Effect of Hindered Phenol Crystallization on Properties of Organic Hybrid Damping Materials
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Preparation of the Composites
2.3. Characterization
3. Results and Discussion
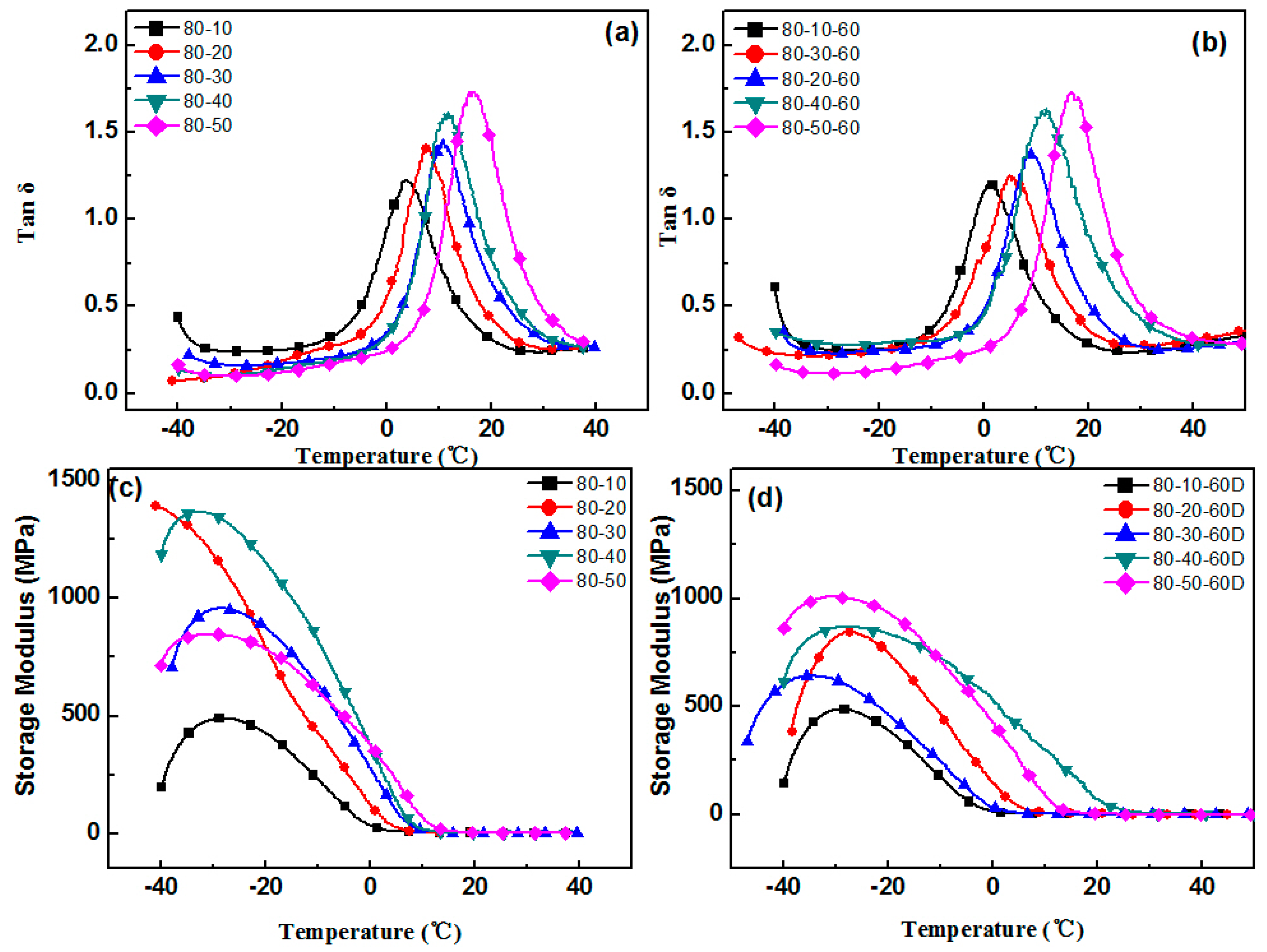
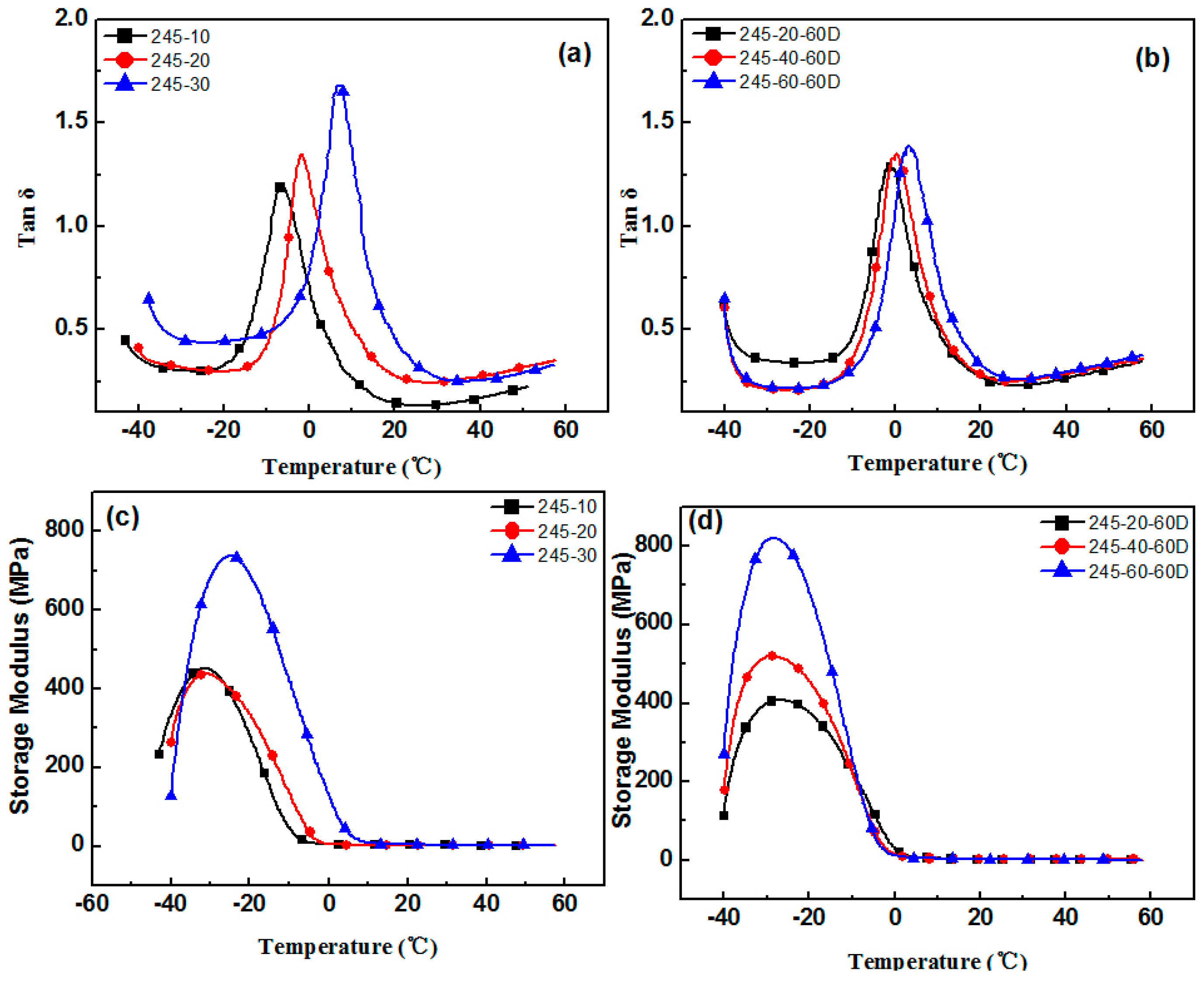
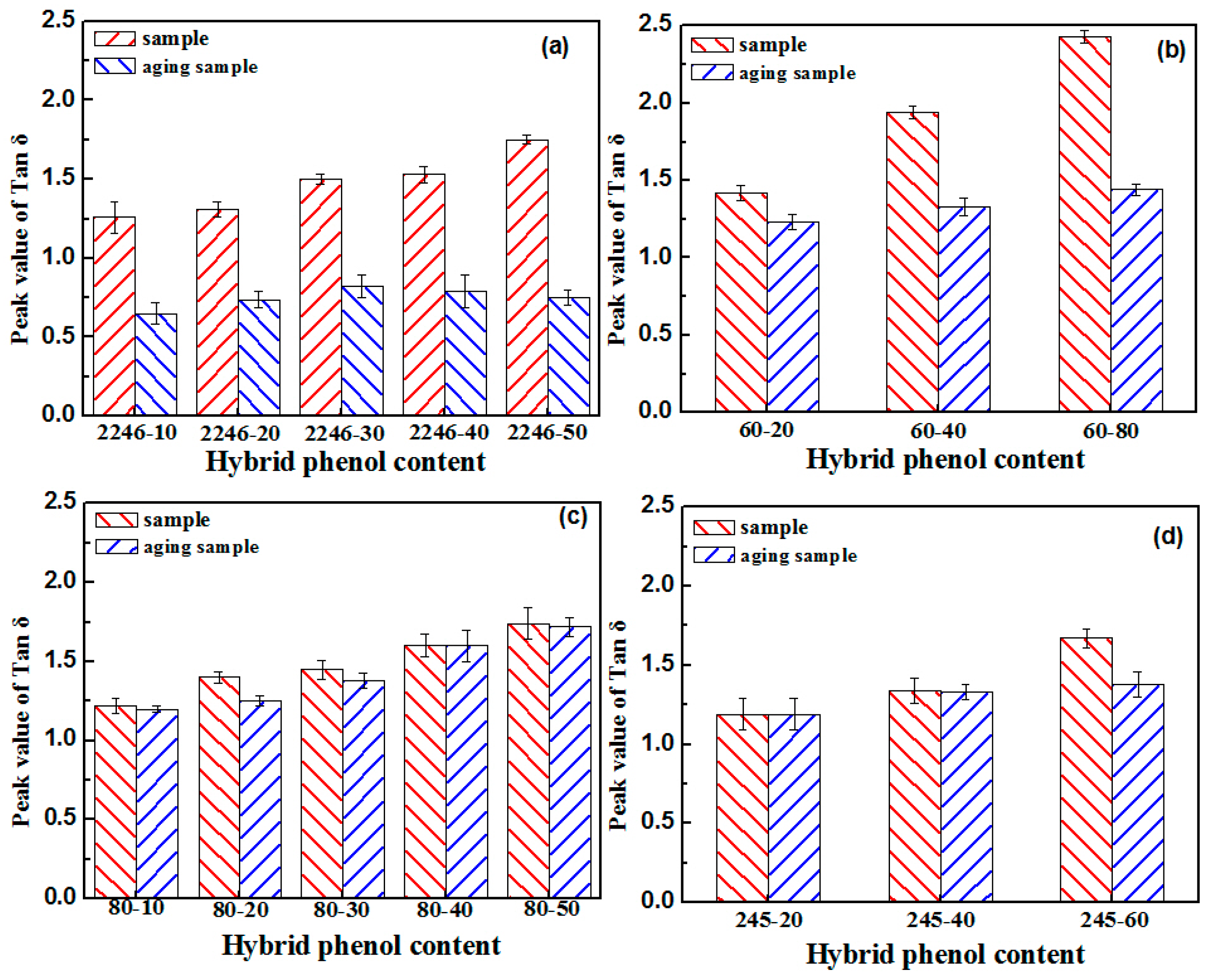
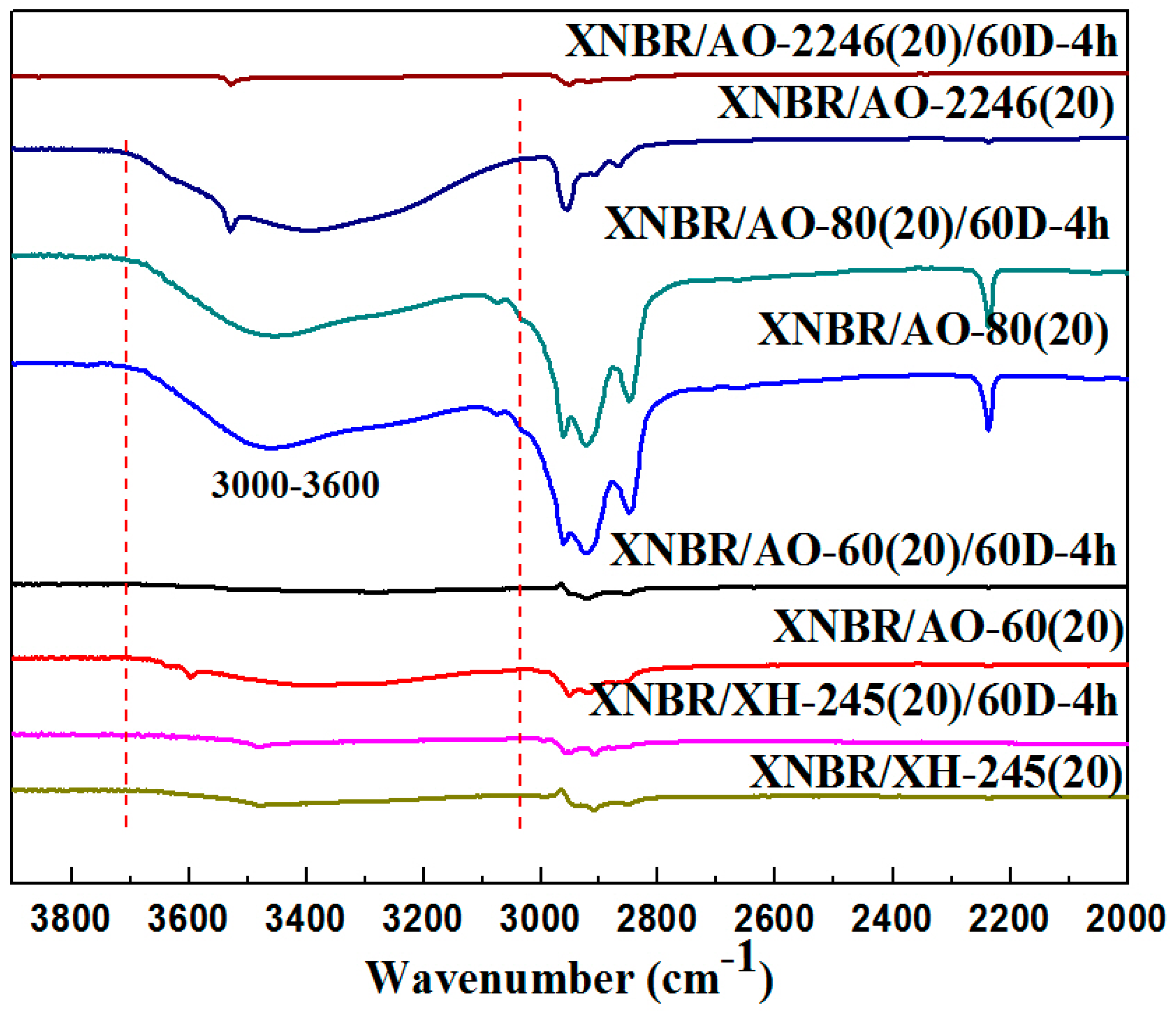
3.1. Aging Properties of Hindered Phenolic Organic Hybrid Materials
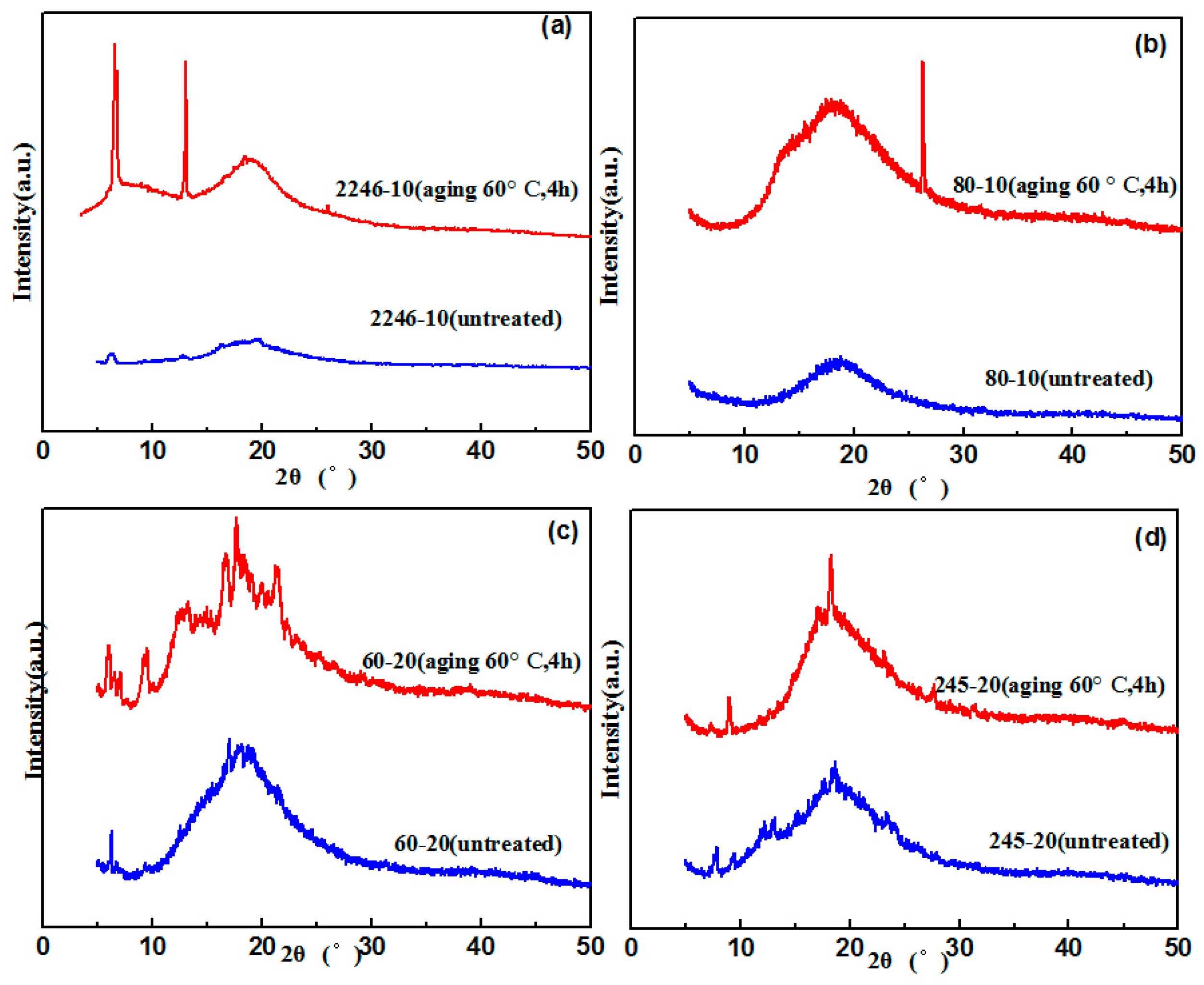
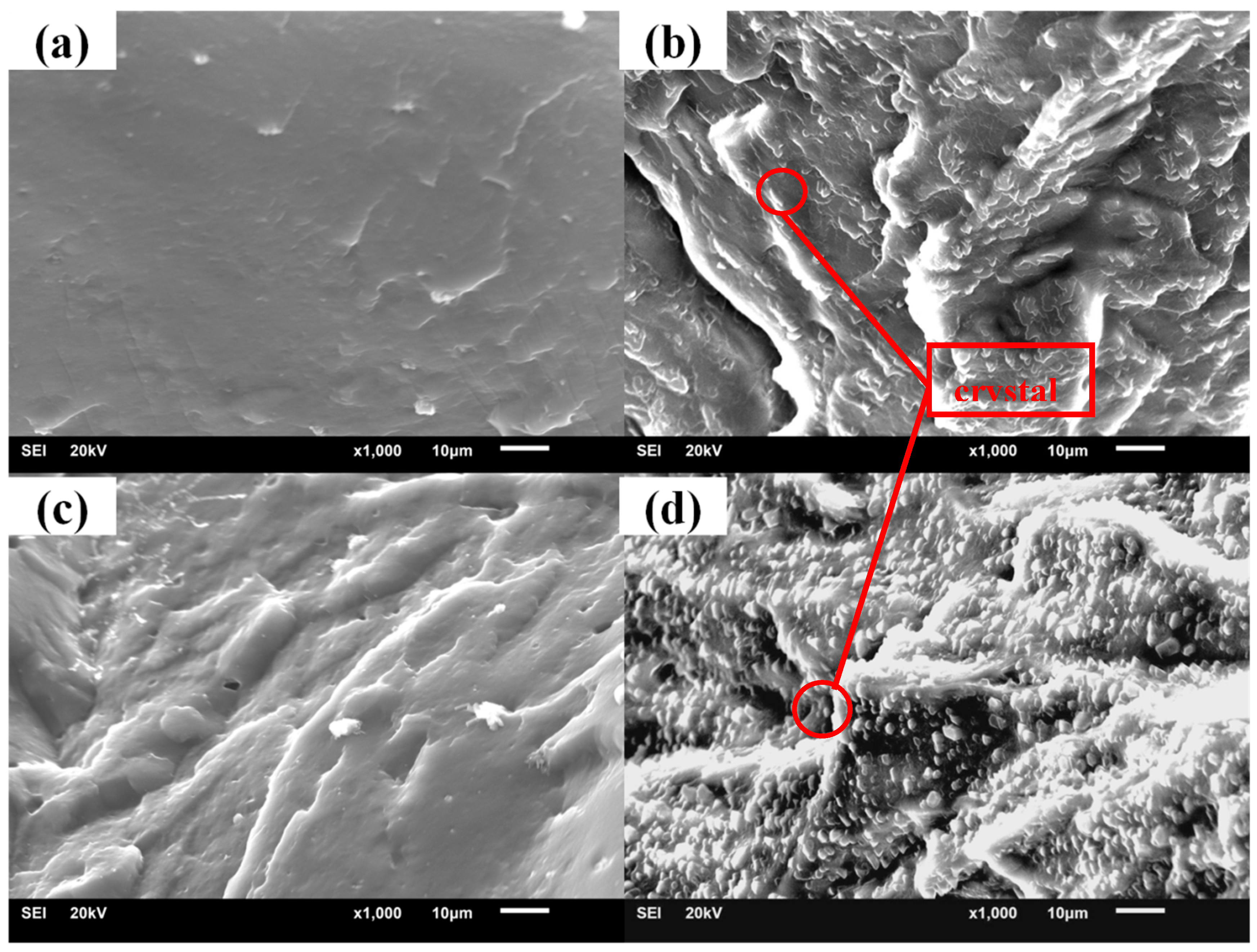
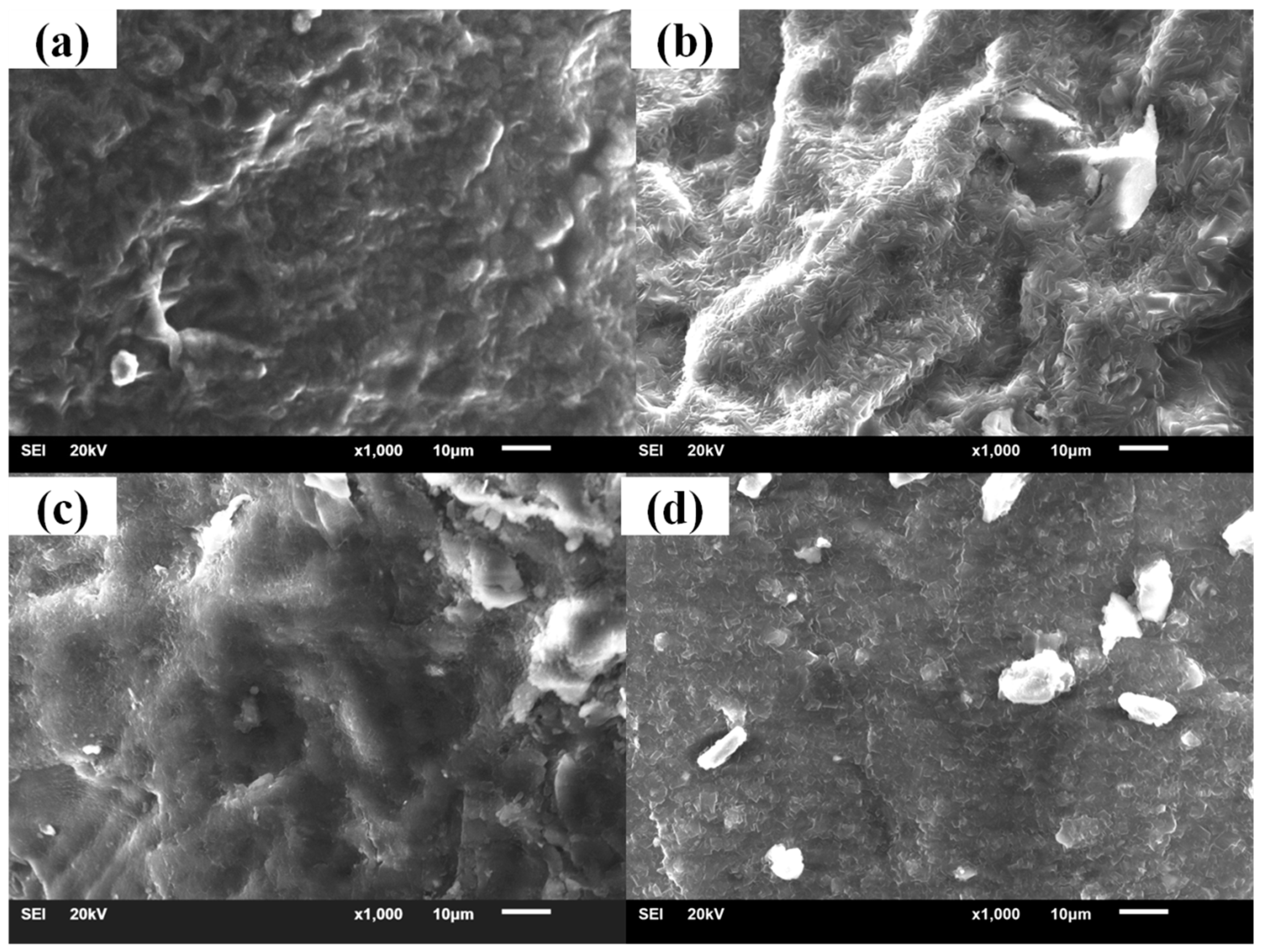
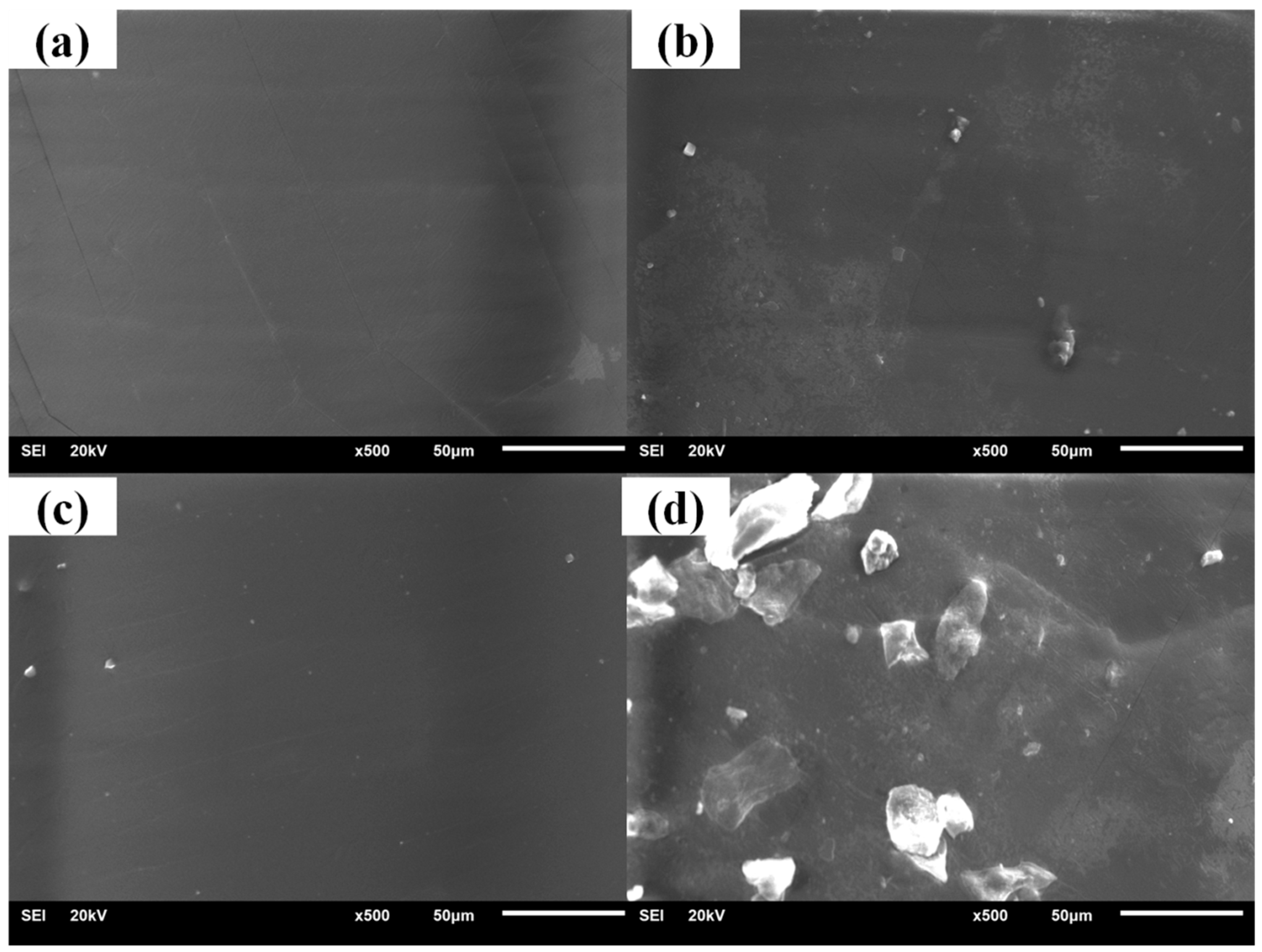
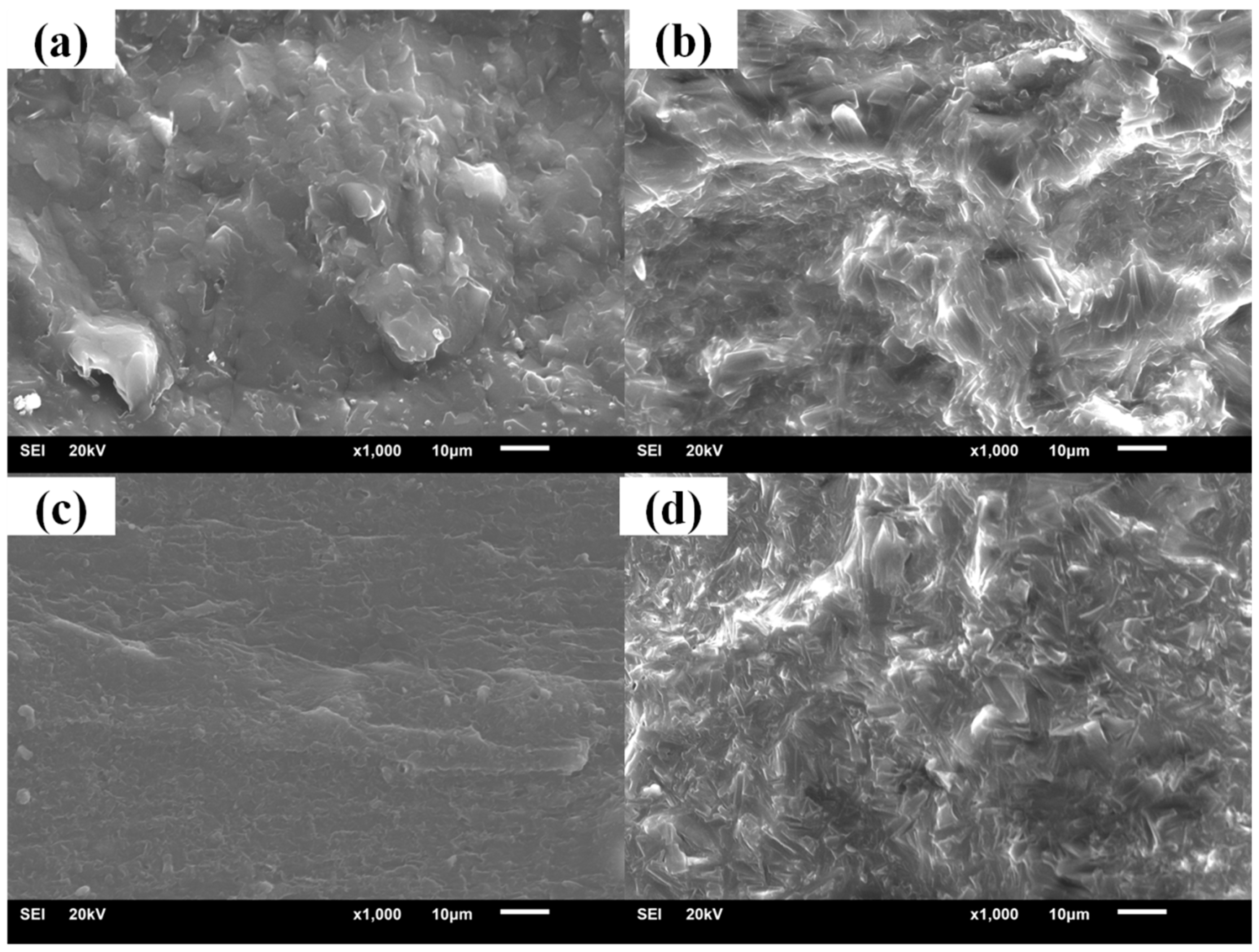
3.2. Crystallization Properties of the Hindered Phenol in Compounds
3.3. Mechanism of Organic Hybrid Crystallization
3.4. Discussion
4. Conclusions
- (1)
- The high damping capacity of organic hybrid damping materials is attributed to hydrogen bonding, and the crystallization of hindered phenols can lead to the disappearance of hydrogen bonds in the material system. Thus, the crystals can decrease the material properties.
- (2)
- The molecular structure of the hindered phenol had an influence on the properties of organic hybrid damping materials, and hindered phenol molecules with linear and branching structure had better performances.
- (3)
- The molecular structures of AO-2246 and AO-60 are more symmetrical and regular than that of linear hindered phenols, which makes crystallization easier. There are more hydrogen bonds between the hindered phenol itself in the matrix, thus promoting agglomeration and crystallization.
- (4)
- AO-80 and XH-245 had good crystallization resistance properties. There was a greater number of hydrogen bonds between the hindered phenol and XNBR matrix. When XH-245 was crystallized at a higher content, the crystal had a lamellar structure similar to that of sheet graphite, which was conducive to maintaining the material properties.
Author Contributions
Funding
Conflicts of Interest
References
- Kriston, I.; Orbán-Mester, Á.; Nagy, G.; Staniek, P.; Főldes, E.; Pukánszky, B. Melt stabilisation of Phillips type polyethylene, Part II: Correlation between additive consumption and polymer properties. Polym. Degrad. Stabil. 2009, 94, 1448–1456. [Google Scholar] [CrossRef]
- Rattanasom, N. Effect of Mastication, Antioxidants and Crosslink Density on the Mechanical Properties of Gum and Black-Filled Natural Rubber Vulcanizates. Ph.D. Thesis, The University of Akron, Akron, OH, USA, 1999. [Google Scholar]
- Tochacek, J.; Matuska, R.; Polacek, P.; Stohandl, J. The role of organic phosphite primary structure in the overall stabilization performance in polypropylene. Polym. Test. 2017, 57, 126–132. [Google Scholar] [CrossRef]
- Catania, G.; Strozzi, M. Damping oriented design of thin-walled mechanical components by means of multi-layer coating technology. Coatings 2018, 8, 73. [Google Scholar] [CrossRef]
- Yu, L.; Ma, Y.; Zhou, C.; Xu, H. Damping efficiency of the coating structure. Int. J. Solids Struct. 2005, 42, 3045–3058. [Google Scholar] [CrossRef]
- Rongong, J.A.; Goruppa, A.A.; Buravalla, V.R.; Tomlinson, G.R.; Jones, F.R. Plasma deposition of constrained layer damping coatings. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2004, 218, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Tolinski, M. Additives for Polyolefins: Getting the Most Out of Polypropylene, Polyethylene and TPO; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Zweifelschielly, B.; Suter, W. Performance of GPS telemetry collars for red deer cervus elaphus in rugged alpine terrain under controlled and free-living conditions. Wildl. Biol. 2013, 13, 299–312. [Google Scholar] [CrossRef]
- Wu, C.; Yamagishi, T.; Nakamoto, Y.; Ishida, S. Organic hybrid of chlorinated polyethylene and hindered phenol. I dynamic mechanical properties. J. Polym. Sci. Part B Polym. Phys. 2015, 38, 2285–2295. [Google Scholar]
- Xiao, D.; Zhao, X.; Feng, Y.; Xiang, P.; Zhang, L. The structure and dynamic properties of thermoplastic polyurethane elastomer/hindered phenol hybrids. J. Appl. Polym. Sci. 2010, 116, 2143–2150. [Google Scholar] [CrossRef]
- He, Y.; Asakawa, N.; Inoue, Y. Studies on poly(ε-caprolactone)/thiodiphenol blends: The specific interaction and the thermal and dynamic mechanical properties. J. Polym. Sci. Part B Polym. Phys. 2015, 14, 1848–1859. [Google Scholar] [CrossRef]
- Daga, V.K.; Watkins, J.J. Hydrogen-bond-mediated phase behavior of complexes of small molecule additives with poly(ethylene oxide-b-propylene oxide-b-ethylene oxide) triblock copolymer surfactants. Macromolecules 2010, 43, 9990–9997. [Google Scholar] [CrossRef]
- Yin, X.; Liu, C.; Lin, Y.; Guan, A.; Wu, G. Influence of hydrogen bonding interaction on the damping properties of poly(n-butyl methacrylate)/small molecule hybrids. J. Appl. Polym. Sci. 2015, 132, 41954. [Google Scholar] [CrossRef]
- Liu, Q.X.; Ding, X.B.; Zhang, H.P.; Yan, X. Preparation of high-performance damping materials based on carboxylated nitrile rubber: Combination of organic hybridization and fiber reinforcement. J. Appl. Polym. Sci. 2009, 114, 2655–2661. [Google Scholar] [CrossRef]
- Sajjayanukul, T.; Saeoui, P.; Sirisinha, C. Experimental analysis of viscoelastic properties in carbon black-filled natural rubber compounds. J. Appl. Polym. Sci. 2005, 97, 2197–2203. [Google Scholar] [CrossRef]
- Omayu, A.; Ueno, T.; Matsumoto, A. The role of intermolecular hydrogen bonding on thermal properties of maleimide–isobutene alternating copolymers with polar groups. Macromol. Chem. Phys. 2008, 209, 1503–1514. [Google Scholar] [CrossRef]
- Perera, M.C.S.; Ishiaku, U.S.; Ishak, Z.A.M. Characterisation of PVC/NBR and PVC/ENR50 binary blends and PVC/ENR50/NBR ternary blends by DMA and solid state NMR. Eur. Polym. J. 2001, 37, 167–178. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liu, C.Y.; Liu, Y.B.; Zhang, W.G. Correlation between hydrogen bonding strength and numbers of polymer/hindered phenol hybrids and damping Properties. J. East China Univ. Sci. Technol. 2017, 1, 23–27. [Google Scholar]
- Cao, Y.; Li, Q.; Zhang, L.; Wu, G.; Wu, C. Viscoelasticity of a vitrified hindered phenol compound during thermal annealing. J. Non-Cryst. Solids 2007, 353, 4232–4235. [Google Scholar] [CrossRef]
- Zhao, X.; Xiang, P.; Zhang, L. Structure and properties of hindered phenol/nitrile-butadiene rubber composites. Acta Mater. Comp. Silica 2007, 24, 44–49. [Google Scholar] [CrossRef]
- Song, M.; Zhao, X.; Li, Y.; Hu, S.; Zhang, L. Molecular dynamics simulations and microscopic analysis of the damping performance of hindered phenol AO-60/nitrile-butadiene rubber composites. RSC Adv. 2014, 4, 6719–6729. [Google Scholar] [CrossRef]
- Sacristan, J.; Mijangos, C. Free volume analysis and transport mechanisms of PVC modified with fluorothiophenol compounds. A molecular simulation study. Macromolecules 2010, 43, 7357–7367. [Google Scholar] [CrossRef]
- Majeed, K.; Jawaid, M.; Hassan, A.; Bakar, A.A.; Khalil, H.A.; Salema, A.A.; Inuwa, I. Potential materials for food packaging from nanoclay/natural fibres filled hybrid composites. Mater. Des. 2013, 46, 391–410. [Google Scholar] [CrossRef] [Green Version]
- Peter, C.; Kremer, K. Multiscale simulation of soft matter systems from the atomistic to the coarse-grained level and back. Soft Matter 2009, 22, 4357–4366. [Google Scholar] [CrossRef]
- Zhang, J.; Chaisombat, K.; He, S.; Wang, C.H. Hybrid composite laminates reinforced with glass/carbon woven fabrics for lightweight load bearing structures. Mater. Des. 2012, 36, 75–80. [Google Scholar] [CrossRef]
- Zuo, K.; Zhang, L.; Peng, J.; Cai, Z.; Zhu, M. Dynamic mechanic performances of nitrile butadiene rubber/hindered phenol AO-2246/sericite blends. Polym. Mater. Sci. Eng. 2015, 31, 83–86. [Google Scholar]


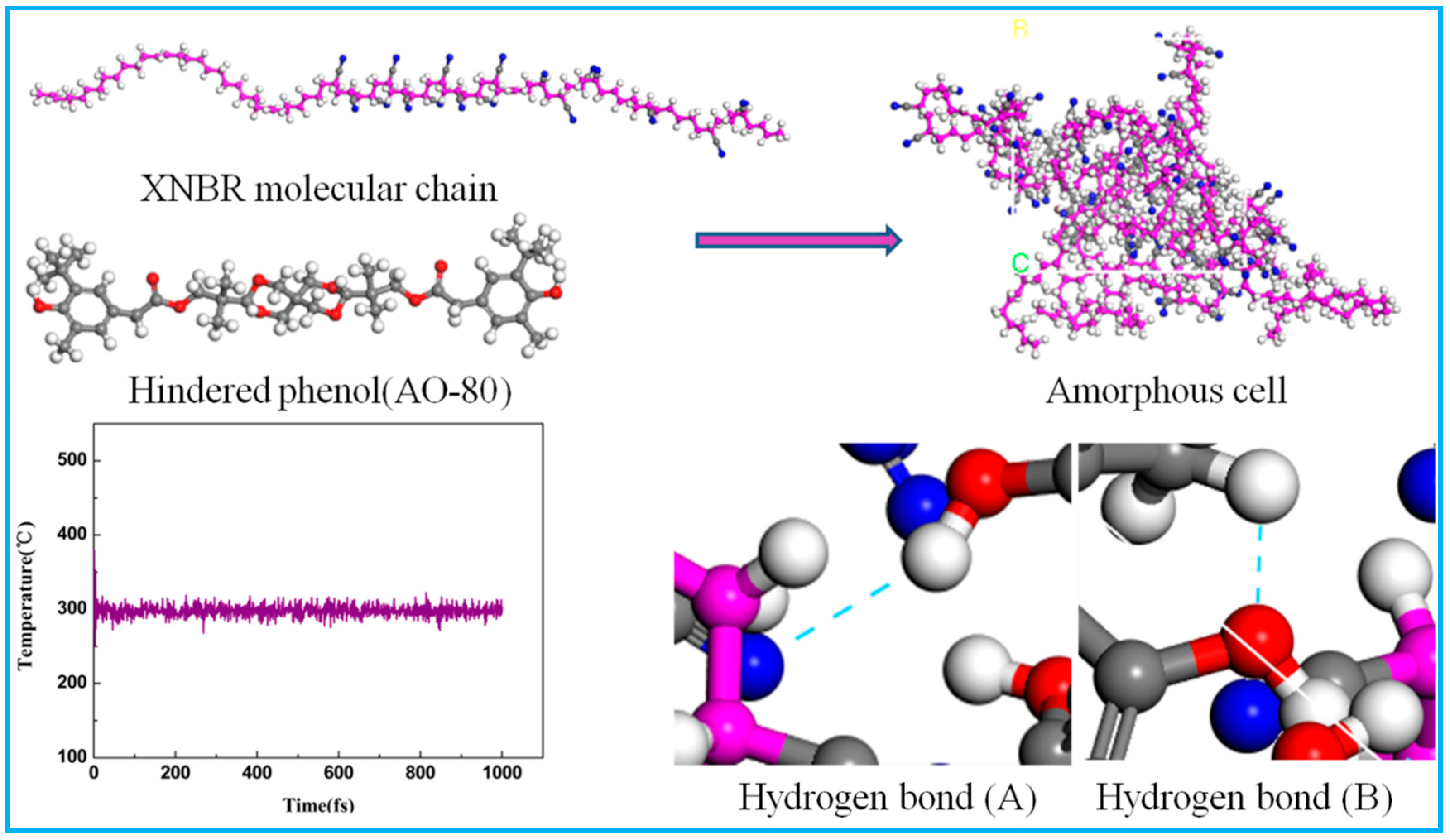
| Materials | Content (Parts Per Hundreds of Rubber) | |||
|---|---|---|---|---|
| XNBR/AO-80 | XNBR/AO-2246 | XNBR/AO-60 | XNBR/XH-245 | |
| XNBR | 100 | 100 | 100 | 100 |
| AO-80 | 10/20/30/40/50 | 0 | 0 | 0 |
| AO-2246 | 0 | 10/20/30/40/50 | 0 | 0 |
| AO-60 | 0 | 0 | 20/40/60 | 0 |
| XH-245 | 0 | 0 | 0 | 20/40/60 |
| Samples | Content | Hydrogen Bond (A) | Hydrogen Bond (B) |
|---|---|---|---|
| AO-2246 | XNBR/AO-2246(10) | 1 | 2 |
| XNBR/AO-2246(20) | 2 | 4 | |
| XNBR/AO-2246(30) | 2 | 4 | |
| XNBR/AO-2246(40) | 3 | 5 | |
| XNBR/AO-2246(50) | 3 | 6 | |
| AO-80 | XNBR/AO-80(10) | 1 | 0 |
| XNBR/AO-80(20) | 2 | 0 | |
| XNBR/AO-80(30) | 2 | 0 | |
| XNBR/AO-80(40) | 3 | 1 | |
| XNBR/AO-80(50) | 4 | 2 | |
| AO-60 | XNBR/AO-60(20) | 2 | 1 |
| XNBR/AO-60(40) | 4 | 2 | |
| XNBR/AO-60(60) | 5 | 3 | |
| XH-245 | XNBR/XH-245(20) | 1 | 0 |
| XNBR/XH-245(40) | 2 | 1 | |
| XNBR/XH-245(60) | 4 | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chen, D.; Fan, X.; Cai, Z.; Zhu, M. Effect of Hindered Phenol Crystallization on Properties of Organic Hybrid Damping Materials. Materials 2019, 12, 1008. https://doi.org/10.3390/ma12071008
Zhang L, Chen D, Fan X, Cai Z, Zhu M. Effect of Hindered Phenol Crystallization on Properties of Organic Hybrid Damping Materials. Materials. 2019; 12(7):1008. https://doi.org/10.3390/ma12071008
Chicago/Turabian StyleZhang, Lin, Duoli Chen, Xiaoqiang Fan, Zhenbing Cai, and Minhao Zhu. 2019. "Effect of Hindered Phenol Crystallization on Properties of Organic Hybrid Damping Materials" Materials 12, no. 7: 1008. https://doi.org/10.3390/ma12071008
APA StyleZhang, L., Chen, D., Fan, X., Cai, Z., & Zhu, M. (2019). Effect of Hindered Phenol Crystallization on Properties of Organic Hybrid Damping Materials. Materials, 12(7), 1008. https://doi.org/10.3390/ma12071008






