Effect of Phenolic Resin on Micropores Development in Carbon Foam with High Performance
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Carbon Foam from Modified CTP
2.2. Characterization
3. Results and Discussions
3.1. Composition of Pristine CF and Modified mCF Materials
3.2. Optical Structure of Pristine CF and Modified mCF Materials
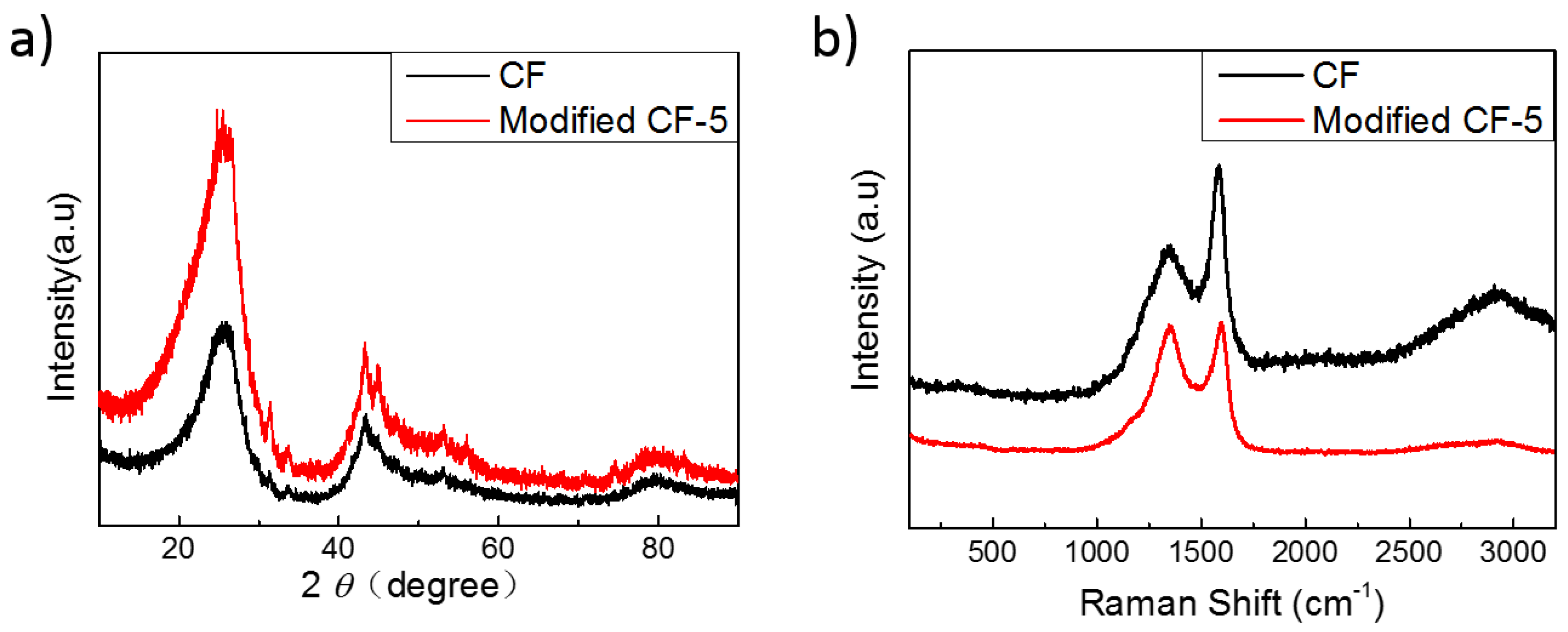
3.3. XRD and Raman Spectra of Pristine CF and Modified mCF Materials
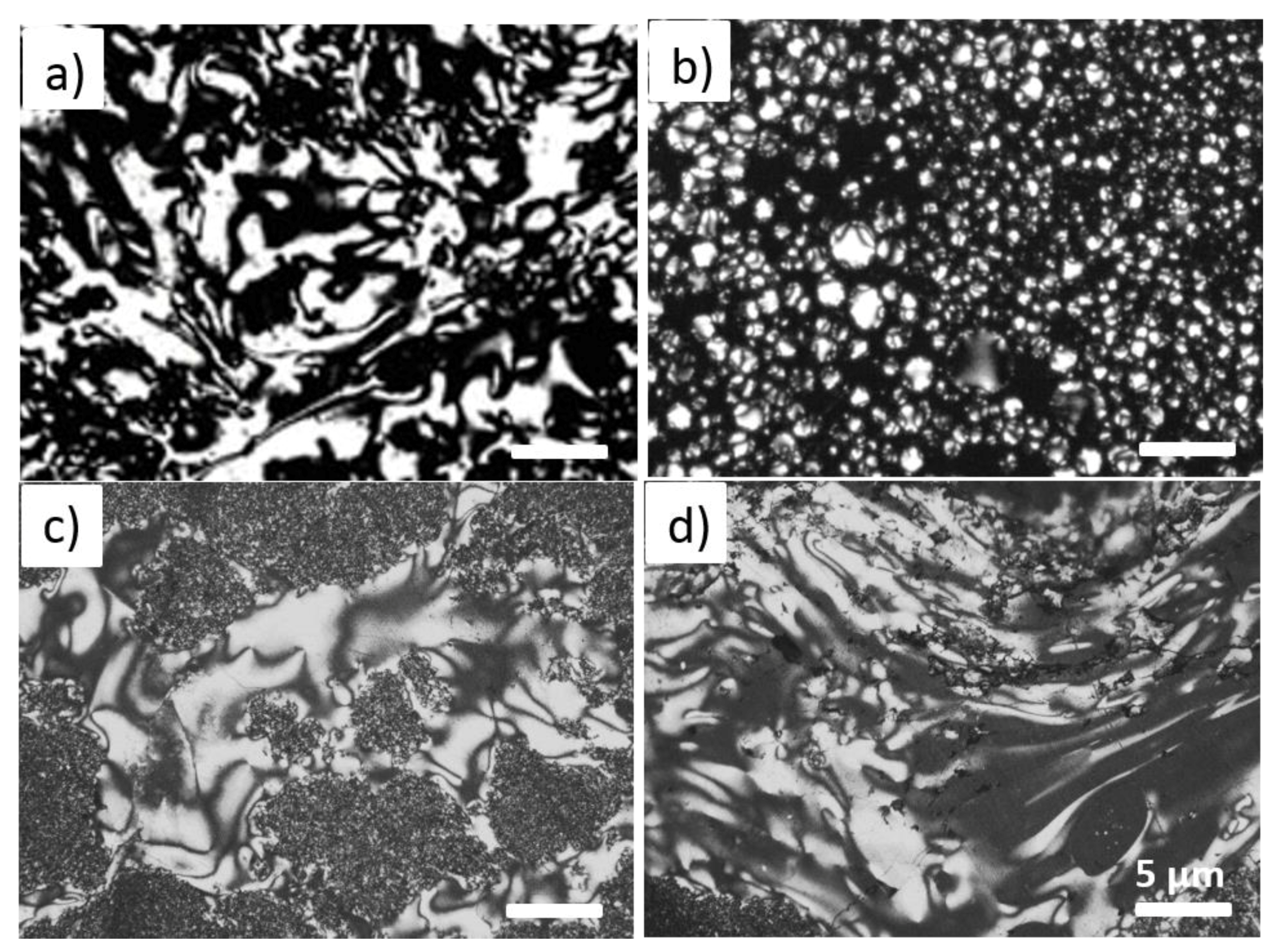
3.4. Morphologies of Pristine CF and Modified mCF Materials
3.5. Compression Strength of Pristine CF and Modified mCF Materials
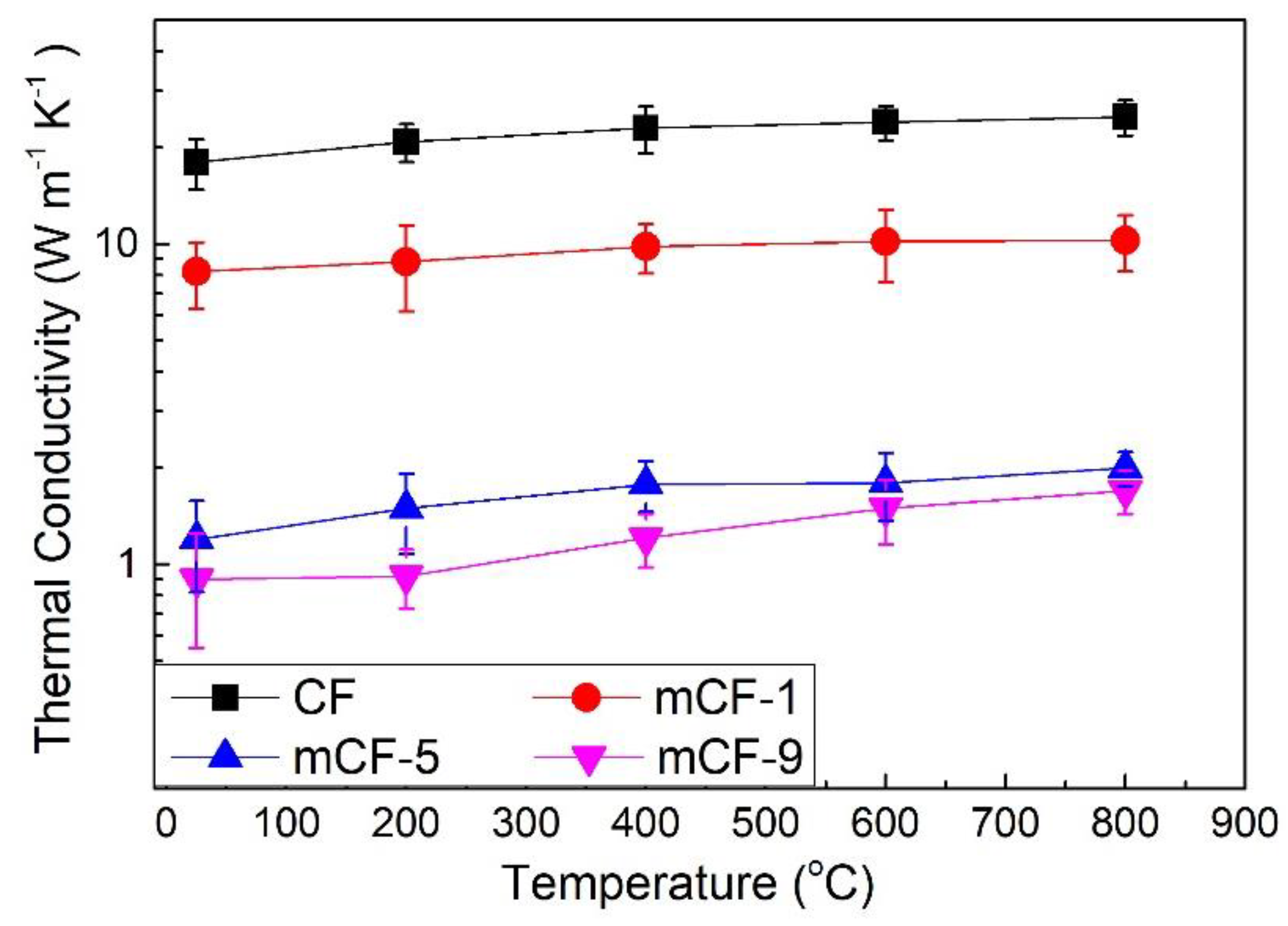
3.6. Thermal Conductivity of Pristine CF and Modified mCF Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banhart, J. Light-metal foams-history of innovation and technological challenges. Adv. Funct. Mater. 2013, 15, 82–111. [Google Scholar] [CrossRef]
- Linul, E.; Movahedi, N.; Marsavina, L. On the lateral compressive behavior of empty and ex-situ aluminum foam filled tubes at high temperature. Materials 2018, 11, 554. [Google Scholar] [CrossRef]
- Kováčik, J.; Marsavina, L.; Linul, E. Poisson’s Ratio of closed-cell aluminum foams. Materials 2018, 11, 1904. [Google Scholar] [CrossRef]
- Lehmhus, D.; Baumeister, J.; Stutz, L.; Schneider, E.; Stöbener, K.; Avalle, M.; Peroni, L.; Peroni, M. Mechanical Characterization of particulate aluminum foams-strain-rate density and matrix alloy versus adhesive effects. Adv. Eng. Mater. 2010, 12, 596–603. [Google Scholar] [CrossRef]
- Banhart, J. Metal foams: Production and stability. Adv. Eng. Mater. 2016, 8, 781–794. [Google Scholar] [CrossRef]
- Stoia, D.I.; Linul, E.; Marsavina, L. Influence of manufacturing parameters on mechanical properties porous materials by selective laser sintering. Materials 2019, 12, 871. [Google Scholar] [CrossRef]
- Movahedi, N.; Taherishargh, M.; Belova, I.V.; Murch, G.E.; Fiedler, T. Mechanical and microstructural characterization of an AZ91-activated carbon syntactic foam. Materials 2019, 12, 3. [Google Scholar] [CrossRef]
- Orbulov, I.N. Metal matrix syntactic foams produced by pressure infiltration-the effect of infiltration parameters. Mater. Sci. Eng. A 2013, 583, 11–19. [Google Scholar] [CrossRef]
- Labella, M.; Zeltmann, S.E.; Shunmugasamy, V.C.; Gupta, N.; Rohatgi, P.K. Mechanical and thermal properties of fly ash/vinyl ester syntactic foams. Fuel 2014, 121, 240–249. [Google Scholar] [CrossRef]
- Lin, T.C.; Gupta, N.; Talalayev, A. Thermoanalytical characterization of epoxy matrix-glass microballon syntactic foams. J. Mater. Sci. 2009, 44, 1520–1527. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, Y.S.; Kwac, L.K.; Chae, S.H.; Shin, H.K. Synthesis of carbon foam from waste artificial marble powder and carboxymethyl cellulose via electron beam irradiation and its characterization. Materials 2018, 11, 469. [Google Scholar] [CrossRef]
- Yu, Y.X.; Wang, Y.F.; Xu, P.P.; Chang, J.M. Preparation and characterization of phenolic foam modified with bio-oil. Materials 2018, 11, 2228. [Google Scholar] [CrossRef]
- Elsayed, H.; Rincón, A.R.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Bioactive glass-ceramic scaffolds from novel ‘inorganic gel casting’ and sinter-crystallization. Materials 2017, 10, 171. [Google Scholar] [CrossRef]
- Elsayed, H.; Rincón, A.R.; Molino, G.; Vitale, B.C.; Bernardo, E. Bioactive glass-ceramic foam scaffolds from ‘inorganic gel casting’ and sinter-crystallization. Materials 2018, 11, 349. [Google Scholar] [CrossRef]
- Tan, J.; Li, W.; Ma, C.H.; Wu, Q.; Xu, Z.; Liu, S.X. Synthesis of honeycomb-like carbon foam from larch sawdust as efficient absorbents for oil spills cleanup and recovery. Materials 2018, 11, 1106. [Google Scholar] [CrossRef]
- Inagaki, M.; Qiu, J.S.; Guo, Q.G. Carbon foam: Preparation and application. Carbon 2015, 87, 128–152. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.L.; Zhan, L. Carbon foams prepared from coal tar pitch for building thermal insulation materials with low cost. Chinese. J. Chem. Eng. 2018, 26, 415–420. [Google Scholar]
- Dang, A.L.; Li, T.H.; Xiong, C.Y.; Zhao, T.K.; Shang, Y.D.; Liu, H.G.; Chen, X.D.; Li, H.; Zhuang, Q.; Zhang, S.Z. Long-life electrochemical supercapacitor based on a novel hierarchically carbon foam templated carbon nanotube electrode. Compos. Part B Eng. 2018, 141, 250–257. [Google Scholar] [CrossRef]
- Kumar, R.; Dhakate, S.R.; Saini, P.; Mathur, R.B. Improved electromagnetic interference shielding effectiveness of light weight carbon foam by ferrocene accumulation. RSC Adv. 2013, 3, 4145–4151. [Google Scholar] [CrossRef]
- Liu, H.G.; Wu, J.S.; Zhuang, Q.; Dang, A.L.; Li, T.H.; Zhao, T.K. Preparation and the electromagnetic interference shielding in the X-band of carbon foams with Ni-Zn ferrite additive. J. Eur. Ceram. Soc. 2016, 36, 3939–3946. [Google Scholar] [CrossRef]
- Daems, N.; Sheng, X.; Vankelecom, I.F.J.; Pescarmona, P.P. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4085–4110. [Google Scholar] [CrossRef]
- Ribeiro, R.P.; Sauer, T.P.; Lopes, F.V.; Moreira, R.F.; Grande, C.A.; Rodrigues, A.E. Adsorption of CO2, CH4, and N2 in activated carbon honeycomb monolith. J. Chem. Eng. Data 2008, 53, 2311–2317. [Google Scholar] [CrossRef]
- Gallego, N.C.; Klett, J.W. Carbon foams for thermal management. Carbon 2003, 41, 1461–1466. [Google Scholar] [CrossRef] [Green Version]
- Ford, W.D. Method of Making Cellular Refractory Thermal Insulating Material. U.S. Patent 3121050, 11 February 1964. [Google Scholar]
- Gao, S.; Ge, L.; Rufford, T.; Zhu, Z. The preparation of activated carbon discs from tar pitch and coal power for adsorption of CO2, CH4 and N2. Microporous Mesoporous Mater. 2017, 238, 19–26. [Google Scholar] [CrossRef]
- Focke, W.; Badenhorst, H.; Ramjee, S.; Kruger, H.; Schalkwyk, R.; Rand, B. Graphite foam from pitch and expandable graphite. Carbon 2014, 73, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Villacorta, B.; Ge, L.; Steel, K.; Rufford, T.; Zhu, Z. Effect of rheological properties of mesophase pitch and coal mixtures on pore development in activated carbon discs with high compressive strength. Fuel Process. Technol. 2018, 177, 219–227. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, J.; Wang, Y.; Yu, M. A study of the properties of carbon foam reinforced by clay. Carbon 2006, 44, 1560–1564. [Google Scholar] [CrossRef]
- Gao, S.; Villacorta, B.; Ge, L.; Rufford, T.; Zhu, Z. Effect of sonication and hydrogen peroxide oxidation of carbon nanotube modifiers on the microstructure of pitch-derived activated carbon foam discs. Carbon 2017, 124, 142–151. [Google Scholar] [CrossRef]
- Liu, H.G.; Li, T.; Huang, T.; Zhao, X. Effect of multi-walled carbon nanotube additive on the microstructure and properties of pitch-derived carbon foams. J. Mater. Sci. 2015, 23, 7583–7590. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Chen, T.; Lin, Q.; Huang, X. Preparation and properties of graphene nanosheets/carbon foam composites. J. Anal. Appl. Pyrol. 2016, 117, 290–295. [Google Scholar] [CrossRef]
- Wu, X.; Luo, R.; Ni, Y.; Xiang, Q. Microstructure and mechanical properties of carbon foams and fibers reinforced carbon composites densified by CLVI and pitch impregnation. Compos. Part A Appl. Sci. Manuf. 2009, 40, 225–231. [Google Scholar] [CrossRef]
- Kumar, R.; Dhakate, S.; Mathur, R. The role of ferrocene on the enhancement of the mechanical and electrochemical properties of coal tar pitch-based carbon foams. J. Mater. Sci. 2013, 48, 7071–7080. [Google Scholar] [CrossRef]
- Liu, H.; Li, T.; Wang, X.; Zhang, W.; Zhao, T. Preparation and characterization of carbon foams with high mechanical strength using modified coal tar pitch. J. Anal. Appl. Pyrol. 2014, 110, 442–447. [Google Scholar] [CrossRef]
- Tsyntsarski, B.; Petrova, B.; Budinova, T.; Petrov, N.; Velasco, L.; Parra, J.; Ania, C. Porosity development during steam activation of carbon foams from chemically modified pitch. Microporous Mesoporous Mater. 2012, 154, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Grint, A.; Swietlik, U.; Marsh, H. Carbonization and liquid-crystal (mesophase) development. 9. The co-carbonization of vitrains with Ashland A200 petroleum pitch. Fuel 1979, 58, 642–650. [Google Scholar] [CrossRef]
- Dang, A.; Li, H.; Li, T.; Zhao, T.; Xiong, C.; Zhuang, Q.; Shang, Y.; Chen, X.; Ji, X. Preparation and Pyrolysis of Modified Coal Tar Pitch as C/C Composites Matrix Precursor. J. Anal. Appl. Pyrol. 2016, 119, 18–23. [Google Scholar] [CrossRef]
- Liu, H.; Li, T.; Shi, Y.; Wang, X.; Lv, J.; Zhang, W. Effect of different secondary quinoline insoluble content on the cellular structure of carbon foam derived from coal tar pitch. J. Anal. Appl. Pyrol. 2014, 108, 310–315. [Google Scholar] [CrossRef]
- Mochida, I.; Marsh, H.; Grint, A. Carbonization and liquid-crystal (mesophase) development. 8. The co-carbonization of coals with acenaphthylene and decacyclene. Fuel 1979, 58, 633–641. [Google Scholar] [CrossRef]
- Mochida, I.; Harry, M. Carbonization and liquid-crystal (mesophase) development. 13. Kinetic considerations of the co-carbonization of coals with organic additives. Fuel 1979, 58, 809–814. [Google Scholar] [CrossRef]
- Tsyntsarski, B.; Petrova, B.; Budinova, T.; Petrov, N.; Krzesinska, M.; Pusz, S.; Majewska, J.; Tzvetkov, P. Carbon foam derived from pitches modified with mineral acids by a low pressure foaming process. Carbon 2010, 48, 3523–3530. [Google Scholar] [CrossRef]
- Yu, M.; Zhong, C.; Zhang, Y.; Chen, Q.; Ao, X.; Lei, X.; Li, C. Pyrolysis behavior and chemical composition of the precursor influence on structure and properties of coal-tar-pitch-based carbon foams. J. Anal. Appl. Pyrol. 2018, 134, 293–300. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Zhong, Y.; Yan, X.; Song, Y.; Guo, Q.; Shi, J.; Liu, L. Formation mechanism of carbon foams derived from mesophase pitch. Carbon 2011, 49, 618–624. [Google Scholar] [CrossRef]
- Klett, J.; Hardy, R.; Romine, E.; Walls, C.; Burchell, T. High-thermal-conductivity, mesophase-pitch-derived carbon foams: Effect of precursor on structure and properties. Carbon 2000, 38, 953–973. [Google Scholar] [CrossRef]




| Samples | TS (wt%) a | QI (wt%) b |
|---|---|---|
| CTP | 79.2 | 3.9 |
| CTP-1 | 77.4 | 4.4 |
| CTP-3 | 75.8 | 5.8 |
| CTP-5 | 74.8 | 6.5 |
| CTP-7 | 75.8 | 5.6 |
| CTP-9 | 76.4 | 5.3 |
| Sample ID | XRD d002 (nm) | Graphitization Degree (%) | Raman (ID/IG) |
|---|---|---|---|
| Pristine CF | 0.352 | 93.02 | 0.51 |
| mCF-5 | 0.350 | 69.80 | 0.99 |
| Samples | Amount of Additive of PR (wt%) | Bulk Density (g/cm3) | Compressive Strength (MPa) |
|---|---|---|---|
| Pristine CF | 0 | 0.53 ± 0.1 | 6.5 ± 0.7 |
| mCF-1 | 1 | 0.64 ± 0.1 | 9.3 ± 1.2 |
| mCF-5 | 5 | 0.72 ± 0.1 | 12.8 ± 0.5 |
| mCF-9 | 9 | 0.74 ± 0.1 | 13.1 ± 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, A.; Zhao, Z.; Tang, C.; Fang, C.; Kong, S.; Khan, M.; Li, T.; Zhao, T.; Li, H. Effect of Phenolic Resin on Micropores Development in Carbon Foam with High Performance. Materials 2019, 12, 1213. https://doi.org/10.3390/ma12081213
Dang A, Zhao Z, Tang C, Fang C, Kong S, Khan M, Li T, Zhao T, Li H. Effect of Phenolic Resin on Micropores Development in Carbon Foam with High Performance. Materials. 2019; 12(8):1213. https://doi.org/10.3390/ma12081213
Chicago/Turabian StyleDang, Alei, Zhao Zhao, Chen Tang, Chenglin Fang, Siyuan Kong, Muhammad Khan, Tiehu Li, Tingkai Zhao, and Hao Li. 2019. "Effect of Phenolic Resin on Micropores Development in Carbon Foam with High Performance" Materials 12, no. 8: 1213. https://doi.org/10.3390/ma12081213






