Effect of Potassium Ions on the Formation of Mixed-Valence Manganese Oxide/Graphene Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO
2.3. Preparation of MnOx/rGO Nanocomposites
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
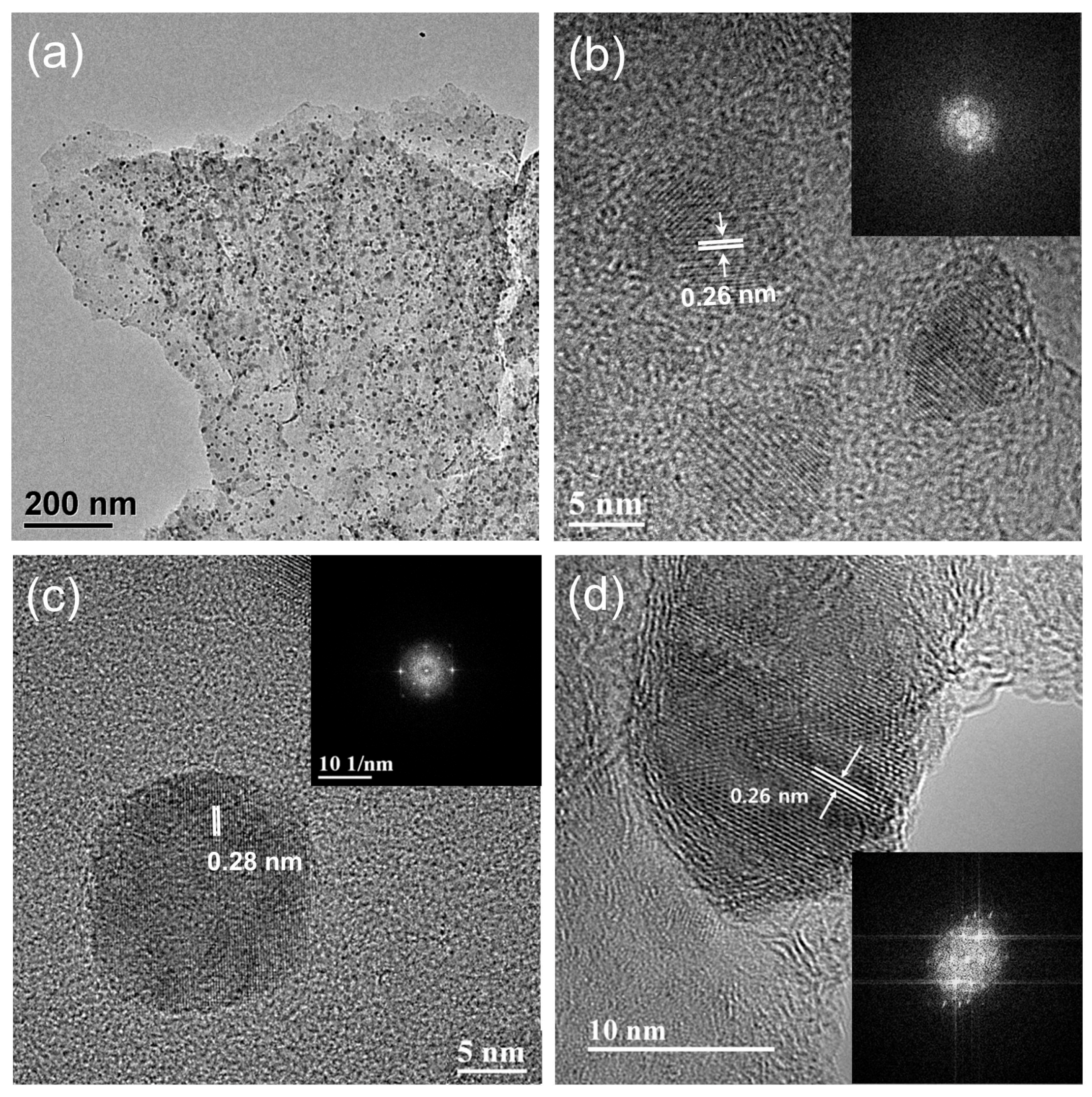
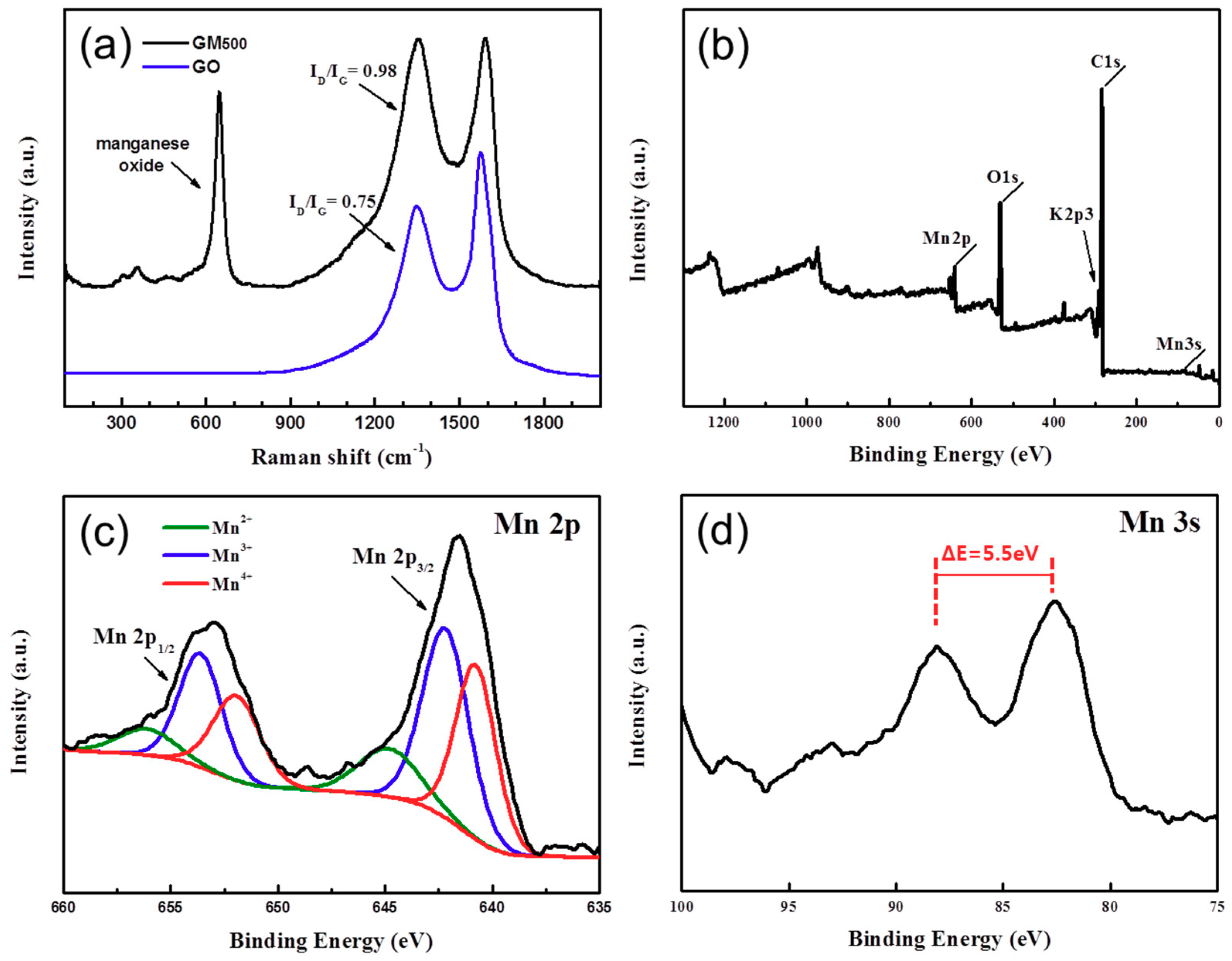
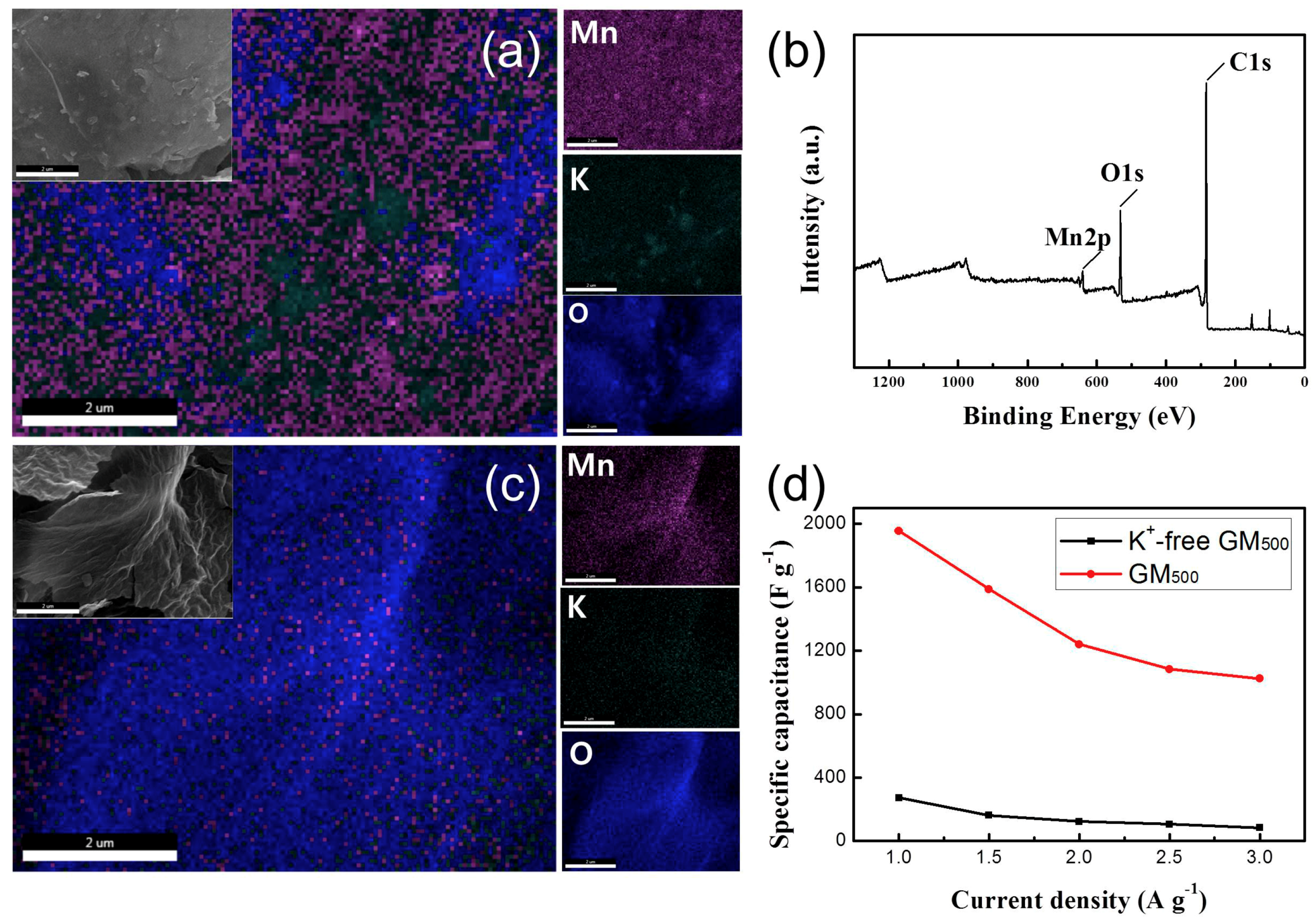
3.1. Materials Characterization
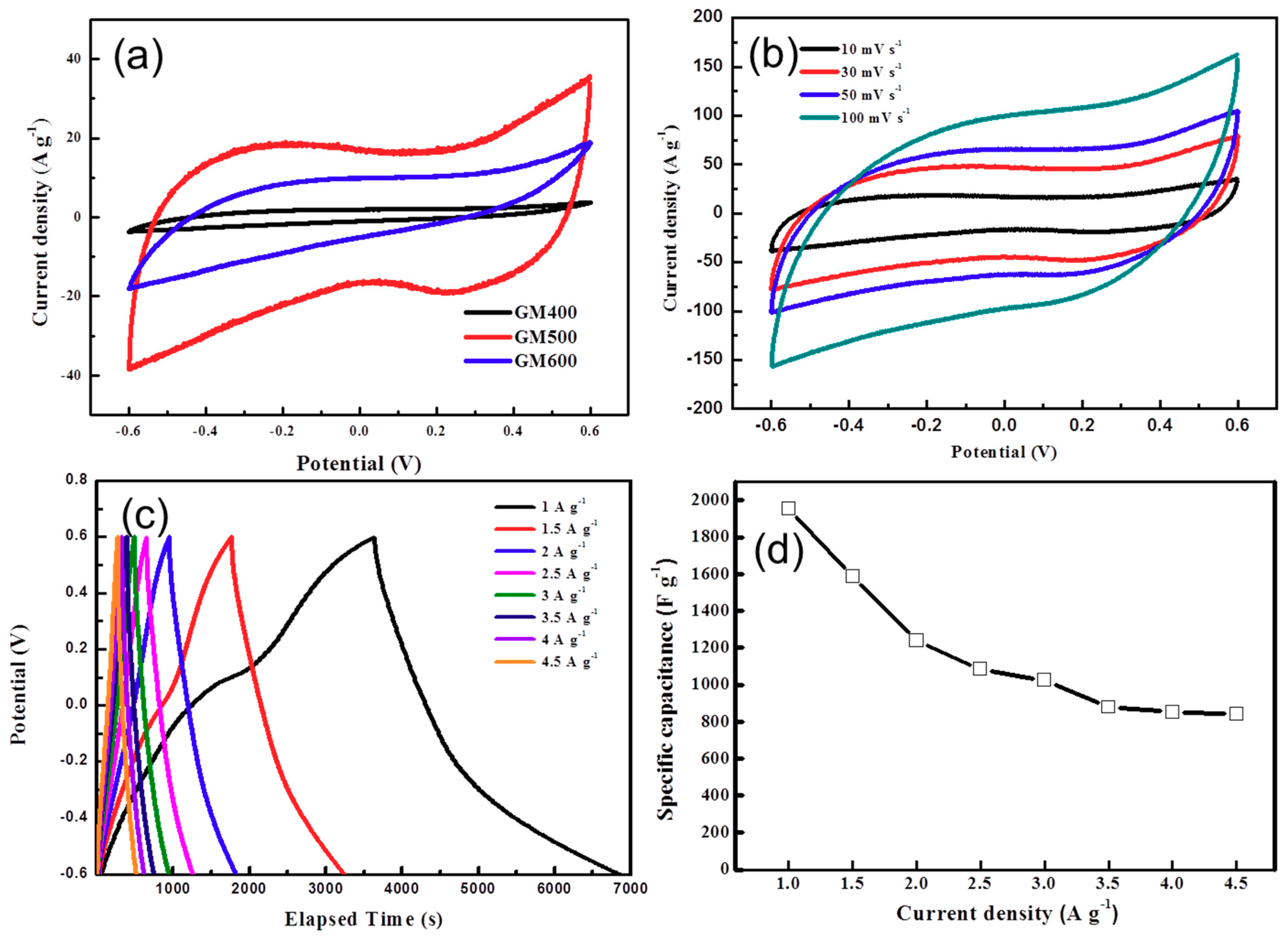
3.2. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Xiao, F.; Qian, L.; Xiao, J.; Wang, S.; Liu, Y. Facile synthesis of 3D MnO2-graphene and carbon nanotube-graphene composite networks for high-performance, flexible, all-solid-state asymmetric supercapacitors. Adv. Energy Mater. 2012, 4, 1400064. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Murali, S.; Stoller, M.D.; Zhang, Q.; Zhu, Y.; Ruoff, R.S. Incorporation of manganese dioxide within ultraporous activated graphene for high-performance electrochemical capacitors. ACS Nano 2012, 6, 5404–5412. [Google Scholar] [CrossRef] [PubMed]
- Sumboja, A.; Foo, C.Y.; Wang, X.; Lee, P.S. Large Areal Mass, Flexible and free-standing reduced graphene oxide/manganese dioxide paper for asymmetric supercapacitor device. Adv. Mater. 2013, 25, 2809–2815. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Hu, L.; Vosgueritchian, M.; Wang, H.; Xie, X.; McDonough, J.R.; Cui, X.; Cui, Y.; Bao, Z. Solution-processed Graphene/MnO2 nanostructured textiles for high-performance electrochemical capacitors. Nano Lett. 2011, 11, 2905–2911. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Q.; Yan, Z.; Hu, X.; Kang, L.; Lei, Z.; Liu, Z.-H. High performance graphene/manganese oxide hybrid electrode with flexible holey structure. Electrochim. Acta 2014, 129, 237–244. [Google Scholar] [CrossRef]
- Staiti, P.; Lufrano, F. Study and optimization of manganese oxide-based electrodes for electrochemical supercapacitors. J. Power Sources 2009, 187, 284–289. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Liu, G.-J.; Zhao, J.-C.; Wu, J.-H.; Lin, J.-M.; Huo, J.-H.; Liu, L. Facile one-step hydrothermal syntheses and supercapacitive performances of reduced graphene oxide/MnO2 composites. Compos. Sci. Technol. 2014, 103, 113–118. [Google Scholar] [CrossRef]
- Lei, W.; He, P.; Wang, Y.; Zhang, X.; Xia, A.; Dong, F. Solvothermal preparation of microspherical shaped cobalt-manganese oxide as electrode materials for supercapacitors. Compos. Sci. Technol. 2014, 102, 82–86. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, R.D. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser Scribing of high-performance and flexible Graphene-based electrochemical capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-based nanocomposites for energy storage. Adv. Mater. 2016, 6, 1502159. [Google Scholar] [CrossRef]
- Hassan, M.; Reddy, K.R.; Haque, E.; Faisal, S.N.; Ghasemi, S.; Minett, A.I.; Gomes, V.G. Hierarchical assembly of graphene/polyaniline nanostructures to synthesize free-standing supercapacitor electrode. Compos. Sci. Technol. 2014, 98, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Ni, T.; Dai, T.; Lu, Y. One-step synthesis of iodine doped polyaniline-reduced graphene oxide composite hydrogel with high capacitive properties. Compos. Sci. Technol. 2015, 109, 12–17. [Google Scholar] [CrossRef]
- Pullini, D.; Siong, V.; Tamvakos, D.; Ortega, B.L.; Sgroi, M.F.; Veca, A.; Glanz, C.; Kolaric, I.; Pruna, A. Enhancing the capacitance and active surface utilization of supercapacitor electrode by graphene nanoplatelets. Compos. Sci. Technol. 2015, 112, 16–21. [Google Scholar] [CrossRef]
- Moussa, M.; El-Kady, M.F.; Abdel-Azeim, S.; Kaner, R.B.; Majewski, P.; Ma, J. Compact, flexible conducting polymer/graphene nanocomposites for supercapacitors of high volumetric energy density. Compos. Sci. Technol. 2018, 160, 50–59. [Google Scholar] [CrossRef]
- Belanger, D.; Brousse, T.; Long, J.W. Manganese oxides: battery materials make the leap to electrochemical capacitors. Electrochem. Soc. Interface 2008, 17, 49–52. [Google Scholar]
- Lee, J.W.; Hall, A.S.; Kim, J.-D.; Mallouk, T.E. ChemInform Abstract: A Facile and template-free hydrothermal synthesis of Mn3O4 nanorods on graphene sheets for supercapacitor electrodes with long cycle stability. Chem. Mater. 2012, 43, 1158–1164. [Google Scholar] [CrossRef]
- Park, K.W. Carboxylated graphene oxide-Mn2O3 nanorod composites for their electrochemical characteristics. J. Mater. Chem. A 2014, 2, 4292–4298. [Google Scholar] [CrossRef]
- Song, M.-K.; Cheng, S.; Chen, H.; Qin, W.; Nam, K.-W.; Xu, S.; Yang, X.-Q.; Bongiorno, A.; Lee, J.; Bai, J.; et al. Anomalous pseudocapacitive behavior of a nanostructured, mixed-valent manganese oxide film for electrical energy storage. Nano Lett. 2012, 12, 3483–3490. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Alonso, E.; Hutter, C.; Romero, M.; Steinfeld, A.; Gonzalez-Aguilar, J. Kinetics of Mn2O3-Mn3O4 and Mn3O4-MnO redox reactions performed under concentrated thermal radiative flux. Energy Fuels 2013, 27, 4884–4890. [Google Scholar] [CrossRef]
- Dang, T.-D.; Banerjee, A.N.; Joo, S.W.; Min, B.-K. Effect of potassium ions on the formation of crystalline manganese oxide nanorods via acidic reduction of potassium permanganate. Ind. Eng. Chem. Res. 2013, 52, 14154–14159. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Xia, F.; Huang, Y. Reconstruction of conformal nanoscale MnO on graphene as a high-capacity and long-life anode Material for lithium ion batteries. Adv. Funct. Mater. 2013, 23, 2436–2444. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Banerjee, D. Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am. Miner. 1998, 83, 305–315. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Huang, X.; Hu, N.; Gao, R.; Yu, Y.; Wang, Y.; Yang, Z.; Kong, E.S.-W.; Wei, H.; Zhang, Y. Reduced graphene oxide-polyaniline hybrid: Preparation, characterization and its applications for ammonia gas sensing. J. Mater. Chem. 2012, 22, 22488–22495. [Google Scholar] [CrossRef]
- Adelkhani, H.; Ghaemi, M.; Jafari, S.M. Novel nanostructured MnO2 prepared by pulse electrodeposition: Characterization and electrokinetics. J. Mater. Sci. Technol. 2008, 24, 857–862. [Google Scholar]
- Yu, H.; Xin, G.; Ge, X.; Bulin, C.; Li, R.; Xing, R.; Zhang, B. Porous graphene-polyaniline nanoarrays composite with enhanced interface bonding and electrochemical performance. Compos. Sci. Technol. 2018, 154, 76–84. [Google Scholar] [CrossRef]
- Ruetschi, P. Cation-vacancy model for MnO2. J. Electrochem. Soc. 1984, 131, 2737–2744. [Google Scholar] [CrossRef]
- Mefford, J.T.; Hardin, W.G.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat. Mater. 2014, 13, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.Q.; Minhas-Khan, A.; Tian, X.; Hercule, K.M.; Zhao, Y.L.; Lin, X.; Xu, X. Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance. Nat. Commun. 2013, 4, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, X.; Lozano, K.; Mao, Y. Asymmetric supercapacitors with dominant pseudocapacitance based on manganese oxide nanoflowers in a neutral aqueous electrolyte. RSC Adv. 2013, 3, 24886–24890. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.; Jeon, D.-Y.; Lee, Y.-S.; Koo, H.Y. Effect of Potassium Ions on the Formation of Mixed-Valence Manganese Oxide/Graphene Nanocomposites. Materials 2019, 12, 1245. https://doi.org/10.3390/ma12081245
Jang W, Jeon D-Y, Lee Y-S, Koo HY. Effect of Potassium Ions on the Formation of Mixed-Valence Manganese Oxide/Graphene Nanocomposites. Materials. 2019; 12(8):1245. https://doi.org/10.3390/ma12081245
Chicago/Turabian StyleJang, Wooree, Dae-Young Jeon, Youn-Sik Lee, and Hye Young Koo. 2019. "Effect of Potassium Ions on the Formation of Mixed-Valence Manganese Oxide/Graphene Nanocomposites" Materials 12, no. 8: 1245. https://doi.org/10.3390/ma12081245



