Highly Active AuCu-Based Catalysts for Acetylene Hydrochlorination Prepared Using Organic Aqua Regia
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterisation
2.3. Catalytic Test
3. Results and Discussion
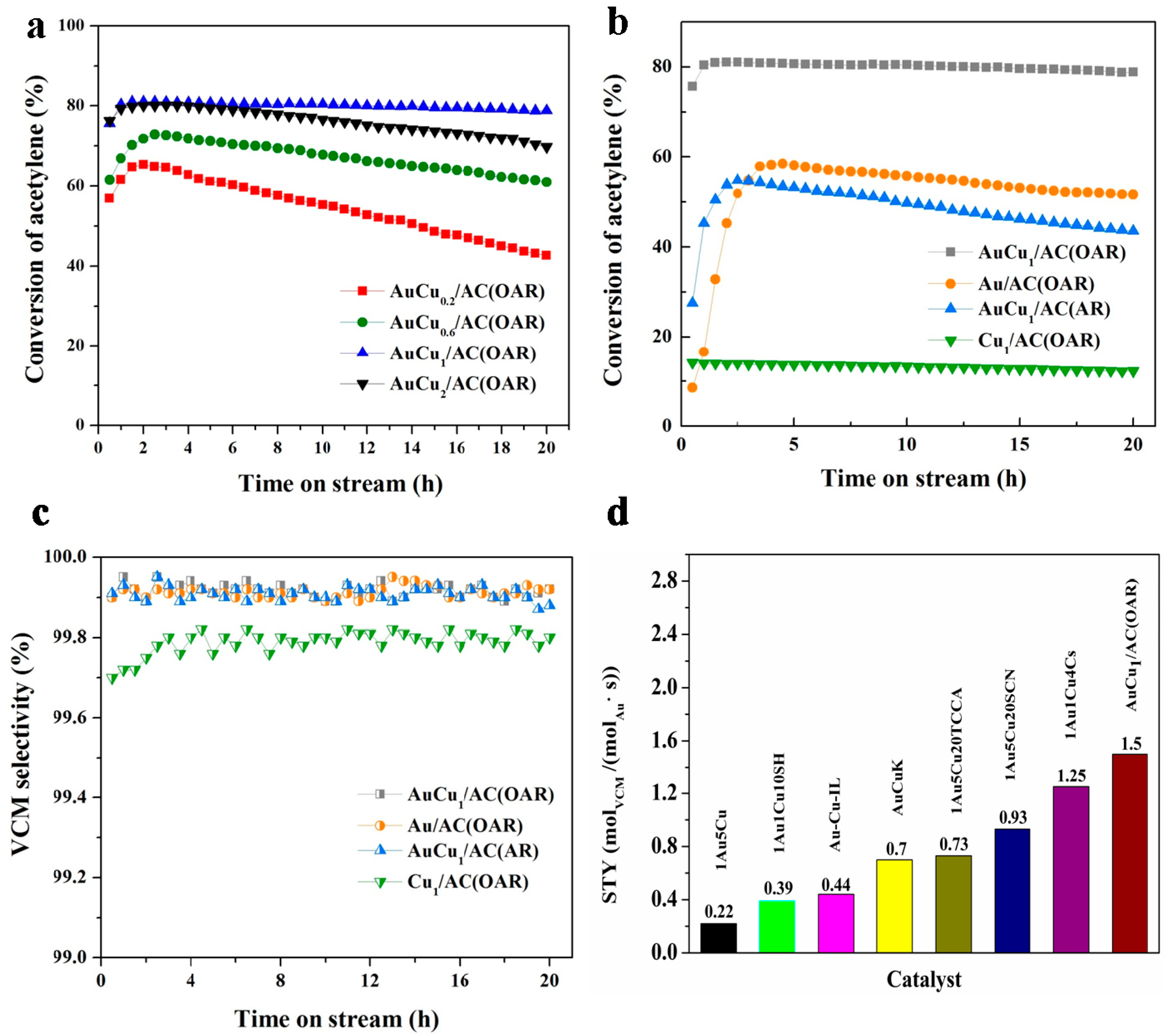
3.1. Optimisations for the Bimetallic Au-Based Catalysts
3.2. Effect of the OAR on the AuCu/AC(OAR) Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schobert, H. Production of acetylene and acetylene-based chemicals from coal. Chem. Rev. 2014, 114, 1743–1760. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.; Hutchings, G.J. Gold catalysis. Angew. Chem. 2006, 45, 7896–7936. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.J.; Miedziak, P.J.; Brett, G.L.; Hutchings, G.J. Vinyl chloride monomer production catalysed by gold: A review. Chin. J. Catal. 2016, 37, 1600–1607. [Google Scholar] [CrossRef]
- Malta, G.; Kondra, S.A.; Freakley, S.J.; Davies, C.J.; Lu, L.; Dawson, S.; Thetford, A.; Gibson, E.K.; Morgan, D.J.; Jones, W. Identification of single site gold catalysis in acetylene hydrochlorination. Science 2017, 355, 1399–1403. [Google Scholar] [CrossRef]
- Nkosi, B.; Adams, M.D.; Coville, N.J.; Hutchings, G.J. Hydrochlorination of Acetylene Using Carbon-Supported Gold Catalysts: A Study of Catalyst Reactivation. J. Catal. 1991, 128, 378–386. [Google Scholar] [CrossRef]
- Malta, G.; Freakley, S.J.; Kondrat, S.A.; Hutchings, G.J. Acetylene hydrochlorination using Au/carbon: A journey towards single site catalysis. Chem. Commun. 2017, 53, 11733–11746. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Carley, A.F.; Hutchings, G.J. Reactivation of a Carbon-supported Gold Catalyst for the Hydrochlorination of Acetylene. Catal Lett. 2008, 124, 165–167. [Google Scholar] [CrossRef]
- Conte, M.; Davies, C.; Morgan, D.; Davies, T.; Elias, D.; Carley, A.; Johnston, P.; Hutchings, G.J. Aqua regia activated Au/C catalysts for the hydrochlorination of acetylene. J. Catal. 2013, 297, 128–136. [Google Scholar] [CrossRef]
- Liu, X.; Conte, M.; Elias, D.; Lu, L.; Morgan, D.J.; Freakley, S.J.; Johnston, P.; Kiely, C.J.; Hutchings, G.J. Investigation of the active species in the carbon-supported gold catalyst for acetylene hydrochlorination. Catal. Sci. Technol. 2016, 6, 5144–5153. [Google Scholar] [CrossRef]
- Conte, M.; Davies, C.J.; Morgan, D.J.; Carley, A.F.; Johnston, P.; Hutchings, G.J. Characterization of Au3+ Species in Au/C Catalysts for the Hydrochlorination Reaction of Acetylene. Catal. Lett. 2013, 144, 1–8. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Lin, R.; Mitchell, S.; Fako, E.; Krumeich, F.; Hauert, R.; Safonova, O.V.; Kondratenko, V.A.; Kondratenko, E.V.; Collins, S.M. Controlling the speciation and reactivity of carbon-supported gold nanostructures for catalysed acetylene hydrochlorination. Chem. Sci. 2019, 10, 359–369. [Google Scholar] [CrossRef]
- Malta, G.; Kondrat, S.A.; Freakley, S.J.; Davies, C.; Dawson, S.; Liu, X.; Lu, L.; Dymkowski, K.; Fernandez-Alonso, F.; Mukhopadhyay, S. Deactivation of a Single-Site Gold-on-Carbon Acetylene Hydrochlorination Catalyst: An X-ray Absorption and Inelastic Neutron Scattering Study. ACS Catal. 2018, 8, 8493–8505. [Google Scholar] [CrossRef]
- Zhao, J.; Yue, Y.; Sheng, G.; Wang, B.; Lai, H.; Di, S.; Zhai, Y.; Guo, L.; Li, X. Supported ionic liquid-palladium catalyst for the highly effective hydrochlorination of acetylene. Chem. Eng. J. 2019, 360, 38–46. [Google Scholar] [CrossRef]
- Li, P.; Ding, M.; He, L.; Tie, K.; Ma, H.; Pan, X.; Bao, X. The activity and stability of PdCl2/C-N catalyst for acetylene hydrochlorination. Sci. Chi. Chem. 2018, 61, 444–448. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J. Enhanced stability of hydrochlorination of acetylene using polyaniline-modified Pd/HY catalysts. Catal. Commun. 2016, 74, 55–59. [Google Scholar] [CrossRef]
- Panova, S.A.; Shestakov, G.K.; Temkin, O.N. Supported liquid-phase rhodium catalyst for acetylene hydrochlorination. J. Chem. Soc. Chem. Commun. 1994, 7, 977. [Google Scholar] [CrossRef]
- Mitchenko, S.A.; Khomutov, E.V.; Shubin, A.A.; Shul’ga, Y.M. Catalytic hydrochlorination of acetylene by gaseous HCl on the surface of mechanically pre-activated K2PtCl6 salt. J. Mol. Catal. A: Chem. 2004, 212, 345–352. [Google Scholar] [CrossRef]
- Sil’chenko, L.A.; Panova, S.A.; Shestakov, G.K.; Temkin, O.N. Acetylene hydrochlorination in Pt(II) complex solutions: II. The kinetics of acetylene hydrochlorination in Pt(II) complex solutions. Kinet. Catal. 1998, 39, 24–28. [Google Scholar]
- Mitchenko, S.A.; Khomutov, E.V.; Shubin, A.A.; Shul’ga, Y.M. Mechanochemical Activation of K2PtCl6: Heterogeneous Catalyst for Gas-Phase Hydrochlorination of Acetylene. Theor. Exp. Chem. 2003, 39, 255–258. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.; Yue, Y.; Di, S.; Zhai, Y.; He, H.; Sheng, G.; Lai, H.; Zhu, Y.; Guo, L. Towards a greener approach for the preparation of highly active gold/carbon catalyst for the hydrochlorination of ethyne. J. Catal. 2018, 365, 153–162. [Google Scholar] [CrossRef]
- Zhou, K.; Si, J.; Jia, J.; Huang, J.; Zhou, J.; Luo, G.; Wei, F. Reactivity enhancement of N-CNTs in green catalysis of C2H2 hydrochlorination by a Cu catalyst. RSC Adv. 2014, 4, 7766–7769. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, M.; Dai, B. The Preparation of Cu-g-C3N4/AC Catalyst for Acetylene Hydrochlorination. Catalysts 2016, 6, 193. [Google Scholar] [CrossRef]
- Wang, B.; Lai, H.; Yue, Y.; Sheng, G.; Deng, Y.; He, H.; Guo, L.; Zhao, J.; Li, X. Zeolite Supported Ionic Liquid Catalysts for the Hydrochlorination of Acetylene. Catalysts 2018, 8, 351. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhao, J.; Di, X.; Di, S.; Wang, B.; Yue, Y.; Sheng, G.; Lai, H.; Guo, L.; Wang, H. Carbon supported perovskite-like CsCuCl3 nanoparticles A highly active and cost-effective heterogeneous catalyst in the hydrochlorination of acetylene to vinyl chloride. Catal. Sci. Technol. 2018, 8, 2901–2908. [Google Scholar] [CrossRef]
- Li, X.; Pan, X.; Yu, L.; Ren, P.; Wu, X.; Sun, L.; Jiao, F.; Bao, X. Silicon carbide-derived carbon nanocomposite as a substitute for mercury in the catalytic hydrochlorination of acetylene. Nat. Commun. 2014, 5, 3688–3694. [Google Scholar] [CrossRef]
- Zhou, K.; Li, B.; Zhang, Q.; Huang, J.Q.; Tian, G.L.; Jia, J.C.; Zhao, M.Q.; Luo, G.H.; Su, D.S.; Wei, F. The Catalytic Pathways of Hydrohalogenation over Metal-Free Nitrogen-Doped Carbon Nanotubes. ChemSusChem. 2014, 7, 723–728. [Google Scholar] [CrossRef]
- Li, P.; Li, H.; Pan, X.; Tie, K.; Cui, T.; Ding, M.; Bao, X. Catalytically Active Boron Nitride in Acetylene Hydrochlorination. ACS Catal. 2017, 7, 8572–8577. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Pan, X.; Ma, H.; Bao, X. Deactivation mechanism and regeneration of carbon nanocomposite catalyst for acetylene hydrochlorination. Appl. Catal. B Environ. 2017, 210, 116–120. [Google Scholar] [CrossRef]
- Dong, X.; Chao, S.; Wan, F.; Guan, Q.; Wang, G.; Li, W. Sulfur and nitrogen co-doped mesoporous carbon with enhanced performance for acetylene hydrochlorination. J. Catal. 2018, 359, 161–170. [Google Scholar] [CrossRef]
- Lan, G.; Wang, Y.; Qiu, Y.; Wang, X.; Liang, J.; Han, W.; Tang, H.; Liu, H.; Liu, J.; Li, Y. Wheat flour-derived N-doped mesoporous carbon extrudate as superior metal-free catalysts for acetylene hydrochlorination. Chem. Commun. 2018, 54, 623–626. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.; Yue, Y.; Sheng, G.; Lai, H.; Wang, S.; Yu, L.; Zhang, Q.; Feng, F.; Hu, Z.; Li, X. Nitrogen- and phosphorus-codoped carbon-based catalyst for acetylene hydrochlorination. J. Catal. 2019, 373, 240–249. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Yue, Y.; Sheng, G.; Lai, H.; Rui, J.; He, H.; Hu, Z.; Feng, F.; Zhang, Q. Carbon with Surface-Enriched Nitrogen and Sulfur Supported Au Catalysts for Acetylene Hydrochlorination. ChemCatChem 2019, 11, 1002–1009. [Google Scholar] [CrossRef]
- Wittanadecha, W.; Laosiripojana, N.; Ketcong, A.; Ningnuek, N.; Praserthdam, P.; Monnier, J.R.; Assabumrungrat, S. Preparation of Au/C catalysts using microwave-assisted and ultrasonic-assisted methods for acetylene hydrochlorination. Appl. Catal. A Gen. 2014, 475, 292–296. [Google Scholar] [CrossRef]
- Zhou, K.; Jia, J.; Li, C.; Xu, H.; Zhou, J.; Luo, G.; Wei, F. A low content Au-based catalyst for hydrochlorination of C2H2 and its industrial scale-up for future PVC processes. Green Chem. 2015, 17, 356–364. [Google Scholar] [CrossRef]
- Tian, X.; Hong, G.; Jiang, B.; Lu, F.; Liao, Z.; Wang, J.; Yang, Y. Efficient Au0/C catalyst synthesized by a new method for acetylene hydrochlorination. RSC Adv. 2015, 5, 46366–46371. [Google Scholar] [CrossRef]
- Zhao, J.; Gu, S.; Xu, X.; Zhang, T.; Yu, Y.; Di, X.; Ni, J.; Pan, Z.; Li, X. Supported ionic-liquid-phase-stabilized Au(iii) catalyst for acetylene hydrochlorination. Catal. Sci. Technol. 2016, 6, 3263–3270. [Google Scholar] [CrossRef]
- Yin, X.; Huang, C.; Kang, L.; Zhu, M.; Dai, B. Novel AuCl3–thiourea catalyst with a low Au content and an excellent catalytic performance for acetylene hydrochlorination. Catal. Sci. Technol. 2016, 6, 4254–4259. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, W.; Zhao, Z.; Luo, G.; Miller, J.T.; Wong, M.S.; Wei, F. Synergistic Gold–Bismuth Catalysis for Non-Mercury Hydrochlorination of Acetylene to Vinyl Chloride Monomer. ACS Catal. 2014, 4, 3112–3116. [Google Scholar] [CrossRef]
- Wittanadecha, W.; Laosiripojana, N.; Ketcong, A.; Ningnuek, N.; Praserthdam, P.; Monnier, J.R.; Assabumrungrat, S. Development of Au/C catalysts by the microwave-assisted method for the selective hydrochlorination of acetylene. Reac. Kinet. Mech. Cat. 2014, 112, 189–198. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, Y.; Xu, X.; Di, S.; Wang, B.; Xu, H.; Ni, J.; Guo, L.; Pan, Z.; Li, X. Stabilizing Au(III) in supported-ionic-liquid-phase (SILP) catalyst using CuCl2 via a redox mechanism. Appl. Catal. B Environ. 2017, 206, 175–183. [Google Scholar] [CrossRef]
- Nkosi, B.; Coville, N.; Hutchings, G.J.; Adams, M.; Friedl, J.; Wagner, F. Hydrochlorination of Acetylene Using Gold Catalysts: A Study of Catalyst Deactivation. J. Catal. 1991, 128, 366–377. [Google Scholar] [CrossRef]
- Zhang, J.; He, Z.; Li, W.; Han, Y. Deactivation mechanism of AuCl3 catalyst in acetylene hydrochlorination reaction: A DFT study. RSC Adv. 2012, 2, 4814–4821. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Xu, J.; Zhang, T.; Di, X.; Ni, J.; Li, X. Enhancement of Au/AC acetylene hydrochlorination catalyst activity and stability via nitrogen-modified activated carbon support. Chem. Eng. J. 2015, 262, 1152–1160. [Google Scholar] [CrossRef]
- Lan, G.; Yang, Y.; Wang, X.; Han, W.; Tang, H.; Liu, H.; Li, Y. Direct synthesis of mesoporous nitrogen doped Ru-carbon catalysts with semi-embedded Ru nanoparticles for acetylene hydrochlorination. Microporous Mesoporous Mater. 2018, 264, 248–253. [Google Scholar] [CrossRef]
- Lin, R.; Kaiser, S.K.; Hauert, R.; Pérez-Ramírez, J. Descriptors for High-Performance Nitrogen-Doped Carbon Catalysts in Acetylene Hydrochlorination. ACS Catal. 2018, 8, 1114–1121. [Google Scholar] [CrossRef]
- Gong, W.; Zhao, F.; Kang, L. Novel nitrogen-doped Au-embedded graphene single-atom catalysts for acetylene hydrochlorination: A density functional theory study. Comput. Theor. Chem. 2018, 1130, 83–89. [Google Scholar] [CrossRef]
- Di, X.X.; Zhao, J.; Yu, Y.; Xu, X.L.; Gu, S.C.; He, H.H.; Zhang, T.T.; Li, X.N. One-pot synthesis of nitrogen and sulfur co-doped activated carbon supported AuCl3 as efficient catalysts for acetylene hydrochlorination. Chin. Chem. Lett. 2016, 27, 1567–1571. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, R.; Zhou, Q.; Wang, H.; Gao, X.; Zhang, J. Boron-modified activated carbon supporting low-content Au-based catalysts for acetylene hydrochlorination. J. Catal. 2017, 348, 223–232. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Xu, J.; Ni, J.; Zhang, T.; Xu, X.; Li, X. Activated-Carbon-Supported Gold-Cesium(I) as Highly Effective Catalysts for Hydrochlorination of Acetylene to Vinyl Chloride. Chem. Plus Chem. 2015, 80, 196–201. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, T.; Di, X.; Xu, J.; Xu, J.; Feng, F.; Ni, J.; Li, X. Nitrogen-modified activated carbon supported bimetallic gold–cesium(i) as highly active and stable catalyst for the hydrochlorination of acetylene. RSC Adv. 2015, 5, 6925–6931. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, B.; Wang, X.; Li, W.; Han, Y.; Gu, J.; Zhang, J. Non-mercury catalytic acetylene hydrochlorination over bimetallic Au–Co(iii)/SAC catalysts for vinyl chloride monomer production. Green Chem. 2013, 15, 829–836. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, B.; Wang, X.; Xu, L.; Zhu, M. Hydrochlorination of acetylene to vinyl chloride monomer over bimetallic Au–La/SAC catalysts. J. Ind. Eng. Chem. 2012, 18, 49–54. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, J.; Wang, X.; Zhang, H.; Yu, L.; Dong, Y.; Li, W. Bimetallic Au–Ni/CSs catalysts for acetylene hydrochlorination. Catal. Sci. Technol. 2014, 4, 4426–4432. [Google Scholar] [CrossRef]
- Li, G.; Li, W.; Zhang, J. Non-mercury catalytic acetylene hydrochlorination over activated carbon-supported Au catalysts promoted by CeO2. Catal. Sci. Technol. 2016, 6, 1821–1828. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, T.; Di, X.; Xu, J.; Gu, S.; Zhang, Q.; Ni, J.; Li, X. Activated carbon supported ternary gold–cesium(i)–indium(iii) catalyst for the hydrochlorination of acetylene. Catal. Sci. Technol. 2015, 5, 4973–4984. [Google Scholar] [CrossRef]
- Zhao, J.; Gu, S.; Xu, X.; Zhang, T.; Di, X.; Pan, Z.; Li, X. Promotional effect of copper(ii) on an activated carbon supported low content bimetallic gold–cesium(i) catalyst in acetylene hydrochlorination. RSC Adv. 2015, 5, 101427–101436. [Google Scholar] [CrossRef]
- Wang, S.; Shen, B.; Song, Q. Kinetics of Acetylene Hydrochlorination over Bimetallic Au–Cu/C Catalyst. Catal. Lett. 2010, 134, 102–109. [Google Scholar] [CrossRef]
- Wang, L.; Shen, B.; Zhao, J.; Bi, X. Trimetallic Au-Cu-K/AC for acetylene hydrochlorination. Can. J. Chem. Eng. 2017, 95, 1069–1075. [Google Scholar] [CrossRef]
- Ma, J.; Wang, S.; Shen, B. Study on the effects of acetylene on an Au–Cu/C catalyst for acetylene hydrochlorination using Monte Carlo and DFT methods. Reac. Kinet. Mech. Cat. 2013, 110, 177–186. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, B.; Li, W.; Wang, X.; Zhang, J.; Zhu, M.; Gu, J. Non-mercury catalytic acetylene hydrochlorination over spherical activated-carbon-supported Au–Co(III)–Cu(II) catalysts. J. Catal. 2014, 316, 141–148. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, J.; Cheng, X.; Wang, L.; Yang, H.; Shen, B. An Au–Cu bimetal catalyst for acetylene hydrochlorination with renewable γ-Al2O3 as the support. RSC Adv. 2015, 5, 16727–16734. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, K.; Si, J.; Li, C.; Luo, G. A ligand coordination approach for high reaction stability of an Au–Cu bimetallic carbon-based catalyst in the acetylene hydrochlorination process. Catal. Sci. Technol. 2016, 6, 1357–1366. [Google Scholar] [CrossRef]
- Du, Y.; Hu, R.; Jia, Y.; Zhou, Q.; Meng, W.; Yang, J. CuCl2 promoted low-gold-content Au/C catalyst for acetylene hydrochlorination prepared by ultrasonic-assisted impregnation. J. Ind. Eng. Chem. 2016, 37, 32–41. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.; Xu, X.; Yu, Y.; Di, S.; Xu, H.; Zhai, Y.; He, H.; Guo, L.; Pan, Z. Alternative solvent to aqua regia to activate Au/AC catalysts for the hydrochlorination of acetylene. J. Catal. 2017, 350, 149–158. [Google Scholar] [CrossRef]
- Johnston, P.; Carthey, N.; Hutchings, G.J. Discovery, Development, and Commercialization of Gold Catalysts for Acetylene Hydrochlorination. J. Am. Chem. Soc. 2015, 137, 14548–14557. [Google Scholar] [CrossRef]
- Hong, G.; Tian, X.; Jiang, B.; Liao, Z.; Wang, J.; Yang, Y.; Zheng, J. Improvement of performance of a Au–Cu/AC catalyst using thiol for acetylene hydrochlorination reaction. RSC Adv. 2016, 6, 3806–3814. [Google Scholar] [CrossRef]
- Horikawa, T.; Sakao, N.; Sekida, T.; Hayashi, J.i.; Do, D.D.; Katoh, M. Preparation of nitrogen-doped porous carbon by ammonia gas treatment and the effects of N-doping on water adsorption. Carbon 2012, 50, 1833–1842. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, R.; Sun, X.; Désilets, S.; Abou-Rachid, H.; Jaidann, M.; Lussier, L.S. Structural and morphological control of aligned nitrogen-doped carbon nanotubes. Carbon 2010, 48, 1498–1507. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-Doped Graphene as an Efficient Metal-free Cathode Catalyst for Oxygen Reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef]
- Pensa, E.; Cortes, E.; Corthey, G.; Carro, P.; Vericat, C.; Fonticelli, M.H.; Benitez, G.; Rubert, A.A.; Salvarezza, R.C. The Chemistry of the Sulfur–Gold Interface: In Search of a Unified Model. Acc. Chem. Res. 2012, 45, 1183–1192. [Google Scholar] [CrossRef]
- Li, G.; Li, W.; Zhang, J.; Zhang, W.; Zhou, H.; Si, C.L. The Effect of N-Doping in Activated Carbon-Supported Au-Sr Catalysts for Acetylene Hydrochlorination to Vinyl Chloride. ChemistrySelect 2018, 3, 3561–3569. [Google Scholar] [CrossRef]


| Catalysts | Surface Elemental Composition (wt%) | ||||||
|---|---|---|---|---|---|---|---|
| Au4f | C1s | Cl2p | O1s | S2p | N1s | Cu2p | |
| Fresh AuCu1/AC(OAR) | 0.22 | 89.68 | 1.95 | 4.42 | 1.35 | 1.41 | 0.97 |
| Fresh AuCu1/AC(AR) | 0.21 | 91.53 | 2.28 | 4.75 | 0.00 | 0.31 | 0.92 |
| Used AuCu1/AC(OAR) | 0.19 | 90.07 | 1.89 | 4.33 | 1.25 | 1.32 | 0.95 |
| Used AuCu1/AC(AR) | 0.20 | 91.33 | 2.54 | 4.65 | 0.00 | 0.36 | 0.92 |
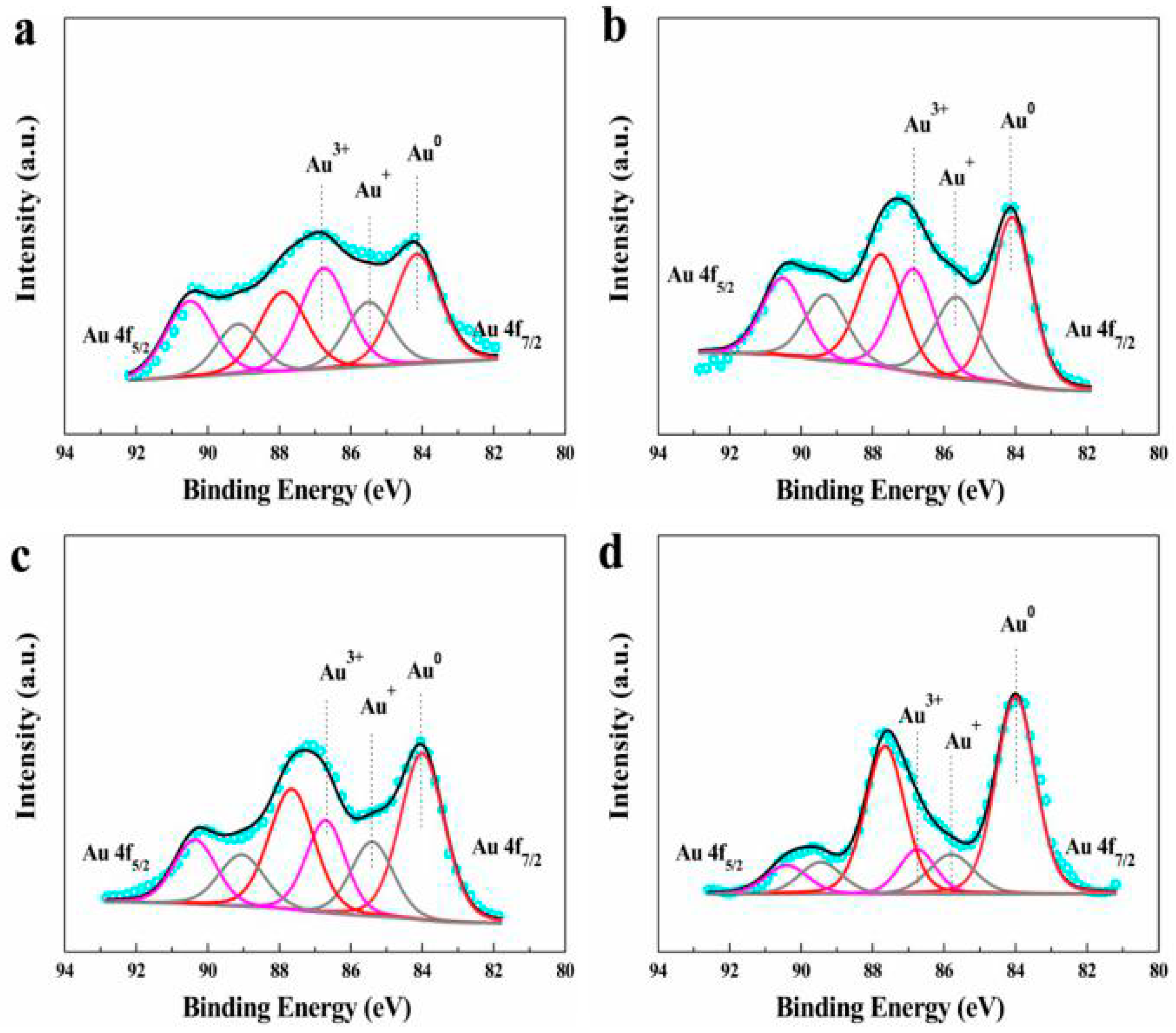
| Catalysts | Au Species (%) | Binding Energies (eV) | ||||
|---|---|---|---|---|---|---|
| Au3+ | Au+ | Au0 | Au3+ | Au+ | Au0 | |
| Fresh AuCu1/AC(OAR) | 26.8 | 36.6 | 36.6 | 86.7 | 85.4 | 84.1 |
| Fresh AuCu1/AC(AR) | 29.8 | 25.4 | 44.8 | 87.0 | 85.5 | 84.0 |
| Used AuCu1/AC(OAR) | 25.8 | 22.4 | 51.8 | 86.8 | 85.6 | 84.1 |
| Used AuCu1/AC(AR) | 14.5 | 16.1 | 69.4 | 86.8 | 85.8 | 84.0 |
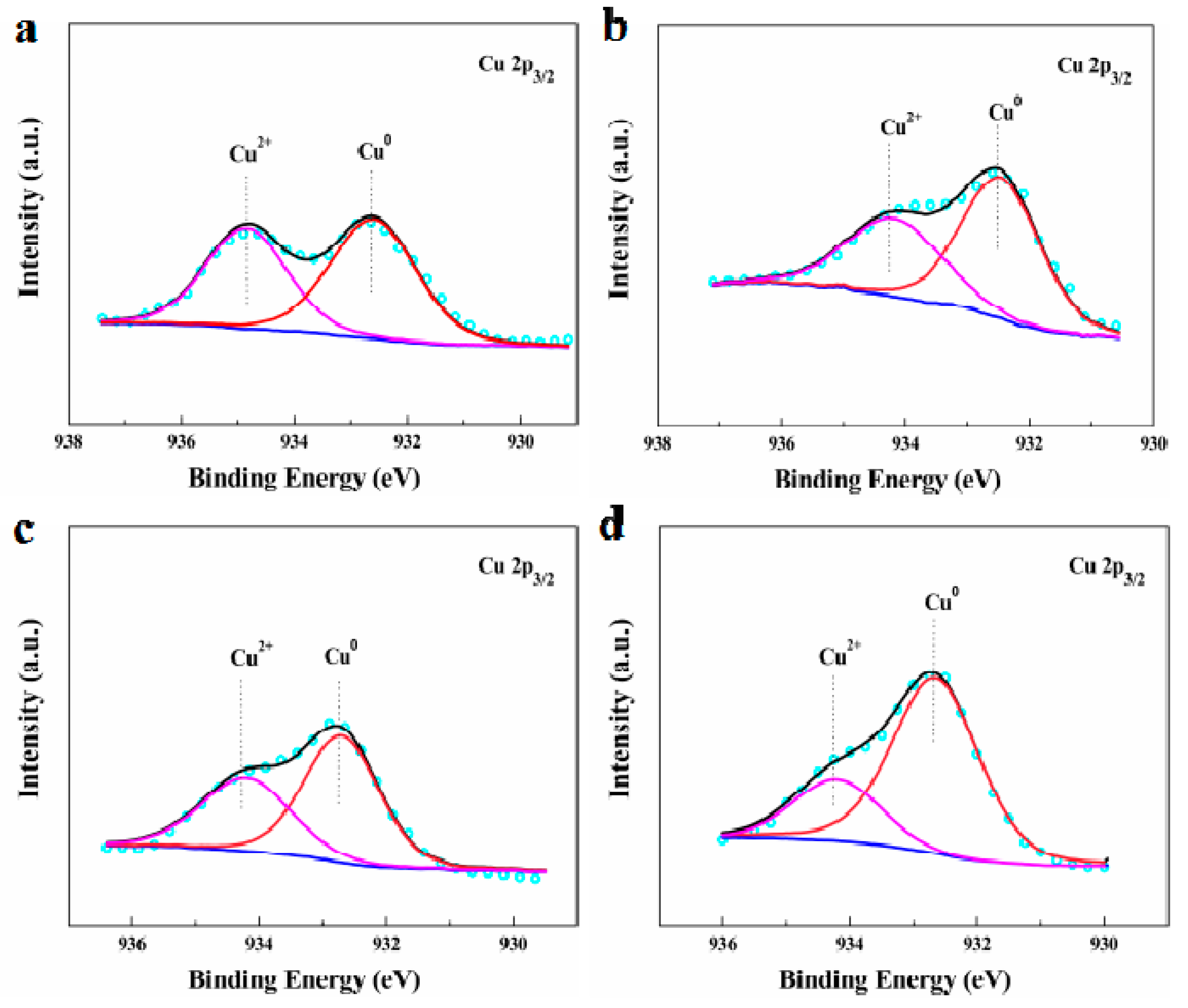
| Catalysts | Cu Species (%) | Binding Energies (eV) | ||
|---|---|---|---|---|
| Cu2+ | Cu0 | Cu2+ | Cu0 | |
| Fresh AuCu1/AC(OAR) | 44.4 | 55.6 | 934.9 | 932.8 |
| Fresh AuCu1/AC(AR) | 40.5 | 59.5 | 934.4 | 932.6 |
| Used AuCu1/AC(OAR) | 39.8 | 60.2 | 934.4 | 932.8 |
| Used AuCu1/AC(AR) | 24.0 | 76.0 | 934.2 | 932.6 |
| Catalysts | SBET (m2 g−1) | ∆SBET (m2 g−1) | |
|---|---|---|---|
| Fresh | Used | ||
| AC | 1162.1 | / | / |
| AuCu1/AC(AR) | 1005.3 | 839.1 | 166.2 |
| AuCu1/AC(OAR) | 1067.6 | 986.6 | 81.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Zhao, J.; Wang, B.; Yue, Y.; Sheng, G.; Wang, Q.; Yu, L.; Hu, Z.-T.; Li, X. Highly Active AuCu-Based Catalysts for Acetylene Hydrochlorination Prepared Using Organic Aqua Regia. Materials 2019, 12, 1310. https://doi.org/10.3390/ma12081310
He H, Zhao J, Wang B, Yue Y, Sheng G, Wang Q, Yu L, Hu Z-T, Li X. Highly Active AuCu-Based Catalysts for Acetylene Hydrochlorination Prepared Using Organic Aqua Regia. Materials. 2019; 12(8):1310. https://doi.org/10.3390/ma12081310
Chicago/Turabian StyleHe, Haihua, Jia Zhao, Bolin Wang, Yuxue Yue, Gangfeng Sheng, Qingtao Wang, Lu Yu, Zhong-Ting Hu, and Xiaonian Li. 2019. "Highly Active AuCu-Based Catalysts for Acetylene Hydrochlorination Prepared Using Organic Aqua Regia" Materials 12, no. 8: 1310. https://doi.org/10.3390/ma12081310
APA StyleHe, H., Zhao, J., Wang, B., Yue, Y., Sheng, G., Wang, Q., Yu, L., Hu, Z.-T., & Li, X. (2019). Highly Active AuCu-Based Catalysts for Acetylene Hydrochlorination Prepared Using Organic Aqua Regia. Materials, 12(8), 1310. https://doi.org/10.3390/ma12081310