2018, 204, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, D.; Zhou, L.; Zhao, Y.; Chen, J.; Chen, Z.; Wang, F. Polypyrrole/reduced graphene oxide aerogel particle electrodes for high-efficiency electro-catalytic synergistic removal of Cr(VI) and bisphenol A. Chem. Eng. J. 2018, 336, 690–700. [Google Scholar] [CrossRef]
- Wang, R.; Diao, P.; Chen, Q.; Wu, H.; Xu, N.; Duan, S. Identification of novel pathways for biodegradation of bisphenol A by the green alga Desmodesmus sp.WR1, combined with mechanistic analysis at the transcriptome level. Chem. Eng. J. 2017, 321, 424–431. [Google Scholar] [CrossRef]
- Moussavi, G.; Haddad, F.A. Bacterial peroxidase-mediated enhanced biodegradation and mineralization of bisphenol A in a batch bioreactor. Chemosphere 2019, 222, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Agarose-chitosan hydrogel-immobilized horseradish peroxidase with sustainable bio-catalytic and dye degradation properties. Int. J. Biol. Macromol. 2019, 124, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, P.; Shukla, G.; Raj, G.; Ferreira, L.F.R.; Bharagava, R.N. Microbial manganese peroxidase: A ligninolytic enzyme and its ample opportunities in research. SN Appl. Sci. 2019, 1, 45. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.; Yan, Y. Peroxidases-assisted removal of environmentally-related hazardous pollutants with reference to the reaction mechanisms of industrial dyes. Sci. Total Environ. 2018, 644, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Inukai, K.; Fujikura, K.; Kasuga, T. Effective encapsulation of laccase in an aluminium silicate nanotube hydrogel. New J. Chem. 2014, 38, 3591–3599. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kumar, V.; Chaudhary, B.; Kaith, B.S.; Kalia, S.; Swart, H.C. Application of biodegradable superabsorbent hydrogel composite based on gum ghatti-co-poly(acrylic acid-aniline) for controlled drug delivery. Polym. Degrad. Stab. 2016, 124, 101–111. [Google Scholar] [CrossRef]
- Cao, L.; Cao, B.; Lu, C.; Wang, G.; Yu, L.; Ding, J. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J. Mater. Chem. B 2015, 3, 1268–1280. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gassara-Chatti, F.; Brar, S.K.; Ajila, C.M.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Encapsulation of ligninolytic enzymes and its application in clarification of juice. Food Chem. 2013, 137, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food. Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Davidson, D.W.; Verma, M.S.; Gu, F.X. Controlled root targeted delivery of fertilizer using an ionically crosslinked carboxymethyl cellulose hydrogel matrix. SpringerPlus 2013, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, N.B.; Ristić, M.Đ.; Perić-Grujić, A.A.; Filipović, J.M.; Štrbac, S.B.; Rakočević, Z.L.; Krušić, M.T.K. Sorption of zinc by novel pH-sensitive hydrogels based on chitosan, itaconic acid and methacrylic acid. J. Hazard. Mater. 2011, 192, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Guareño, E.; Santiago-Gutiérrez, F.; Morán-Quiroz, J.L.; Hernandez-Olmos, S.L.; Soto, V.; De la Cruz, W.; Manríquez, R.; Gomez-Salazar, S. Removal of Cu (Ⅱ) ions from aqueous streams using poly(acrylic acid-co-acrylamide) hydrogels. J. Colloid Interface Sci. 2010, 349, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.T.; Belfiore, L.A.; Kubota, L.T.; Muniz, E.C.; Tambourgi, E.B. Efficiency of hydrogels based on natural polysaccharides in the removal of Cd2+ ions from aqueous solutions. Chem. Eng. J. 2011, 168, 68–76. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Ray, S.K. Adsorption of industrial dyes by semi-ipn hydrogels of acrylic copolymers and sodium alginate. J. Ind. Eng. Chem. 2015, 22, 92–102. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Mittal, H.; Mishra, S.B.; Mishra, A.K. Gum ghatti and acrylic acid based biodegradable hydrogels for the effective adsorption of cationic dyes. J. Ind. Eng. Chem. 2015, 22, 171–178. [Google Scholar] [CrossRef]
- Sharma, R.; Kaith, B.S.; Kalia, S.; Pathania, D.; Kumar, A.; Sharma, N.; Street, R.M.; Schauer, C. Biodegradable and conducting hydrogels based on guar gum polysaccharide for antibacterial and dye removal applications. J. Environ. Manag. 2015, 162, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Koklukaya, S.Z.; Sezer, S.; Aksoy, S.; Hasirci, N. Polyacrylamide-based semi-interpenetrating networks for entrapment of laccase and their use in azo dye decolorization. Biotechnol. Appl. Biochem. 2016, 63, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancement of catalytic, reusability, and long-term stability features of Trametes versicolor IBL-04 laccase immobilized on different polymers. Int. J. Biol. Macromol. 2017, 95, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancing catalytic functionality of Trametes versicolor IBL-04 laccase by immobilization on chitosan microspheres. Chem. Eng. Res. Des. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Fabrication of nanobiocatalyst using encapsulated laccase onto chitosan-nanobiochar composite. Int. J. Biol. Macromol. 2019, 124, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Murugesan, K.; Lee, C.S.; Vu, C.H.; Chang, Y.S.; Jeon, J.R. Degradation of synthetic pollutants in real wastewater using laccase encapsulated in core–shell magnetic copper alginate beads. Bioresour. Technol. 2016, 216, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, H.; Huang, W.; Zhang, S. Immobilization of laccase in a sponge-like hydrogel for enhanced durability in enzymatic degradation of dye pollutants. J. Colloid Interface Sci. 2015, 450, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Han, D.J.; Azad, M.R.; Park, M.; Seo, T.S. Enzyme incorporated microfluidic device for in-situ glucose detection in water-in-air microdroplets. Biosen. Bioelectron. 2015, 65, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; García, A.J. Methods for generating hydrogel particles for protein delivery. Ann. Biomed. Eng. 2016, 44, 1946–1958. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A.; Dubovik, A.S.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y. Temperature-sensitive chitosan-poly(n-isopropylacrylamide) interpenetrated network with enhanced loading capacity and controlled release properties. J. Control. Release 2005, 102, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Enrica Caló, V.V.K. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Ter Schiphorst, J.; Coleman, S.; Stumpel, J.E.; Ben Azouz, A.; Diamond, D.; Schenning, A.P. Molecular design of light-responsive hydrogels, for in situ generation of fast and reversible valves for microfluidic applications. Chem. Mater. 2015, 27, 5925–5931. [Google Scholar] [CrossRef]
- Bahram, M.; Hoseinzadeh, F.; Farhadi, K.; Saadat, M.; Najafi-Moghaddam, P.; Afkhami, A. Synthesis of gold nanoparticles using pH-sensitive hydrogel and its application for colorimetric determination of acetaminophen, ascorbic acid and folic acid. Colloids Surf. A Physicochem. Eng. Asp. 2013, 441, 517–524. [Google Scholar] [CrossRef]
- Pistone, L.; Ottolina, G.; De, S.; Romero, A.A.; Martins, L.O.; Luque, R. Encapsulated laccases for the room-temperature oxidation of aromatics: Towards synthetic low-molecular-weight lignins. ChemSusChem 2016, 9, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Rekuć, A.; Bryjak, J.; Szymańska, K.; Jarzębski, A.B. Very stable silica-gel-bound laccase biocatalysts for the selective oxidation in continuous systems. Bioresour. Technol. 2010, 101, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Laccase immobilization on titania nanoparticles and titania-functionalized membranes. J. Membr. Sci. 2014, 452, 229–240. [Google Scholar] [CrossRef]
- Behrens, A.M.; Sikorski, M.J.; Li, T.; Wu, Z.J.; Griffith, B.P.; Kofinas, P. Blood-aggregating hydrogel particles for use as a hemostatic agent. Acta Biomater. 2014, 10, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, A. Granular hydrogel initiated by Fenton reagent and their performance on Cu(II) and Ni(II) removal. Chem. Eng. J. 2012, 200–202, 601–610. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Jiang, M. Effects of the multilayer structures on Exenatide release and bioactivity in microsphere/thermosensitive hydrogel system. Colloids Surf. B Biointerfaces 2018, 171, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Umeyama, S.; Nagai, K.; Onoe, H.; Takinoue, M. Controlled construction of stable network structure composed of honeycomb-shaped microhydrogels. Life 2018, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Krutkramelis, K.; Oakey, J. Oxygen-purged microfluidic device to enhance cell viability in photopolymerized PEG hydrogel microparticles. Biomacromolecules 2016, 17, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Knystautas, E.J.; Verma, M.; Surampalli, R.Y. Degradation of chlortetracycline using immobilized laccase on polyacrylonitrile-biochar composite nanofibrous membrane. Sci. Total Environ. 2017, 605–606, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Chi, C.; Li, F.; Zhang, B. Laccase-polyacrylonitrile nanofibrous membrane: Highly immobilized, stable, reusable, and efficacious for 2,4,6-trichlorophenol removal. ACS Appl. Mater. Interfaces 2013, 5, 12554–12560. [Google Scholar] [CrossRef] [PubMed]
- Gokgoz, M.; Altinok, H. Immobilization of laccase on polyacrylamide and polyacrylamide–k–carragennan-based semi-interpenetrating polymer networks. Artif. Cells Blood Substit. Biotechnol. 2012, 40, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.N.M.; Felgueiras, H.P.; Gouveia, I.; Zille, A. Synergistically enhanced stability of laccase immobilized on synthesized silver nanoparticles with water-soluble polymers. Colloids Surf. B Biointerfaces 2017, 154, 210–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Takahashi, A.; Kashiwada, A.; Yamada, K. Removal of bisphenol A and its derivatives from aqueous medium through laccase-catalyzed treatment enhanced by addition of polyethylene glycol. Environ. Technol. 2016, 37, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Hai, F.I.; Dosseto, A.; Richardson, C.; Price, W.E.; Nghiem, L.D. Continuous adsorption and biotransformation of micropollutants by granular activated carbon-bound laccase in a packed-bed enzyme reactor. Bioresour. Technol. 2016, 210, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, M.; Zou, D.; Yang, Y.; Ren, X.; Qin, C.; Piao, Y. Multi-Functional Laccase Immobilized Hydrogel Microparticles for Efficient Removal of Bisphenol A. Materials 2019, 12, 704. https://doi.org/10.3390/ma12050704
Piao M, Zou D, Yang Y, Ren X, Qin C, Piao Y. Multi-Functional Laccase Immobilized Hydrogel Microparticles for Efficient Removal of Bisphenol A. Materials. 2019; 12(5):704. https://doi.org/10.3390/ma12050704
Chicago/Turabian StylePiao, Mingyue, Donglei Zou, Yuesuo Yang, Xianghao Ren, Chuanyu Qin, and Yunxian Piao. 2019. "Multi-Functional Laccase Immobilized Hydrogel Microparticles for Efficient Removal of Bisphenol A" Materials 12, no. 5: 704. https://doi.org/10.3390/ma12050704



