Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications
Abstract
:1. Introduction
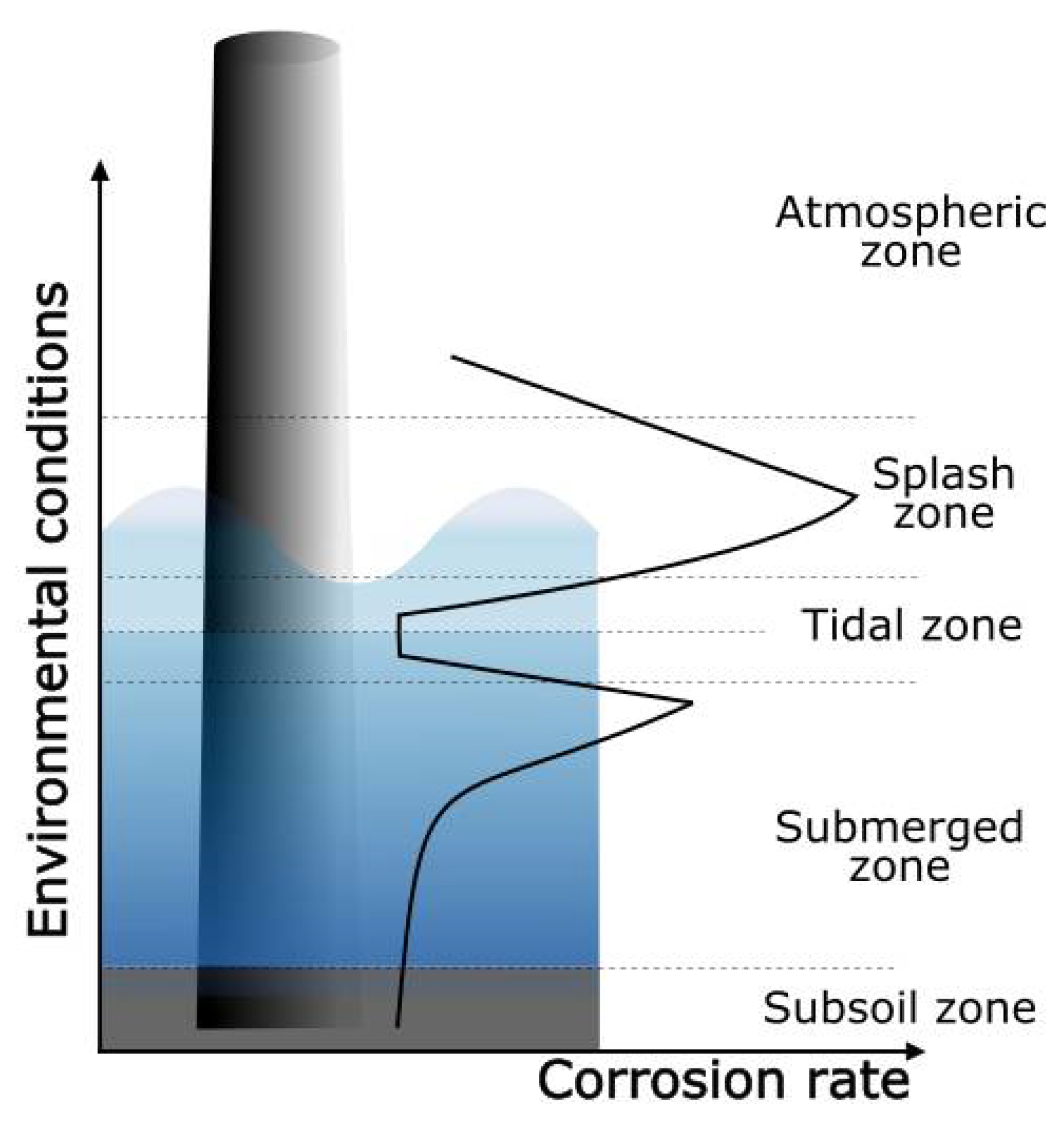
- Atmospheric zone: This zone is located above the sea level, and the severity of corrosion is related to the time of wetness, during which electrochemical processes take place. There is a direct relationship between atmospheric salt content and corrosion rate. Materials are also exposed to solar radiation, which deteriorates the performance of organic coatings.
- Splash zone: This section in the structure is intermittently wetted, due to tides and the wind. The corrosion rate of metals in this zone is the highest, due to the aerated condition, which makes the access of dissolved oxygen for electrochemical reactions easy. Since it is continuously being wetted, chlorides can concentrate on the surface while the water films dry. Furthermore, the impinging of seawater containing sand and other flowing matter adds a mechanical component to the materials’ deterioration in this exposure zone.
- Tidal zone: The materials are alternately submerged and exposed to the splash zone, as the tide fluctuates. In the submerged condition, materials are exposed to a well-aerated seawater, which favors the attachment and growth of biofouling. The corrosion rate is influenced by the tidal flow, with higher corrosion rates with increasing movements.
- Submerged zone: The section of the structure that is always immersed in the sea. The corrosion rate in this zone depends on the availability of oxygen to be transported to the cathodic sites of the materials’ surfaces. As oxygen concentration varies with depth, decreasing with increasing distance to the surface, the corrosion rate is also slower at higher depths.
- Subsoil: In the buried structure, the oxygen concentration is low, and hydrogen sulfide may be present.
- Low (L): 2 to 5 years
- Medium (M): 5 to 15 years
- High (H): 15 to 25 years
- Very High (VH): >25 years
2. Experimental Procedure
2.1. Materials and Sample Preparation
- Thermally sprayed carbide (WC–CrCo) with an organic sealant (C1)
- Arc thermally sprayed aluminum (TSA) with an organic topcoat (C2)
- Epoxy-based organic coating reinforced with ceramic particles (C3)
2.2. Corrosion Aging Tests
2.2.1. Immersion Tests (ISO 2812-2)
2.2.2. Water Condensation Tests (ISO 6270)
- Condensation atmosphere with constant humidity (CH)
- Condensation atmosphere with alternating humidity and air temperature (AHT)
- Condensation atmosphere with alternating air temperature (AT)
2.2.3. Salt Spray Tests (ISO 9227)
2.2.4. Combined Aging Test (NORSOK M-501, ISO 12944-9)
3. Results
3.1. Immersion Tests (ISO 2812-2)
- C3-sample No.1: 4 (S4)
- C3-sample No.2: 2 (S4) (removed from the chamber after 504 h of immersion)
- C3-sample No.2: 4 (S5)
3.2. Water Condensation Tests (ISO 6270)
- C3-sample No.1: 2 (S3)
- C3-sample No.2: 2 (S4)
- C3-sample No.2: 2 (S4)
3.3. Salt Spray Tests (ISO 9227)
3.4. Combined Aging Test (NORSOK M-501, ISO 12944-9)
4. Discussion
5. Conclusions
- From the immersion tests (ISO 2812-2), the only coating that did not reach 4000 h of immersion was the C3 coating. Blisters were detected to appear after 504 h in one of the samples, and after 672 h in the other two samples. The C1 and C2 coatings were classified as IM2 (high), overcoming the 4000 h of exposure without the appearance of any defect.
- Regarding the water condensation tests (ISO 6270), the C1 and C2 coatings obtained the C5-M (high) category in the three water atmospheres. However, C3 was classified as C5-M (high) in the AHT and AT atmospheres, and as C4 (high)/C5-M (medium) for the CH test due to the apparition of blisters after 614 h of exposure.
- In the case of the salt spray tests (ISO 9227), C2 was the only coating reaching the C5-M (high) category. The C1 and C3 coatings were classified as C4 (medium)/C5-M (low), after the detection of ferric corrosion (480 h) in the C1 coating and blisters (972 h) in the C3 coating. The adherence that was measured for the C2 and C3 coatings in the samples with incision was 4A/5A, according to the ASTM D3359 standard. The adherence of the C1 coating was not measured, since the incision could not be made in the hard carbide layer.
- With respect to the combined aging tests (NORSOK M-501, ISO 12944), the C1 coating did not pass the test, since the surface of the samples was covered by a considerable amount of ferric corrosion at the end of the last test cycle. On the other hand, the C2 and C3 coatings passed the test, showing just some discoloration and loss of brightness, maintaining the protective properties.
- Finally, the only coating that reached the higher category and overtook all the test atmospheres was the C2 coating. The combination of both the aluminum layer and the organic topcoat providing a two-layered protection by means of a sacrificial aluminum anode and the high corrosion resistance of the organic layer.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhanak, M.R.; Xiros, N.I. Springer Handbook of Ocean Engineering; Springer: Berlin, Germany, 2016; ISBN 978-319-16648-3. [Google Scholar]
- Revie, R.W. Corrosion and Corrosion Control—An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-471-73279-2. [Google Scholar]
- Baboian, R. ASTM Corrosion Tests and Standards. Application and Interpretation, 2nd ed.; ASTM International: West Conshohocken, PA, USA, 2006; ISBN 0-8031-2098-2. [Google Scholar]
- ASM Handbook. Volume 13. Corrosion; ASM International: Russell Township, OH, USA, 1987; ISBN 0-87170-007-7. [Google Scholar]
- Melchers, R.E.; Moan, T.; Gao, Z. Corrosion of working chains continuously immersed in seawater. J. Mar. Sci. Technol. 2007, 12, 102–110. [Google Scholar] [CrossRef]
- Momber, A.; Plagemann, P.; Stenzel, V. Performance and integrity of protective coating systems for offshore wind power structures after three years under offshore site conditions. Renew. Energy 2015, 74, 606–617. [Google Scholar] [CrossRef]
- López, A.; Bayon, R.; Pagano, F.; Igartua, A.; Arredondo, A.; Arana, J.; González, J. Tribocorrosion behaviour of mooring high strength low alloy steels in synthetic seawater. Wear 2015, 338, 1–10. [Google Scholar] [CrossRef]
- López-Ortega, A.; Bayón, R.; Arana, J.L.; Arredondo, A.; Igartua, A. Influence of temperature on the corrosion and tribocorrosion behavior of high-strength low-alloy steels used in offshore applications. Tribol. Int. 2018, 121, 341–352. [Google Scholar] [CrossRef]
- Santos, D.; Brites, C.; Costa, M.; Santos, M. Performance of paint systems with polyurethane topcoats, proposed for atmospheres with very high corrosivity category. Prog. Org. Coat. 2005, 54, 344–352. [Google Scholar] [CrossRef]
- Kutz, M. Handbook of Environmental Degradation of Materials, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-1-4377-3455-3. [Google Scholar]
- Baboian, R. NACE Corrosion Engineer’s Reference Book, 3rd ed.; NACE International: Houston, TX, USA, 2002; ISBN 978-1-57590-127-5. [Google Scholar]
- Iannuzzi, M.; Barnoush, A.; Johnsen, R. Materials and corrosion trends in offshore and subsea oil and gas production. npj Mater. Degrad. 2017, 1, 2. [Google Scholar] [CrossRef]
- Weng, Y.; Dong, H.; Gan, Y. Advanced Steels. The Recent Scenario in Steel Science and Technology; Springer: Berlin, Germany, 2011; ISBN 978-3-642-17664-7. [Google Scholar]
- Ault, J.P. The use of coatings for corrosion control on offshore oil structures. J. Prot. Coat. Linings 2006, 23, 42–47. [Google Scholar]
- ISO 12944:1-8. Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- NORSOK M-501. Surface Preparation and Protective Coating, 6th ed.; Standard No.: Lysaker, Norway, 2012. [Google Scholar]
- ISO 9223. Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation, 2nd ed.; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- ISO 12944-9. Paints and Varnishes. Corrosion Protection of Steel Structures by Protective Paint Systems. Part 9: Protective Paint Systems and Laboratory Performance Test Methods for Offshore and Related Structures, 1st ed.; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Mühlberg, K. Corrosion protection of offshore wind turbines—A challenge for the steel builder and paint applicator. J. Prot. Coat. Linings 2010, 20, 29. [Google Scholar]
- López-Ortega, A.; Arana, J.L.; Bayón, R. Evaluation of protective coatings for offshore applications. Corrosion and tribocorrosion behaviour in synthetic seawater. Surf. Coat. Technol. 2018, 349, 1083–1097. [Google Scholar]
- López-Ortega, A.; Arana, J.L.; Rodríguez, E.; Bayón, R. Corrosion, wear and tribocorrosion performance of a thermally sprayed aluminum coating modified by plasma electrolytic oxidation technique for offshore submerged components protection. Corros. Sci. 2018, 143, 258–280. [Google Scholar] [CrossRef]
- Offshore Standard DNVGL-OS E302. Offshore Mooring Chains; DNV GL: Bærum, Norway, 2018. [Google Scholar]
- ISO 2812-2. Paints and Varnishes—Determination of Resistance to Liquids, 2nd ed.; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- ISO 6270. Paints and Varnishes—Determination of Resistance to Humidity, 2nd ed.; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- ISO 9227. Corrosion Tests in Artificial Atmospheres—Salt Spray Test, 4th ed.; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- ISO 16474-3. Paints and varnishes. Methods of Exposure to Laboratory Light Sources—Fluorescence UV Lamps; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- ISO 4628 1-5. Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Density of Uniform Changes in Appearance, 4th ed.; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- ISO 17872. Paints and Varnishes—Guidelines for the Introduction of Scribe Marks through Coatings on Metallic Panels for Corrosion Testing, 1st ed.; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- ASTM D3359-17. Standard Test Methods for Rating Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Lee, H.S.; Singh, J.; Ismail, M.; Bhattacharya, C. Corrosion resistance properties of aluminum coating applied by arc thermal metal spray in SAE J2334 solution with exposure periods. Metals 2016, 6, 55. [Google Scholar] [CrossRef]
- Deshpande, S.; Kulkarni, A.; Sampath, S.; Herman, H. Application of image analysis for characterization of porosity in thermal spray coatings and correlation with small angle neutron scattering. Surf. Coat. Technol. 2004, 187, 6–16. [Google Scholar] [CrossRef]
- Fauchais, P.; Vardelle, A. Thermal sprayed coatings used against corrosion and corrosive wear. In Advanced Plasma Spray Applications; InTech: London, UK, 2012; ISBN 978-953-51-0349-3. [Google Scholar]
- Sun, X.; Huang, D.; Wu, G. The current state of offshore wind energy technology development. Energy 2012, 41, 298–312. [Google Scholar] [CrossRef]
- Alam, M.A.; Sherif, E.S.M.; Al-Zahrani, S.M. Fabrication of various epoxy coatings for offshore applications and evaluating their mechanical properties and corrosion behavior. Int. J. Electrochem. Sci. 2013, 8, 3121–3131. [Google Scholar]











| Exposure Zone | Category (ISO 12944) | Coating System | Desirable Coating Properties | |
|---|---|---|---|---|
| Atmospheric | C5-M | Zinc-rich epoxy primer | (60–100 µm) | Corrosion-resistant, erosion-resistant, anti-icing, UV-resistant |
| Epoxy intermediate layer | (100–120 µm) | |||
| Polyurethane top coat | (50–80 µm) | |||
| Splash and tidal | C5-M and Im2 | Two or three epoxy-based coats | (>1000 µm in total) | Combination of atmospheric and submerged coatings´ properties |
| Polyurethane top-coat | (50–80 µm) | |||
| Submerged | Im2 | Two or three epoxy-based coats | (>450 µm in total) | Corrosion-resistant, antifouling, wear-resistant |
| Coating | Thickness (µm) | Porosity (%) | |
|---|---|---|---|
| Organic Sealant | Sprayed Metal | ||
| C1 | 9 ± 3 | 191 ± 14 | 1.9 ± 0.4 |
| C2 | 239 ± 8 | 284 ± 14 | 4.7 ± 0.3 |
| C3 | 430 ± 15 * | - | |
| Category | Durability Ranges | ISO 2812-2 [23] (Immersion in Water) | ISO 6270-2 [24] (Water Condensation) | ISO 9227 [25] (Salt Spray Test) |
|---|---|---|---|---|
| C5-M | Low | - | 240 h | 480 h |
| Medium | - | 480 h | 720 h | |
| High | - | 720 h | 1440 h | |
| Im2 | Low | - | - | - |
| Medium | 2000 h | - | 720 h | |
| High | 3000 h | - | 1440 h |
| Standard | Test Type | Cycle Duration | Conditions in the Cabinet | Total Duration of the Test | ||||
|---|---|---|---|---|---|---|---|---|
| Test Periods | Total | Temperature | Relative Humidity | |||||
| ISO 2812 [23] | Immersion test | - | (40 ± 3) °C | - | 3000 h | |||
| ISO 6270 [24] | Constant humidity condensation atmosphere (CH) | From warm-up to end of exposure | (40 ± 3) °C | 100% | 720 h | |||
| Alternating condensation atmosphere | Alternating humidity and air temperature (AHT) | 8 h including warm-up | 24 h | (40 ± 3) °C | 100% | 720 h | ||
| 16 h including warm-up | 18–28 °C | Ambient | ||||||
| Alternating air temperature (AT) | 8 h including warm-up | 24 h | (40 ± 3) °C | 100% | 720 h | |||
| 16 h including warm-up | 18–28 °C | 100% | ||||||
| ISO 9227 [25] | Salt spray | - | (35 ± 3) °C | - | 1440 h | |||
| NORSOK M-501 [16] ISO 12944-9 [18] | Ultraviolet radiation and condensation (ISO 16474-3 [26]) | 4 h UV radiation (0.77 W/m2) | 72h | 168 h | (60 ± 3) °C | - | 720 h | |
| 4 h condensation | (50 ± 3) °C | 100% | ||||||
| Salt spray (ISO 9227 [25]) | 72 h | (35 ± 3) °C | - | |||||
| Low temperature | 24 h | (−20 ± 2) °C | - | |||||
| Coating | Oxidation Grade | Blistering Grade | ||||||
|---|---|---|---|---|---|---|---|---|
| CH | AHT | AH | CH | AHT | AH | |||
| C1 | Sample 1 | 3% (*E) | 2% (E) | 3% (E) | 0% | 0% | 0% | |
| Sample 2 | 3% (E) | 2% (E) + 1% (**C) | 2% (E) | 0% | 0% | 0% | ||
| Sample 3 | 3% (E) | 1% (E) | - | 0% | 0% | - | ||
| C2 | Sample 1 | 1% (***WS) | 0% | 0% | 0% | 0% | 0% | |
| Sample 2 | 1% (WS) | 0% | 1% (WS) | 0% | 0% | 0% | ||
| Sample 3 | 1% (WS) | 0% | - | 0% | 0% | - | ||
| C3 | Sample 1 | 0% | 0% | 0% | 20% | 0% | 0% | |
| Sample 2 | 0% | 0% | 0% | 30% | 0% | 0% | ||
| Sample 3 | 0% | 0% | - | 30% | 0% | - | ||
| Coating | Incision | Sample No. | Exposure Time (h) | Adhesion Test (ASTM D3359) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 504 | 672 | 840 | 1008 | 1178 | 1370 | 1440 | ||||
| C1 | No | 1 | ~15% FC (*) | 25% FC | 25% FC | 25% FC | 25% FC | 25% FC | 25% FC | - |
| No | 2 | ~5% FC | 5% FC | 5% FC | 5% FC | 5–10% FC | 5–10% FC | 5–10% FC | - | |
| No | 3 | 15-20% FC | 25% FC | 25% FC | 25% FC | 25–30% FC | ~30% FC | ~30% FC | - | |
| No | 4 | 15% FC | 20% FC | ~20% FC | ~20% FC | ~30% FC | ~30% FC | ~30% FC | - | |
| No | 5 | Isolated pits | <5% FC | <5% FC | <5% FC | <5% FC | <5% FC | <5% FC | - | |
| No | 6 | 10–15% FC | 15–20% FC | 20–25% FC | 20–25% FC | 20–25% FC | 20–25% FC | 20–25% FC | - | |
| C2 | No | 1 | Unaltered | Ws (*) + P (*) | Ws + P | Ws + P | <5% Ws | <5% Ws | 5-10% Ws | - |
| No | 2 | Unaltered | Unaltered | Unaltered | Ws + P | <5% Ws | <5% Ws | 5% Ws | - | |
| No | 3 | Unaltered | Ws + P | Ws + P | Ws + P | <5% Ws | <5% Ws | 5–10% Ws | - | |
| Yes | 4 | Ws + Np (*) | Ws + Np | Ws + Np | Ws + Np | Ws + Np | Ws + Np | Ws + Np | 5A | |
| Yes | 5 | Ws + Np | Ws + Np | Ws + Np | Ws + Np | Ws + Np | Ws + Np | Ws + Np | 4A | |
| Yes | 6 | Ws + Np | Ws + Np | Ws + Np | Ws + Np | Ws + Np | Ws + Np | Ws + Np | 5A | |
| C3 | No | 1 | Unaltered | Unaltered | 1 blister | 1 blister | 1 blister | 1 blister | 1 blister | - |
| No | 2 | Unaltered | Unaltered | 1 blister | 1 blister | 1 blister | 1 blister | 1 blister | - | |
| No | 3 | Unaltered | Unaltered | Unaltered | Unaltered | Unaltered | Unaltered | Unaltered | - | |
| Yes | 4 | 0.4-mm PFC (*) | 0.6-mm PFC | 0.6-mm PFC | 0.8-mm PFC | 0.8-mm PFC | 2-mm PFC | 2-mm PFC | 4A | |
| Yes | 5 | 0.5-mm PFC | 0.8-mm PFC | 0.8-mm PFC | 0.8-mm PFC | 0.8-mm PFC | 1.2-mm PFC | 1.2-mm PFC | 5A | |
| Yes | 6 | 0.5-mm PFC | 0.9-mm PFC | 0.9-mm PFC | 1-mm PFC | 1-mm PFC | 2.6-mm PFC + 1 blister | 2.6-mm PFC + 1 blister | 5A | |
| Coating | Oxidation Grade | Blistering Grade | |
|---|---|---|---|
| C1 | Sample 1 | 16% | 0% |
| Sample 2 | 4% | 0% | |
| Sample 3 | 12% | 0% | |
| C2 | Sample 1 | 2% (Inclusions) | 0% |
| Sample 2 | 2% (Inclusions) | 0% | |
| Sample 3 | 3% (Inclusions) | 0% | |
| C3 | Sample 1 | 0% | 0% |
| Sample 2 | 0% | 0% | |
| Sample 3 | 0% | 0% | |
| Test/Standard | Coating Category (ISO 12944-6) | |||
|---|---|---|---|---|
| C1 | C2 | C3 | ||
| Immersion (ISO 2812) | Im2 (High) | Im2 (High) | No pass (Blistering) | |
| Condensation (ISO 6270) | CH | C5-M (High) | C5-M (High) | C4 (High)/C5-M (Medium) |
| AHT | C5-M (High) | C5-M (High) | C5-M (High) | |
| AT | C5-M (High) | C5-M (High) | C5-M (High) | |
| Salt spray (ISO 9227) | C4 (Medium)/C5-M (Low) | C5-M (High) | C4 (Medium)/C5-M (Low) | |
| Combined aging (ISO 12944-9, NORSOK M-501) | No passed | Passed | Passed | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Ortega, A.; Bayón, R.; Arana, J.L. Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications. Materials 2019, 12, 1325. https://doi.org/10.3390/ma12081325
López-Ortega A, Bayón R, Arana JL. Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications. Materials. 2019; 12(8):1325. https://doi.org/10.3390/ma12081325
Chicago/Turabian StyleLópez-Ortega, Ainara, Raquel Bayón, and José Luís Arana. 2019. "Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications" Materials 12, no. 8: 1325. https://doi.org/10.3390/ma12081325
APA StyleLópez-Ortega, A., Bayón, R., & Arana, J. L. (2019). Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications. Materials, 12(8), 1325. https://doi.org/10.3390/ma12081325







