The Expansion Cracks of Dolomitic Aggregates Cured in TMAH Solution Caused by Alkali–Carbonate Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Concrete Microbars and Rock Prisms
2.3. Testing and Characterization
3. Results and Discussion
3.1. The Expansion of Concrete Microbars and Rock Prisms Cured in TMAH Solution
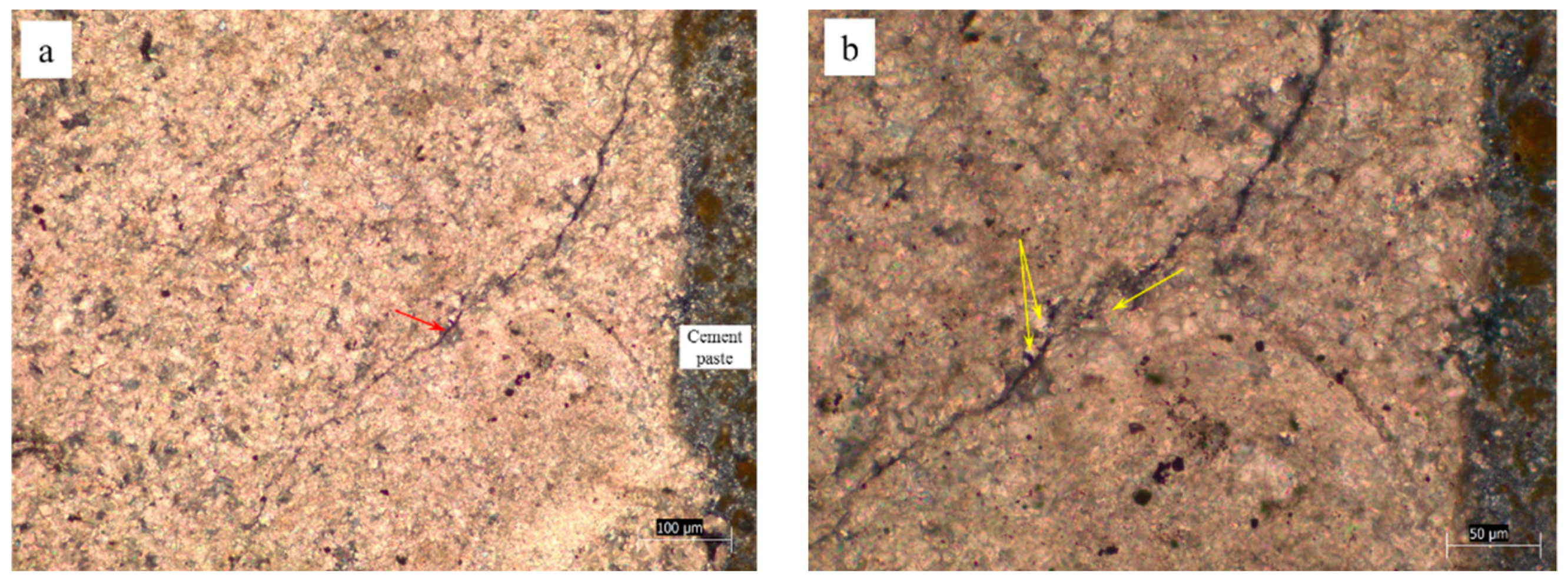
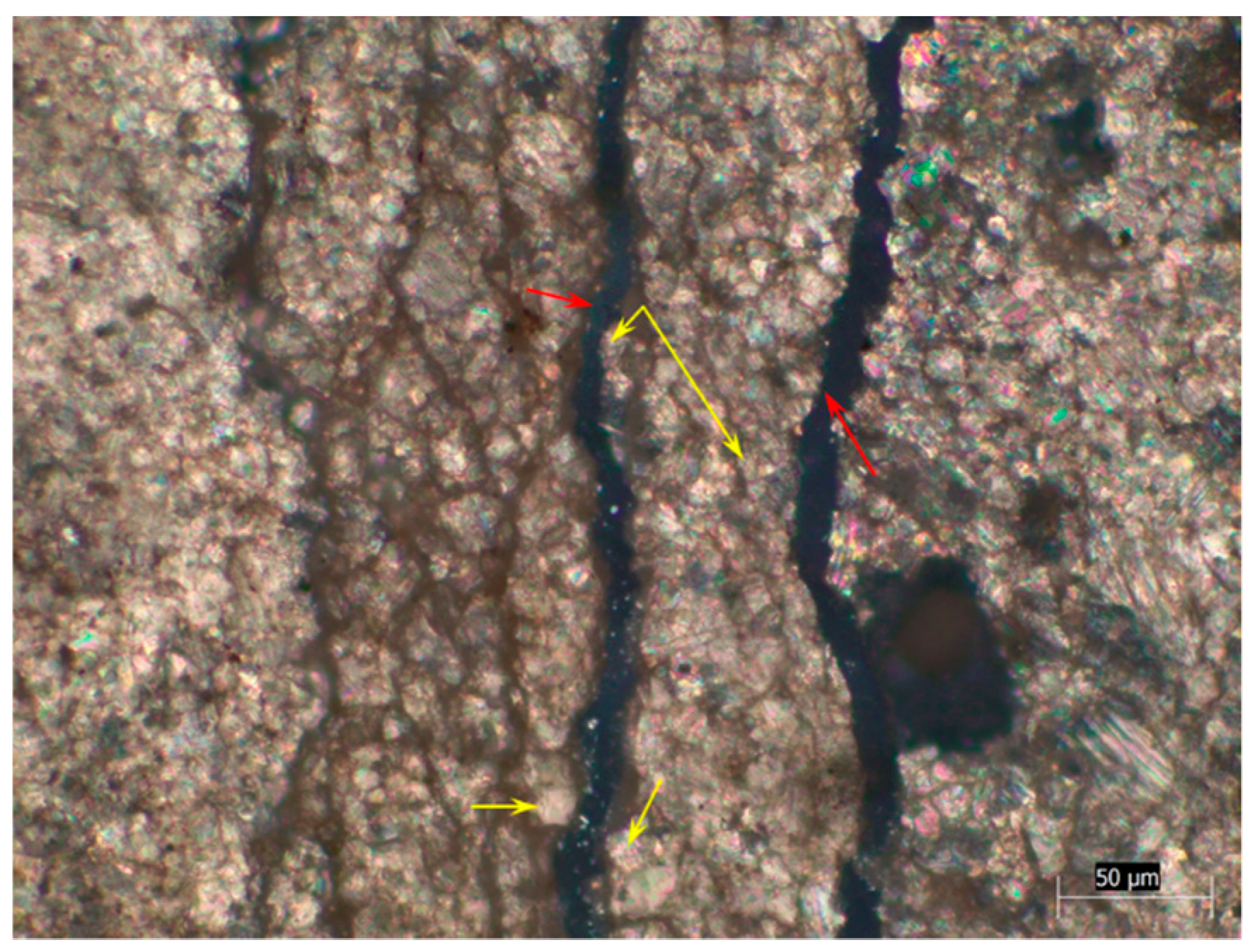
3.2. Crack Characteristics
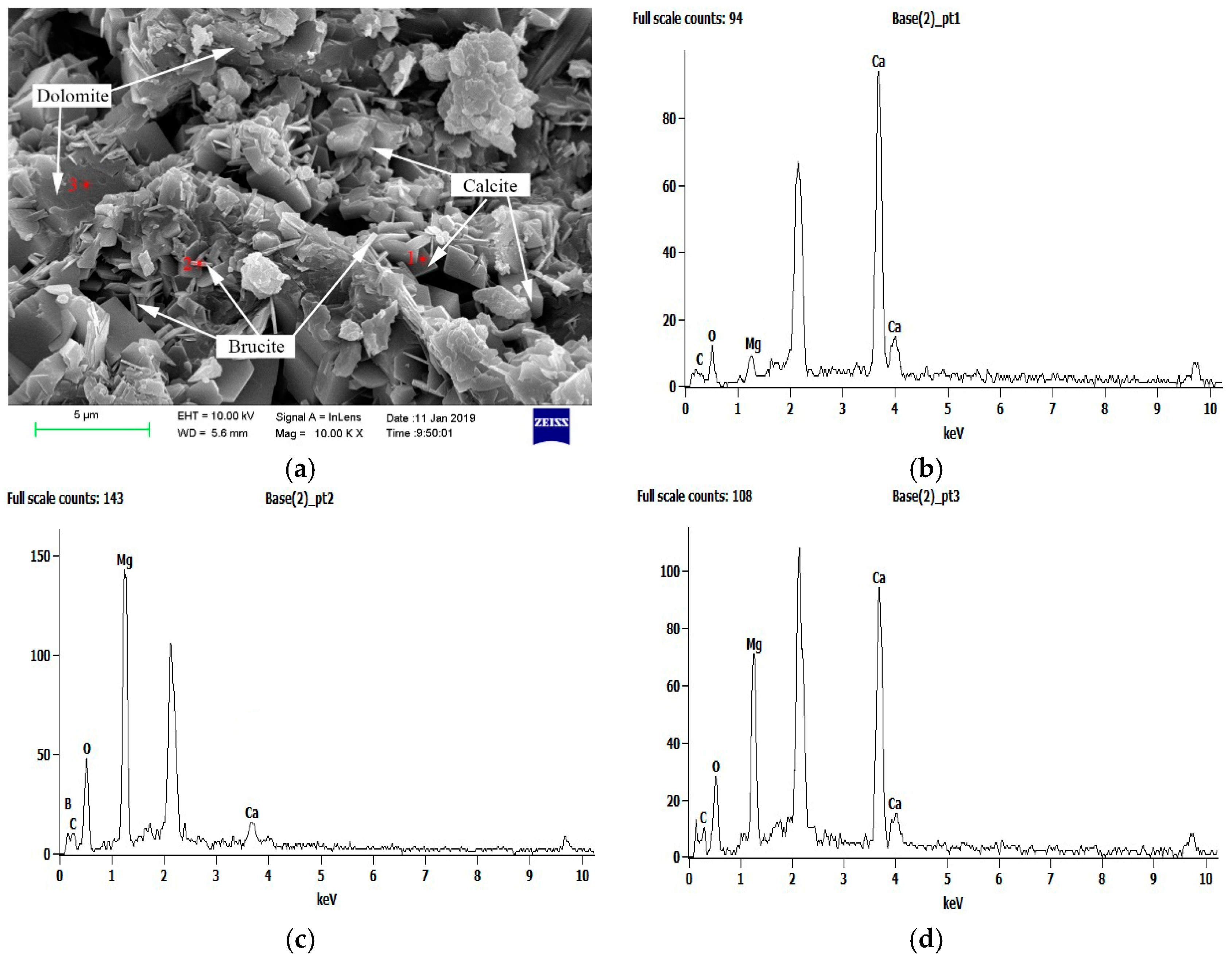
3.3. Products Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feng, X.; Feng, N. Expansion Mechanism of Alkali Carbonate Reaction. J. Chin. Ceram. Soc. 2005, 33, 912–915. [Google Scholar]
- Katayama, T. The so-called alkali-carbonate reaction (ACR)—Its mineralogical and geochemical details, with special reference to ASR. Cem. Concr. Res. 2010, 40, 643–675. [Google Scholar] [CrossRef]
- Pagano, M.A.; Candy, P.D. A chemical approach to the problem of alkali-reactive carbonate aggregates. Cem. Concr. Res. 1982, 12, 1–12. [Google Scholar] [CrossRef]
- Tong, L.; Tang, M. Correlation between reaction and expansion of alkali-carbonate reaction. Cem. Concr. Res. 1995, 25, 470–476. [Google Scholar] [CrossRef]
- Katayama, T. How to identify carbonate rock reactions in concrete. Mater. Charact. 2004, 53, 85–104. [Google Scholar] [CrossRef]
- López-Buendia, A.M.; Climent, V.; Verdú, P. Lithological influence of aggregate in the alkali-carbonate reaction. Cem. Concr. Res. 2006, 36, 1490–1500. [Google Scholar] [CrossRef]
- Swenson, E.G. A reactive a aggregate undetected by ASTM test. Astm Bull. 1957, 226, 48–51. [Google Scholar]
- Grattan-Bellew, P.E.; Mitchell, L.D.; Margeson, J.; Min, D. Is alkali-carbonate reaction just a varian of alkali-silica reaction ACR=ASR? Cem. Concr. Res. 2010, 40, 556–562. [Google Scholar] [CrossRef]
- Grattan-Bellew, P.E.; Chan, G. Comparison of the morphology of alkali-silica gel formed in limestones in concrete affected by the so-called alkali-carbonate reaction (ACR) and alkali-silica reaction (ASR). Cem. Concr. Res. 2013, 47, 51–54. [Google Scholar] [CrossRef]
- Fecteau, P.L.; Fournier, B. Contribution to the understanding of the so-called alkali-carbonate reaction. In Proceedings of the 14th ICAAR in Concrete, Austin, TX, USA, 20–25 May 2012. [Google Scholar]
- Prinčič, T.; Štukovnik, P.; Pejovnik, S.; de Schutter, G.; Bosiljkov, V.B. Observations on dedolomitization of carbonate concrete aggregates, Implications for ACR and expansion. Cem. Concr. Res. 2013, 54, 151–160. [Google Scholar] [CrossRef]
- Gali, S.; Ayora, C.; Alfonso, P.; Tauler, E.; Labrador, M. Kinetics of dolomite-portlandite reaction—Application to Portland cement concrete. Cem. Concr. Res. 2001, 31, 933–939. [Google Scholar] [CrossRef]
- Milanesi, C.A.; Marfil, S.A.; Batic, O.R.; Maiza, P.J. The alkali-carbonate reaction and its reaction products an experience with Argentinean dolomite rocks. Cem. Concr. Res. 1996, 26, 1579–1591. [Google Scholar] [CrossRef]
- Katayama, T. A critical review of carbonate rock reactions—Is their reactivity useful or harmful? In Proceedings of the Ninth International Conference on Alkali–Aggregate Reaction in Concrete, London, UK, 27–31 July 1992; Volume 1, pp. 508–518. [Google Scholar]
- Mather, B. Developments in specifications and control. Cem. Aggreg. 1974, 525, 38–42. [Google Scholar]
- Duke, E.F. Reactions between dolomite and cement paste in deteriorated concrete. In Proceedings of the USA-Australia Workshop on High Performance Concrete, Sydney, Australia, 20–23 August 1997. [Google Scholar]
- Milanesi, C.A.; Batic, O.R. Alkali reactivity of dolomitic rocks from Argentina. Cem. Concr. Res. 1994, 24, 1073–1084. [Google Scholar] [CrossRef]
- Milanesi, C.A.; Marfil, S.A.; Batic, O.R. An expansive dolostone from Argentinean-The common dilemma: ACR or another variant of ASR? In Proceedings of the 14th International Conference on Alkali Aggregate Reactions in Concrete, Austin, TX, USA, 20–25 May 2012. [Google Scholar]
- Chen, B.; Deng, M.; Lan, X.; Xu, L. Behaviors of reactive silica and dolomite in tetramethyl ammonium hydroxide solutions. In Proceedings of the 15th International Conference on Alkali-Aggregate Reactions in Concrete, St Paul, Brazil, 3–7 July 2016. [Google Scholar]
- Pu, Y. Removal of Crystal Water from Tetramethylammonium Hydroxide Pentahydrate and Determination of Its Solubility; Zhengzhou University: Zhengzhou, China, 2011. [Google Scholar]
- Thong, J.T.L.; Choi, W.K. TMAH etching of silicon and the interaction of etching parameters. Sens. Actuators 1997, 243–249. [Google Scholar] [CrossRef]
- RILEM Recommendation. AAR-5: Rapid preliminary screening test for carbonate aggregates. Mater. Struct. 2005, 38, 787–792. [Google Scholar]
- RILEM Recommendation. AAR-2: Detection of potential alkali-reactivity of aggregates-The ultra-accelerated mortar-bar test. Mater. Struct. 2000, 33, 226. [Google Scholar]
- ASTM C586-11: Standard Test Method for Potential Alkali Reactivity of Carbonate Rocks as Concrete Aggregates. Available online: https://www.astm.org/Standards/C586.htm (accessed on 1 November 2011).







| Rock | Composition (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| LOI | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | ||
| 1 | CG1 | 29.26 | 22.47 | 2.59 | 6.59 | 31.70 | 4.29 |
| 2 | JT1 | 28.60 | 23.36 | 2.84 | 7.25 | 31.15 | 3.58 |
| 3 | YMS1 | 26.48 | 25.02 | 2.87 | 7.82 | 29.50 | 3.09 |
| 4 | CX1 | 33.94 | 15.21 | 1.81 | 4.30 | 38.26 | 2.92 |
| 5 | CX2 | 35.41 | 12.95 | 1.94 | 3.96 | 40.63 | 3.12 |
| 6 | SJW1 | 37.91 | 9.10 | 1.16 | 2.96 | 42.75 | 4.29 |
| 7 | SJW2 | 36.72 | 11.69 | 1.43 | 3.82 | 39.27 | 4.58 |
| CaCO3 | SiO2 | Al2O3 | Fe3O4 | Total |
|---|---|---|---|---|
| 78.18 | 14.03 | 4.40 | 3.39 | 100 |
| C3S | C2S | C4AF | C3A | f-CaO | f-MgO |
|---|---|---|---|---|---|
| 64.0 | 11.9 | 10.9 | 13.2 | 0.1 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yang, B.; Mao, Z.; Deng, M. The Expansion Cracks of Dolomitic Aggregates Cured in TMAH Solution Caused by Alkali–Carbonate Reaction. Materials 2019, 12, 1228. https://doi.org/10.3390/ma12081228
Chen X, Yang B, Mao Z, Deng M. The Expansion Cracks of Dolomitic Aggregates Cured in TMAH Solution Caused by Alkali–Carbonate Reaction. Materials. 2019; 12(8):1228. https://doi.org/10.3390/ma12081228
Chicago/Turabian StyleChen, Xiaoxiao, Bin Yang, Zhongyang Mao, and Min Deng. 2019. "The Expansion Cracks of Dolomitic Aggregates Cured in TMAH Solution Caused by Alkali–Carbonate Reaction" Materials 12, no. 8: 1228. https://doi.org/10.3390/ma12081228
APA StyleChen, X., Yang, B., Mao, Z., & Deng, M. (2019). The Expansion Cracks of Dolomitic Aggregates Cured in TMAH Solution Caused by Alkali–Carbonate Reaction. Materials, 12(8), 1228. https://doi.org/10.3390/ma12081228





